Abstract
Breast reconstruction with tissue expanders and implants offers patients satisfying aesthetic results with no donor site morbidity. This article provides a practical guide for successful reconstruction using current techniques and available devices. Preoperative planning is discussed, emphasizing close collaboration with medicial and surgical oncology colleagues. Special concerns regarding adjuvant radiation are also addressed. Intraoperative techniques that optimize the final result are presented, along with a reliable and reasoned approach to the management of complications. Following these guidelines, aesthetically pleasing results with few complications can be obtained consistently.
Keywords: Breast reconstruction, tissue expanders, breast implants, breast cancer
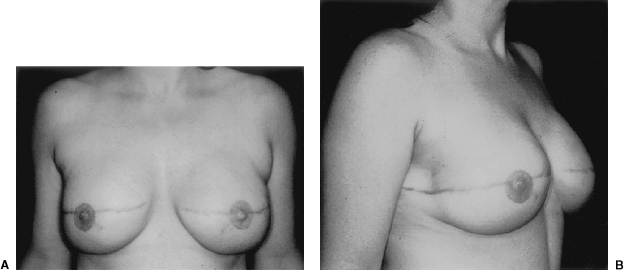
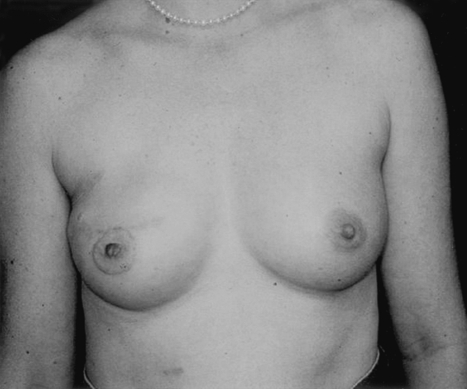
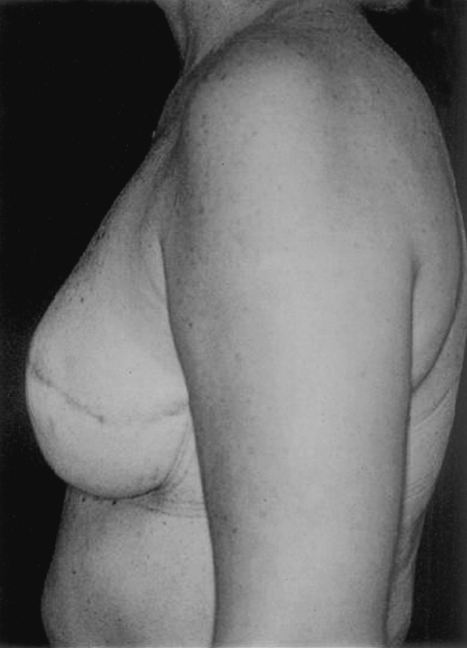
Implant breast reconstruction has evolved over the past two decades into a highly successful and satisfying method of reconstruction. Although conceptually simple, it is now a precise and demanding art that requires the integration of many variables.1 These include preoperative consideration of patient expectations, communication with oncology colleagues regarding possible adjuvant therapy, and decision making about expander/implant selection. Operatively, close collaboration with the surgical oncologist is essential,2 as is a clear appreciation of the technical aspects of device placement. Finally, careful postoperative management is crucial to appropriately manage complications and minimize the incidence of explantation. With careful attention to detail, implant breast reconstruction offers patients a very satisfactory restoration of body form with minimal morbidity.3 For both patient and surgeon, the results can be tremendously gratifying (Fig. 1).
Figure 1.
(A,B) This woman had immediate bilateral reconstruction. Note the natural appearing breast ptosis.
PREOPERATIVE CONSIDERATIONS
Women contemplating breast reconstruction, either in the immediate or delayed setting, must assimilate a considerable amount of information. Clear communication regarding the nature of the reconstructive process is essential. In the preoperative consultation, it is important to set reasonable expectations regarding the likely aesthetic outcome and possible complications.4 This discussion should also include options and timing for management of the contralateral breast. For immediate reconstruction patients, the implications of possible adjuvant chemotherapy or radiation should be delineated.5,6
Implant reconstruction is contraindicated in the presence of an inadequate skin envelope. This may be due to previous biopsies or to locally advanced disease necessitating a large skin excision at the time of mastectomy. Previous radiation is a relative contraindication to implant reconstruction because of the increased risk of extrusion, poor expansion, and capsular contracture.7 The implications of pre- and postoperative radiation are discussed later in this article (under Special Considerations).
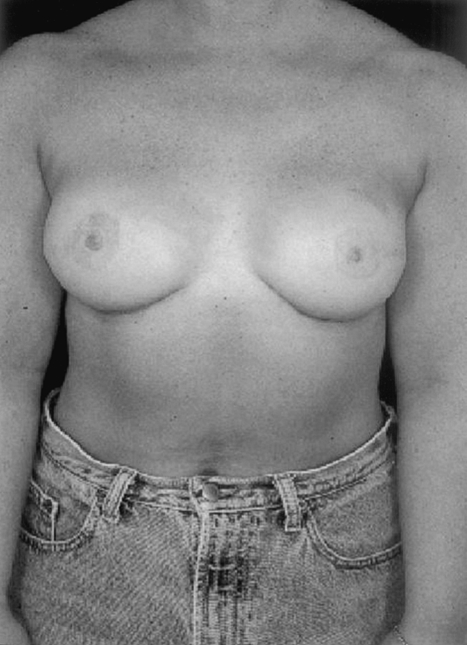
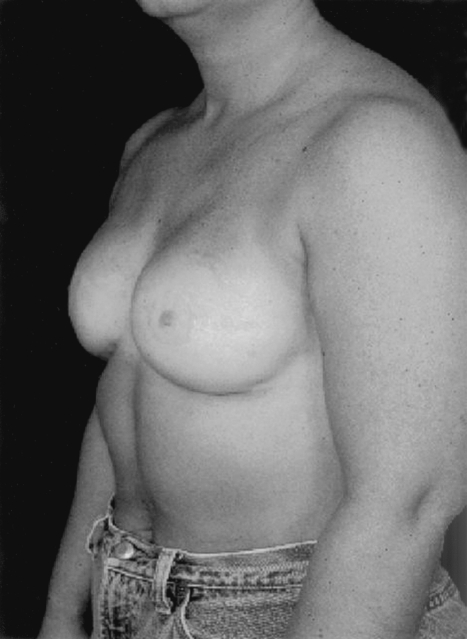
The success of implant reconstruction is significantly influenced by the manner in which the oncological surgery is performed. Placement of excision biopsy scars, design of the mastectomy incisions, maintenance of adequate flap thickness and vascularity, and preservation of the inframammary fold all impact heavily on the final reconstructive outcome (Figs. 2 and 3). Close collaboration with oncological surgery colleagues is fundamental. In general, biopsy incisions should be as close to the areola as possible and parallel to the eventually mastectomy incision. Whenever possible, a skin-sparing mastectomy should be performed and excision of lower pole skin avoided (Figs. 4 and 5). This will result in a short, laterally placed transverse scar. The inframammary fold should be well-marked preoperatively and respected as the inferior limit of the mastectomy. In addition, maintenance of the fascia overlying the inferomedial border of the pectoralis muscle, the superior edge of the rectus, and the medial extension of the serratus will greatly facilitate musculofascial coverage of the expander.
Figure 2.
Bilateral reconstruction with mastectomy incision has been laterally placed, avoiding any distortion of the medial breast.
Figure 3.
Bilateral reconstruction with mastectomy incision has been laterally placed, avoiding any distortion of the medial breast.
Figure 4.
This patient has had an immediate unilateral reconstruction. A skin-sparing mastectomy with minimal excision of lower pole skin has facilitated this result.
Figure 5.
This patient has had an immediate unilateral reconstruction. A skin-sparing mastectomy with minimal excision of lower pole skin has facilitated this result.
FIRST STAGE
Implant breast reconstruction is generally best performed as a two-stage procedure. Although satisfactory results can be obtained with single-stage surgery, for most patients a more reliable approach involves removal of the expander and placement of the permanent device as a second operation. This second stage allows for (1) precise positioning of the inframammary fold; (2) capsulotomy to release soft tissue, thus increasing breast projection and ptosis; and (3) reevaluation of breast height and width to achieve maximal symmetry with the contralateral breast.
Selection of the optimal tissue expander requires careful assessment of the breast dimensions. In general, an expander should be selected that accurately matches breast width. The height of the device may exceed that of the contralateral breast to permit full expansion of the breast skin flaps. The potential volume of the expander should equal or slightly exceed the estimated breast volume. In the setting of a delayed reconstruction, a review of the previous mastectomy weight/volume may be helpful as an estimate of the contralateral breast volume.
Precise positioning of the expander is critical to the success of the reconstruction. In delayed reconstructions, the lateral one third of the mastectomy incision is reopening to provide access to the subpectoral plane. In immediate reconstruction, the lateral border of the pectoralis is easily elevated. The submuscular dissection is then continued medially to ∼1 cm from the midline. Inferiorly, the dissection proceeds to the junction of the pectoralis fascia with the rectus fascia. As the dissection extends below the inframammary fold, it remains deep to the overlying fascia. Once below the level of the breast resection, the dissection changes to a more superficial plane. This facilitates release of the superficial fascia, which would otherwise hinder the expansion process.
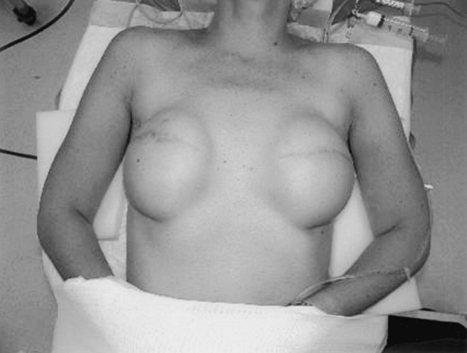
The expander should ideally be placed 1 to 2 cm below the inframammary fold to allow for maximal expansion in the lower pole of the breast (Fig. 6). In bilateral reconstructions, great care should be taken to obtain symmetric expander placement. If at all possible, total musculofascial coverage of the device should be achieved. In the event of skin flap necrosis, total muscle coverage will decrease the risk of device exposure (Fig. 7). Laterally, a flap of serratus fascia and muscle is raised to the anterior axillary line. It is not necessary to raise the entire thickness of the serratus muscle. The serratus flap is then sutured to the lateral border of the pectoralis major muscle. Drains are then placed and a secure, layered skin closure performed. The mastectomy flaps should be tailored to minimize medial and lateral skin excess and to maximize future breast contour.
Figure 6.
Note the position of the expanders below the inframammary fold. The lower pole has been maximally expanded.
Figure 7.
It is important to achieve total muscle coverage of the expander whenever possible.
Intraoperative expansion may be performed if the mastectomy flaps are judged to have adequate laxity and vascularity; 20 to 40% of the expander volume may be placed (Fig. 8). This helps to decrease dead space while maintaining skin tension. In the absence of skin flap necrosis, postoperative expansions may begin 10 to 14 days after surgery. Following this, 60 to 100 mL are injected every 7 days. The final expander volume is usually 20% greater than the planned implant volume or 20% greater than the recommended volume of the tissue expander.
Figure 8.
Intraoperative expansion may be performed if the skin flaps are judged to have adequate laxity and vascularity.
SECOND STAGE
Removal of the expander and placement of the permanent implant may be performed approximately 1 month after completion of the expansion. If the patient has undergone chemotherapy or radiation, the second-stage surgery should be scheduled no sooner than 1 month after completion of adjuvant treatment to minimize the risk of local wound complications. Prior to surgery, the patient should be well informed regarding implant risks and have the opportunity to select either a silicone- or saline-filled implant. In general, silicone implants may give a more natural and pleasing texture to the breast. Use of a saline implant in unilateral reconstructions, on the other hand, presents more options with regard to shape and volume of the implant. This is particularly helpful for difficult or larger-volume reconstructions.
At the time of surgery, a full circumferential capsulotomy is performed. It is important that the release extend into the subcutaneous tissues to fully release the skin envelope. In combination with the implant, the skin envelope predominately determines the shape of the reconstructed breast.8 Additional capsulotomies may be needed to achieve full breast projection. It is also important to release the inferomedial border of the pectoralis muscles just as one does in cosmetic breast augmentation.
The inframammary fold is released with the circumferential capsulotomy. The area of the fold is then advanced and carefully approximated to the anterior chest wall with interrupted, permanent sutures. This step is critical to obtaining appropriate breast shape and ptosis. It is not necessary to suture to periosteum as this causes needless patient discomfort. It is sometimes useful to also close the lateral capsule with interrupted sutures to prevent lateral displacement of the implant.
The shape, volume, and projection of the final implant needs to be carefully considered both preoperatively and intraoperatively. In unilateral reconstructions, implant selection is guided by the dimensions of the contralateral breast and in bilateral reconstruction, by the proportions of the chest wall. Breast height and width tends to fall into one of several categories. For example, tall, slender patients may have increased breast height with a narrow width. Conversely, older, overweight patients are more likely to have a short, wide breast. Although a round implant may be occasionally used, selection of contoured implants helps to incorporate these dimensions. Depending on the type of expander used, implant volume will generally be ∼80% of the final expander volume. If possible, it is also helpful to consider the original mastectomy weight. Full-projection implants are most commonly used to try to match the projection of a contralateral breast.
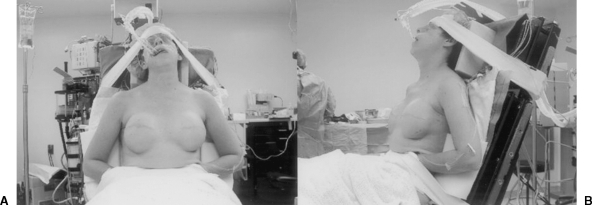
The use of implant sizers is essential to intraoperative implant selection and determination of volume. This applies for both saline and silicone implants. It is also critical that the patient be brought into the full upright sitting position (Fig. 9). The upright position may be considered the “anatomic position” for the breast and should be the point of reference for decisions regarding shape, volume, projection, and position.
Figure 9.
(A,B) At the time of expander removal, it is important that the patient be positioned such that she may be safely brought into an upright sitting position to aid implant placement.
Finally, a careful two-layered closure completes the second stage. The use of drains is strongly recommended to control seromas and maintain the orientation of contoured implants.
MANAGEMENT OF THE CONTRALATERAL BREAST
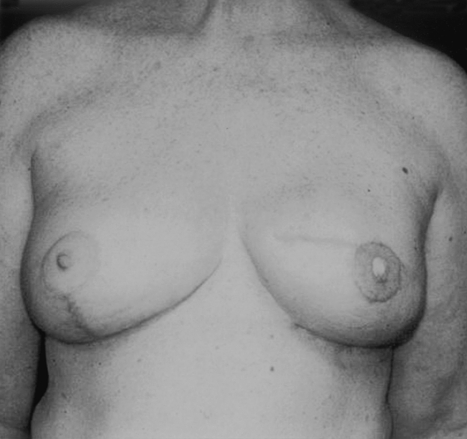
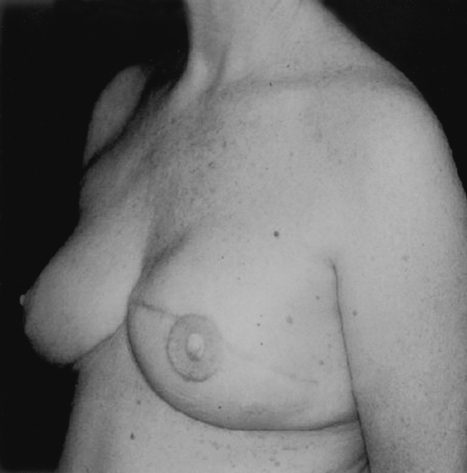
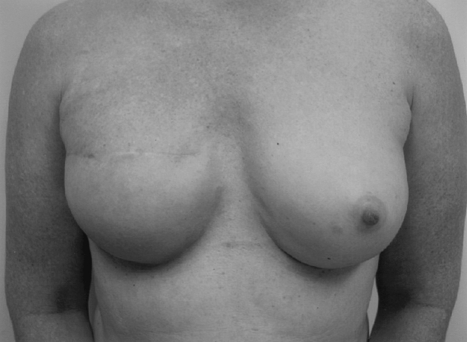
In unilateral reconstructions, procedures performed on the contralateral breast can greatly improve the symmetry of the reconstruction. These procedures can be performed at either the first or second stage. For patients with large breasts who will require contralateral reduction, this surgery is best performed at the time of final implant placement to maximize symmetry of the breasts mounds (Figs. 10 and 11). Similarly for patients requiring a subtle augmentation of the contralateral breast, this is best deferred until final implant placement when the shape and projection of the reconstructed breast has been more clearly delineated.
Figure 10.
This woman had a unilateral reconstruction with a contralateral breast reduction.
Figure 11.
This woman had a unilateral reconstruction with a contralateral breast reduction.
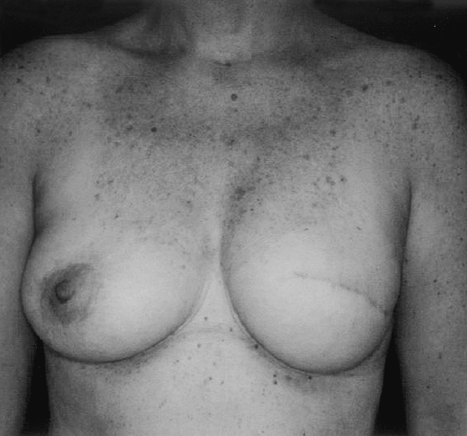
Mastopexy and small reductions can be performed using a periareolar Benelli-type approach (Figs. 12 and 13). This technique tends to decrease breast projection, which is advantageous when the reconstructed breast also has limited projection. Larger reductions are preferably performed using an inferior pedicle, Wise-pattern technique. In contradistinction to vertical scar techniques, the Wise-pattern breast reduction results in less projection relative to the base width of the breast. This outcome more closely mirrors the final appearance of an implant reconstruction. Similarly, for patients requiring contralateral augmentation to improve upper pole fullness, a low-projection implant will provide better symmetry and superior results.
Figure 12.
A periareolar mastopexy has been performed to improve symmetry in this unilateral reconstruction.
Figure 13.
A periareolar mastopexy has been performed to improve symmetry in this unilateral reconstruction.
MANAGING COMPLICATIONS
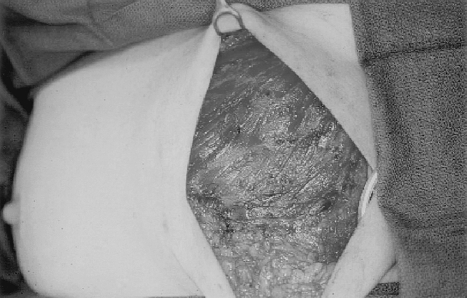
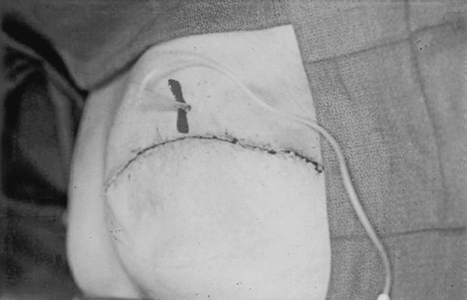
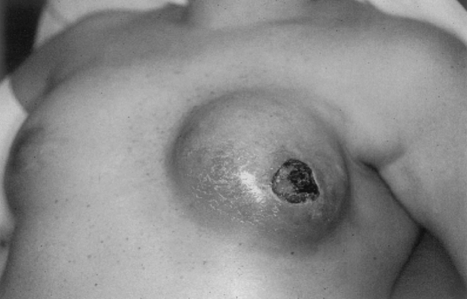
Timely, appropriate management of complications following implant reconstruction can reduce patient morbidity and decrease the incidence of explantation. In the immediate postoperative period, skin flap necrosis is not uncommon, particularly in smokers or when excessively thin mastectomy flaps have been raised. If the flaps appear poorly perfused at the time of surgery, it is prudent to excise back to healthy, bleeding wound edges. It is also important to achieve total muscle coverage of the expander. In the event of postoperative skin necrosis, this will ensure that the device is not exposed. Wound management with conservative debridement and dressing changes can then generally be performed and the wound allowed to heal by secondary intention. If a larger area of necrosis occurs, operative debridement and wound closure is warranted (Fig. 14).
Figure 14.
Skin necrosis and implant extrusion.
Cellulitis should be treated aggressively, first with intravenous antibiotics and failing this, with removal of the device.9 This is particularly true for radiated patients. If a purulent collection is obvious, then early removal of the device is recommended.10 Extrusion of either the expander or the implant may occur early in the postoperative period or later during adjuvant treatment. Extrusion usually requires definitive removal of the device and delayed, flap reconstruction.
Significant capsular contracture occurs in 10% of nonradiated patients and in 40% of radiated patients.11 Open capsulotomy with partial capsulectomy appears to be of benefit. For those patients who develop recurrent contracture following capsulotomy, conversion to flap reconstruction is advisable as further contracture is likely to occur.
SPECIAL CONSIDERATIONS
The optimal approach to immediate reconstruction of patients who may require adjuvant radiotherapy following mastectomy has yet to be determined.12 Radiated patients who seek delayed reconstruction also represent a controversial group. This includes patients who may have undergone breast-conserving surgery and radiotherapy and then subsequently required mastectomy because of local recurrence.
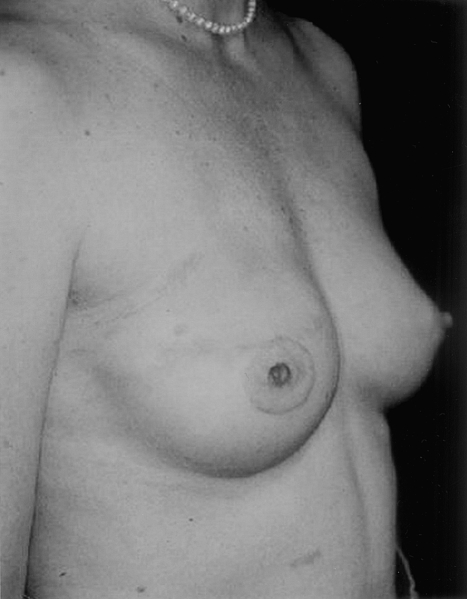
In these subgroups, the incidence of complications following implant reconstruction is clearly higher. Patients are at increased risk of infection, extrusion, and contracture. Nevertheless, a significant proportion will have a satisfactory result and will not require further surgery (Fig. 15).11 Given this, it is important that women be well informed and actively involved in reconstructive decision making. For those who may not be candidates for flap reconstruction or who are unwilling to accept possible donor site morbidity, implant reconstruction remains a valuable option. It is, however, vitally important that these patients recognize the increased risk of complication related to radiation and that they have reasonable expectations regarding the final result.
Figure 15.
This patient had delayed right breast reconstruction. She received postmastectomy radiation before beginning reconstruction.
REFERENCES
- Spear S L, Spittler C J. Breast reconstruction with implants and expanders. Plast Reconstr Surg. 2001;107:177–187. doi: 10.1097/00006534-200101000-00029. [DOI] [PubMed] [Google Scholar]
- Vandeweyer E, Hertens D, Nogaret J M, Deraemaecker R. Immediate breast reconstruction with saline-filled implants: no interference with the oncologic outcome? Plast Reconstr Surg. 2001;107:1409–1412. doi: 10.1097/00006534-200105000-00013. [DOI] [PubMed] [Google Scholar]
- Clough K B, O'Donoghue J M, Fitoussi A D, Nos C, Falcou M C. Prospective evaluation of late cosmetic results following breast reconstruction: I. Implant reconstruction. Plast Reconstr Surg. 2001;107:1702–1709. doi: 10.1097/00006534-200106000-00010. [DOI] [PubMed] [Google Scholar]
- Lin K Y, Johns F R, Gibson J, Long M, Drake D B, Moore M M. An outcome study of breast reconstruction: presurgical identification of risk factors for complications. Ann Surg Oncol. 2001;8:586–591. doi: 10.1007/s10434-001-0586-3. [DOI] [PubMed] [Google Scholar]
- Ragaz J, Jackson S M, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337:956–962. doi: 10.1056/NEJM199710023371402. [DOI] [PubMed] [Google Scholar]
- Overgaard M, Hansen P S, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337:949–955. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- Krueger E A, Wilkins E G, Strawderman M, et al. Complications and patient satisfaction following expander/implant breast reconstruction with and without radiotherapy. Int J Radiat Oncol Biol Phys. 2001;49:713–721. doi: 10.1016/s0360-3016(00)01402-4. [DOI] [PubMed] [Google Scholar]
- Hudson D A. Factors determining shape and symmetry in immediate breast reconstruction. Ann Plast Surg. 2004;52:15–21. doi: 10.1097/01.sap.0000099962.79156.16. [DOI] [PubMed] [Google Scholar]
- Nahabedian M Y, Tsangaris T, Momen B, Manson P N. Infectious complications following breast reconstruction with expanders and implants. Plast Reconstr Surg. 2003;112:467–476. doi: 10.1097/01.PRS.0000070727.02992.54. [DOI] [PubMed] [Google Scholar]
- Disa J J, Ad E, Cohen S M, Cordeiro P G, Hidalgo D A. The premature removal of tissue expanders in breast reconstruction. Plast Reconstr Surg. 1999;104:1662–1665. doi: 10.1097/00006534-199911000-00008. [DOI] [PubMed] [Google Scholar]
- Cordeiro P G, Pusic A L, Disa J J, McCormick B, VanZee K J. Irradiation after immediate tissue expander/implant breast reconstruction: complications, aesthetic results and patient satisfaction among 156 patients. Plast Reconstr Surg. 2004;113:877–881. doi: 10.1097/01.prs.0000105689.84930.e5. [DOI] [PubMed] [Google Scholar]
- Evans G R, Schusterman M A, Kroll S S, et al. Reconstruction and the radiated breast: is there a role for implants? Plast Reconstr Surg. 1995;96:1111–1115. [PubMed] [Google Scholar]