ABSTRACT
This article reviews the authors' experience over the last decade in the multidisciplinary management of children with brachial plexus birth injuries. When compared with the results of a study of 91 children who received nonoperative treatment, the results of surgical intervention can improve the functional outcome in properly selected infants.
Keywords: Erb's palsy, brachial plexus injury
In early 1991, three of the authors (JAIG, AEP, LR) were instrumental in the development of a brachial plexus program at New York University Medical Center. In 1996, the unit relocated to Miami Children's Hospital and merged with a long-standing neurology-based program (IA, OP) already at that institution. The resulting multidisciplinary program (peripheral nerve surgery, pediatric orthopedics, neurology, neuroradiology, neuropathology, rehabilitation medicine) evaluates and provides comprehensive management of over 150 new cases of brachial plexus birth trauma each year. This article summarizes some of our experiences to date with various aspects related to the care of children with brachial plexus birth injuries.
EVALUATION OF TREATMENT
The senior author (JAIG) had the opportunity during his training in the early 1980s to spend time with the late Professor A. Narakas,1 who strongly impressed upon him the value of careful and precise documentation of the various clinical parameters related to the treatment of patients with brachial plexus injuries and the importance of evaluating one's results using straightforward and reproducible methods. All patients seen in this program are evaluated with a routine clinical examination, including active and passive motion and sensibility testing when possible, combined with standardized videos suitable for later data analysis. The latter has been greatly aided over the past 5 years by digital technology.
In 2001, after a decade of work, we began an ongoing detailed evaluation of surgical results. It became apparent that the potential existed to improve upon the various inherent weaknesses in the commonly accepted systems for evaluation of the shoulder (Gilbert [Table 1], Mallet [Fig. 1]) and hand (Gilbert-Raimondi [Table 2]) functions.2,3
Table 1.
Gilbert Shoulder Classification
| Grade (Function) | Clinical Finding | |
|---|---|---|
| 0 (none) | Completely flail shoulder | |
| 1 (poor) | Abduction = 45 | No active external rotation |
| 2 (fair) | Abduction < 90 | No external rotation |
| 3 (satisfactory) | Abduction = 90 | Weak external rotation |
| 4 (good) | Abduction < 120 | Incomplete external rotation |
| 5 (excellent) | Abduction > 120 | Active external rotation |
Figure 1.
Mallet classification of shoulder following obstetrical brachial plexus injury. Total score from all columns: 0–4 indicates minimal function (grade 0); 5–9, poor (grade 1); 10–13, fair (grade 2); 14–17, satisfactory (grade 3); 18–22, good (grade 4); and 22–25, excellent (grade 5). (Adapted from Grossman JAI, Ramos LE, Shumway S, Alfonso I. Management strategies for children with obstetrical brachial plexus injuries. Int Pediatr 1997;12:82–86.)
Table 2.
Gilbert-Raimondi Classification of Impairment of the Hand in Patients with Obstetric Palsy
| Grade (Function) | Criteria |
|---|---|
| 0 (none) | Complete paralysis or slight finger flexion of no use, useless thumb—no pinch, some or no sensation |
| 1 (poor) | Limited active flexion of fingers; no extension of wrist or fingers; possibility of thumb lateral pinch |
| 2 (fair) | Active extension of wrist with passive flexion of fingers (tenodesis)—passive lateral pinch of thumb (pronation) |
| 3 (satisfactory) | Active complete flexion of wrists and fingers—mobile thumb with partial abduction—opposition intrinsic balance—no active supination; good possibilities for palliative surgery |
| 4 (good) | Active complete flexion of wrist and fingers; active wrist extension—weak or absent finger extensor; good thumb opposition with active ulnar intrinsics; partial prosupination |
| 5 (excellent) | Hand IV with finger extension and almost complete prosupination |
In addition to the preceding systems, we currently use our own shoulder score (Table 3), which takes into account the individual components of active elevation, active external rotation, and the presence of an internal rotation contracture. This makes up for certain weaknesses with choosing a grade in the Gilbert system (i.e., elevation 90 degrees; external rotation > 60 degrees). We have tested this system in a group of 35 randomly selected surgical cases of brachial plexus birth trauma. The shoulder function for each child was evaluated using Gilbert, Mallet, and Miami scales. The data were computer analyzed using Spearman's rho nonparametric correlations. A weak correlation (0.603345) between the results with Gilbert and Mallet was found. The Miami shoulder score had a higher and similar correlation between both Gilbert (0.784656) and Mallet (0.722301), suggesting that this method is useful for both accurate documentation of motion data and assessing functional outcome. Although this finding in no way reduces the proven value of both the Gilbert and Mallet systems,4 in our hands it appears to provide easier use, especially with video analysis, and more reliable reproducibility. In addition, the scapular instability pattern is recorded using the classification of Price (Fig. 2A–C).
Table 3.
Miami Shoulder Classification
| Range |
Total Score | Grade | |
|---|---|---|---|
| Average Active Abduction/Forward Flexion | Active External Rotation* | ||
| Total the scores for active abduction/forward flexion and active external rotation. | |||
| 0 degrees | Fixed deformity | 0 | 0 (no function) |
| < 45 degrees | Full passive < 10 degrees | 1–2 | 1 (poor) |
| < 90 degrees | < 30 degrees | 3–4 | 2 (fair) |
| 90–120 degrees | 30–45 degrees | 5–6 | 3 (satisfactory) |
| 120–150 degrees | 45–90 degrees | 7–8 | 4 (good) |
| > 150 degrees | 90 degrees | 9–10 | 5 (excellent) |
Maximum shoulder score = 10; decrease score by 1 point for a contracture > 20 degrees.
Figure 2.
(A–C) Patterns of scapular instability.
NATURAL HISTORY OF BRACHIAL PLEXUS BIRTH INJURIES
The timing and indications for surgical treatment of brachial plexus birth injuries remain somewhat controversial.5,6,7,8 In addition, considerable doubt has existed within the pediatric community concerning the natural history when these cases are allowed to recover spontaneously and whether early operative intervention can favorably alter the final impairment in certain injuries.9 One of the authors (PDT), in collaboration with the Hospital Materno Infantil in Mar del Plato, Argentina, has carefully evaluated and followed over a minimum 2-year period 91 consecutive infants born with a brachial plexus birth injury during a 7-year period (38,589 live births).10 All of the patients received only weekly occupational and physical therapy and underwent sequential motor examinations at 3- to 4-month intervals by the same examiners. Recovery of critical marker muscles (deltoid, biceps, triceps, and wrist extensors) was carefully documented at each follow-up using the British Medical Research Council Muscle Grading Scale. Shoulder and hand functions were evaluated after 2 years using the Miami Shoulder Scale and the Gilbert-Raimondi Hand Evaluation.
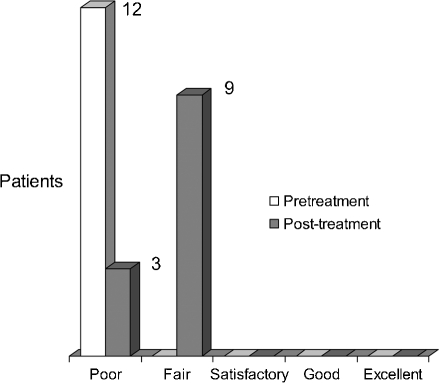
Three subsets of patients' outcomes were found. Type I (n = 12) infants presented at birth with a global palsy including a flail and insensate limb. At 6 months of age, no patient had greater than M1 deltoid or biceps, all patients lacked active wrist extension, and all patients had persistent extensive hand paralysis. Long-term follow-up showed extensive limitations throughout the extremity with minimal functional use (Figs. 3 and 4).
Figure 3.
Miami Classification. Shoulder Evaluation: Global Palsy (n = 12).
Figure 4.
Gilbert-Raimondi Classification. Hand Evaluation: Global Palsy (n = 12).
The type II subset of infants (n = 63) presented at birth with a typical Erb's palsy posture: shoulder paralysis with the arm held in adduction/internal rotation, absent biceps, weak triceps, and wrist extension. At 4 to 6 months, biceps and deltoid recovered M4-M5. At 3 to 6 months, triceps and the radial wrist extensors recovered M5. At final evaluation, no significant limitations in hand and wrist function were found. Mild limitations in shoulder range of motion were seen (Figs. 5 and 6).
Figure 5.
Miami Classification. Shoulder Evaluation: C5, C6 (n = 63).
Figure 6.
Gilbert-Raimondi Classification. Hand Evaluation: C5, C6 (n = 63).
The type III infants (n = 16) had an initial presentation at birth that was similar to that of the 63 infants with a satisfactory spontaneous recovery. However, minimal subsequent recovery was noted at 6 months; deltoid and biceps remained M1-M2 and wrist extension M0-M1. At the final evaluation, all children had a persistent deformity and functional loss (Figs. 7 and 8). In five children, recovery of active wrist extension with reasonable hand function occurred. In 11 children, the deficit involved the shoulder, forearm, wrist, and hand.
Figure 7.
Miami Classification. Shoulder Evaluation: C5, C6, C7 (n = 16).
Figure 8.
Gilbert-Raimondi Classification. Hand Evaluation: C5, C6, C7 (n = 12).
These findings clearly document the need to try to improve the outcome in types I and III and have provided us with a group of patients to compare with our surgically treated children.
EARLY NEUROSURGICAL TREATMENT
Pre- and Intraoperative Assessment of Spinal Nerve Integrity
A major challenge in the surgical management of brachial plexus injuries in infants is assessment of spinal nerve integrity. Knowing the spinal nerve integrity is crucial information for neurosurgical management and planning. A false interpretation of the spinal nerve integrity has a direct negative impact on the surgical outcome. To overcome this problem, we undertook a prospective study designed to evaluate the various modalities used for determining intra/extraforaminal spinal nerve integrity and continuity with the spinal cord.
One hundred consecutive spinal nerves from 58 infants with various types of brachial plexus birth injury, ages between 5 months and 25 months (mean age 8.75 months, standard deviation 4.34 months), were evaluated during a 2-year period by (1) preoperative high-resolution magnetic resonance imaging11; (2) preoperative subjective clinical evaluation; (3) intraoperative neurophysiology, including somatosensory evoked potentials to examine sensory pathways of the spinal nerve and intraoperative motor evoked potentials to examine motor pathways of the spinal nerve; and (4) intraoperative histopathology of the spinal nerve using a trichrome stain to obtain objective information on the amount of preserved myelinated fibers. There was no correlation between any pair of multidisciplinary modalities using chi-square statistical analysis (p = 0.05). No single modality provides consistent, reliable functional information on the spinal cord. Use of multiple modalities to determine spinal nerve integrity seems to provide a high rate of accurate determination as documented by our surgical results. This requires multidisciplinary input into surgical management.
Global Palsy
The data on the natural history of global palsy discussed earlier further confirm the opinion of all experienced surgeons who manage these cases that early operative intervention offers the best opportunity to restore some degree of motor and sensory function to the limb.12,13 Our approach is to operate between 3 and 5 months of age if possible. Although each case is individualized based on operative findings, we generally focus the surgical efforts on regaining shoulder stability and elbow flexion and providing for recovery of basic prehension and median sensibility. This often requires long sural nerve grafts passed beneath the clavicle to the lateral and posterior cords. During the period from 1997 to 2000, 40 cases of global palsy were operated and 36 were available for a recent follow-up. Shoulder function was graded by both the Miami and Gilbert shoulder scales as fair in 22%, satisfactory/good in 72.2%, and excellent in 5.5%. All children recovered functional elbow flexion (hand-to-mouth). Hand function as evaluated by the Gilbert-Raimondi system was fair in 19.4%, satisfactory in 58.3%, good in 16.6%, and excellent in 5.5%. When compared with the smaller series of nonoperated cases presented earlier in this article, the difference in the outcomes appears significant.
C5/C6/±C7 Lesions
This group encompasses a wide range of both clinical and intraoperative findings. In our practice, the decision to operate is based on sequential motor examinations focusing on recovery against gravity of biceps, deltoid, and wrist extensors. Precise early nerve conduction studies performed according to the protocol of Smith and Birch14 at the Royal National Orthopaedic Hospital in London have provided supportive data with a positive correlation in about 75% of our cases (n = 100). Nonetheless, the clinical examination still remains the “gold standard.”
For this report we reviewed C5/C6 (N = 34) and C5/C6/C7 (N = 43) surgical cases performed during the 4-year-period 1997 to 2000. There were 22 C5/C6 cases and 30 C5/C6/C7 cases available for a minimum 3-year follow-up. Shoulder function was evaluated according to the Miami scale without any interval secondary procedure. The results are C5/C6 4.5% fair, 13.6% satisfactory, 26.6% good, 45.4% excellent. For C5/C6/C7 C6 lesions we rated 3.3% fair, 30% satisfactory, 56.6% good, 10% excellent.
LATE NEUROSURGICAL INTERVENTION
In 1997 we performed a careful neurolysis with grafting into the suprascapular nerve in two children who presented after 1 year of age with persistent significant shoulder deficits but full passive mobility resulting from an upper plexus lesion. Subsequently, we were inspired by Birch's report of several successful cases of late grafting into the suprascapular nerve15 and in late 1998 we began to perform routinely a “late” neurosurgical procedure on infants seen after 10 months of age with persistent shoulder sequelae following an upper plexus birth injury. To date, over 40 infants have undergone a brachial plexus reconstruction between 12 and 30 months of age. More than 70% of these cases have presented with a severe internal rotation contracture and posterior subluxation of the shoulder that has been treated simultaneously with both nerve repair and a subscapularis slide and posterior capsuloplasty when needed.
We recently reviewed the charts of 19 infants who underwent a simultaneous plexus and shoulder procedure.16 The average follow-up is now greater than 4 years. Ten patients had a shoulder score of good and nine excellent. Only four patients required a secondary shoulder procedure prior to the last evaluation.
Another eight patients have been evaluated a minimum of 2 years following only late neurolysis with bypass grafting into the suprascapular nerve performed between 9 and 21 months of age with a minimum follow-up of 30 months. Shoulder function by the Miami scale was rated good in all eight patients. Two patients had undergone a secondary shoulder procedure prior to the final evaluation.
We believe that these results suggest that undertaking a late nerve reconstruction in properly selected infants with persistent severe shoulder sequelae following a birth injury to the upper plexus can be beneficial.
We have also had limited experience in two patients with a sensory nerve transfer. Both cases involved untreated global palsies with chronic problematic trophic charges and absent sensibility on the ulnar aspect of the hand. In both children, a transfer of the lateral antebrachial cutaneous nerve into the ulnar nerve in the midforearm corrected the tropic charges with return of sweating and pulp wrinkling during immersion in water17 and provided a degree of protective sensibility within 1 year. The long-term benefit of such a procedure remains to be seen.
SURGICAL COMPLICATIONS
Our experience has been decidedly different from the experience in some recent literature that suggests a significant rate of surgical complications for plexus repairs in infants.18 Subsequent to our earlier report of no significant perioperative complications in 100 consecutive cases,19 we have fortunately not encountered any major problems. We attribute this in large part to the expertise of the anesthesia staff at Miami Children's Hospital who are directly involved in the preoperative evaluation and postoperative management.
SECONDARY RECONSTRUCTION PROCEDURES
The benefits of carefully planned secondary musculoskeletal procedures to maximize extremity function cannot be overemphasized.20,21,22 In our experience, approximately 25% of children undergoing a primary nerve procedure benefit from an additional shoulder procedure that includes a contracture release, muscle transfer, or both to improve both active elevation and external rotation. Occasionally, an external rotational osteotomy of the humerus or a glenoid osteotomy is required.
For this report we reviewed the records of 68 children who had undergone a major shoulder reconstruction (contracture release and muscle transfer) over a 10-year period from 1991 to 2001. At the last evaluation, the Miami shoulder score showed 5.8% fair, 23.5% satisfactory, 54.5% good, and 16.1% excellent. There were no surgical complications or reoperations.
Limitations and deformities of forearm rotation are common in this population of patients. In an earlier report we presented our unique successful experience in the treatment of pronation deformities of the forearm.23 Over the past decade, we have also used a similar individualized surgical approach in 10 children with a supination deformities. As with other authors,24 postoperatively in all cases we have found a significant functional improvement combined with a high degree of parental and patient satisfaction.
ANCILLARY THERAPEUTIC MODALITIES
Comprehensive rehabilitation services are an integral part of our patient management program.25 Both occupational and physical therapy techniques are preferably combined by a single therapist. Regardless of their location all patients are either directly treated or a local therapist is advised on our approach by two of the authors (LR and DB).
Several orthotic and bracing techniques have proved particularly useful. The soft, figure-of-eight shoulder harness is useful for prompting shoulder movements with increased scapular stability (Fig. 9). Taping techniques have been successfully employed to manage the various patterns of scapular instability (Fig. 10). A semisoft or rigid intermittent pediatric elbow extension splint not only controls elbow flexion postures but also encourages more normal patterns of shoulder elevation (Fig. 11).
Figure 9.
Figure-of-eight shoulder harness.
Figure 10.
Taping technique aids in scapular stabilization during recovery.
Figure 11.
Elbow splint for promoting shoulder elevation and controlling flexion posture.
All patients are treated with a carefully supervised program of neuromuscular electrical stimulation. Parents are provided with a home unit and individualized instruction on use for their child's particular needs. We have encountered no complications with a protocol of daily use and a large number of children have been followed for over a decade by one of the authors (LR). We hypothesize that the maintenance of muscle bulk during the early postoperative reinnervation period leads to better long-term functional recovery and appears in many cases to limit permanent atrophy.
Another useful therapeutic modality is botulinum toxin A. This is frequently used to assist in the management of shoulder internal rotation-adduction contractures. Often, the imbalance of the shoulder internal/external rotators seen after surgical treatment of the upper plexus lesion can be managed by a gentle manipulation under anesthesia and administration of botulinum toxin A into the pectoralis major and latissimus dorsi at a total dose of 10 units/kg. We have used this modality in over 60 children without complication. In more than 80% of cases, the goal of the chemoneurolysis was realized. We have had limited experience in using this modality to treat triceps-biceps contractions.26,27
ACKNOWLEDGMENT
Supported by a grant from the Dr. Ralph and Marian C. Falk Medical Research Trust.
REFERENCES
- Egloff D. Algimantas Otonas Narakas (1927–1993). A biographical note. Hand Clin. 1995;11:535–537. [PubMed] [Google Scholar]
- Grossman J AI, Ramos L E, Shumway S, Alfonso I. Management strategies for children with obstetrical brachial plexus injuries. International Pediatrics. 1997;12:82–85. [Google Scholar]
- Muhlig R S, Blaauw G, Slooff A CJ, Kortleve J W, Tonino A J. In: Gilbert A, editor. Brachial Plexus Surgery. London: Martin Dunitz; 2001. Conservative treatment of obstetrical brachial plexus palsy (OBPP) and rehabilitation. pp. 173–189.
- Bae D S, Waters P M, Zurakowski D. Reliability of three classification systems measuring active motion in brachial plexus birth palsy. J Bone Joint Surg Am. 2003;85-A:1733–1738. doi: 10.2106/00004623-200309000-00012. [DOI] [PubMed] [Google Scholar]
- Gilbert A. In: Gilbert A, editor. Brachial Plexus Injuries. London: Martin Dunitz; 2001. Indications and strategy. pp. 205–210.
- Waters P M. Comparison of the natural history, the outcome of microsurgical repair, and the outcome of operative reconstruction in brachial plexus birth palsy. J Bone Joint Surg Am. 1999;81:649–659. doi: 10.2106/00004623-199905000-00006. [DOI] [PubMed] [Google Scholar]
- Al-Qattan M M. The outcome of Erb's palsy when the decision to operate is made at 4 months of age. Plast Reconstr Surg. 2000;106:1461–1465. doi: 10.1097/00006534-200012000-00003. [DOI] [PubMed] [Google Scholar]
- Clarke H M, Curtis C G. An approach to obstetrical brachial plexus injuries. Hand Clin. 1995;11:563–581. [PubMed] [Google Scholar]
- Gilbert A. Long-term evaluation of brachial plexus surgery in obstetrical palsy. Hand Clin. 1995;11:583–594. 594–595. discussion. [PubMed] [Google Scholar]
- DiTaranto P, Campagna L, Price A E, Grossman J AI. Outcome following nonoperative treatment of brachial plexus birth injuries. J Child Neurol. 2004;19:87–90. doi: 10.1177/08830738040190020101. [DOI] [PubMed] [Google Scholar]
- Birchansky S, Altman N. Imaging the brachial plexus and peripheral nerves in infants and children. Semin Pediatr Neurol. 2000;7:15–25. doi: 10.1016/s1071-9091(00)80006-6. [DOI] [PubMed] [Google Scholar]
- Gilbert A. In: Gilbert A, editor. Brachial Plexus Surgery. London: Martin Dunitz; 2001. Results of repair to the obstetrical plexus. pp. 205–217.
- Anand P, Birch R. Restoration of sensory function and lack of long-term chronic pain syndromes after brachial plexus injury in human neonates. Brain. 2002;125(Pt 1):113–122. doi: 10.1093/brain/awf017. [DOI] [PubMed] [Google Scholar]
- Birch R. Obstetric brachial plexus palsy. J Hand Surg [Br] 2002;27:3–8. doi: 10.1054/jhsb.2001.0722. [DOI] [PubMed] [Google Scholar]
- Birch R, Bonney G, Wynn Parry C B. In: Birch R, Bonney G, Wynn Parry CB, editor. Surgical Disorders of the Peripheral Nerves. 1st ed. Edinburgh: Churchill Livingstone; 1998. Birth lesions of the brachial plexus. pp. 209–233.
- Grossman J AI, Price A E, Tidwell M A, Ramos L E, Alfonso I, Yaylali I. Outcome after late combined brachial plexus and shoulder surgery following birth trauma. J Bone Joint Surg Br. 2003;85:1166–1168. doi: 10.1302/0301-620x.85b8.14246. [DOI] [PubMed] [Google Scholar]
- Lister G. The Hand: Diagnosis and Indications. 3rd ed. London: Churchill Livingstone; 1993.
- La Scala G C, Rice S B, Clarke H M. Complications of microsurgical reconstruction of obstetrical brachial plexus palsy. Plast Reconstr Surg. 2003;111:1383–1388. doi: 10.1097/01.PRS.0000049110.65510.10. [DOI] [PubMed] [Google Scholar]
- Grossman J A, Price A E, Sadeghi P. Perioperative complications associated with brachial plexus repair in infants. J Hand Surg [Br] 2003;28:274–275. doi: 10.1016/s0266-7681(02)00353-4. [DOI] [PubMed] [Google Scholar]
- Price A, Tidwell M, Grossman J AI. Improving shoulder and elbow function in children with Erb's palsy. Semin Pediatr Neurol. 2000;7:44–51. doi: 10.1016/s1071-9091(00)80009-1. [DOI] [PubMed] [Google Scholar]
- Waters P M, Paljovich A E. Shoulder reconstruction in patients with chronic brachial plexus birth palsy: a case-control study. Clin Orthop. 1999;364:144–152. doi: 10.1097/00003086-199907000-00019. [DOI] [PubMed] [Google Scholar]
- Goddard N J, Fixsen J A. Rotation osteotomy of the humerus for birth injuries of the brachial plexus. J Bone Joint Surg Br. 1984;66:257–259. doi: 10.1302/0301-620X.66B2.6707064. [DOI] [PubMed] [Google Scholar]
- Liggio F J, Tham S, Price A, Ramos L E, Mulloy E, Grossman J AI. Outcome of surgical treatment for forearm pronation deformities in children with obstetric brachial plexus injuries. J Hand Surg [Br] 1999;24:43–45. doi: 10.1016/s0266-7681(99)90023-2. [DOI] [PubMed] [Google Scholar]
- Bahm J, Gilbert A. Surgical correction of supination deformity in children with obstetric brachial plexus palsy. J Hand Surg [Br] 2002;27:20–23. doi: 10.1054/jhsb.2001.0647. [DOI] [PubMed] [Google Scholar]
- Ramos L E, Zell J P. Rehabilitation program for children with brachial plexus and peripheral nerve injury. Semin Pediatr Neurol. 2000;7:52–57. doi: 10.1016/s1071-9091(00)80010-8. [DOI] [PubMed] [Google Scholar]
- Rollnik J D, Hierner R, Schubert M, et al. Botulinum toxin treatment of cocontractions after birth-related brachial plexus lesions. Neurology. 2000;55:112–114. doi: 10.1212/wnl.55.1.112. [DOI] [PubMed] [Google Scholar]
- Hierner D, Berger A C. In: Gilbert A, editor. Brachial Plexus Injuries. London: Martin Dunitz; 2001. Treatment of co-contraction. pp. 303–314.