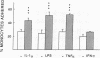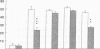Abstract
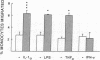
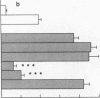
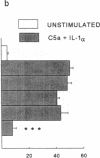
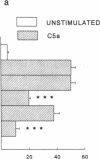
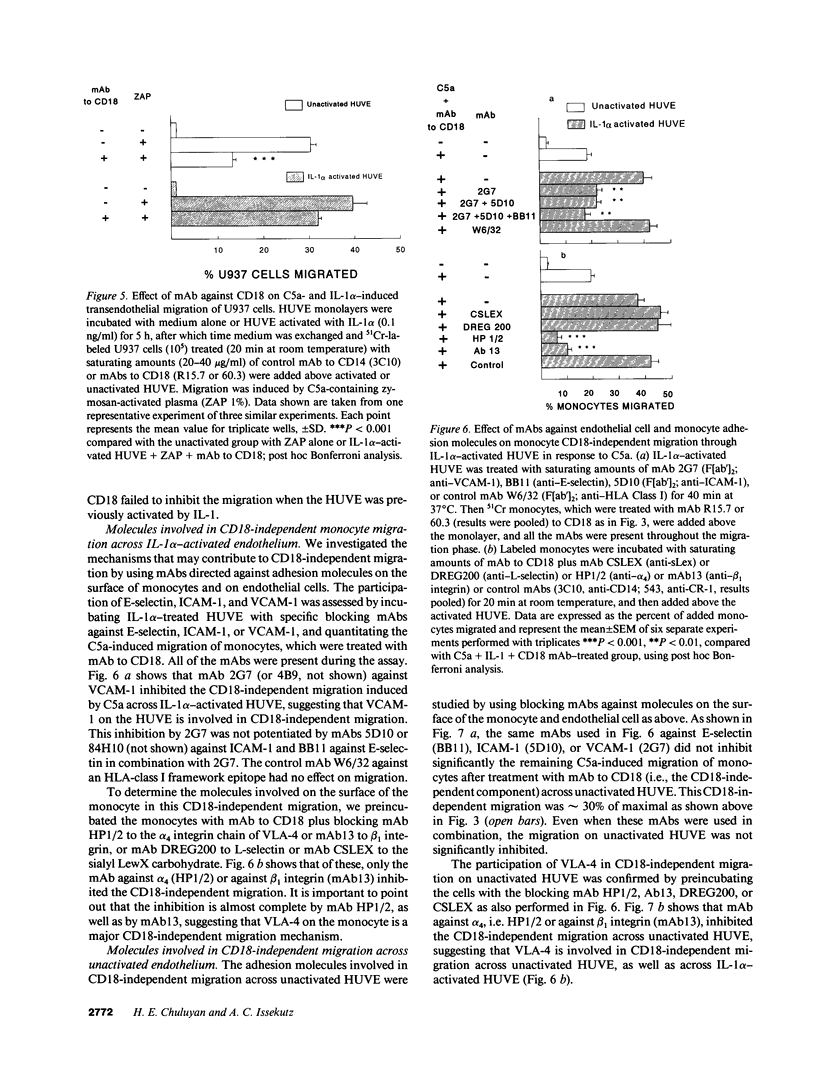
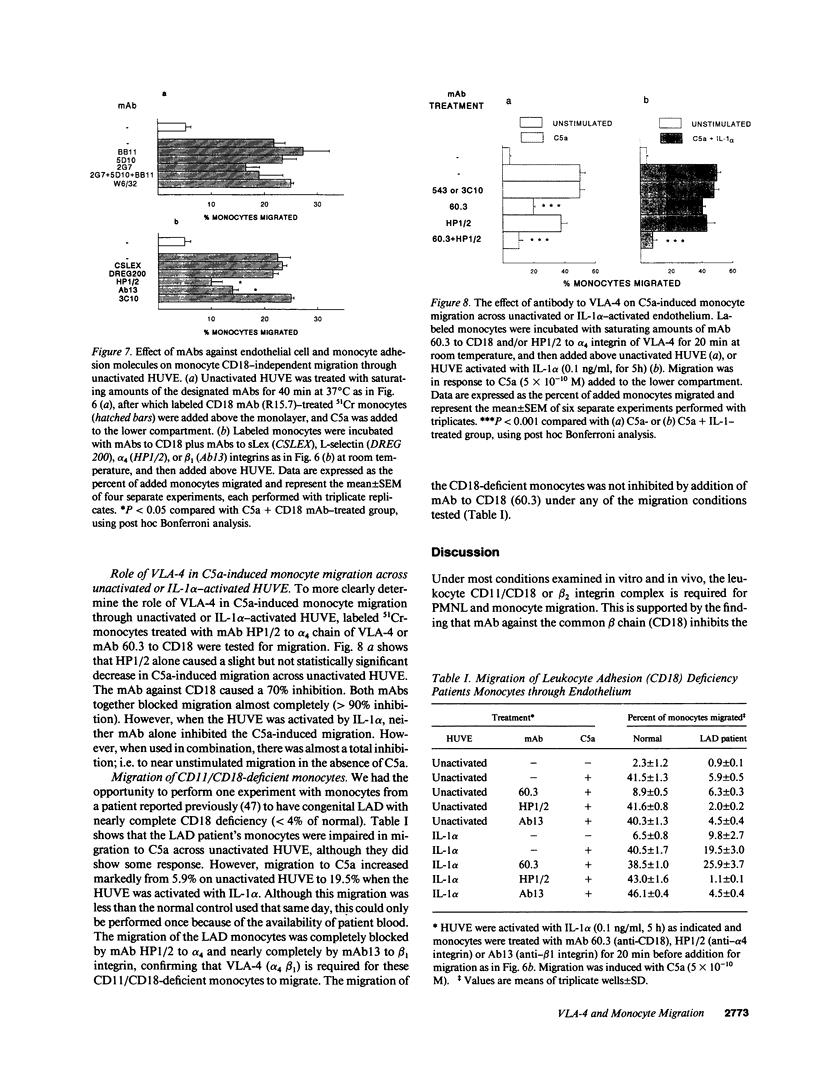
The migration of human monocytes across unactivated and activated human umbilical vein endothelium (HUVE) in response to chemotactic factors was studied, and the adhesion molecules involved were characterized. Migration of blood monocytes or U937 cell line-derived monocytes across unactivated HUVE induced by C5a, was partially inhibited (by 75%) by mAbs (R15.7 or 60.3) to CD18 of the CD11/CD18 complex on the monocyte. However, when the HUVE was pretreated for 5 h with IL-1 alpha (0.1 ng/ml), TNF-alpha (100 U/ml), or LPS (1 ng/ml), migration induced by C5a was no longer inhibited; i.e., migration became CD18 independent. The monocyte CD18-independent migration was completely blocked by mAbs against alpha 4 or beta 1 integrin chains of VLA-4. This migration was also partially inhibited by mAbs against vascular cell adhesion molecule-1 (VCAM-1), a major counter-receptor on HUVE for VLA-4, but not by mAbs to E-selectin or intercellular adhesion molecule-1. The significant CD18-independent migration across "unactivated" HUVE was also inhibited by mAbs against alpha 4 or beta 1 chains of VLA-4, although mAbs against VCAM-1 did not inhibit under these conditions. Finally, considerable VLA-4-dependent transendothelial migration to C5a was also observed with monocytes from a patient with CD18 deficiency (leukocyte adhesion deficiency). These results suggest that (a) there is a major CD18-independent component in monocyte chemotactic factor-dependent migration across activated and unactivated endothelium; (b) that VLA-4 integrin on the monocyte has a major role in this migration; and (c) that VCAM-1 on activated endothelium functions as a counter-receptor in this process, but other ligands for VLA-4, especially on unactivated endothelium, may also be involved.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S. K., Yamada S. S., Chen W. T., Yamada K. M. Analysis of fibronectin receptor function with monoclonal antibodies: roles in cell adhesion, migration, matrix assembly, and cytoskeletal organization. J Cell Biol. 1989 Aug;109(2):863–875. doi: 10.1083/jcb.109.2.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander-Miller M. A., Burke K., Koszinowski U. H., Hansen T. H., Connolly J. M. Alloreactive cytotoxic T lymphocytes generated in the presence of viral-derived peptides show exquisite peptide and MHC specificity. J Immunol. 1993 Jul 1;151(1):1–10. [PubMed] [Google Scholar]
- Anderson D. C., Schmalsteig F. C., Finegold M. J., Hughes B. J., Rothlein R., Miller L. J., Kohl S., Tosi M. F., Jacobs R. L., Waldrop T. C. The severe and moderate phenotypes of heritable Mac-1, LFA-1 deficiency: their quantitative definition and relation to leukocyte dysfunction and clinical features. J Infect Dis. 1985 Oct;152(4):668–689. doi: 10.1093/infdis/152.4.668. [DOI] [PubMed] [Google Scholar]
- Argenbright L. W., Letts L. G., Rothlein R. Monoclonal antibodies to the leukocyte membrane CD18 glycoprotein complex and to intercellular adhesion molecule-1 inhibit leukocyte-endothelial adhesion in rabbits. J Leukoc Biol. 1991 Mar;49(3):253–257. doi: 10.1002/jlb.49.3.253. [DOI] [PubMed] [Google Scholar]
- Beatty P. G., Ledbetter J. A., Martin P. J., Price T. H., Hansen J. A. Definition of a common leukocyte cell-surface antigen (Lp95-150) associated with diverse cell-mediated immune functions. J Immunol. 1983 Dec;131(6):2913–2918. [PubMed] [Google Scholar]
- Beekhuizen H., Corsèl-Van Tilburg A. J., Blokland I., Van Furth R. Characterization of the adherence of human monocytes to cytokine-stimulated human macrovascular endothelial cells. Immunology. 1991 Dec;74(4):661–669. [PMC free article] [PubMed] [Google Scholar]
- Benjamin C., Dougas I., Chi-Rosso G., Luhowskyj S., Rosa M., Newman B., Osborn L., Vassallo C., Hession C., Goelz S. A blocking monoclonal antibody to endothelial-leukocyte adhesion molecule-1 (ELAM1). Biochem Biophys Res Commun. 1990 Aug 31;171(1):348–353. doi: 10.1016/0006-291x(90)91400-m. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Stengelin S., Gimbrone M. A., Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989 Mar 3;243(4895):1160–1165. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- Bochner B. S., Luscinskas F. W., Gimbrone M. A., Jr, Newman W., Sterbinsky S. A., Derse-Anthony C. P., Klunk D., Schleimer R. P. Adhesion of human basophils, eosinophils, and neutrophils to interleukin 1-activated human vascular endothelial cells: contributions of endothelial cell adhesion molecules. J Exp Med. 1991 Jun 1;173(6):1553–1557. doi: 10.1084/jem.173.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner H. M., Lauffenburger D. A., Zigmond S. H. Measurement of leukocyte motility and chemotaxis parameters with the Millipore filter assay. J Immunol Methods. 1989 Sep 29;123(1):25–37. doi: 10.1016/0022-1759(89)90026-4. [DOI] [PubMed] [Google Scholar]
- Bøyum A. Isolation of human blood monocytes with Nycodenz, a new non-ionic iodinated gradient medium. Scand J Immunol. 1983 May;17(5):429–436. doi: 10.1111/j.1365-3083.1983.tb00809.x. [DOI] [PubMed] [Google Scholar]
- Carlos T. M., Schwartz B. R., Kovach N. L., Yee E., Rosa M., Osborn L., Chi-Rosso G., Newman B., Lobb R., Rosso M. Vascular cell adhesion molecule-1 mediates lymphocyte adherence to cytokine-activated cultured human endothelial cells. Blood. 1990 Sep 1;76(5):965–970. [PubMed] [Google Scholar]
- Carlos T., Kovach N., Schwartz B., Rosa M., Newman B., Wayner E., Benjamin C., Osborn L., Lobb R., Harlan J. Human monocytes bind to two cytokine-induced adhesive ligands on cultured human endothelial cells: endothelial-leukocyte adhesion molecule-1 and vascular cell adhesion molecule-1. Blood. 1991 May 15;77(10):2266–2271. [PubMed] [Google Scholar]
- Chan B. M., Kassner P. D., Schiro J. A., Byers H. R., Kupper T. S., Hemler M. E. Distinct cellular functions mediated by different VLA integrin alpha subunit cytoplasmic domains. Cell. 1992 Mar 20;68(6):1051–1060. doi: 10.1016/0092-8674(92)90077-p. [DOI] [PubMed] [Google Scholar]
- Darby H., Brown K. A., Anderson R. A., Williams B. T., Dumonde D. C. Transendothelial chemotaxis in vitro of human monocytes. J Immunol Methods. 1988 Oct 26;113(2):157–163. doi: 10.1016/0022-1759(88)90328-6. [DOI] [PubMed] [Google Scholar]
- Diamond M. S., Staunton D. E., Marlin S. D., Springer T. A. Binding of the integrin Mac-1 (CD11b/CD18) to the third immunoglobulin-like domain of ICAM-1 (CD54) and its regulation by glycosylation. Cell. 1991 Jun 14;65(6):961–971. doi: 10.1016/0092-8674(91)90548-d. [DOI] [PubMed] [Google Scholar]
- Elices M. J., Osborn L., Takada Y., Crouse C., Luhowskyj S., Hemler M. E., Lobb R. R. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990 Feb 23;60(4):577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- Fernandez H. N., Henson P. M., Otani A., Hugli T. E. Chemotactic response to human C3a and C5a anaphylatoxins. I. Evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo conditions. J Immunol. 1978 Jan;120(1):109–115. [PubMed] [Google Scholar]
- Firestein G. S. Cytokines in autoimmune diseases. Concepts Immunopathol. 1992;8:129–160. [PubMed] [Google Scholar]
- Fischer A., Lisowska-Grospierre B., Anderson D. C., Springer T. A. Leukocyte adhesion deficiency: molecular basis and functional consequences. Immunodefic Rev. 1988;1(1):39–54. [PubMed] [Google Scholar]
- Furie M. B., Tancinco M. C., Smith C. W. Monoclonal antibodies to leukocyte integrins CD11a/CD18 and CD11b/CD18 or intercellular adhesion molecule-1 inhibit chemoattractant-stimulated neutrophil transendothelial migration in vitro. Blood. 1991 Oct 15;78(8):2089–2097. [PubMed] [Google Scholar]
- Gavison R., Matzner Y., Fibach E. Differential induction of monocytic functions by dibutyryl cyclic AMP and retinoic acid in a human monoblast cell line U937. Isr J Med Sci. 1988 Dec;24(12):697–701. [PubMed] [Google Scholar]
- Graber N., Gopal T. V., Wilson D., Beall L. D., Polte T., Newman W. T cells bind to cytokine-activated endothelial cells via a novel, inducible sialoglycoprotein and endothelial leukocyte adhesion molecule-1. J Immunol. 1990 Aug 1;145(3):819–830. [PubMed] [Google Scholar]
- Hakkert B. C., Kuijpers T. W., Leeuwenberg J. F., van Mourik J. A., Roos D. Neutrophil and monocyte adherence to and migration across monolayers of cytokine-activated endothelial cells: the contribution of CD18, ELAM-1, and VLA-4. Blood. 1991 Nov 15;78(10):2721–2726. [PubMed] [Google Scholar]
- Hemler M. E. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- Issekutz A. C., Lopes N. Endotoxin activation of endothelium for polymorphonuclear leucocyte transendothelial migration and modulation by interferon-gamma. Immunology. 1993 Aug;79(4):600–607. [PMC free article] [PubMed] [Google Scholar]
- Issekutz T. B., Wykretowicz A. Effect of a new monoclonal antibody, TA-2, that inhibits lymphocyte adherence to cytokine stimulated endothelium in the rat. J Immunol. 1991 Jul 1;147(1):109–116. [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. L., Warren J. S. Monocyte chemoattractant protein 1 in a rat model of pulmonary granulomatosis. Lab Invest. 1992 Apr;66(4):498–503. [PubMed] [Google Scholar]
- Jonjic N., Jílek P., Bernasconi S., Peri G., Martìn-Padura I., Cenzuales S., Dejana E., Mantovani A. Molecules involved in the adhesion and cytotoxicity of activated monocytes on endothelial cells. J Immunol. 1992 Apr 1;148(7):2080–2083. [PubMed] [Google Scholar]
- Keizer G. D., Te Velde A. A., Schwarting R., Figdor C. G., De Vries J. E. Role of p150,95 in adhesion, migration, chemotaxis and phagocytosis of human monocytes. Eur J Immunol. 1987 Sep;17(9):1317–1322. doi: 10.1002/eji.1830170915. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. K., Jutila M. A., Butcher E. C. Identification of a human peripheral lymph node homing receptor: a rapidly down-regulated adhesion molecule. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2244–2248. doi: 10.1073/pnas.87.6.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. E., Burrows J. C., Haines G. K., Carlos T. M., Harlan J. M., Leibovich S. J. Immunolocalization of endothelial and leukocyte adhesion molecules in human rheumatoid and osteoarthritic synovial tissues. Lab Invest. 1991 Mar;64(3):313–320. [PubMed] [Google Scholar]
- Lasky L. A. Selectins: interpreters of cell-specific carbohydrate information during inflammation. Science. 1992 Nov 6;258(5084):964–969. doi: 10.1126/science.1439808. [DOI] [PubMed] [Google Scholar]
- Luscinskas F. W., Cybulsky M. I., Kiely J. M., Peckins C. S., Davis V. M., Gimbrone M. A., Jr Cytokine-activated human endothelial monolayers support enhanced neutrophil transmigration via a mechanism involving both endothelial-leukocyte adhesion molecule-1 and intercellular adhesion molecule-1. J Immunol. 1991 Mar 1;146(5):1617–1625. [PubMed] [Google Scholar]
- Migliorisi G., Folkes E., Pawlowski N., Cramer E. B. In vitro studies of human monocyte migration across endothelium in response to leukotriene B4 and f-Met-Leu-Phe. Am J Pathol. 1987 Apr;127(1):157–167. [PMC free article] [PubMed] [Google Scholar]
- Morzycki W., Sadowska J., Issekutz A. C. Interleukin-1 and tumour necrosis factor alpha induced polymorphonuclear leukocyte-endothelial cell adhesion and transendothelial migration in vitro: the effect of apical versus basal monolayer stimulation. Immunol Lett. 1990 Sep;25(4):331–340. doi: 10.1016/0165-2478(90)90204-4. [DOI] [PubMed] [Google Scholar]
- Moser R., Fehr J., Bruijnzeel P. L. IL-4 controls the selective endothelium-driven transmigration of eosinophils from allergic individuals. J Immunol. 1992 Aug 15;149(4):1432–1438. [PubMed] [Google Scholar]
- Moser R., Fehr J., Olgiati L., Bruijnzeel P. L. Migration of primed human eosinophils across cytokine-activated endothelial cell monolayers. Blood. 1992 Jun 1;79(11):2937–2945. [PubMed] [Google Scholar]
- Moser R., Schleiffenbaum B., Groscurth P., Fehr J. Interleukin 1 and tumor necrosis factor stimulate human vascular endothelial cells to promote transendothelial neutrophil passage. J Clin Invest. 1989 Feb;83(2):444–455. doi: 10.1172/JCI113903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movat H. Z., Cybulsky M. I. Neutrophil emigration and microvascular injury. Role of chemotaxins, endotoxin, interleukin-1 and tumor necrosis factor alpha. Pathol Immunopathol Res. 1987;6(3):153–176. doi: 10.1159/000157043. [DOI] [PubMed] [Google Scholar]
- Muller W. A., Weigl S. A. Monocyte-selective transendothelial migration: dissection of the binding and transmigration phases by an in vitro assay. J Exp Med. 1992 Sep 1;176(3):819–828. doi: 10.1084/jem.176.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. S., Wilson G. P., Todd R. F., Smith C. W., Anderson D. C., Varani J., Issekutz T. B., Miyasaka M., Tamatani T., Myasaka M. Role of beta 1, beta 2 integrins and ICAM-1 in lung injury after deposition of IgG and IgA immune complexes. J Immunol. 1993 Mar 15;150(6):2407–2417. [PubMed] [Google Scholar]
- Oppenheim J. J., Zachariae C. O., Mukaida N., Matsushima K. Properties of the novel proinflammatory supergene "intercrine" cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- Oppenheimer-Marks N., Davis L. S., Bogue D. T., Ramberg J., Lipsky P. E. Differential utilization of ICAM-1 and VCAM-1 during the adhesion and transendothelial migration of human T lymphocytes. J Immunol. 1991 Nov 1;147(9):2913–2921. [PubMed] [Google Scholar]
- Oppenheimer-Marks N., Davis L. S., Lipsky P. E. Human T lymphocyte adhesion to endothelial cells and transendothelial migration. Alteration of receptor use relates to the activation status of both the T cell and the endothelial cell. J Immunol. 1990 Jul 1;145(1):140–148. [PubMed] [Google Scholar]
- Osborn L., Hession C., Tizard R., Vassallo C., Luhowskyj S., Chi-Rosso G., Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989 Dec 22;59(6):1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- Osborn L., Vassallo C., Benjamin C. D. Activated endothelium binds lymphocytes through a novel binding site in the alternately spliced domain of vascular cell adhesion molecule-1. J Exp Med. 1992 Jul 1;176(1):99–107. doi: 10.1084/jem.176.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepinsky B., Hession C., Chen L. L., Moy P., Burkly L., Jakubowski A., Chow E. P., Benjamin C., Chi-Rosso G., Luhowskyj S. Structure/function studies on vascular cell adhesion molecule-1. J Biol Chem. 1992 Sep 5;267(25):17820–17826. [PubMed] [Google Scholar]
- Pober J. S., Cotran R. S. Cytokines and endothelial cell biology. Physiol Rev. 1990 Apr;70(2):427–451. doi: 10.1152/physrev.1990.70.2.427. [DOI] [PubMed] [Google Scholar]
- Pober J., Cotran R. S. What can be learned from the expression of endothelial adhesion molecules in tissues? Lab Invest. 1991 Mar;64(3):301–305. [PubMed] [Google Scholar]
- Pulido R., Elices M. J., Campanero M. R., Osborn L., Schiffer S., García-Pardo A., Lobb R., Hemler M. E., Sánchez-Madrid F. Functional evidence for three distinct and independently inhibitable adhesion activities mediated by the human integrin VLA-4. Correlation with distinct alpha 4 epitopes. J Biol Chem. 1991 Jun 5;266(16):10241–10245. [PubMed] [Google Scholar]
- Recalde H. R. A simple method of obtaining monocytes in suspension. J Immunol Methods. 1984 Apr 13;69(1):71–77. doi: 10.1016/0022-1759(84)90278-3. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Marlin S. D., Rothlein R., Toman C., Anderson D. C. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest. 1989 Jun;83(6):2008–2017. doi: 10.1172/JCI114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spertini O., Luscinskas F. W., Gimbrone M. A., Jr, Tedder T. F. Monocyte attachment to activated human vascular endothelium in vitro is mediated by leukocyte adhesion molecule-1 (L-selectin) under nonstatic conditions. J Exp Med. 1992 Jun 1;175(6):1789–1792. doi: 10.1084/jem.175.6.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Sáez-Llorens X., Jafari H. S., Olsen K. D., Nariuchi H., Hansen E. J., McCracken G. H., Jr Induction of suppurative arthritis in rabbits by Haemophilus endotoxin, tumor necrosis factor-alpha, and interleukin-1 beta. J Infect Dis. 1991 Jun;163(6):1267–1272. doi: 10.1093/infdis/163.6.1267. [DOI] [PubMed] [Google Scholar]
- Van Epps D. E., Potter J., Vachula M., Smith C. W., Anderson D. C. Suppression of human lymphocyte chemotaxis and transendothelial migration by anti-LFA-1 antibody. J Immunol. 1989 Nov 15;143(10):3207–3210. [PubMed] [Google Scholar]
- Vonderheide R. H., Springer T. A. Lymphocyte adhesion through very late antigen 4: evidence for a novel binding site in the alternatively spliced domain of vascular cell adhesion molecule 1 and an additional alpha 4 integrin counter-receptor on stimulated endothelium. J Exp Med. 1992 Jun 1;175(6):1433–1442. doi: 10.1084/jem.175.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Detmers P. A., Aida Y., Adamowski R., Anderson D. C., Chad Z., Kabbash L. G., Pabst M. J. CD18-deficient cells respond to lipopolysaccharide in vitro. J Immunol. 1990 Apr 1;144(7):2566–2571. [PubMed] [Google Scholar]
- Yednock T. A., Cannon C., Fritz L. C., Sanchez-Madrid F., Steinman L., Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992 Mar 5;356(6364):63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- Yoshimura T., Leonard E. J. Human monocyte chemoattractant protein-1: structure and function. Cytokines. 1992;4:131–152. [PubMed] [Google Scholar]