Abstract
The era of breast conserving treatment of early-stage breast carcinoma has created reconstructive challenges for the plastic surgeon. Although good to excellent cosmetic outcomes occur in the majority of patients, a significant number could benefit from additional reconstructive measures. Because of the need for continuing surveillance following breast-conserving therapy, estimated at 5–10% after fifteen years, plastic surgeons should choose techniques that do not interfere with the detection of recurrent breast carcinoma. Myocutaneous flaps-in particular, the latissimus dorsi and transverse rectus abdominis—have fulfilled the reconstructive needs of these patients by providing well-vascularized soft tissue. Postoperative radiological evaluation has demonstrated that these flaps are radiolucent, unlike breast implants that can obscure accurate mammographic interpretation.
Myocutaneous flaps have been used for both immediate and delayed reconstruction of post-breast conservation deformities. The delayed approach offers the benefit of an established contour deformity that usually involves cutaneous, parenchymal, and nipple-areolar components. Moderate overcorrection of the defect has been advocated in anticipation of ongoing postradiation wound contraction and fibrosis. Immediate reconstruction of lumpectomy and partial mastectomy defects permits wider initial excision of the breast lesion, but can be compromised by positive histological margins. Long-term results suggest stability of the aesthetic outcome following reconstruction of delayed deformities.
Keywords: Reconstruction, breast, conservation, deformity
The treatment of breast cancer has evolved from an extreme to a more conservative approach since the early 1970s. Halsted's radical mastectomy was the primary treatment of breast cancer for most of the 20th century.1 Modified radical mastectomy with preservation of the pectoralis major muscle gained more popularity in the 1970s when it was realized that cancers were being detected at an earlier stage than ever before and that the majority of treatment failures occurred due to systemic rather than local recurrence.2 The most recent development has been toward conservation therapy.3
Breast conservation therapy (BCT), defined as tumor excision and radiation treatment, has now become the preferred treatment for women with stage I and stage II breast carcinoma. BCT preserves innate breast sensation and avoids the psychological distress associated with mastectomy. Before BCT could gain widespread acceptance as a valid oncologic treatment, its efficacy to mastectomy had to be proven. Veronesi and colleagues and Fisher and associates both published landmark studies in the mid 1980s that demonstrated no decrease in overall survival after BCT.4 Twenty-year follow-up studies by both of these groups were published in the New England Journal of Medicine in October 2002. After 20 years, the Veronesi group found the rate of death from all causes to be 41.7% in the breast conservation arm and 41.2% in the mastectomy arm. The rate of death from breast cancer was 26.1% and 24.3%, respectively. The 20-year incidence of local recurrence was 8.8% in the breast conservation group and 2.3% in the mastectomy group.5 The Fisher study found the overall survival of the BCT group to be 46% versus 47% in the mastectomy group.6 With its efficacy proven, more women are choosing BCT as a less-invasive form of therapy.
Given the increased prevalence of BCT, attention has now focused on the cosmetic outcome that it provides. In general, most patients have a good to excellent result following BCT. One study found that physicians rated the overall cosmetic results obtained by BCT as excellent in 77% of cases, good in 9%, fair in 9%, and poor in 5%. A fair or poor cosmetic result was found to be correlated with the severity of breast retraction.7 Another study found that 81% of patients had a good to excellent cosmetic result at 3 years following BCT. A scoring method and breast retraction assessment were used to assess cosmetic outcome in this study.8 Another study published in Plastic and Reconstructive Surgery in 1990 demonstrated that the evaluation of cosmetic results depended on who was the judge. Patients tended to be more lenient judges than plastic surgeons. In the study, 80% of patients judged their own result to be good or excellent in comparison to 50% when the results were judged by a plastic surgeon.9 This seems to indicate that the retention of one's own breast has a strong influence on the evaluation of outcome. The general consensus of these and other studies is that ∼80% of patients develop a good to excellent result following BCT.10,11,12 Despite the high level of cosmetic outcome following BCT, several factors found in these and other studies negatively influence outcome.
One of the most significant factors affecting cosmetic outcome is the size of the excision. One study demonstrated that the weight of the tumor specimen had a direct, negative impact on cosmesis.13 Therefore, quadrantectomy, defined as excision of 2 to 3 cm of normal tissue around the tumor plus underlying fascia, creates a more devastating cosmetic problem than lumpectomy that removes the tumor mass with only a small margin of normal tissue.14 Controversy remains, however, as to the optimum extent of surgical resection. Reducing the extent of resection from quadrantectomy to lumpectomy increased the local recurrence rate by three times in one study.15 Several other studies contradict this finding. One investigation showed that 5-year freedom from local recurrence was equivalent between lumpectomy and quadrantectomy at 92% and 93%, respectively. This study also found that the most unfavorable cosmetic results occurred following quadrantectomy.16 Another study echoed these results, finding that lumpectomy and quadrantectomy provided comparable results with respect to local control and overall survival.17 Most centers in the United States practice lumpectomy given that it provides optimum cosmesis with no decrease in overall survival and questionable increase in local recurrence.
Another factor that influences the result of BCT is breast size. Patients with smaller breasts tend to have a worse cosmetic result than those with larger breasts. One paper showed that 94% of patients with cup size D had a good to excellent result following BCT, but only 33% of patients with cup size A had similar results.13 Perhaps more pertinent, however, is the size of the tumor relative to the breast. For example, a large tumor in a small breast is likely to have a poor cosmetic outcome following BCT due to the relative deformity conferred.
Radiation therapy has a significant impact on cosmesis following BCT. Radiation therapy may induce skin telangiectasias and edema while contributing to breast retraction and nipple asymmetry. The overall cosmetic result, however, varies depending on the radiation technique applied. Radiation boost, increasing number of radiation fields, and total radiation dose to the breast all seem to have a negative impact on cosmetic outcome following BCT. In one study, 22% of patients treated with a radiation boost had a fair or poor result compared with none of the patients without a boost, after 4 years.18 Similarly, a study of the cosmetic outcome in the European Organization for Research and Treatment of Cancer “boost versus no boost” trial found that 86% of patients in the no-boost group had an excellent or good outcome compared with 71% of patients in the boost group. Evaluation using a breast retraction assessment showed that boost was associated with an increase in breast retraction.19 One report found that 89% of patients treated with a tangent pair technique had an excellent cosmetic result versus only 69% of patients treated with a three-field technique.20 Other studies21,22 also found that using a radiation technique with a higher number of radiation fields was associated with a worse cosmetic outcome. One investigation analyzed radiation fibrosis with quantitative measurements. Radiation doses greater than 75 Gy were associated with very poor results in 30% of patients. Above 50 Gy, increases in dose of 1 Gy correlated with an average nipple displacement of 1 mm in upward direction and 0.75 mm medially.23
Although it appears the use of radiation itself has a negative impact on cosmesis, the overall effect is dependent upon the radiation technique applied.
Chemotherapy has also been found to influence cosmetic result following BCT. The mechanism whereby this influence is exerted is not clearly understood. One can surmise, however, that the immunosuppressive effects of the chemotherapy agents hinder wound healing. One study demonstrated that adjuvant chemotherapy adversely affected the cosmetic outcome of BCT but only when chemotherapy was concurrently administered with radiation. If the chemotherapy was given sequentially, the cosmetic impact was only slight.24 Another study also found that concomitant adjuvant chemotherapy produced a worse aesthetic outcome.25 These studies suggest that to improve cosmetic outcome following BCT, radiation and chemotherapy should be given sequentially rather than in concert.
SURVEILLANCE AFTER BCT
The local recurrence rate following BCT has been found to be ∼10 to 15%. Given that 1 in 10 women will develop a recurrence after her treatment, these patients must be followed closely with physical exam and mammography. Physical exam following breast conservation therapy reveals mild thickening of the breast tissue. Sometimes areas of fibrosis in the breast that develop after excision and radiation therapy may be difficult to distinguish from recurrence.26 Posttreatment change tends to stabilize by 1 year following therapy. Changes in physical exam that develop more than a few years after BCT, however, are more likely to represent recurrence than posttreatment change. Similarly, it may be difficult to distinguish benign BCT-induced change from malignancy on a mammogram. Skin thickening, calcifications, and increased density may all develop after BCT27; however, calcifications or densities that develop in different quadrants from the initial tumor are more likely to be malignant.28 Increased glandular density, coarse calcifications, and architectural distortion within the tumor bed appear to be more related to postsurgical change. Fine calcifications that vary in size and shape must be viewed as suspicious. A spiculated, irregular mass is to be considered malignant until proven otherwise. Although challenging, an experienced radiologist can usually distinguish between postsurgical change and local recurrence.
IMMEDIATE RECONSTRUCTION OF PARTIAL MASTECTOMY DEFECTS
There is a subset of patients not considered appropriate for BCT because the defect left after BCT would be too cosmetically deforming. These patients with large tumors or medium-sized tumors in small breasts would previously been offered only a mastectomy. Now surgeons, especially in Europe, are offering reconstruction at the time of partial mastectomy. This practice has expanded the option of BCT to more women. Some of the techniques used for immediate reconstruction include local glandular flaps, areola transposition, mastopexy, or reduction mammoplasty.29 Bold and colleagues published a series of nine patients treated with BCT using local rotational flaps. The patients in this series were deemed inappropriate for standard BCT because of predicted cosmetic outcome. Cosmesis was found to be good to excellent in eight of the nine patients in this study. Within the 2-year follow-up period, one of the nine patients had developed a recurrence.30 Local flaps are problematic, however, in that they lack adequate vascularity and pose initial healing concerns. These flaps have an increased risk for infection because of their poor blood supply.
Another technique used for immediate reconstruction is reduction mammoplasty. The breast cancer is excised using a breast reduction technique and the opposite breast is reduced by the same volume. This creates symmetry and improves cosmesis. One prospective study of 101 patients treated with this “oncoplastic” procedure found the 5-year local recurrence rate to be 9.4%, comparable to that of BCT alone.31 One benefit of the reduction mammoplasty technique is the ability to examine the contralateral breast for occult breast carcinoma. In the prospective study mentioned above, one patient was found to have a contralateral breast carcinoma. Other studies have reported as high as a 4.5% incidence of occult breast cancer in the opposite breast found during reduction mammoplasty for breast reconstruction.32 There are several disadvantages to the technique in that final surgical margins are not available at the time of the reduction, requiring further surgery for completion. In one retrospective review of 20 women who underwent partial mastectomy with reduction mammoplasty, 20% of the patients had positive final surgical margins requiring return to the operating room for reexcision.33 Techniques that involve rearrangement of tissue such as this make reexcision difficult and may not be oncologically sound. Additionally, the final contour of the affected breast cannot be predicted at the time of reduction because the patient has yet to undergo radiation therapy. It then becomes difficult to adequately assess symmetry at the time of operation. Radiation can cause atrophy and fat necrosis, both of which can affect the final result and cause dissymmetry.
IMMEDIATE RECONSTRUCTION USING AUTOGENOUS FLAPS
Another technique being used to improve cosmesis following BCT is immediate volume replacement with autogenous flaps. Proponents of this technique argue that it allows for wider local excision because the defect is immediately corrected. The thought is that the surgeon is less constrained by the eventual cosmetic deficit and will perform a more extensive resection. Furthermore, advocates claim that immediate volume replacement may extend the indications for BCT and even prevent some patients from undergoing a mastectomy.
Some of the flaps used for immediate reconstruction include the latissimus dorsi flap with adipose tissue, the latissimus dorsi myocutaneous flap, and the lateral thoracic adipose tissue flap.34 One study looked at 20 patients who underwent immediate latissimus dorsi mini flap reconstruction compared with 38 patients with wide local excision alone and found that the reconstructed patients had larger tumors and underwent wider specimen excisions. Cosmetic outcome was judged using a breast retraction assessment and panel assessment and cosmetic failure was found to be 34% after wide local excision but 10% following reconstruction.35 A study of 62 patients having undergone immediate volume replacement using the latissimus dorsi miniflap found a local recurrence rate of 8%, comparable to that of BCT alone. Additionally, four of the five patients who recurred had not received radiation therapy. These patients received radiation treatment for their recurrence and other patient underwent a mastectomy.36 A study of 101 patients who underwent lateral tissue flaps following quadrantectomy had a 4% local recurrence rate.37 In another study, surgical margins were examined histologically at the time of surgery and, if involved, further tissue was extracted. If the reexcision specimen was positive, a mastectomy was performed instead of wide local excision with autogenous flap. Using this technique, the local recurrence rate was found to be 0.7% in the immediate volume replacement group.38
Although immediate volume replacement appears advantageous, there are several disadvantages to this technique. One is that the final pathology result of surgical margins is often not known at the time of reconstruction. This may require a return to the operating room for reexcision and disruption of the flap. Additionally, the flap must undergo postoperative radiation therapy. Radiation therapy has been shown to cause fat necrosis, volume loss, and flap contracture in autogenous flaps.39 This adversely affects the eventual outcome.
DELAYED RECONSTRUCTION USING AUTOGENOUS FLAPS
Although the surgical defect is known to have the most effect on cosmetic outcome following BCT, radiation treatment and chemotherapy both contribute negatively to the eventual result. Often, stabilization of breast appearance does not occur until 1 to 2 years following BCT. In fact, significant breast contracture may not occur until years after treatment. For this reason, it seems prudent to reconstruct these deformities in a delayed fashion. Furthermore, the final pathology of the tumor specimen is known at the time of reconstruction and the autogenous flap would not be required to withstand radiation therapy.
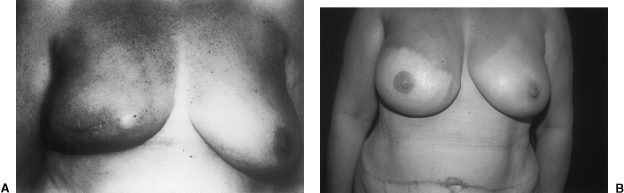
The latissimus dorsi flap appears to be the best choice for delayed reconstruction of a partial mastectomy defect. It can be used with a skin island if there is a deficiency of skin and parenchyma or inverted and deepithelialized if there is no shortage of overlying skin.40 Donor site morbidity is much less significant with the latissimus flap compared with the transverse rectus abdominis myocutaneous (TRAM) flap. The most common complication following the harvest of the latissimus flap is seroma formation, whereas abdominal wall weakness or hernia may develop following TRAM flap harvest. The skin and parenchyma provided by the latissimus dorsi flap is usually sufficient to correct the defect created by partial mastectomy and the additional skin and soft tissue provided by the TRAM flap is unnecessary. Additionally, given the local recurrence rate of ∼10% for patients receiving BCT as well as an increase in risk of breast cancer in the contralateral breast, it seems wise to preserve the TRAM flap for potential need. The TRAM flap, however, may be a good choice for those patients with a significant lower pole defect (Fig. 1). A disadvantage of the latissimus myocutaneous flap is that the skin it provides is of slightly different color and texture to that of the breast.41
Figure 1.
(A) Preoperative view of a 56-year-old woman who had excision of right nipple-areolar complex and quadrantectomy for breast cancer. (B) Result 1 year after surgery following reconstruction of the partial mastectomy defect with an ipsilateral midabdominal transverse rectus abdominis myocutaneous flap.
IMPLANTS
Although the use of submuscular implants to correct partial mastectomy deformities has been suggested, this has several disadvantages. The placement of an implant into a defect that has been irradiated may lead to implant extrusion, infection, and chronic wound drainage. Additionally, the ability to detect cancer recurrence may be impaired as the implant may obscure some areas of breast parenchyma on mammogram. Given the risk of recurrence following BCT, this does not seem appropriate for surveillance.
RECURRENCE AFTER DELAYED RECONSTRUCTION
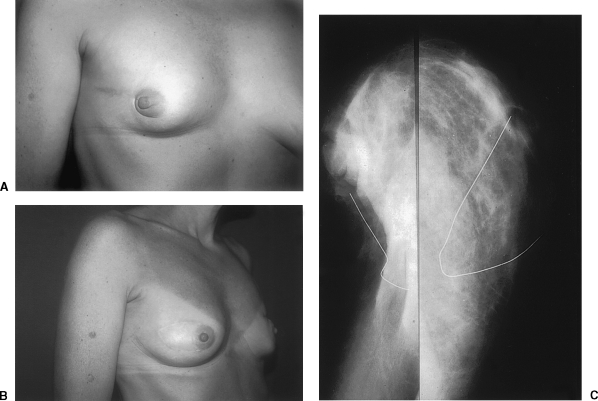
Given that there is a 10 to 15% local recurrence rate after BCT, the patients who have received delayed reconstruction following this treatment must be monitored closely. A concern regarding reconstruction after BCT is that the flap may obscure breast tissue and make surveillance difficult. Autogenous musculocutaneous flaps are radiolucent, however, and quite amenable to mammography (Fig. 2).42 Some of the typical mammographic findings with a musculocutaneous flap are surgical clips, the vascular pedicle, and surgical scars that produce radiopaque lines. Abnormal findings include irregular masses, pleomorphic calcifications, and masses associated with calcifications. These findings are suspicious for malignancy. In one study, a fibroadenoma that had been seen on a preoperative mammogram was again visualized despite the placement of a flap. This suggests that autologous myocutaneous flaps do not obscure visualization of malignancy.43
Figure 2.
(A) Preoperative view of a 37-year-old woman with right breast loss of volume and tethering dislocation of nipple-areolar complex. (B) Postoperative view 10 years after reconstruction with a right latissimus dorsi myocutaneous flap. (C, left side) Preoperative mammographic image on left demonstrates breast conservation defect with volume loss and increased opacification of the breast caused by scar tissue. (C, right side) Postoperative mammographic appearance after placement of latissimus dorsi myocutaneous flap (enclosed by wire). The flap has restored breast volume and contour, and the flap appears as fibrofatty tissue radiologically.
As 1 in 10 patients will experience recurrence, it is imperative that there be treatment options for those patients. Patients who have failed conservation therapy are candidates for salvage mastectomy as are patients who have failed BCT with reconstruction. Using the latissimus dorsi musculocutaneous flap for reconstruction after BCT preserves the TRAM flap for use in the event of recurrence. If, however, the TRAM flap was used for reconstruction after BCT, other distant flaps may be used to fill the mastectomy defect.
SURGICAL MANAGEMENT
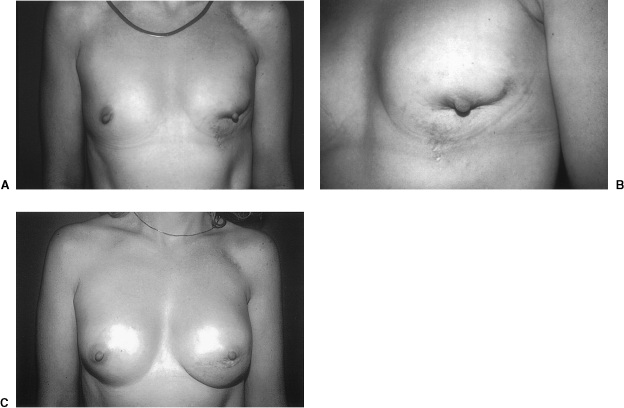
The latissimus dorsi flap is the preferred tissue for reconstruction of the partial mastectomy defect. To proceed with the latissimus dorsi flap, the patient should be placed in either the supine or in the lateral decubitus position. The first step is to prepare the recipient site. This should be done through the partial mastectomy incision site. All scar tissue should be excised down to pectoralis fascia. Preparation of the recipient bed first allows for accurate determination of donor tissue needed. Often, the defect is much larger than first expected. The tissue resected should be sent to pathology for evaluation and the margins analyzed. Measurements of the defect are taken and attention is then turned to mobilization of the latissimus dorsi flap. The patient may remain in the lateral decubitus position for this or, if the patient was supine, may be repositioned prone. The skin island is fashioned in a transverse or transverse oblique direction and care is taken to remain posterior to the posterior axillary fold. This is done so that the donor site scar is not visible from the anterior. The latissimus muscle is mobilized and the subcutaneous tunnel is created. When estimating how much flap tissue is necessary to fill the defect, it is recommended that an overcorrection of ∼10 to 20% be performed. This is to allow for the late contracture that occurs in radiated wounds. Once the appropriate amount of donor tissue has been mobilized and brought through the subcutaneous tunnel, the donor site may be closed. At this point, the patient is repositioned in the supine position and the latissimus flap may be folded and deepithelialized as necessary to appropriately fill the defect. The flap should be secured into place by tacking the muscle component of the flap to the pectoralis muscle. The skin island should precisely fit into the defect (Fig. 3). If it is taut, there is a risk of wound separation. If it is too large, there will be redundant skin that must be excised at a later date. The skin island is sutured into place and the reconstruction is complete.44
Figure 3.
(A) Preoperative view of a 37-year-old female with breast conservation defect characterized by nipple-areolar retraction, significant parenchymal volume loss, and skin stigmata with discoloration and telangiectasia. (B) Preoperative close-up view of the left breast conservation defect. (C) Appearance of the patient 1 year following reconstruction of the left breast conservation deformity with a left latissimus dorsi myocutaneous flap. No implant was placed in the left, but a subpectoral breast augmentation was performed in the right.
INDICATION FOR CONVERSION TO MASTECTOMY AT THE TIME OF RECONSTRUCTION
When the recipient site is being prepared for flap placement, the scar tissue removed is sent to pathology for assessment. If any of the biopsies sent should demonstrate a malignancy, the surgery should be converted to a total mastectomy. This possibility should be discussed with the patient prior to surgery. The patient may undergo reconstruction of the mastectomy site immediately or in a delayed fashion depending on the discussion made with the patient.
LONG-TERM AESTHETIC RESULTS AFTER FLAP RECONSTRUCTION
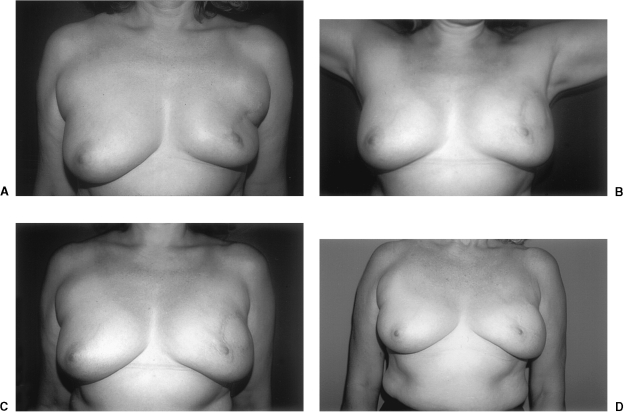
Although there are few long-term studies of the aesthetic results following reconstruction of the breast conservation deformity, postoperative assessments at 10 and 15 years demonstrate significant stability of the breast contour (Fig. 4). Myocutaneous flaps such as the latissimus dorsi and rectus abdominis undergo few changes in size or surface characteristics. Despite a tendency of the muscle component to become fibrofatty tissue over time, the mass of the flap shows little change, possibly as a result of preserved motor innervation in the case of the latissimus. In our series, patient follow-up extended to 16 years, with remarkable persistence of contour, size, shape, and consistency of the soft tissues.
Figure 4.
(A) Preoperative view of a 48-year-old woman with a lateral breast defect after breast conservation. The patient developed restricted range of motion of the left shoulder following radiation therapy. (B) Appearance of the patient 1 year after surgery. Range of motion at the left shoulder was improved after reconstruction of the breast conservation defect with a left latissimus dorsi myocutaneous flap. (C) Postoperative view 1 year after reconstruction. (D) Postoperative view 15 years after reconstruction.
REFERENCES
- Halsted W S. The results of radical operations for the cure of carcinoma of the breast. Ann Surg. 1907;66:1. doi: 10.1097/00000658-190707000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher B, Redmond C, Fisher E R, et al. Ten-year results of a randomized clinical trial comparing radical mastectomy and total mastectomy with or without radiation. N Engl J Med. 1985;312:674–681. doi: 10.1056/NEJM198503143121102. [DOI] [PubMed] [Google Scholar]
- Jacobson J A, Danforth D N, Cowan K H, et al. Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N Engl J Med. 1995;332:907–911. doi: 10.1056/NEJM199504063321402. [DOI] [PubMed] [Google Scholar]
- Veronesi U, Banfi A, Delvecchio M, et al. Comparison of Halsted mastectomy with quadrantectomy, axillary dissection and radiotherapy in early breast cancer: long term results. Eur J Cancer Clin Oncol. 1986;22:1085–1089. doi: 10.1016/0277-5379(86)90011-8. [DOI] [PubMed] [Google Scholar]
- Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- Beadle G F, Silver B, Botnick L, Hellman S, Harris J R. Cosmetic results following primary radiation therapy for early breast cancer. Cancer. 1984;54:2911–2918. doi: 10.1002/1097-0142(19841215)54:12<2911::aid-cncr2820541216>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Fujishiro S, Misumori M, Kokubo M, et al. Cosmetic results and complications after breast conserving therapy for early breast cancer. Breast Cancer. 2000;7:57–63. doi: 10.1007/BF02967189. [DOI] [PubMed] [Google Scholar]
- Matory W E, Wertheimer M, Fitzgerald T J, Walton R L, Love S, Mato W E. Aesthetic results following partial mastectomy and radiation therapy. Plast Reconstr Surg. 1990;85:739–746. doi: 10.1097/00006534-199005000-00014. [DOI] [PubMed] [Google Scholar]
- Rose M A, Olivotto I, Cady B, et al. Conservative surgery and radiation therapy for early breast cancer. Long-term cosmetic results. Arch Surg. 1989;124:153–157. doi: 10.1001/archsurg.1989.01410020023002. [DOI] [PubMed] [Google Scholar]
- Amichetti M, Busana L, Caffo O. Long-term cosmetic outcome and toxicity in patients treated with quadrantectomy and radiation therapy for early-stage breast cancer. Oncology. 1995;52:177–181. doi: 10.1159/000227454. [DOI] [PubMed] [Google Scholar]
- Steeves R A, Phromratanapongse P, Wolberg W H, Tormey D C. Cosmesis and local control after irradiation in women treated conservatively for breast cancer. Arch Surg. 1989;124:1369–1373. doi: 10.1001/archsurg.1989.01410120015004. [DOI] [PubMed] [Google Scholar]
- Al-Ghazal S K, Blamey R W, Stewart J, Morgan A A. The cosmetic outcome in early breast cancer treated with breast conservation. Eur J Surg Oncol. 1999;25:566–570. doi: 10.1053/ejso.1999.0707. [DOI] [PubMed] [Google Scholar]
- Veronesi U, Volterrani F, Luini A, et al. Quadrantectomy versus lumpectomy for small size breast cancer. Eur J Cancer. 1990;26:671–673. doi: 10.1016/0277-5379(90)90114-9. [DOI] [PubMed] [Google Scholar]
- Veronesi U, Luini A, Galimberti V, Zurrida S. Conservation approaches for the management of stage I/II carcinoma of the breast: Milan Cancer Institute trials. World J Surg. 1994;18:70–75. doi: 10.1007/BF00348194. [DOI] [PubMed] [Google Scholar]
- Fagundes M A, Fagundes H M, Brito C S, Fagundes M H, Daudt A, Bruno L A, Azevedo S J, Fagundes L A. Breast-conserving surgery and definitive radiation: a comparison between quadrantectomy and local excision with special focus on local-regional control and cosmesis. Int J Radiat Oncol Biol Physiol. 1993;27:553–560. doi: 10.1016/0360-3016(93)90379-a. [DOI] [PubMed] [Google Scholar]
- Arcangeli G, Micheli A, D'Angelo L, et al. Conservative surgery and radiotherapy in early stage breast cancer: a comparison between tumourectomy and quadrantectomy. Radiother Oncol. 1997;45:39–45. doi: 10.1016/s0167-8140(97)00109-6. [DOI] [PubMed] [Google Scholar]
- Beadle G F, Silver B, Botnick L, Hellman S, Harris J R. Cosmetic results following primary radiation therapy for early breast cancer. Cancer. 1984;54:2911–2918. doi: 10.1002/1097-0142(19841215)54:12<2911::aid-cncr2820541216>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Vrieling C, Collette L, Fourquet A, et al. The influence of the boost in breast-conserving therapy on cosmetic outcome in EORTC “boost versus no boost” trial. EORTC Radiotherapy and Breast Cancer Cooperative Groups. European Organization for Research and Treatment of Cancer. Int J Radiation Oncol Biol Physiol. 1999;45:677–685. doi: 10.1016/s0360-3016(99)00211-4. [DOI] [PubMed] [Google Scholar]
- Olivotto I A, Rose M A, Osteen R T, et al. Late cosmetic outcome after conservative surgery and radiotherapy: an analysis of causes of cosmetic failure. Int J Radiat Oncol Biol Physiol. 1989;17:747–753. doi: 10.1016/0360-3016(89)90061-8. [DOI] [PubMed] [Google Scholar]
- Wazer D E, DiPetrillo T, Schmidt-Ullrich R, et al. Factors influencing cosmetic outcome and complication risk after conservation surgery and radiotherapy for early-stage breast carcinoma. J Clin Oncol. 1992:356–363. doi: 10.1200/JCO.1992.10.3.356. [DOI] [PubMed] [Google Scholar]
- Taylor M E, Perez C A, Halverson K J, et al. Factors influencing cosmetic result after conservation therapy for breast cancer. Int J Radiat Oncol Biol Physiol. 1995;31:753–764. doi: 10.1016/0360-3016(94)00480-3. [DOI] [PubMed] [Google Scholar]
- Limbergen E Van, Rijnders A, der Schueren E van, Lerut T, Christieaens R. Cosmetic evaluation of breast conserving treatment for mamma cancer. Radiother Oncol. 1989;16:253–267. doi: 10.1016/0167-8140(89)90037-6. [DOI] [PubMed] [Google Scholar]
- Abner A L, Recht A, Vicini F A, et al. Cosmetic results after surgery, chemotherapy, and radiation therapy for early breast cancer. Int J Radiat Oncol Biol Physiol. 1991;21:331–338. doi: 10.1016/0360-3016(91)90779-4. [DOI] [PubMed] [Google Scholar]
- Moro G, Stasi M, Borca V C. Does concomitant chemoradiotherapy influence cosmetic outcome in conservative treatment of breast cancer? Tumori. 1997;83:743–747. doi: 10.1177/030089169708300406. [DOI] [PubMed] [Google Scholar]
- Recht A, Sadowsky N L, Cady B. Clinical problems in follow-up of patients following conservative surgery and radiotherapy. Surg Clin N Am. 1990;70:1179–1186. doi: 10.1016/s0039-6109(16)45238-2. [DOI] [PubMed] [Google Scholar]
- Brenner R J, Pfaff J M. Mammographic features after conservation therapy for malignant breast disease: serial findings standardized by regression analysis. AJR Am J Roentgenol. 1996;167:171–178. doi: 10.2214/ajr.167.1.8659366. [DOI] [PubMed] [Google Scholar]
- Solin L J, Fowble B L, Troupin R H, Goodman R L. Biopsy results of new calcifications in the postirradiated breast. Cancer. 1989;63:1956–1961. doi: 10.1002/1097-0142(19890515)63:10<1956::aid-cncr2820631015>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Petit J Y, Garusi C, Greuse M, et al. One hundred and eleven cases of breast conservation treatment with simultaneous reconstruction at the European Institute of Oncology. Tumori. 2002;88:41–47. [PubMed] [Google Scholar]
- Bold R J, Kroll S S, Baldwin B J, Ross M I, Singletary S E. Local rotational flaps for breast conservation therapy as an alternative to mastectomy. Ann Surg Oncol. 1997;4:540–544. doi: 10.1007/BF02305533. [DOI] [PubMed] [Google Scholar]
- Clough K B, Lewis J S, Couturaud B, Fitoussi A, Nos C, Falcou M-C. Oncoplastic techniques allow extensive resections for breast-conserving therapy of breast carcinoma. Ann Surg. 2003;237:26–34. doi: 10.1097/00000658-200301000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit J Y, Rietjens M, Contesso G, Bertin F, Gilles R. Contralateral mastoplasty for breast reconstruction: a good opportunity for glandular exploration and occult carcinomas diagnosis. Ann Surg Oncol. 1997;4:511–515. doi: 10.1007/BF02303678. [DOI] [PubMed] [Google Scholar]
- Losken A, Elwood E, Styblo T, Bostwick J. The role of reduction mammoplasty in reconstructing partial mastectomy defects. Plast Reconstr Surg. 2002;109:968–975. doi: 10.1097/00006534-200203000-00025. [DOI] [PubMed] [Google Scholar]
- Noguchi M, Taniya T, Tsugawa K, Miwa K. Expanding the role of breast-conserving therapy using immediate volume replacement. Breast Cancer. 1998;5:219–226. doi: 10.1007/BF02966700. [DOI] [PubMed] [Google Scholar]
- Raja M A, Straker V F, Rainsbury R M. Extending the role of breast conserving surgery to immediate volume replacement. Br J Surg. 1997;84:101–105. [PubMed] [Google Scholar]
- Rainsbury R M, Paramanathan N. Recent progress with breast-conserving volume replacement using latissimus dorsi miniflaps in UK patients. Breast Cancer. 1998;5:139–147. doi: 10.1007/BF02966686. [DOI] [PubMed] [Google Scholar]
- Ohuchi N, Harada Y, Ishida T, Kiyohara H, Satomi S. Breast-conserving surgery for primary breast cancer: immediate volume replacement using lateral tissue flaps. Breast Cancer. 1997;4:135–141. doi: 10.1007/BF02967067. [DOI] [PubMed] [Google Scholar]
- Noguchi M, Tsugawa K, Taniya T, Miwa K. The outcome of patients who underwent breast conserving treatment with or without immediate volume replacement with autogenous tissue. Breast Cancer. 1999;5:43–50. doi: 10.1007/BF02966905. [DOI] [PubMed] [Google Scholar]
- Tran N V, Chang D W, Gupta A, Kroll S, Robb G. Comparison of immediate and delayed free tram flap breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg. 2001;108:78–82. doi: 10.1097/00006534-200107000-00013. [DOI] [PubMed] [Google Scholar]
- Abramson D L, Cooper S, Wait R B. Inverted deepithelialized latissimus dorsi flap for the correction of lumpectomy defects in the irradiated breast. Ann Plast Surg. 1998;40:664–667. doi: 10.1097/00000637-199806000-00017. [DOI] [PubMed] [Google Scholar]
- Clough K B, Kroll S S, Audretsch W. An approach to the repair of partial mastectomy defects. Plast Reconstr Surg. 1999;104:409–420. doi: 10.1097/00006534-199908000-00014. [DOI] [PubMed] [Google Scholar]
- Hogge J P, Zuurbier R A, De Paredes E S. Mammography of autologous musculocutaneous flaps. Radiographics. 1999;19:S63–S72. doi: 10.1148/radiographics.19.suppl_1.g99oc12s63. [DOI] [PubMed] [Google Scholar]
- Slavin S, Love S, Sadowsky N. Reconstruction of the radiated partial mastectomy defect. Plast Reconstr Surg. 1992;90:854–864. [PubMed] [Google Scholar]
- Slavin S. Breast reconstruction after lumpectomy-radiation and quadrantectomy-radiation. Operative Techniques in Plastic and Reconstructive Surgery. 1994;1:28–34. [Google Scholar]