Abstract
Several trends have influenced autologous breast reconstruction in the last decade. The development of the skin-sparing mastectomy has markedly improved the aesthetic results of autologous breast reconstruction. Modifications have included purse-stringing periareolar incisions and vertical reduction pattern incisions. The increasing use of postmastectomy has had a negative impact on transverse rectus abdominis musculocutaneous (TRAM) flap reconstruction. Delayed reconstruction may be the best option when adjuvant radiation is planned. Careful anatomic studies of the blood supply to the abdominal wall and critical outcome analyses have resulted in many refinements in TRAM flap breast reconstruction. Careful patient selection is critical to avoid complications. Obesity, tobacco smoking, a history of chest wall radiation, and abdominal scars are known risk factors for wound complications. TRAM flap reconstruction should be considered a two-stage procedure regardless of nipple reconstruction. The first stage is building the foundation and framework of the breast. The second stage is essential for final adjustments to the volume, contour, and position of the breast mound.
Keywords: Skin sparing mastectomy, radiation, TRAM flap
Breast reconstruction has undergone a marked evolution since the introduction of the silicone gel implants in 1963.1 The original goals of reducing the psychosexual trauma associated with total mastectomy, freeing the patient of an external appliance, and improving the appearance in clothes have been met. As the experience with breast reconstruction has increased, so have the aesthetic demands to match the remaining breast in dimensions, position, and contour. The improvement in the quality of breast reconstructions can be attributed in part to a refinement in mastectomy technique. There is an increasing emphasis on individualizing skin incisions, which allow for preservation of native skin and the inframammary fold. Refinements in autologous tissue reconstruction, specifically the transverse rectus abdominis musculocutaneous (TRAM) flap, have markedly improved the aesthetic results of breast reconstruction. Despite these advances, implant-based reconstruction is still the preferred method by the majority of plastic surgeons. A recent survey of the American Society of Plastic Surgeons found that two thirds of all breast reconstructions reported employed the use of implants. TRAM flap reconstruction, which many feel is the “gold standard” in breast reconstruction, comprised 20% of total breast reconstructions performed. Free flap reconstruction, advocated by a handful of mostly academic surgeons in the United States, comprised only 5% of the total. This trend continues despite the controversy over the safety of silicone gel implants and the increasing use of postmastectomy radiation therapy. Surgeon training and patient preference certainly account for some of these differences but one would be naïve to think that reimbursement does not play a factor in the decision-making process. Spear and colleagues recently did a cost comparison of implant-based and TRAM flap breast reconstruction. A total of 140 patients (implant 76, TRAM flap 64) were evaluated.2 Operating room time for the complete multistage reconstructive process was nearly twice as long for TRAM flap reconstruction. The average cost was found to be significantly less for implant-based reconstruction.
Implant reconstruction generally has low morbidity and can produce good results in properly selected patients. Several operations are required and symmetry procedures are often required on the opposite breast. At least a quarter of patients will require reoperative surgery for implant-related complications like deflation, capsular contraction, and malposition. In my experience, implant reconstruction is not as durable as autologous tissue reconstruction. Clough and colleagues followed the cosmetic outcome of 334 patients who completed implant reconstruction.3 An objective 5-point global scale was used to evaluate results. They found that the overall cosmetic outcome deteriorated in a linear fashion from an initial acceptable result of 86% 2 years after patients completed reconstruction to only 54% at 5 years. They proposed that the possible reason for their poor results was late asymmetry produced by the failure of both breasts to undergo symmetrical ptosis with aging. They also reviewed the cosmetic outcome of 171 patients who completed TRAM flap reconstruction.4 In contrast to implant reconstruction, the cosmetic outcome of TRAM flap reconstruction remained stable. An acceptable result was seen in 96.4% of patients at 2 years and in 94.2% of patients at 5 years.
Several trends have influenced breast reconstruction in the last decade. The Federal Drug Administration's moratorium on the use of silicone gel implants made many patients reluctant to undergo implant reconstruction. At the same time, health care reform reduced the reimbursement for autologous breast reconstruction, making implant reconstruction more attractive to the reconstructive breast surgeon. The development of the skin-sparing mastectomy (SSM) markedly improved the aesthetic results of autologous breast reconstruction. Finally, the increasing use of postmastectomy radiotherapy has impacted all forms of immediate reconstruction.
SKIN-SPARING MASTECTOMY
The term “skin-sparing mastectomy” was introduced into the medical lexicon in 1991.5,6 Preservation of the native skin and the inframammary fold (IMF) markedly enhances the aesthetic results of immediate breast reconstruction (Fig. 1). It reduces the amount of autologous tissue required for breast reconstruction; it facilitates breast shaping and reduces symmetry procedures on the contralateral breast. There were initial concerns that this compromised the completeness of the mastectomy. Anatomical and clinical studies found that the IMF region contained minimal breast tissue and its preservation did not alter the outcome of a SSM.7 The local recurrence rate of breast cancer after SSM has been reported to be similar to more traditional forms of total mastectomy.8,9,10 Despite the increasing body of literature supporting the use of SSM in the treatment of breast cancer, there appears to be a lack of understanding among treating physicians. A recent worldwide survey of over 1000 respondents found that 78% had knowledge that SSM does not have a higher local disease recurrence than modified radical mastectomy.11 Surprisingly, 25% of these individuals did not believe the literature.
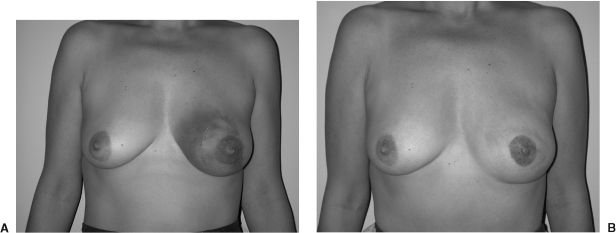
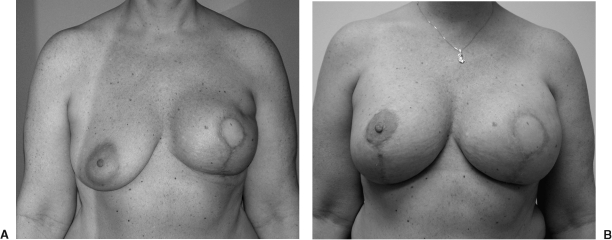
Figure 1.
(A) Preoperative view of a 42-year-old patient after a left breast biopsy revealed ductal carcinoma in situ (DCIS). (B) Postoperative view after a SSM type II and a unipedicle TRAM flap reconstruction.
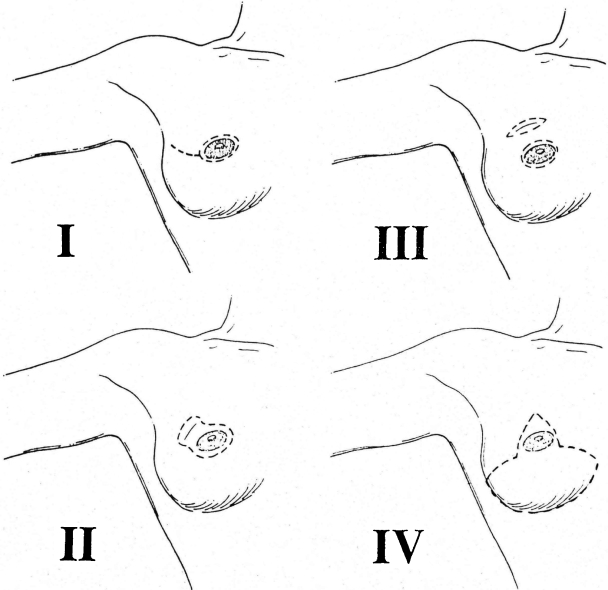
SSMs have been stratified into four types based on the type of incision used and the amount of native skin resected (Fig. 2). Type IV SSM is a Wise-pattern reduction incision when a reduction is planned on the opposite breast. The types of SSM, preoperative radiation, and tobacco smoking influence the incidence of native skin flap necrosis (Table 1). Modifications of the SSM have included purse-stringing periareolar incisions and avoiding the horizontal incision in the type IV SSM in an attempt to reduce the incidence of skin loss (Fig. 3). Small areas of native skin loss can be managed conservatively in autologous reconstruction. Larger areas of ischemic skin should be treated aggressively with prompt excision. The best solution is avoidance by resecting areas of native skin that are marginally perfused. I have not found fluoroscein injection useful and rely on my intraoperative clinical assessment of skin viability to avoid this complication.
Figure 2.
Types of skin-sparing mastectomy.
Table 1.
Risk Factors for Native Skin Flap Necrosis after SSM
| Factor | Total, n (%) | Native Skin Flap Necrosis, n (%) |
|---|---|---|
| SSM type | ||
| Type I | 232 (36.7) | 22 (9.5) |
| Type II | 293 (46.2) | 28 (13) |
| Type III | 40 (6.3) | 10 (25) |
| Type IV | 68 (10.8) | 18 (26.5) |
| Tobacco | 79 (12.5) | 16 (20.3) |
| Radiation | 21 (3.3) | 5 (23.8) |
| Overall | 633 (100) | 88 (13.9) |
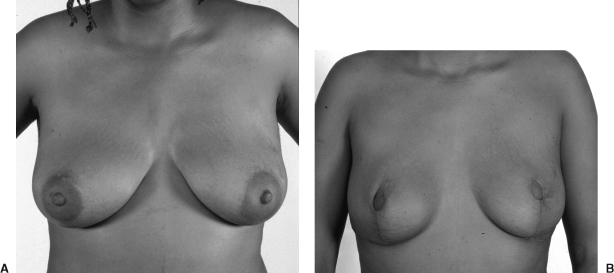
Figure 3.
(A) Preoperative view of a 34-year-old patient with a strong family history of breast cancer. (B) Postoperative view after modified SSMs type IV and TRAM flap reconstruction. The SSMS were performed via a vertical incision and purse-stringing of the areola excision.
POSTMASTECTOMY RADIATION THERAPY
Postmastectomy radiation therapy is increasingly being administered in patients with early breast cancer. It reduces the risk of locoregional recurrence of breast cancer by ∼67% but the survival benefit has been largely offset by an increase in cardiac deaths secondary to radiation. Two recent randomized trials have shown a survival benefit for postmastectomy radiotherapy in patients with one to three metastatic lymph nodes.12,13 The NIH Consensus Statement on the Adjuvant Therapy for Breast Cancer released in November 2000 concluded that women with four or more positive lymph nodes or advanced primary tumor would benefit from postmastectomy radiotherapy.14 It stated that the role of postmastectomy radiotherapy for women with one to three positive lymph nodes remains uncertain.
Chest wall irradiation after implant reconstructions results in an increase incidence of capsular contraction and implant exposure.15,16,17 The dense scarring makes tissue expansion difficult. Because of this, many authors feel that implant reconstruction is contraindicated when postmastectomy radiation is planned.
There are only a few reports in the literature on the effects of radiation therapy on TRAM flap breast reconstruction (Table 2). My colleagues at Emory University and I recently reviewed 41 patients who received radiation therapy after pedicled TRAM flap reconstruction. A control group consisted of 402 nonirradiated pedicled TRAM flap. The incidence of fat necrosis was 22.6% in the control group and 46.3% in the irradiated group (P < 0.005) (Fig. 4). Fibrosis, defined as a generalized decrease in the size of the breast mound associated with a decreased elasticity of the skin, was not noted in the control group but occurred in 32.4% of the irradiated group.
Table 2.
Effects of Radiation Therapy on TRAM Flap Reconstruction
Figure 4.
(A) Patient who underwent a unipedicle TRAM flap reconstruction. She is 6 weeks postradiation therapy. Skin excoriation is noted. (B) Three months postradiation. The flap has become fibrotic and there is a large area of fat necrosis in the superior pole.
It is intuitive that better flap vascularity would afford better resistance to the fibrosis and fat necrosis induced by radiation. A case has been made to perform free TRAM flap reconstruction to maximize flap blood supply and reduce the effects of radiation. Tran and associates reviewed the M.D. Anderson Cancer Center experience of TRAM flap irradiation, which addressed this issue.18 The review included 41 TRAM flap (free TRAM 32, pedicled TRAM 9). Ten patients (24%) required an additional flap to correct radiation-induced contracture. Nine patients (22%) maintained normal breast volume and palpable fat necrosis was noted in 34% of flaps.
Rogers and Allen demonstrated similar deleterious effects of radiation on deep inferior epigastric perforator (DIEP) flap reconstruction.19 They examined 30 DIEP flaps that received radiation therapy. A control group consisted of 30 nonirradiated DIEP flaps. They reported a 23.3% incidence of fat necrosis in the radiated group versus 0% incidence in the control group. Radiation fibrosis was seen in 56.7% of cases with 5 (16.7%) requiring surgical revision.
Tran and colleagues compared immediate and delayed free TRAM flap reconstruction in patients receiving postmastectomy radiation therapy.20 The study group included 32 immediate and 70 delayed TRAM flaps. The incidence of early complications did not differ between the groups. However, the incidence of late complications was significantly higher in the immediate reconstruction group. Nine patients (28%) in the immediate group required an additional flap to correct distorted contour. The authors concluded that in patients who need postmastectomy radiation therapy, TRAM flap reconstruction should be delayed until radiation therapy is completed.
TRAM RECONSTRUCTION
Hartrampf, Scheflan, and Black introduced the TRAM flap in 1982.21 The skin island is oriented transversely across the abdomen to camouflage the scar and doubles as an abdominoplasty. It has become the method of choice for autologous tissue reconstruction. The ellipse of skin and underlying fat of the lower abdomen are supplied by perforators from the underlying rectus abdominis muscles supplied by the superior and inferior epigastric vessels. The TRAM flap produces the most natural-looking and -feeling breast but the results must be balanced against the magnitude of the procedure and its potential morbidity.
Careful anatomic studies of the blood supply to the abdominal wall and critical outcome analyses have resulted in many refinements in TRAM flap breast reconstruction. Flap modifications have been developed to increase flap vascularity and reduce abdominal wall morbidity. Bipedicle TRAM flaps were initially advocated to enhance flap blood supply but it became apparent that this was obtained at the cost of abdominal wall morbidity. Hartrampf reviewed his experience with 300 pedicled TRAM flap reconstructions. He found that 17% patients with single-pedicle and 64% those with double-pedicle flaps lost the ability to do sit-ups after surgery.22 Lejour and Dome tested the abdominal wall function of 57 patients after TRAM flap reconstruction.23 They concluded the functional compromise after single-pedicle TRAM flap was acceptable but restricted the use of the bipedicle flap. There are many surgeons who feel that for unilateral breast reconstruction, the abdominal wall morbidity of a bipedicle TRAM flap is prohibitive.
Free TRAM flap breast reconstruction gained popularity in an attempt to increase flap vascularity and decrease the abdominal wall morbidity seen with the conventional pedicled flap. The free TRAM flap utilizes the inferior epigastric vessels, which are the main blood supply to the lower rectus muscle and overlying skin. A large skin island can be utilized based on one pedicle and designed lower in the abdomen to hide the incision. Microvascular anastomoses are performed to the thoracodorsal or internal mammary vessels. Vein grafts are seldom necessary because of the long pedicle length. The free TRAM flap has better vascularity than a unipedicle TRAM flap when zones II and IV are needed. It requires less muscle harvest, thus reducing abdominal wall morbidity. Patients undergoing free TRAM flap reconstruction are generally more likely to retain the ability to perform sit-ups than are patients following unipedicle TRAM flap.24 However, dynamometric assessment of abdominal wall strength after TRAM flap reconstruction has not been shown to be different in patients undergoing pedicled versus free TRAM flap reconstruction.25,26,27 Unipedicle TRAM flap reconstruction rarely interferes with the activities of daily living in my experience.
The main disadvantage of free TRAM flap reconstruction is the dependence on microsurgery. The development of a vein coupling device has simplified the procedure but there is still a learning curve. A total flap loss of 1 to 6% has been reported in large series of experienced surgeons.28,29,30
The DIEP flap is a refinement in the conventional musculocutaneous free flap. The skin and fat are perfused by transmuscular perforators without muscle sacrifice. This flap was first reported by Allen and Treece in 1994.31 The perforator flap is popular in Europe but has gained slow acceptance in the United States. Reports indicate the DIEP flap reconstruction preserves abdominal wall function compared with the free TRAM flap.32,33 There are several potential disadvantages including a tedious dissection that increases the harvest time 1 to 2 hours over a free TRAM flap. The DIEP flap appears to have vascularity somewhere between a pedicled and free TRAM flap. It is intuitive that the vascularity may be less than a free TRAM flap because of the small size and number of the perforating vessels in some patients. Kroll compared the incidence of flap necrosis and fat necrosis in free TRAM flaps and DIEP flaps.34 He concluded that the incidence of partial flap loss and fat necrosis is higher in the DIEP flaps than in the free TRAM flaps, probably because the blood supply to the former is less robust.
Patient Selection
A careful preoperative history and physical examination is essential in evaluating patients for possible TRAM flap reconstruction. Watterson and colleagues reviewed 556 patients who underwent TRAM flap reconstruction.35 The overall complication rate was 23.7%. Multivariate analysis revealed that smoking, history of chest wall irradiation, significant abdominal scarring, and obesity were associated with an increased complication rate. Among unipedicle reconstructions, patients with multiple risk factors had three times the incidence of fat necrosis (24.7 versus 8.3%) compared with patients with one or no risk factors.
Active smoking predisposes to flap and abdominal wall necrosis and is a contraindication to performing the flap in many centers. Smoking also increases the risk of mastectomy skin flap necrosis as previously discussed. I have patients stop smoking for at least 2 weeks prior to unipedicle TRAM flap reconstruction. If this is not possible, a bipedicle or free TRAM flap is indicated to improve vascularity. Some authors have advocated delaying the TRAM flap in high-risk patients.36 The deep and superficial inferior epigastric vessels are ligated 7 to 21 days prior to the TRAM flap. Studies have shown that this preliminary surgical delay significantly increases the area of flap survival.36,37,38
Kroll and Netscher examined the effect of obesity on the complication rate.39 They found a 15.4% incidence of complications in thin patients compared with a 41.7% complication rate in those that were morbidly obese. I have found an autologous latissimus dorsi musculocutaneous flap a good option in morbidly obese patients.40 Donor site seroma formation can be problematic but the overall morbidity is much lower than a TRAM flap in this patient population.
Subcostal scars and multiple abdominal scars predispose to skin-healing problems in the abdomen and decrease blood supply to the flap. Losken and coworkers reviewed 26 patients with a right subcostal incisions and 126 age- and risk-matched patients who underwent TRAM flap reconstruction.41 Abdominal wall skin necrosis in patients with a subcostal incision (25%) was significantly higher than those patients without abdominal scars (5%, P = 0.02).
Complications
Any form of TRAM flap reconstruction is a large, complex operation that requires a 5- to 7-day hospitalization and 4- to 6-week convalescence. The overall complication has been reported to be 16 to 28%.22,39,42 Partial flap loss has been reported in 6 to 31% of pedicled TRAM flap cases and abdominal herniation or weakness in 0.3 to 13%.22,24,42,43,44
In my experience, the use of the skin-sparing mastectomy technique has permitted more liberal use of the unipedicle TRAM flap for reconstruction. Areas of ischemic flap instead of being excised are often buried beneath the native skin and can present as areas of fat necrosis. We have defined fat necrosis as any firm area of the flap usually with cytological confirmation made by fine-needle aspiration. The incidence in 402 nonirradiated pedicled TRAM was 22.6%. This seems high but most are small, thickened areas that do not require excision.
Caveats
FLAP SELECTION
The key consideration in TRAM flap reconstruction is to match the tissue requirements of the opposite breast. The base width, height, and projection must be noted in the preoperative evaluation. Patients' risk factors for complications must be considered prior to TRAM flap reconstruction. An infraumbilical midline incision would preclude a unipedicle or free TRAM flap if zones II and IV are required. If only the tissue directly over the muscle (zone I) and the adjacent tissue on the same side of the lower abdomen (zone III) are required, I prefer an ipsilateral unipedicle TRAM flap. Abdominal morbidity is not significantly worse than free TRAM and it avoids the potential for total flap loss. If more tissue is required, especially in delayed reconstruction, a free flap or bipedicled TRAM flap should be considered.
UNIPEDICLE FLAP DESIGN
Many authors advocate preservation of a lateral strip of rectus muscle to reduce abdominal morbidity and facilitate fascial closure. This strip of preserved muscle has been shown to atrophy because segmental intercostals nerves and vessels enter the middle third of muscle. I routinely harvest the entire muscle because it appears to improve the blood supply to the flap. Harris and colleagues did intraoperative blood flow studies of unipedicle TRAM flaps.45 They found that occlusion of the medial and lateral thirds of the isolated rectus muscle decreased the mean arterial blood pressure in the flap an average of 19% in 80% of the individuals studied. This data supports the technique of harvesting the entire rectus muscle.
The contralateral pedicle was preferred for a long time over an ipsilateral flap because of concerns of potential folding of the pedicle, which would compromise venous flow. Several recent studies compared the safety of both procedures.46,47 Intraoperative monitoring of the deep inferior epigastric pedicle revealed a significantly higher venous pressure in the contralateral rotation and inset compared with the ipsilateral group. The ipsilateral pedicle permits better flap mobility and less tension on the pedicle, making flap shaping easier.
FLAP INSET
The ipsilateral pedicled flap is brought up through a subcutaneous tunnel made through the central portion of the inframammary fold. The medial and lateral attachments of the fold are preserved. The ipsilateral pedicle folds on itself, making a smooth contour that has minimal bulge over costal margin. This eliminates the epigastric bulge seen with a contralateral pedicle. Sectioning of the seventh intercostal nerve along the costal margin will result in muscle atrophy.
The “hemi-TRAM” flap is oriented on the chest wall with zone III in the axilla. This places the area most prone to fat or flap necrosis in a relatively inconspicuous area. Tissue loss in this area can be easily dealt with by local tissue rearrangement or a small latissimus flap transposition. The reconstructed side is made larger than the native breast to account for postoperative atrophy of the rectus muscle. The final flap shaping and volume correction is reserved for the second stage.
Secondary Procedures
TRAM flap reconstruction should be considered a two-stage procedure regardless of nipple reconstruction. I counsel patients that the first stage is building the foundation and framework of the breast. The second stage is performed at least 3 months after the initial operation. This stage is essential for final adjustments to the volume, contour, and position of the breast mound. This delay allows for flap shrinkage and settling. Nipple reconstruction is also performed during this stage.
Volume adjustment can be performed both by direct excision and suction-assisted lipectomy. If major flap revisions are performed, I delay nipple reconstruction until a third stage to ensure its proper position. Areas of fat necrosis can become very painful, especially if located medially against the chest wall. These should be excised but can leave a defect if large.
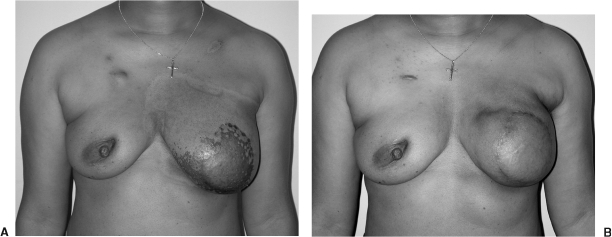
If the flap has not been suspended from the chest wall with sutures, an infraclavicular hollow often develops, which is hard to correct. Small soft defects can be corrected by mobilizing the flap off the native skin and chest wall and resuspending it superiorly. Larger infraclavicular defects are best corrected with a breast implant placed under the TRAM flap (Fig. 5). A contralateral breast augmentation is often necessary to achieve symmetry. A lateral convexity along the anterior axillary fold can be corrected with a small latissimus muscle flap elevated through the axilla.
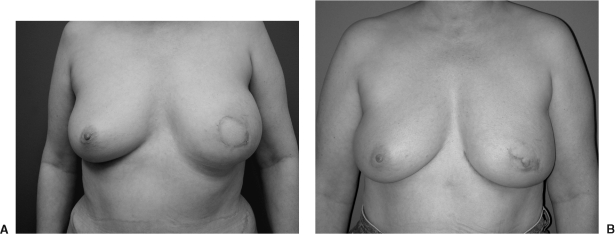
Figure 5.
(A) Postoperative view of a patient after a unipedicle TRAM flap reconstruction. She underwent an SSM using a vertical reduction pattern skin incision. (B) Postoperative view after placement of an implant under her TRAM flap as well as a augmentation mastopexy on the contralateral side.
Loss of definition of the inframammary fold is not an uncommon problem. This usually results from failure to close a large subcutaneous tunnel around the pedicle. Small defects in the IMF can be resuspended with internal sutures approached through the periareolar incision. Suction lipectomy is helpful in moderate-sized defects. Larger defects with total loss of the IMF definition may require an external approach (Fig. 6). A deepithelialized Ryan flap may be advanced and sutured to the chest wall after excising excess soft tissue.
Figure 6.
(A) Postoperative view after ipsilateral unipedicle TRAM flap reconstruction TRAM flap. The inframammary fold has lost definition. (B) Postoperative view after revision of the IMF via an external inframammary incision.
REFERENCES
- Cronin T, Gerow F. A new “natural feel” prothesis. Amsterdam: In: Transactions of the Third International Congress of Plastic and Reconstructive Surgery; Excerpta Medica; 1963.
- Spear S L, Mardini S, Ganz J C. Resource cost comparison of implant-based breast reconstruction versus TRAM flap breast reconstruction. Plast Reconstr Surg. 2003;112:101–105. doi: 10.1097/01.PRS.0000066007.06371.47. [DOI] [PubMed] [Google Scholar]
- Clough K B, O'Donoghue J M, Fitoussi A D, Nos C, Falcou M C. Prospective evaluation of late cosmetic results following breast reconstruction: I. Implant reconstruction. Plast Reconstr Surg. 2001;107:1702–1709. doi: 10.1097/00006534-200106000-00010. [DOI] [PubMed] [Google Scholar]
- Clough K B, O'Donoghue J M, Fitoussi A D, Nos C, Falcou M C. Prospective evaluation of late cosmetic results following breast reconstruction: II. Tram flap reconstruction. Plast Reconstr Surg. 2001;107:1710–1716. doi: 10.1097/00006534-200106000-00011. [DOI] [PubMed] [Google Scholar]
- Kroll S S, Ames F, Singletary S E, Schusterman M A. The oncologic risks of skin preservation at mastectomy when combined with immediate reconstruction of the breast. Surg Gynecol Obstet. 1991;172:17–20. [PubMed] [Google Scholar]
- Toth B, Lappert P. Modified skin incisions for mastectomy: the need for plastic surgical input in preoperative planning. Plast Reconstr Surg. 1991;87:1048–1053. [PubMed] [Google Scholar]
- Carlson G W, Grossl N, Lewis M M, Temple J R, Styblo T M. Preservation of the inframammary fold: what are we leaving behind? Plast Reconstr Surg. 1996;98:447–450. doi: 10.1097/00006534-199609000-00012. [DOI] [PubMed] [Google Scholar]
- Carlson G W, Styblo T M, Lyles R H, et al. Local recurrence after skin-sparing mastectomy: tumor biology or surgical conservatism? Ann Surg Oncol. 2003;10:108–112. doi: 10.1245/aso.2003.03.053. [DOI] [PubMed] [Google Scholar]
- Kroll S S, Khoo A, Singletary S E, et al. Local recurrence risk after skin-sparing and conventional mastectomy: a 6-year follow-up. Plast Reconstr Surg. 1999;104:421–425. doi: 10.1097/00006534-199908000-00015. [DOI] [PubMed] [Google Scholar]
- Simmons R M, Fish S K, Gayle L, et al. Local and distant recurrence rates in skin-sparing mastectomies compared with non-skin-sparing mastectomies. Ann Surg Oncol. 1999;6:676–681. doi: 10.1007/s10434-999-0676-1. [DOI] [PubMed] [Google Scholar]
- Bleicher R J, Hansen N M, Giuliano A E. Skin-sparing mastectomy. Cancer. 2003;98:2316–2321. doi: 10.1002/cncr.11801. [DOI] [PubMed] [Google Scholar]
- Overgaard M, Hansen P S, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337:949–955. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- Ragaz J, Jackson S M, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337:956–962. doi: 10.1056/NEJM199710023371402. [DOI] [PubMed] [Google Scholar]
- Adjuvant Therapy for Breast Cancer In: NIH Consensus Statement. Bethesda, MD: National Institutes of Health; 2000.
- Schuster R H, Kuske R R, Young V L, Fineberg B. Breast reconstruction in women treated with radiation therapy for breast cancer: cosmesis, complications, and tumor control. Plast Reconstr Surg. 1992;90:445–452. discussion 453–454. [PubMed] [Google Scholar]
- Spear S L, Majidian A. Immediate breast reconstruction in two stages using textured, integrated-valve tissue expanders and breast implants: a retrospective review of 171 consecutive breast reconstructions from 1989 to 1996. Plast Reconstr Surg. 1998;101:53–63. doi: 10.1097/00006534-199801000-00010. [DOI] [PubMed] [Google Scholar]
- Dowden R V. Selection criteria for successful immediate breast reconstruction. Plast Reconstr Surg. 1991;88:628–634. doi: 10.1097/00006534-199110000-00011. [DOI] [PubMed] [Google Scholar]
- Tran N V, Evans G R, Kroll S S, et al. Postoperative adjuvant irradiation: effects on tranverse rectus abdominis muscle flap breast reconstruction. Plast Reconstr Surg. 2000;106:313–317. discussion 318–320. doi: 10.1097/00006534-200008000-00011. [DOI] [PubMed] [Google Scholar]
- Rogers N E, Allen R J. Radiation effects on breast reconstruction with the DIEP flap. Plast Reconstr Surg. 2001;109:1919–1924. doi: 10.1097/00006534-200205000-00022. [DOI] [PubMed] [Google Scholar]
- Tran N V, Chang D W, Gupta A, Kroll S S, Robb G L. Comparison of immediate and delayed free TRAM flap breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg. 2001;108:78–82. doi: 10.1097/00006534-200107000-00013. [DOI] [PubMed] [Google Scholar]
- Hartrampf C R, Scheflan M, Black P W. Breast reconstruction with a transverse abdominal island flap. Plast Reconstr Surg. 1982;69:216–225. doi: 10.1097/00006534-198202000-00006. [DOI] [PubMed] [Google Scholar]
- Hartrampf C R, Jr, Bennett G K. Autogenous tissue reconstruction in the mastectomy patient. A critical review of 300 patients. Ann Surg. 1987;205:508–519. doi: 10.1097/00000658-198705000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejour M, Dome M. Abdominal wall function after rectus abdominis transfer. Plast Reconstr Surg. 1991;87:1054–1068. doi: 10.1097/00006534-199106000-00007. [DOI] [PubMed] [Google Scholar]
- Kroll S S, Schusterman M A, Reece G P, Miller M J, Robb G, Evans G. Abdominal wall strength, bulging, and hernia after TRAM flap breast reconstruction. Plast Reconstr Surg. 1995;96:616–619. doi: 10.1097/00006534-199509000-00013. [DOI] [PubMed] [Google Scholar]
- Suominen S, Asko-Seljavaara S, Kinnunen J, Sainio P, Alaranta H. Abdominal wall competence after free transverse rectus abdominis musculocutaneous flap harvest: a prospective study. Ann Plast Surg. 1997;39:229–234. doi: 10.1097/00000637-199709000-00002. [DOI] [PubMed] [Google Scholar]
- Kind G M, Rademaker A W, Mustoe T A. Abdominal-wall recovery following TRAM flap: a functional outcome study. Plast Reconstr Surg. 1997;99:417–428. doi: 10.1097/00006534-199702000-00016. [DOI] [PubMed] [Google Scholar]
- Edsander-Nord A, Jurell G, Wickman M. Donor-site morbidity after pedicled or free TRAM flap surgery: a prospective and objective study. Plast Reconstr Surg. 1998;102:1508–1516. doi: 10.1097/00006534-199810000-00025. [DOI] [PubMed] [Google Scholar]
- Schusterman M A, Kroll S S, Miller M J, et al. The free transverse rectus abdominis musculocutaneous flap for breast reconstruction: one center's experience with 211 consecutive cases. Ann Plast Surg. 1994;32:234–241. discussion 241–242. doi: 10.1097/00000637-199403000-00002. [DOI] [PubMed] [Google Scholar]
- Grotting J C, Urist M M, Maddox W A, Vasconez L O. Conventional TRAM flap versus free microsurgical TRAM flap for immediate breast reconstruction. Plast Reconstr Surg. 1989;83:828–841. discussion 842–844. doi: 10.1097/00006534-198905000-00009. [DOI] [PubMed] [Google Scholar]
- Arnez Z M, Bajec J, Bardsley A F, Scamp T, Webster M H. Experience with 50 free TRAM flap breast reconstructions. Plast Reconstr Surg. 1991;87:470–478. discussion 479–482. [PubMed] [Google Scholar]
- Allen R J, Treece P. Deep inferior epigastric perforator flap for breast reconstruction. Ann Plast Surg. 1994;32:32–38. doi: 10.1097/00000637-199401000-00007. [DOI] [PubMed] [Google Scholar]
- Futter C M, Webster M H, Hagen S, Mitchell S L. A retrospective comparison of abdominal muscle strength following breast reconstruction with a free TRAM or DIEP flap. Br J Plast Surg. 2000;53:578–583. doi: 10.1054/bjps.2000.3427. [DOI] [PubMed] [Google Scholar]
- Blondeel N, Vanderstraeten G G, Monstrey S J, et al. The donor site morbidity of free DIEP flaps and free TRAM flaps for breast reconstruction. Br J Plast Surg. 1997;50:322–330. doi: 10.1016/s0007-1226(97)90540-3. [DOI] [PubMed] [Google Scholar]
- Kroll S S. Fat necrosis in free transverse rectus abdominis myocutaneous and deep inferior epigastric perforator flaps. Plast Reconstr Surg. 2000;106:576–583. doi: 10.1097/00006534-200009030-00008. [DOI] [PubMed] [Google Scholar]
- Watterson P A, Bostwick J, III, Hester T R, Jr, Bried J T, Taylor G I. TRAM flap anatomy correlated with a 10-year clinical experience with 556 patients. Plast Reconstr Surg. 1995;95:1185–1194. doi: 10.1097/00006534-199506000-00007. [DOI] [PubMed] [Google Scholar]
- Restifo R J, Thomson J G. The preconditioned TRAM flap: preliminary clinical experience. Ann Plast Surg. 1998;41:343–347. doi: 10.1097/00000637-199810000-00001. [DOI] [PubMed] [Google Scholar]
- Ozgentas H E, Shenaq S, Spira M. Study of the delay phenomenon in the rat TRAM flap model. Plast Reconstr Surg. 1994;94:1018–1024. discussion 1025–1026. doi: 10.1097/00006534-199412000-00016. [DOI] [PubMed] [Google Scholar]
- Moon H K, Taylor G I. The vascular anatomy of rectus abdominis musculocutaneous flaps based on the deep superior epigastric system. Plast Reconstr Surg. 1988;82:815–832. doi: 10.1097/00006534-198811000-00014. [DOI] [PubMed] [Google Scholar]
- Kroll S S, Netscher D T. Complications of TRAM flap breast reconstruction in obese patients. Plast Reconstr Surg. 1989;84:886–892. doi: 10.1097/00006534-198912000-00003. [DOI] [PubMed] [Google Scholar]
- Chang D W, Youssef A, Cha S, Reece G P. Autologous breast reconstruction with the extended latissimus dorsi flap. Plast Reconstr Surg. 2002;110:751–759. discussion 760–761. doi: 10.1097/00006534-200209010-00005. [DOI] [PubMed] [Google Scholar]
- Losken A, Carlson G W, Jones G E, Culbertson J H, Schoemann M, Bostwick J., III Importance of right subcostal incisions in patients undergoing TRAM flap breast reconstruction. Ann Plast Surg. 2002;49:115–119. doi: 10.1097/00000637-200208000-00001. [DOI] [PubMed] [Google Scholar]
- Paige K T, Bostwick J, III, Bried J T, Jones G. A comparison of morbidity from bilateral, unipedicled and unilateral, unipedicled TRAM flap breast reconstructions. Plast Reconstr Surg. 1998;101:1819–1827. doi: 10.1097/00006534-199806000-00007. [DOI] [PubMed] [Google Scholar]
- McCraw J B, Horton C E, Grossman J A, Kaplan I, McMellin A. An early appraisal of the methods of tissue expansion and the transverse rectus abdominis musculocutaneous flap in reconstruction of the breast following mastectomy. Ann Plast Surg. 1987;18:93–113. doi: 10.1097/00000637-198702000-00003. [DOI] [PubMed] [Google Scholar]
- Carlson G W, Losken A, Moore B, et al. Results of immediate breast reconstruction after skin-sparing mastectomy. Ann Plast Surg. 2001;46:222–228. doi: 10.1097/00000637-200103000-00003. [DOI] [PubMed] [Google Scholar]
- Harris N R, 2nd, Webb M S, May J W., Jr Intraoperative physiologic blood flow studies in the TRAM flap. Plast Reconstr Surg. 1992;90:553–558. discussion 559–561. [PubMed] [Google Scholar]
- Clugston P A, Lennox P A, Thompson R P. Intraoperative vascular monitoring of ipsilateral vs. contralateral TRAM flaps. Ann Plast Surg. 1998;41:623–628. doi: 10.1097/00000637-199812000-00007. [DOI] [PubMed] [Google Scholar]
- Clugston P A, Gingrass M K, Azurin D, Fisher J, Maxwell G P. Ipsilateral pedicled TRAM flaps: the safer alternative? Plast Reconstr Surg. 2000;105:77–82. doi: 10.1097/00006534-200001000-00013. [DOI] [PubMed] [Google Scholar]
- Rogers N E, Allen R J. Radiation effects on breast reconstruction with the deep inferior epigastric perforator flap. Plast Reconstr Surg. 2002;109:1919–1924. discussion 1925–1926. doi: 10.1097/00006534-200205000-00022. [DOI] [PubMed] [Google Scholar]