ABSTRACT
This article reports preclinical and clinical experience with a type I and type III injectable human collagen product derived from human sources (containing type I and type III collagen in a proportion of 44:56) utilized as a dermal filler. The preclinical study involves experiments characterizing the histological changes observed after type I and type III injectable human collagen injection in rats. The clinical trial is a study of 123 patients treated with type I and type III injectable human collagen for pitting skin deficiencies including furrow, superficial scar, and superficial atrophy. Histological observations from the preclinical study revealed that type I and type III injectable human collagen persisted for at least a year. The clinical trial showed that most patients were satisfied with the results, and a rating of excellent or good was achieved 90.2% of the time. The positive results lasted about 9 months after the first injection, 12 months after the second injection, and 18 months after the third injection. Reported side effects were slight redness and discomfort at or near the injection site. Type I and type III injectable human collagen is a safe and reliable biomaterial that can easily be used for soft tissue augmentation.
Keywords: Soft tissue augmentation, human injectable collagen, animal studies, clinical trials
A plethora of new dermal fillers have been introduced over the past few years. These are synthetic products that have shortcomings in the goal of achieving the ideal dermal filler. The shortcomings can be either adverse events, short duration of effect, or lack of new tissue in growth. The ideal natural dermal filler would offer immediate correction of the dermal defect with no adverse events and stimulate new tissue regeneration as the dermal filler is slowly absorbed.
Collagen, a family of fibrous proteins, is the major structural component of the vertebrate body and the primary protein in skin.1 The normal human dermis consists of about 80% type I collagen and 20% type III collagen.2,3 Type I collagen is responsible for the strength and integrity of the dermis.2,4 Type III collagen allows for the distensibility of the dermis.2,4
Collagen loss causes the aged, wrinkled look of skin and lip atrophy. Collagen composition in the skin changes with age. Type III collagen constitutes 50% of collagen in neonatal skin dermis but only 5% of collagen in aging adult skin. Facial wrinkles are mainly due to loss of collagen, especially type III. Replacing a natural human collagen into the wrinkles allows the most logical and compatible soft tissue replacement.
Age-related changes appear as wrinkles in facial skin and around the lips and mouth. The lip and mouth changes include atrophy of the upper and lower lips, actinic changes of the mucosal skin surface and vermilion, and atrophy at the corners of the mouth, which causes a downturn of the corners of the mouth and a resultant aged appearance.3 Another sign of aging is an increase in the prominence of the nasolabial folds.3 Environmental factors such as sun and smoking contribute to the wrinkled appearance of facial skin.
The appearance of wrinkles is important to people who want to prolong their youthful appearance. Such people often desire skin augmentation to maintain a youthful appearance. Skin augmentation is popular because it restores the consistency and composition of normal tissue without surgery.5
Fine, superficial wrinkles such as facial rhytids or dermal depressions are most effectively treated with “like material” such as dermal collagen.6 Deeper, more subcutaneous wrinkles are approached best from the subcutaneous space.3 Some wrinkles have superficial and deep components, such as the nasolabial fold, crow's feet around the eyes, forehead, and cheeks.3
The ideal filler for soft tissue augmentation should be safe and effective; easy to obtain and administer; have a minimal risk for infection, extrusion, or migration; produce a minimal inflammatory reaction; last for an acceptable period of time6; and be usable without pretesting in patients. It should also be cost effective, show consistency and reproducibility, and yield a highly acceptable result.6 The substance should be approved by the Food and Drug Administration (FDA).3
Because collagen is immunologically benign, resistant to proteolysis, and a natural substrate for cell adhesion, collagen is the ideal biomaterial for soft tissue augmentation. Many collagen-based products are available to correct facial wrinkles, acne scars, and other small areas of skin defects. Bovine injectable collagen (Zyderm I®, Zyderm II®, and Zyplast® collagen implants; Inamed Corporation, Santa Barbara, CA) has been used extensively in the United States, Japan, Europe, and Hong Kong for these purposes.7
With the growing use of bovine collagen implants, questions have been raised regarding their immunogenicity in humans. Although these bovine collagen preparations are of low immunogenicity, they are still foreign proteins. Their antigenicity is not low enough to escape an immunologic response in all patients. The major disadvantage of bovine collagen is a treatment-associated hypersensitivity reaction that consists of redness and swelling at the treatment site.3 These reactions occurred in 1.3 to 3% of patients treated with Zyderm I or II.5 These allergic reactions are believed to be an immunological response to bovine collagen. In contrast, collagen products derived from human cells do not pose an allergy risk.8 Many investigators believe the use of human-based collagen will overcome this shortcoming.7,9
This article describes the preclinical and clinical experience with a type I and type III injectable human collagen product derived from human sources (containing type I and type III collagen in a proportion of 44:56) utilized as a dermal filler. Our results demonstrate that type I and type III injectable human collagen is a safe and reliable biomaterial that can easily be used for soft tissue augmentation.
HUMAN COLLAGEN
Type I and Type III Injectable Human Collagen
Type I and type III injectable human collagen was prepared by the National Institute of Occupational Health and Poison Control, Chinese Center for Disease Control and Prevention. Type I and type III injectable human collagen was made from highly purified human collagen obtained from placentas according to a known method for collagen extraction and purification.10 All placental tissues were collected from mothers who tested negative for acquired immunodeficiency syndrome (AIDS) and hepatitis. The tissues were tested twice to ensure that negative tests for AIDS and hepatitis were obtained. The purity of type I and type III injectable human collagen was evaluated by amino acid analysis, degradation by collagenase, sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and Western blot.11 The proportion between type I and type III collagen was evaluated using interrupted electrophoresis analysis. The collagen concentration was measured as previously described.12 Type I and type III injectable human collagen passed the required bacteriology, virology, and pathology tests. Type I and type III injectable human collagen is packaged in 2-mL syringes in a physiological saline buffer solution containing 0.3% lidocaine at a pH of 7.2. Type I and type III injectable human collagen is stored at 2 to 10°C until use.
Results
Type I and type III injectable human collagen is a highly purified human collagen that is sterile and virus free. The proportion between type I and type III collagen was 44:56 (Fig. 1). The concentration is 35 mg/mL.
Figure 1.
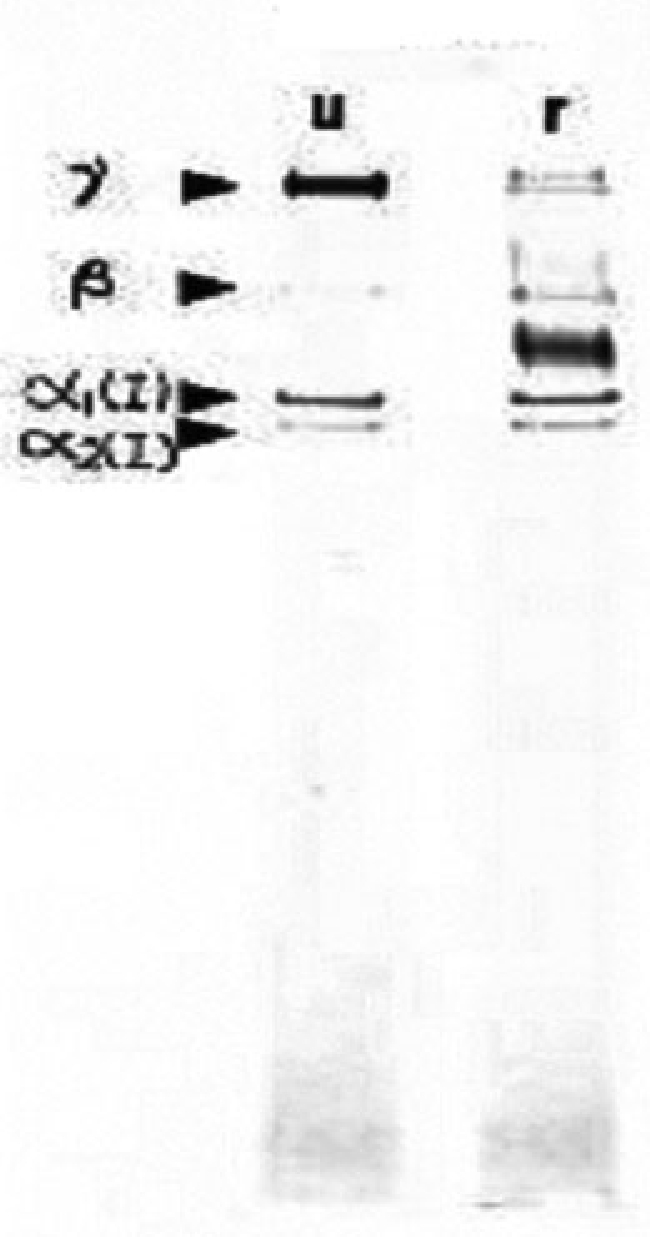
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis profile of type I and type III injectable human collagen. u, unreduced; r, reduced.
Conclusion
The type I and type III injectable human collagen developed by the National Institute of Occupational Health and Poison Control, Chinese Center for Disease Control is highly purified human collagen. Type I and type III injectable human collagen prepared in this manner was used in the animal and clinical experiments described in the following.
PRECLINICAL STUDIES
Materials and Methods
ANIMALS
Twenty male Wistar rats (weight, ∼200 g ± 10 g) were randomly separated into two groups. One group (n = 10) was injected with type I and type III injectable human collagen (0.2 mL, 35 mg/mL, prepared as detailed earlier) and the other group (n = 10) received Zyderm II (0.2 mL, 65 mg/mL). Zyderm II (Inamed Corporation, Santa Barbara, CA) is sterile, purified bovine dermal collagen in phosphate-buffered saline with 0.3% lidocaine.3,5 Each rat received six injections (0.2 mL per site) at three sites on each side of the back (Fig. 2).
Figure 2.
Sites of injection on the back of rats.
Before injection, the back of each rat was shaved and sterilized. After injection, the rats were observed for 1, 6, and 12 months. At the end of 1 and 6 months three rats were sacrificed, and at the end of 12 months four rats were killed for each group to determine the status of the injected collagen and to study the morphological characteristics. All specimens were collected and the sections were prepared for light microscopy and evaluated by hematoxylin-eosin staining analysis.
Results
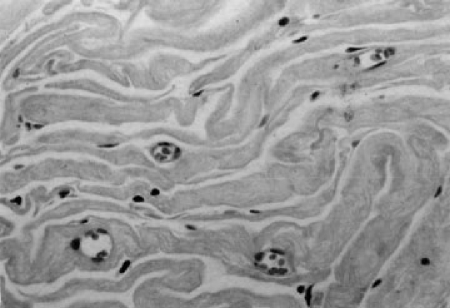
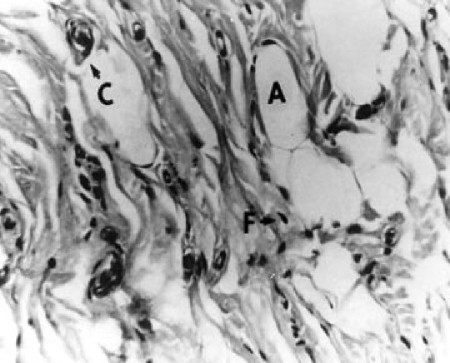
Both groups were observed for 1, 6, and 12 months. In both groups, collagen was found at the sites of injection 1 and 6 months after the injection. Histological observation of these injection sites indicated that collagen had transferred into the injection site, as observed by active collagen synthesis (Fig. 3). One month after implantation, a mass of soft, pliable white tissue was found on biopsy specimens of the subcutaneous injection sites of injected animals. Visible small blood vessels could be seen coursing over the entire surface of the implant. No significant inflammatory response was observed in either group 30 and 180 days after injection. Although the rat dermal collagen appears more dense, the injected collagen could be clearly distinguished because it is homogeneous, pale staining, and eosinophilic. Numerous fibroblasts were observed nested among collagen fibers. A densely stained fibrillar collagen was apparent, indicating that the fibroblasts were secreting new collagen. Adipocytes also appeared on the periphery of the implant and became trapped within it. The amount of collagen produced and the blood vessel supply increased from 1 to 6 months (Fig. 4). Six months after injection, strong collagen bundles formed within the implants. At this time point (6 months) fibroblasts and capillaries were distributed diffusely throughout the implant. Similar results were obtained for both groups. By 12 months after injection, 10% of the collagen was not easily detected. The histological observations revealed that at each time point, the amount of fibroblasts and adipocytes as well as the amount of capillaries remaining was less for the Zyderm II group compared with the type I and type III injectable human collagen group.
Figure 3.
Histology 1 month after injection with type I and type III injectable human collagen.
Figure 4.
Histology 6 months after injection with type I and type III injectable human collagen.
Discussion
In this long-term animal experiment, type I and type III injectable human collagen persisted for at least 1 year. In addition, type I and type III injectable human collagen induced host cells to migrate into the collagen implant and to synthesize their own collagen, which is supported by the growth of capillary vessels at the injection sites. A healthy blood supply is necessary for the injected collagen to incorporate into the animal's tissue, continue to grow, and eventually become the rats' own tissue. The growth performance of induced cells and blood vessels was better with type I and type III injectable human collagen than with Zyderm II. Zyderm II has been shown to be rapidly degraded by tissue collagenases and reabsorbed within months.13 The induced ingrowth of adipocytes that increases over time is an interesting observation that deserves further investigation.
The primary differences between type I and type III injectable human collagen and Zyderm II are the type of collagen (human versus bovine) and the ratio of type I to type III collagen. For type I and type III injectable human collagen, this is 44:56 for type I and type III collagen, respectively. In contrast, Zyderm II is about 95 to 98% type I collagen, with the remainder being type III collagen.3,5
In humans, the major disadvantage of Zyderm II is the adverse reactions reported with its use. Adverse treatment reactions are characterized by urticaria, myalgias, arthralgias, and one anaphylactoid reaction.14,15,16 Such reactions include local hypersensitivity reactions, cystic reactions, and the development of systemic symptoms. Treatment-associated hypersensitivity reactions consist of redness and swelling at the treatment site.3 These allergic reactions are believed to be an immunological response to the foreign source, bovine collagen. About 3% of patients treated with Zyderm II developed positive skin test responses and 1.3% developed transient, localized, adverse reactions.5 Approximately 56% of these locally necrotic events occur in the glabellar area.3 There have been two reports of partial vision loss after Zyderm collagen therapy.3
To avoid these adverse events, skin tests are required before receiving bovine collagen.3 There is a smaller group of allergic patients who develop an allergy to bovine collagen after one, and sometimes two, negative skin tests.6 Zyderm II is contraindicated in patients with autoimmune disease. Patients with lidocaine sensitivity, a history of an anaphylactoid event, or previous sensitivity to bovine collagen are excluded from treatment.3
In contrast, human-based collagen products such as type I and type III injectable human collagen do not pose the allergy risk of bovine collagen and a skin test is not required prior to use. Human-based collagen tends to be replaced with human collagen, which is more biocompatible.
CLINICAL STUDIES
Open Label Trial
MATERIALS AND METHODS
Study Design
A clinical safety trial of highly purified type I and type III injectable human collagen was conducted at three hospitals in China.
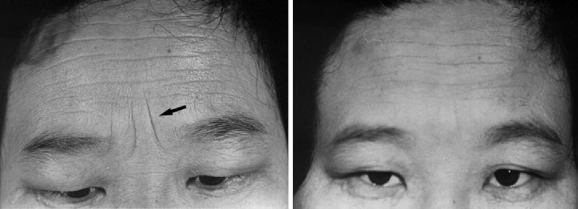
Each patient received two collagen injections: an initial injection and a secondary injection 30 days after the initial injection (Fig. 5). The results were evaluated by both the clinician and the patients. Photographs were taken before and 60 days after the first injection. Thirty days after the second injection, the data were analyzed. The results were classified on the following scale:
Figure 5.
Pictures of typical furrows treated with type I and type III injectable human collagen in this clinical study. Left, Preinjection. Right, 18 months after injection. This patient received an injection 30 days after the initial injection, and this photograph was taken 18 months after the injection.
Excellent: completely attained filling with no observable indentation.
Good: attained satisfactory filling and some observable indentation.
Poor: filling not attained and the indentation is obvious.
This was designed as a safety study of 6 months duration. However, 30 patients were followed for a long term up to 2 years.
Collagen Injections
Type I and type III injectable human collagen was prepared as detailed previously. After the injection site or sites were determined, the type I and type III injectable human collagen was removed from the refrigerator and placed at room temperature for 1 hour. Type I and type III injectable human collagen was injected only after rewarming. Each injection site received 0.2 to 2.0 mL of type I and type III injectable human collagen, depending on the size of the furrow or scar.
Type I and type III injectable human collagen injections require the expertise of a properly trained professional. Type I and type III injectable human collagen was injected superficially into the papillary dermal layer of the furrow in patients lying down or in a semireclining position. The injection continued until the skin turned white and formed a raised area. The best results were obtained from uniform injections. Nonuniform injections may cause a subcutaneous mass or node. After each injection, all physicians completed an observation form.
Study Participants
A total of 123 patients enrolled in the study. All patients were 19 years of age or older. Only patients with pitting skin deficiencies such as furrow, superficial scar, and superficial atrophy were included in this study. Typical examples of furrows and scars treated are seen in Figure 4. Exclusion criteria included immune disease, atopic patients, collagen disease, anaphylaxis, and allergies to lidocaine.
Demographics and Baseline Characteristics of Patients
The 123 patients who received injections of type I and type III injectable human collagen included 17 men and 106 women (Table 1). Most of the participants were women, with the ratio between male and female being 1:6.2. These patients ranged in age from 19 to 59 years, the average being 38.9 years of age. The 31- to 50-year age group accounted for 73.3% of the population of patients. The 123 patients had 151 areas treated. The major defects treated were furrow (87.8%) and superficial scars (12.2%), as indicated in Table 1.
Table 1.
Patient Demographics and Baseline Characteristics
| Parameter | Patient Population |
|---|---|
| Number of patients | 123 |
| Mean age (years) | 38.9 |
| Age ranges (years) | |
| Younger than 20 | 2 |
| 21 to 30 | 17 |
| 31 to 40 | 50 |
| 41 to 50 | 40 |
| 51 to 59 | 14 |
| Gender | |
| Male | 17 |
| Female | 106 |
| Facial areas | |
| Glabellar furrow | 63 |
| Crow's feet | 39 |
| Frontal furrow lines | 33 |
The shortest duration of rhytid (years of skin deficiency present prior to treatment) was half a year, the longest duration of rhytid was 30 years, and the average duration of rhytid was 7.8 years. There were 38 cases with a duration of rhytid of less than 5 years, 46 cases with a duration of rhytid of 6 to 10 years, 18 cases with a duration of rhytid of 11 to 15 years, 14 cases with a duration of rhytid of 16 to 20 years, 4 cases with a duration of rhytid of 21 to 25 years, and 3 cases with a duration of rhytid of 26 to 30 years. Among them, treatment success rate for cases with a duration of rhytid of 1 to 10 years was the highest, observed in 68.25% of the total treatment cases.
RESULTS
Efficacy
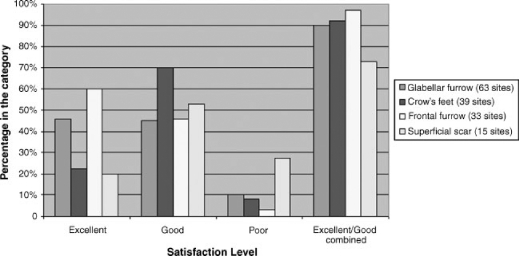
Although this was not an efficacy trial, the results of 123 patients treated with type I and type III injectable human collagen are shown in Table 2. Most of the patients were satisfied with the results, and a rating of excellent or good was achieved 90.2% of the time. The satisfaction level for each type of furrow was equally positive (Fig. 6), with the best results obtained for frontal furrow (96.8%) followed by crow's feet (92.3%) and glabellar furrow (90.5%). Superficial scars received lower ratings compared with all types of furrow: 73.3% versus 93.2%, respectively.
Table 2.
The Overall Rating of Patients Receiving Treatment with Type I and Type III Human Injectable Collagen
| Rating | Number of Patients | Percent |
|---|---|---|
| Excellent | 53 | 43 |
| Good | 58 | 47.2 |
| Poor | 12 | 9.8 |
Figure 6.
Level of patient satisfaction by site treated.
Safety
A slight redness and discomfort at or near the injection site were observed in 20 patients, with an incidence rate of 16.2%. This local reaction typically occurs after needle injection. The redness and discomfort usually resolve within 24 to 48 hours.
This incidence rate of 16.2% included 18 cases of minor subcutaneous hematomas and 2 cases of subcutaneous hemorrhage at the site of injection. All of these responses (small hematomas and hemorrhages) were absorbed into the body and resolved naturally. One patient refused a second injection after the appearance of a subcutaneous hematoma and was removed from the study. No other side effects were observed. There were no differences in the tolerability of the first and subsequent injections.
Long-Term Extension
Thirty patients who participated in the previous open label study were followed up for up to 2 years. The goal of this extension was to determine the long-term safety of type I and type III injectable human collagen injections.
No additional safety problems were observed. It was noted that some clinically significant effect was still present 8 to 9 months after the initial two-injection treatment.
Marketing Experience
Thirty thousand injections of type I and type III injectable human collagen were administered by experienced and properly trained professionals over a period of 10 years. Some patients requested an additional second and even a third injection to obtain a complete correction of rhytids.
No serious adverse experiences were reported. It was observed that the effects lasted on average 9 months after the first injection, 12 months after the second injection, and 18 months after the third injection (personal communication from Bingci Liu, M.D., April 2005).
DISCUSSION
Type III human collagen is becoming recognized as an important component of youthful skin. Type III collagen constitutes 50% of collagen in neonatal skin dermis but only 5% of collagen in aging adult skin. Collagen composition in the skin changes with age and facial rhytids, caused mainly by loss of collagen, especially type III. Replacing a natural human collagen into rhytids allows the most biocompatible soft tissue replacement, in keeping with the plastic surgery premise of replacing lost tissue with similar natural “like” tissue, not synthetic replacements.
Type III collagen induces tissue ingrowth and improves tissue repair, spatial organization, and neovascularization. Type III collagen is angiogenic and encourages neovessel formation and tissue regeneration. It has been shown in preclinical studies to induce fibrocytic ingrowth and neovascularization with no inflammation. Type III atelocollagen is naturally cross-linked by natural disulfide bridges that do not exist in type I atelocollagen.
For these reasons, using a dermal filler with a high type III collagen content may produce better results. In this study, we used type I and type III human injectable collagen, which contains 50% type III collagen. The results of this clinical trial indicated that type I and type III injectable human collagen corrected small skin defects such as furrows with a satisfaction rate of 90% or higher. Type I and type III injectable human collagen is recommended for superficial furrows and scars. This is in agreement with a previous study.8
No adverse or immune reactions were reported for these patients. No immune reactions were observed most likely because type I and type III injectable human collagen is made from human placentas. The safety data presented in this study are similar to the data reported for other countries. The higher number of minor adverse events reported in this study may have been due to the lack of experience of the surgical staff, which was performing this operation for the first time. These minor adverse events were due to the injection needle causing a small hematoma or hemorrhage. The choice of a plastic surgeon or dermatologist experienced in this type of surgery is highly recommended.
As mentioned previously, type I and type III injectable human collagen contains type I and type III collagen in approximately equal proportions (50% type I and 50% type III collagen). The high concentration of type III collagen may be advantageous for the treatment of wrinkles because the loss of type III collagen contributes to the formation of facial wrinkles. In addition, type III collagen induces tissue regeneration, which may be indicated by the length of time needed between retreatments. Patients may require retreatment 6 to 8 months after the first injection, 8 to 12 months after the second injection, and 12 to 18 months after the third injection, if not longer; many patients reported that collagen lasted 2 to 3 years after the first injection (personal communication from Bingci Liu, M.D., April 2005).
CONCLUSION
Type I and type III injectable human collagen from human source is a safe and effective method for correcting skin furrows and superficial scars. It is easy to use and has a very low incidence of minor adverse events of a self-limiting nature related to the injections. No serious adverse events were observed.
The study involving 123 patients resulted in the approval of a highly purified type I and type III injectable human collagen dermal fill product by the State Medicine Authority of China (China's FDA) in 1994. Since that time, more than 30,000 units have been sold with an average persistence of 9 months. This persistence increases to over 1 year with subsequent injections. No allergic reactions have been reported because this is a natural human collagen.
Type I and type III injectable human collagen is closest to the ideal natural dermal filler that stimulates the tissue regenerative effects of the skin. We believe this is due to the high percentage of type III collagen. This is the same type of collagen that is increased in youthful and healthy skin.
FINANCIAL DISCLOSURE
Dr. Harrell is an officer and shareholder in Albiorex, a regenerative medicine biotechnology company that is developing Humallagen®, type I and type III human collagen, for dermal fill application. Dr. Harrell also holds patents for this process.
ACKNOWLEDGMENTS
We gratefully acknowledge the assistance with preparation of the manuscript by Andrea Gwosdow, Ph.D. and Mr. George Chu.
REFERENCES
- Spira M, Rosen T. Injectable soft tissue substitutes. Clin Plast Surg. 1993;20:181–188. [PubMed] [Google Scholar]
- Diegelmann R F. Collagen metabolism. Wounds. 2001;13:177–182. [Google Scholar]
- Klein A W. Collagen substitutes: bovine collagen. Clin Plast Surg. 2001;28:53–62. [PubMed] [Google Scholar]
- Tiollier J, Dumas H, Tardy M, Tayot J-L. Fibroblast behavior on gels of type I, III and IV human placental collagens. Exp Cell Res. 1990;191:95–104. doi: 10.1016/0014-4827(90)90041-8. [DOI] [PubMed] [Google Scholar]
- Sclafani A P, Romo T., 3rd Injectable fillers for facial soft tissue enhancement. Facial Plast Surg. 2000;16:29–34. doi: 10.1055/s-2000-7323. [DOI] [PubMed] [Google Scholar]
- Fagien S. Facial soft-tissue augmentation with injectable autologous and allogeneic human tissue collagen matrix (autologen and dermalogen) Plast Reconstr Surg. 2000;105:362–373. doi: 10.1097/00006534-200001000-00057. [DOI] [PubMed] [Google Scholar]
- Ozgentas H E, Pindur A, Spira M, Liu B, Shenaq S. A comparison of soft-tissue substitutes. Ann Plast Surg. 1994;33:171–177. doi: 10.1097/00000637-199408000-00009. [DOI] [PubMed] [Google Scholar]
- Liu B, Xu Z, Yu R. Experimental and clinical observation on wrinkle correction by medical cosmetic collagen injection. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 1994;16:197–200. [PubMed] [Google Scholar]
- Liu B, Spira M, Xu Z, Ozgentas E, Shenaq S M. A comparative study of gut suture, human amnion collagen, bovine skin collagen and Vicryl suture implants in rats. Chin Med Sci J. 1997;12:26–31. [PubMed] [Google Scholar]
- Jamall I, Finelli V, Que Hee S S. A simple method to determine nanogram levels of 4-hydroxyproline in biological tissues. Anal Biochem. 1981;112:70–75. doi: 10.1016/0003-2697(81)90261-x. [DOI] [PubMed] [Google Scholar]
- Spira M, Liu B, Xu Z, Harrell R, Chahadeh H. Human anion collagen for soft tissue augmentation—biochemical characterizations and animal observations. J Biomed Mater Res. 1994;28:91–96. doi: 10.1002/jbm.820280112. [DOI] [PubMed] [Google Scholar]
- Francis M J, Williams K J, Sykes B C, Smith R. The relative amounts of the collagen chains alpha 1(1), alpha 2 and alpha1(III) in the skin of 31 patients with osteogenesis imperfecta. Clin Sci. 1981;60:617–623. doi: 10.1042/cs0600617. [DOI] [PubMed] [Google Scholar]
- Kligman A M, Armstrong R C. Histologic response to intradermal Zyderm and Zyplast (glutaraldehyde cross-linked) collagen in humans. J Dermatol Surg Oncol. 1986;12:351–357. doi: 10.1111/j.1524-4725.1986.tb01920.x. [DOI] [PubMed] [Google Scholar]
- Burke K E, Naughton G, Cassai N. A histological, immunological, and electron microscopic study of bovine collagen implants in the human. Ann Plast Surg. 1985;14:515–522. doi: 10.1097/00000637-198506000-00004. [DOI] [PubMed] [Google Scholar]
- Siegle R J, McCoy J P, Schade W, et al. Intradermal implantation of bovine collagen: humoral immune responses associated with clinical reactions. Arch Dermatol. 1984;120:183–187. [PubMed] [Google Scholar]
- McCoy J P, Schade W J, Liegle R J, et al. Characterization of the humoral immune response to bovine collagen implants. Arch Dermatol. 1985;121:990–994. [PubMed] [Google Scholar]