Abstract
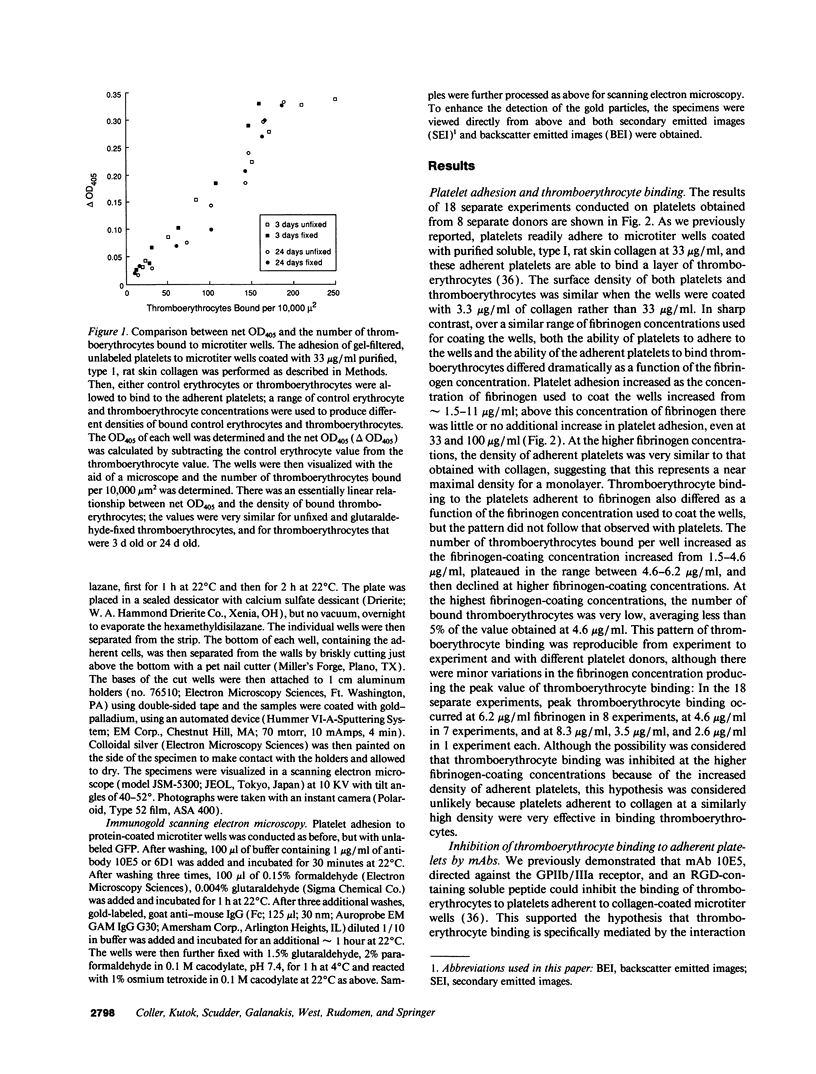
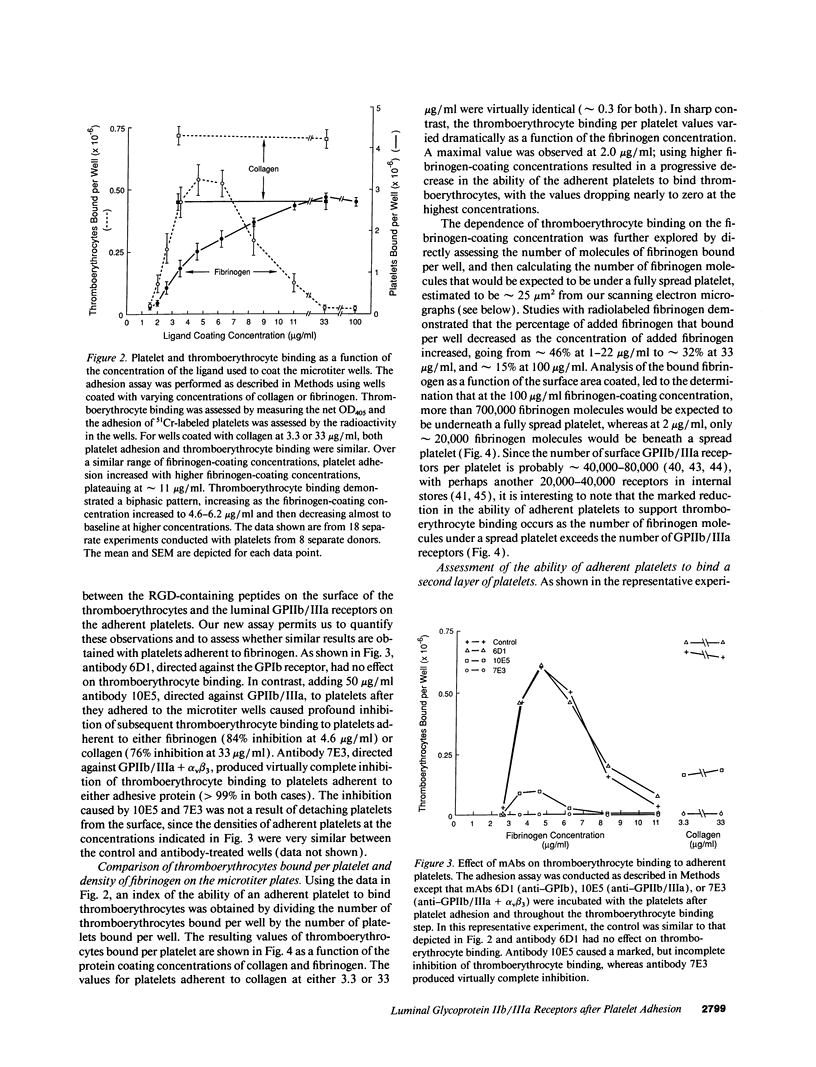
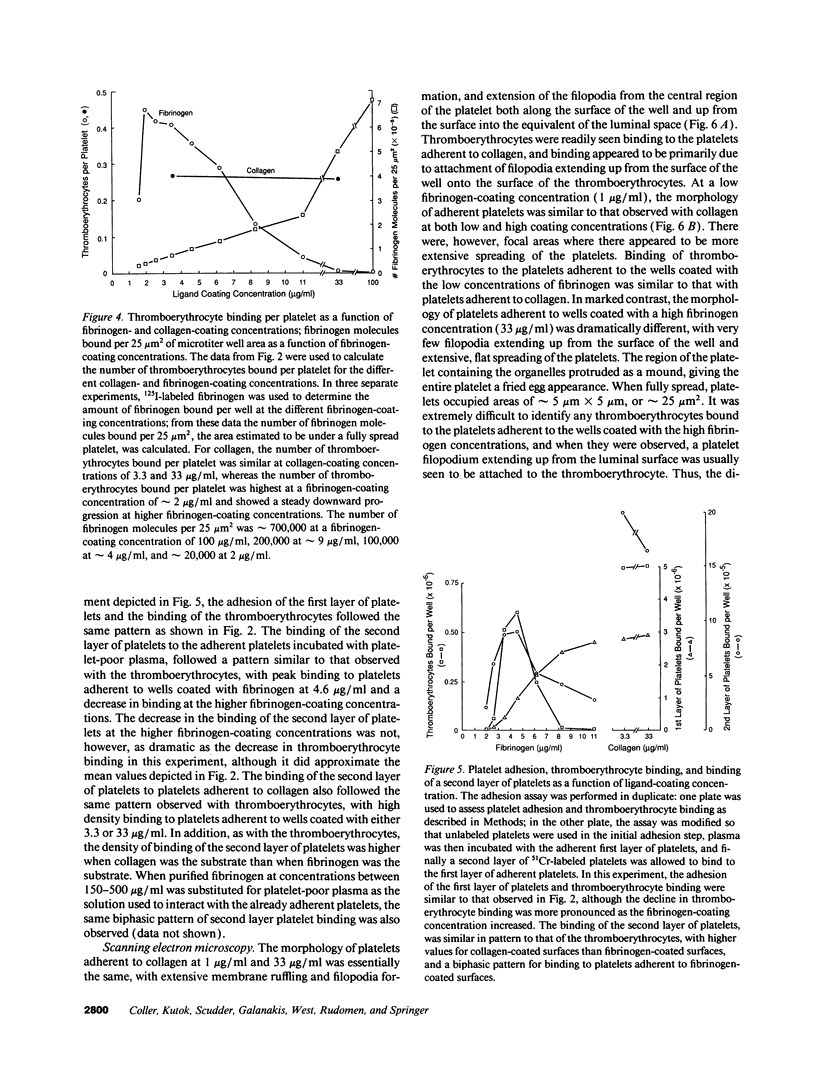
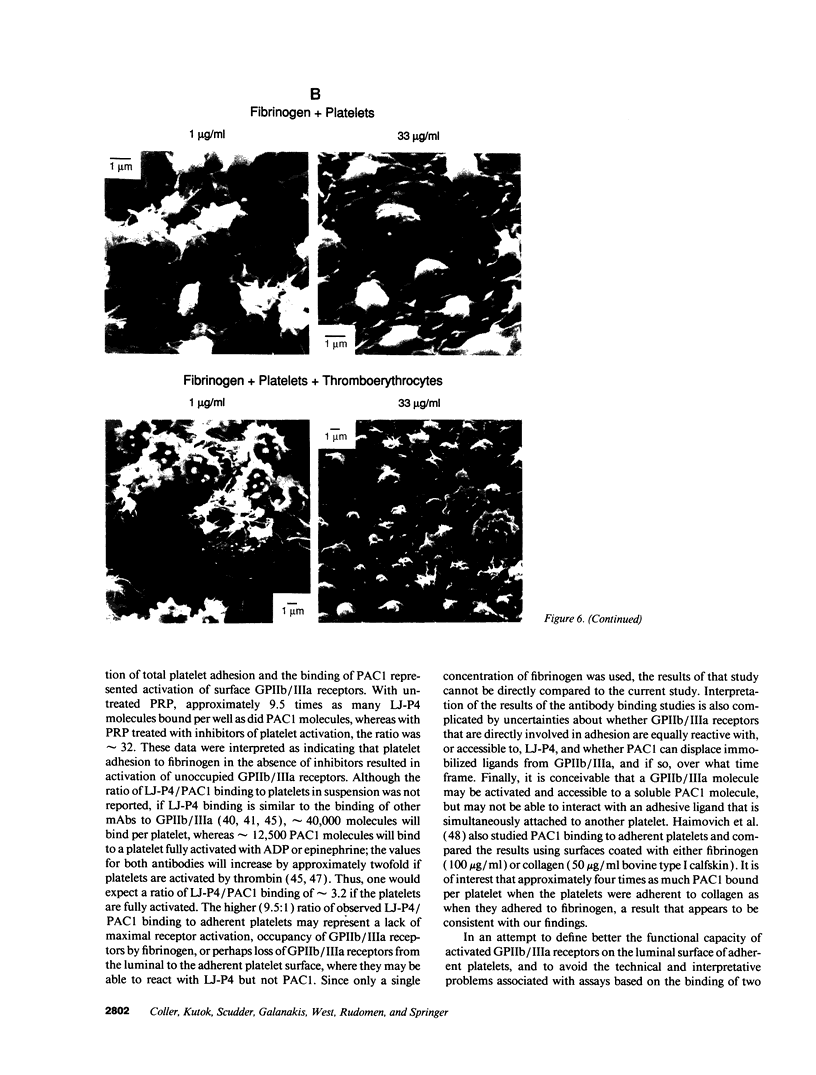
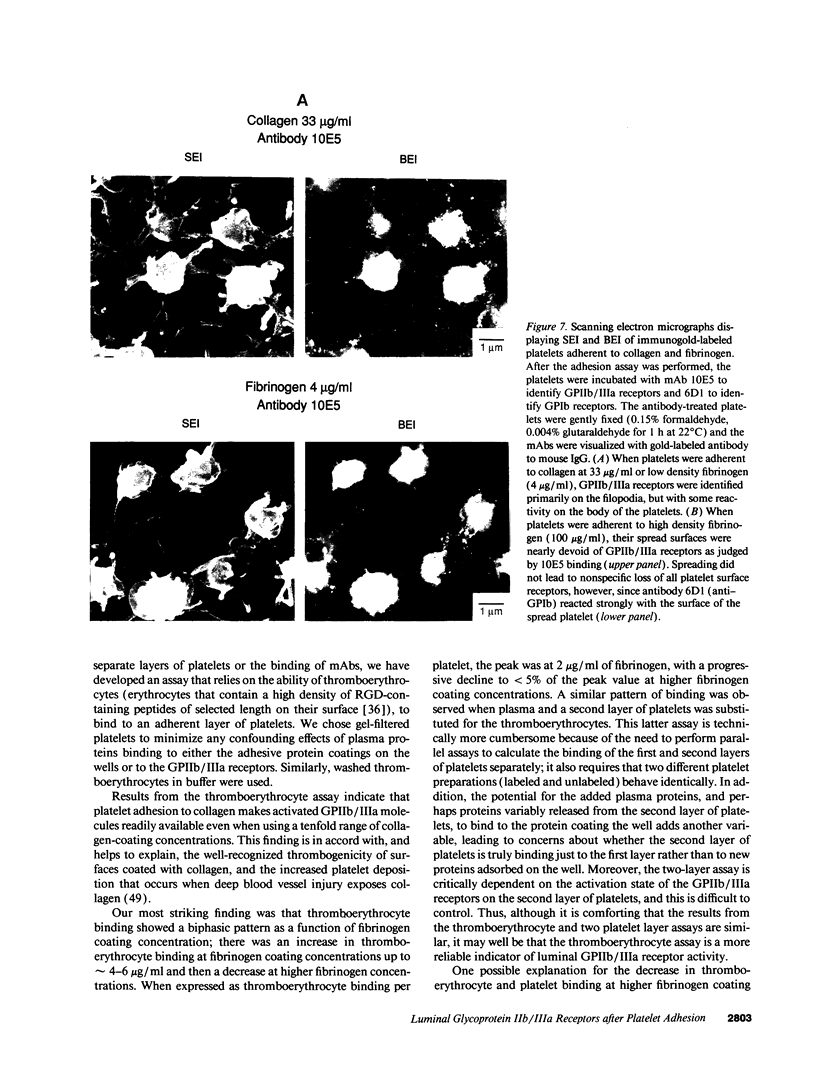
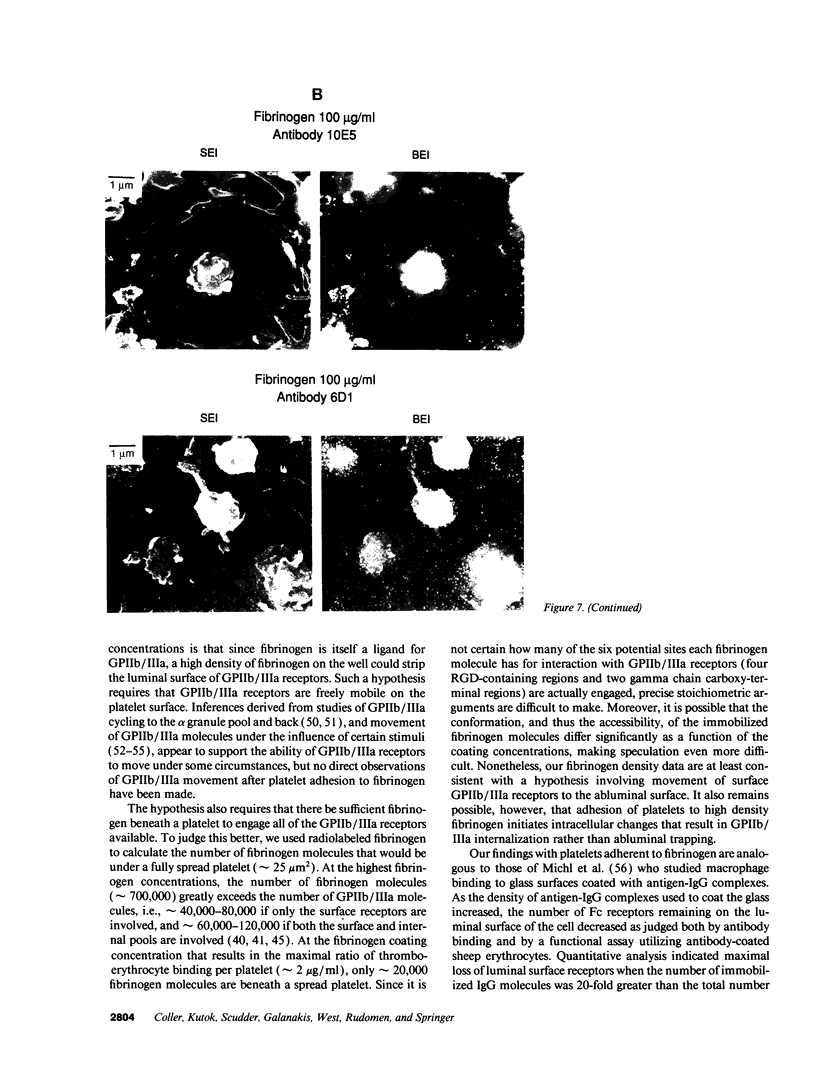
The accessibility of activated GPIIb/IIIa receptors on the luminal surface of platelets adherent to damaged blood vessels or atherosclerotic plaques is likely to play a crucial role in subsequent platelet recruitment. To define better the factors involved in this process, we developed a functional assay to assess the presence of activated, luminal GPIIb/IIIa receptors, based on their ability to bind erythrocytes containing a high density of covalently coupled RGD-containing peptides (thromboerythrocytes). Platelets readily adhered to wells coated with purified type I rat skin collagen and the adherent platelets bound a dense lawn of thromboerythrocytes. With fibrinogen-coated wells, platelet adhesion increased as the fibrinogen-coating concentration increased, reaching a plateau at about 11 micrograms/ml. Thromboerythrocyte binding to the platelets adherent to fibrinogen showed a paradoxical response, increasing at fibrinogen coating concentrations up to approximately 4-6 micrograms/ml and then dramatically decreasing at higher fibrinogen-coating concentrations. Scanning electron microscopy demonstrated that the morphology of platelets adherent to collagen was similar to that of platelets adherent to low density fibrinogen, with extensive filopodia formation and ruffling. In contrast, platelets adherent to high density fibrinogen showed a bland, flattened appearance. Immunogold staining of GPIIb/IIIa receptors demonstrated concentration of the receptors on the filopodia, and depletion of receptors on the flattened portion of the platelets. Thus, there is a paradoxical loss of accessible, activated GPIIb/IIIa receptors on the luminal surface of platelets adherent to high density fibrinogen. Two factors may contribute to this result: engagement of GPIIb/IIIa receptors with fibrinogen on the abluminal surface leading to the loss of luminal receptors, and loss of luminal filopodia that interact with thromboerythrocytes. These data provide insight into the differences in thrombogenicity between surfaces, and may provide a mechanism for purposefully passivating platelet-reactive artificial surfaces.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asch A. S., Barnwell J., Silverstein R. L., Nachman R. L. Isolation of the thrombospondin membrane receptor. J Clin Invest. 1987 Apr;79(4):1054–1061. doi: 10.1172/JCI112918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asch E., Podack E. Vitronectin binds to activated human platelets and plays a role in platelet aggregation. J Clin Invest. 1990 May;85(5):1372–1378. doi: 10.1172/JCI114581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badimon L., Badimon J. J., Turitto V. T., Vallabhajosula S., Fuster V. Platelet thrombus formation on collagen type I. A model of deep vessel injury. Influence of blood rheology, von Willebrand factor, and blood coagulation. Circulation. 1988 Dec;78(6):1431–1442. doi: 10.1161/01.cir.78.6.1431. [DOI] [PubMed] [Google Scholar]
- Beer J. H., Springer K. T., Coller B. S. Immobilized Arg-Gly-Asp (RGD) peptides of varying lengths as structural probes of the platelet glycoprotein IIb/IIIa receptor. Blood. 1992 Jan 1;79(1):117–128. [PubMed] [Google Scholar]
- Charo I. F., Bekeart L. S., Phillips D. R. Platelet glycoprotein IIb-IIIa-like proteins mediate endothelial cell attachment to adhesive proteins and the extracellular matrix. J Biol Chem. 1987 Jul 25;262(21):9935–9938. [PubMed] [Google Scholar]
- Charo I. F., Nannizzi L., Smith J. W., Cheresh D. A. The vitronectin receptor alpha v beta 3 binds fibronectin and acts in concert with alpha 5 beta 1 in promoting cellular attachment and spreading on fibronectin. J Cell Biol. 1990 Dec;111(6 Pt 1):2795–2800. doi: 10.1083/jcb.111.6.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh D. A. Human endothelial cells synthesize and express an Arg-Gly-Asp-directed adhesion receptor involved in attachment to fibrinogen and von Willebrand factor. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6471–6475. doi: 10.1073/pnas.84.18.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller B. S. A new murine monoclonal antibody reports an activation-dependent change in the conformation and/or microenvironment of the platelet glycoprotein IIb/IIIa complex. J Clin Invest. 1985 Jul;76(1):101–108. doi: 10.1172/JCI111931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller B. S., Beer J. H., Scudder L. E., Steinberg M. H. Collagen-platelet interactions: evidence for a direct interaction of collagen with platelet GPIa/IIa and an indirect interaction with platelet GPIIb/IIIa mediated by adhesive proteins. Blood. 1989 Jul;74(1):182–192. [PubMed] [Google Scholar]
- Coller B. S., Cheresh D. A., Asch E., Seligsohn U. Platelet vitronectin receptor expression differentiates Iraqi-Jewish from Arab patients with Glanzmann thrombasthenia in Israel. Blood. 1991 Jan 1;77(1):75–83. [PubMed] [Google Scholar]
- Coller B. S., Peerschke E. I., Scudder L. E., Sullivan C. A. A murine monoclonal antibody that completely blocks the binding of fibrinogen to platelets produces a thrombasthenic-like state in normal platelets and binds to glycoproteins IIb and/or IIIa. J Clin Invest. 1983 Jul;72(1):325–338. doi: 10.1172/JCI110973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller B. S., Springer K. T., Beer J. H., Mohandas N., Scudder L. E., Norton K. J., West S. M. Thromboerythrocytes. In vitro studies of a potential autologous, semi-artificial alternative to platelet transfusions. J Clin Invest. 1992 Feb;89(2):546–555. doi: 10.1172/JCI115619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanakis D. K., Mosesson M. W., Stathakis N. E. Human fibrinogen heterogeneities: distribution and charge characteristics of chains of A alpha origin. J Lab Clin Med. 1978 Sep;92(3):376–386. [PubMed] [Google Scholar]
- Goldsmith H. L., Turitto V. T. Rheological aspects of thrombosis and haemostasis: basic principles and applications. ICTH-Report--Subcommittee on Rheology of the International Committee on Thrombosis and Haemostasis. Thromb Haemost. 1986 Jun 30;55(3):415–435. [PubMed] [Google Scholar]
- Groves H. M., Kinlough-Rathbone R. L., Mustard J. F. Development of nonthrombogenicity of injured rabbit aortas despite inhibition of platelet adherence. Arteriosclerosis. 1986 Mar-Apr;6(2):189–195. doi: 10.1161/01.atv.6.2.189. [DOI] [PubMed] [Google Scholar]
- Groves H. M., Kinlough-Rathbone R. L., Richardson M., Moore S., Mustard J. F. Platelet interaction with damaged rabbit aorta. Lab Invest. 1979 Feb;40(2):194–200. [PubMed] [Google Scholar]
- Hatton M. W., Moar S. L., Richardson M. Deendothelialization in vivo initiates a thrombogenic reaction at the rabbit aorta surface. Correlation of uptake of fibrinogen and antithrombin III with thrombin generation by the exposed subendothelium. Am J Pathol. 1989 Sep;135(3):499–508. [PMC free article] [PubMed] [Google Scholar]
- Heilmann E., Hourdillé P., Pruvost A., Paponneau A., Nurden A. T. Thrombin-induced platelet aggregates have a dynamic structure. Time-dependent redistribution of glycoprotein IIb-IIIa complexes and secreted adhesive proteins. Arterioscler Thromb. 1991 May-Jun;11(3):704–718. doi: 10.1161/01.atv.11.3.704. [DOI] [PubMed] [Google Scholar]
- Houdijk W. P., Sixma J. J. Fibronectin in artery subendothelium is important for platelet adhesion. Blood. 1985 Mar;65(3):598–604. [PubMed] [Google Scholar]
- Isenberg W. M., McEver R. P., Phillips D. R., Shuman M. A., Bainton D. F. The platelet fibrinogen receptor: an immunogold-surface replica study of agonist-induced ligand binding and receptor clustering. J Cell Biol. 1987 Jun;104(6):1655–1663. doi: 10.1083/jcb.104.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunicki T. J., Nugent D. J., Staats S. J., Orchekowski R. P., Wayner E. A., Carter W. G. The human fibroblast class II extracellular matrix receptor mediates platelet adhesion to collagen and is identical to the platelet glycoprotein Ia-IIa complex. J Biol Chem. 1988 Apr 5;263(10):4516–4519. [PubMed] [Google Scholar]
- Lawler J., Hynes R. O. An integrin receptor on normal and thrombasthenic platelets that binds thrombospondin. Blood. 1989 Nov 1;74(6):2022–2027. [PubMed] [Google Scholar]
- Marcus A. J., Safier L. B., Hajjar K. A., Ullman H. L., Islam N., Broekman M. J., Eiroa A. M. Inhibition of platelet function by an aspirin-insensitive endothelial cell ADPase. Thromboregulation by endothelial cells. J Clin Invest. 1991 Nov;88(5):1690–1696. doi: 10.1172/JCI115485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus A. J. Transcellular metabolism of eicosanoids. Prog Hemost Thromb. 1986;8:127–142. [PubMed] [Google Scholar]
- Michl J., Pieczonka M. M., Unkeless J. C., Bell G. I., Silverstein S. C. Fc receptor modulation in mononuclear phagocytes maintained on immobilized immune complexes occurs by diffusion of the receptor molecule. J Exp Med. 1983 Jun 1;157(6):2121–2139. doi: 10.1084/jem.157.6.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michl J., Unkeless J. C., Pieczonka M. M., Silverstein S. C. Modulation of Fc receptors of mononuclear phagocytes by immobilized antigen-antibody complexes. Quantitative analysis of the relationship between ligand number and Fc receptor response. J Exp Med. 1983 Jun 1;157(6):1746–1757. doi: 10.1084/jem.157.6.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niiya K., Hodson E., Bader R., Byers-Ward V., Koziol J. A., Plow E. F., Ruggeri Z. M. Increased surface expression of the membrane glycoprotein IIb/IIIa complex induced by platelet activation. Relationship to the binding of fibrinogen and platelet aggregation. Blood. 1987 Aug;70(2):475–483. [PubMed] [Google Scholar]
- Olorundare O. E., Simmons S. R., Albrecht R. M. Cytochalasin D and E: effects on fibrinogen receptor movement and cytoskeletal reorganization in fully spread, surface-activated platelets: a correlative light and electron microscopic investigation. Blood. 1992 Jan 1;79(1):99–109. [PubMed] [Google Scholar]
- Peerschke E. I. The platelet fibrinogen receptor. Semin Hematol. 1985 Oct;22(4):241–259. [PubMed] [Google Scholar]
- Piotrowicz R. S., Orchekowski R. P., Nugent D. J., Yamada K. Y., Kunicki T. J. Glycoprotein Ic-IIa functions as an activation-independent fibronectin receptor on human platelets. J Cell Biol. 1988 Apr;106(4):1359–1364. doi: 10.1083/jcb.106.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow E. F., Ginsberg M. H. Cellular adhesion: GPIIb-IIIa as a prototypic adhesion receptor. Prog Hemost Thromb. 1989;9:117–156. [PubMed] [Google Scholar]
- Plow E. F., McEver R. P., Coller B. S., Woods V. L., Jr, Marguerie G. A., Ginsberg M. H. Related binding mechanisms for fibrinogen, fibronectin, von Willebrand factor, and thrombospondin on thrombin-stimulated human platelets. Blood. 1985 Sep;66(3):724–727. [PubMed] [Google Scholar]
- Ruggeri Z. M. The platelet glycoprotein Ib-IX complex. Prog Hemost Thromb. 1991;10:35–68. [PubMed] [Google Scholar]
- Sakariassen K. S., Fressinaud E., Girma J. P., Meyer D., Baumgartner H. R. Role of platelet membrane glycoproteins and von Willebrand factor in adhesion of platelets to subendothelium and collagen. Ann N Y Acad Sci. 1987;516:52–65. doi: 10.1111/j.1749-6632.1987.tb33029.x. [DOI] [PubMed] [Google Scholar]
- Salzman E. W., Lindon J., McManama G., Ware J. A. Role of fibrinogen in activation of platelets by artificial surfaces. Ann N Y Acad Sci. 1987;516:184–195. doi: 10.1111/j.1749-6632.1987.tb33040.x. [DOI] [PubMed] [Google Scholar]
- Santoro S. A. Molecular basis of platelet adhesion to collagen. Prog Clin Biol Res. 1988;283:291–314. [PubMed] [Google Scholar]
- Santos M. T., Valles J., Marcus A. J., Safier L. B., Broekman M. J., Islam N., Ullman H. L., Eiroa A. M., Aznar J. Enhancement of platelet reactivity and modulation of eicosanoid production by intact erythrocytes. A new approach to platelet activation and recruitment. J Clin Invest. 1991 Feb;87(2):571–580. doi: 10.1172/JCI115032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage B., Ruggeri Z. M. Selective recognition of adhesive sites in surface-bound fibrinogen by glycoprotein IIb-IIIa on nonactivated platelets. J Biol Chem. 1991 Jun 15;266(17):11227–11233. [PubMed] [Google Scholar]
- Savage B., Shattil S. J., Ruggeri Z. M. Modulation of platelet function through adhesion receptors. A dual role for glycoprotein IIb-IIIa (integrin alpha IIb beta 3) mediated by fibrinogen and glycoprotein Ib-von Willebrand factor. J Biol Chem. 1992 Jun 5;267(16):11300–11306. [PubMed] [Google Scholar]
- Shattil S. J., Hoxie J. A., Cunningham M., Brass L. F. Changes in the platelet membrane glycoprotein IIb.IIIa complex during platelet activation. J Biol Chem. 1985 Sep 15;260(20):11107–11114. [PubMed] [Google Scholar]
- Sonnenberg A., Modderman P. W., Hogervorst F. Laminin receptor on platelets is the integrin VLA-6. Nature. 1988 Dec 1;336(6198):487–489. doi: 10.1038/336487a0. [DOI] [PubMed] [Google Scholar]
- Tandon N. N., Holland E. A., Kralisz U., Kleinman H. K., Robey F. A., Jamieson G. A. Interaction of human platelets with laminin and identification of the 67 kDa laminin receptor on platelets. Biochem J. 1991 Mar 1;274(Pt 2):535–542. doi: 10.1042/bj2740535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon N. N., Kralisz U., Jamieson G. A. Identification of glycoprotein IV (CD36) as a primary receptor for platelet-collagen adhesion. J Biol Chem. 1989 May 5;264(13):7576–7583. [PubMed] [Google Scholar]
- Thiagarajan P., Kelly K. L. Exposure of binding sites for vitronectin on platelets following stimulation. J Biol Chem. 1988 Feb 25;263(6):3035–3038. [PubMed] [Google Scholar]
- Thiagarajan P., Kelly K. Interaction of thrombin-stimulated platelets with vitronectin (S-protein of complement) substrate: inhibition by a monoclonal antibody to glycoprotein IIb-IIIa complex. Thromb Haemost. 1988 Dec 22;60(3):514–517. [PubMed] [Google Scholar]
- Tuszynski G. P., Kowalska M. A. Thrombospondin-induced adhesion of human platelets. J Clin Invest. 1991 Apr;87(4):1387–1394. doi: 10.1172/JCI115144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vane J. R., Anggård E. E., Botting R. M. Regulatory functions of the vascular endothelium. N Engl J Med. 1990 Jul 5;323(1):27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]
- Weisel J. W., Nagaswami C., Vilaire G., Bennett J. S. Examination of the platelet membrane glycoprotein IIb-IIIa complex and its interaction with fibrinogen and other ligands by electron microscopy. J Biol Chem. 1992 Aug 15;267(23):16637–16643. [PubMed] [Google Scholar]
- Wencel-Drake J. D. Plasma membrane GPIIb/IIIa. Evidence for a cycling receptor pool. Am J Pathol. 1990 Jan;136(1):61–70. [PMC free article] [PubMed] [Google Scholar]
- White J. G. Separate and combined interactions of fibrinogen-gold and latex with surface-activated platelets. Am J Pathol. 1990 Oct;137(4):989–998. [PMC free article] [PubMed] [Google Scholar]
- Wilentz J. R., Sanborn T. A., Haudenschild C. C., Valeri C. R., Ryan T. J., Faxon D. P. Platelet accumulation in experimental angioplasty: time course and relation to vascular injury. Circulation. 1987 Mar;75(3):636–642. doi: 10.1161/01.cir.75.3.636. [DOI] [PubMed] [Google Scholar]