Abstract
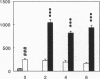
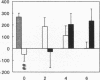
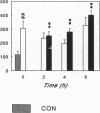
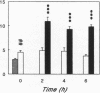
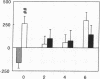
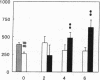
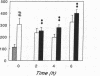
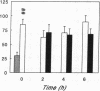
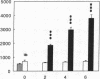
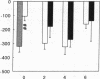
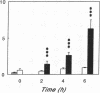
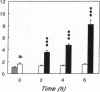
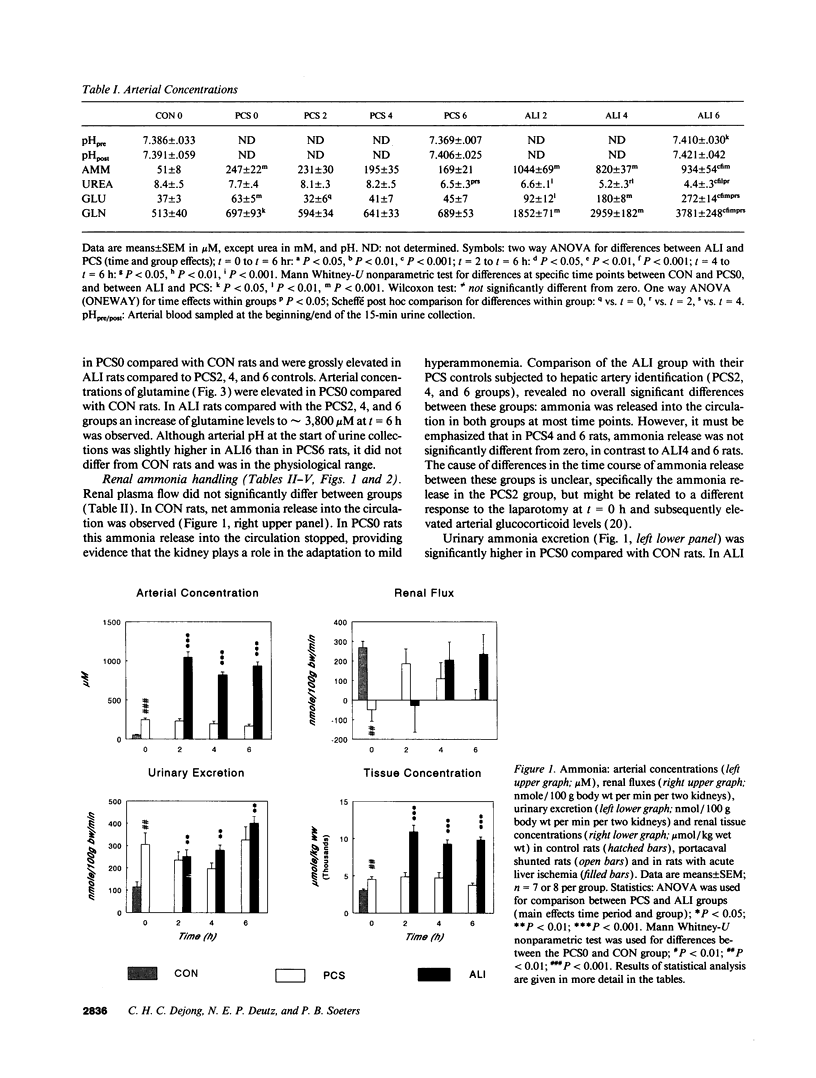
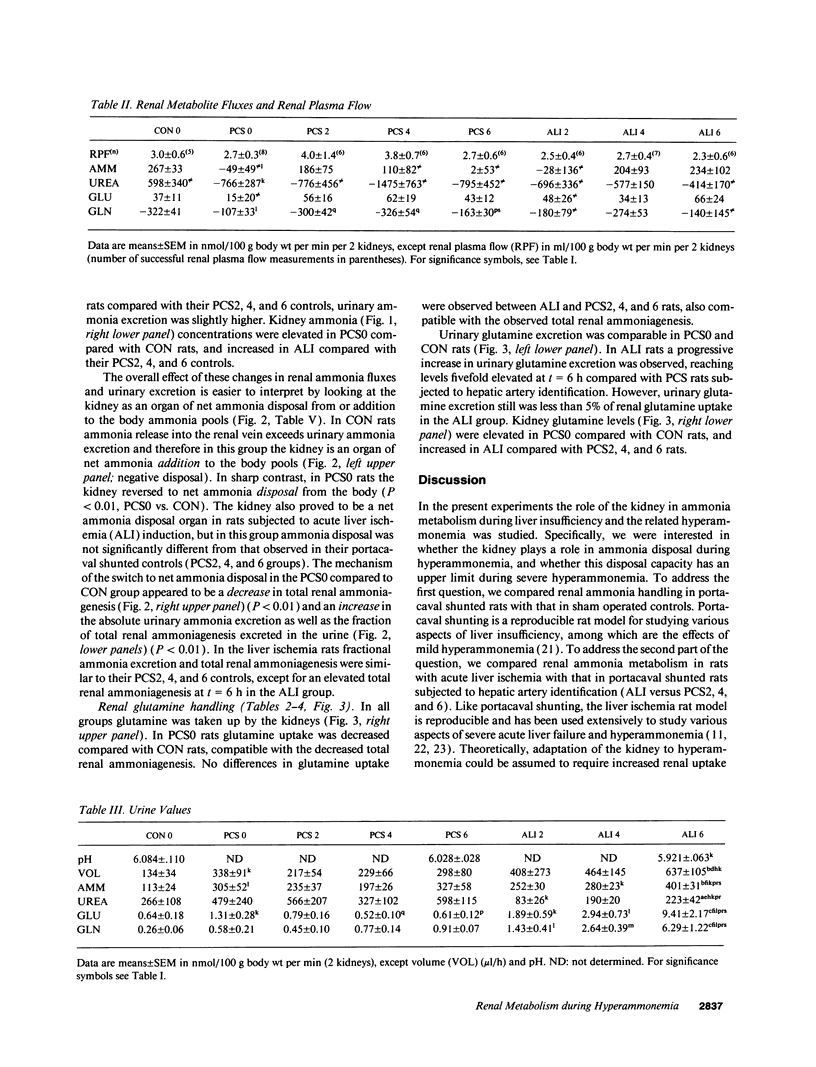
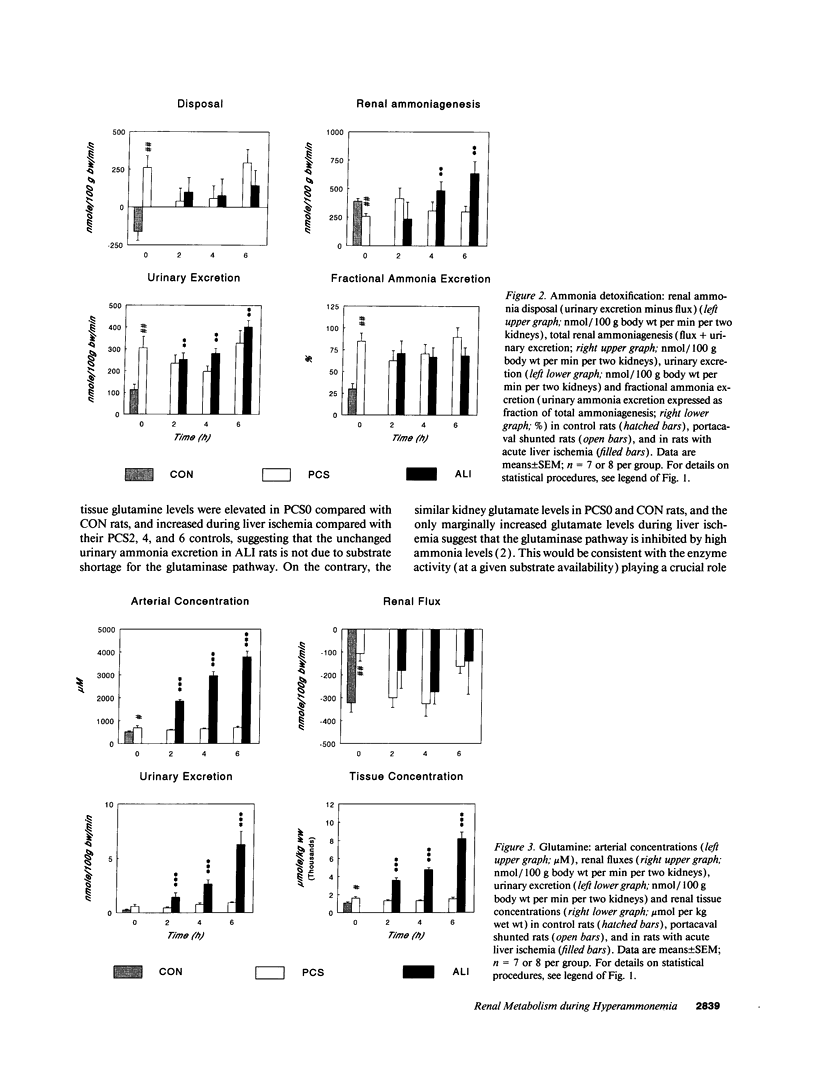
Renal glutamine uptake and subsequent urinary ammonia excretion could be an important alternative pathway of ammonia disposal from the body during liver failure (diminished urea synthesis), but this pathway has received little attention. Therefore, we investigated renal glutamine and ammonia metabolism in midly hyperammonemic, portacaval shunted rats and severely hyperammonemic rats with acute liver ischemia compared to their respective controls, to investigate whether renal ammonia disposal from the body is enhanced during hyperammonemia and to explore the limits of the pathway. Renal fluxes, urinary excretion, and renal tissue concentrations of amino acids and ammonia were measured 24 h after portacaval shunting, and 2, 4, and 6 h after liver ischemia induction and in the appropriate controls. Arterial ammonia increased to 247 +/- 22 microM after portacaval shunting compared to controls (51 +/- 8 microM) (P < 0.001) and increased to 934 +/- 54 microM during liver ischemia (P < 0.001). Arterial glutamine increased to 697 +/- 93 microM after portacaval shunting compared to controls (513 +/- 40 microM) (P < 0.01) and further increased to 3781 +/- 248 microM during liver ischemia (P < 0.001). In contrast to controls, in portacaval shunted rats the kidney net disposed ammonia from the body by diminishing renal venous ammonia release (from 267 +/- 33 to -49 +/- 59 nmol/100 g body wt per min) and enhancing urinary ammonia excretion from 113 +/- 24 to 305 +/- 52 nmol/100 g body wt per min (both P < 0.01). Renal glutamine uptake diminished in portacaval shunted rats compared to controls (-107 +/- 33 vs. -322 +/- 41 nmol/100 g body wt per min) (P < 0.01). However, during liver ischemia, net renal ammonia disposal from the body did not further increase (294 +/- 88 vs. 144 +/- 101 nmol/100 g body wt per min during portacaval shunting versus liver ischemia). Renal glutamine uptake was comparable in both hyperammonemic models. These results indicate that the rat kidney plays an important role in ammonia disposal during mild hyperammonemia. However, during severe liver insufficiency induced-hyperammonemia, ammonia disposal capacity appears to be exceeded.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosman D. K., Deutz N. E., De Graaf A. A., vd Hulst R. W., Van Eijk H. M., Bovée W. M., Maas M. A., Jörning G. G., Chamuleau R. A. Changes in brain metabolism during hyperammonemia and acute liver failure: results of a comparative 1H-NMR spectroscopy and biochemical investigation. Hepatology. 1990 Aug;12(2):281–290. doi: 10.1002/hep.1840120215. [DOI] [PubMed] [Google Scholar]
- Bosman D. K., van den Buijs C. A., de Haan J. G., Maas M. A., Chamuleau R. A. The effects of benzodiazepine-receptor antagonists and partial inverse agonists on acute hepatic encephalopathy in the rat. Gastroenterology. 1991 Sep;101(3):772–781. doi: 10.1016/0016-5085(91)90538-v. [DOI] [PubMed] [Google Scholar]
- Dejong C. H., Kampman M. T., Deutz N. E., Soeters P. B. Altered glutamine metabolism in rat portal drained viscera and hindquarter during hyperammonemia. Gastroenterology. 1992 Mar;102(3):936–948. doi: 10.1016/0016-5085(92)90180-7. [DOI] [PubMed] [Google Scholar]
- Dejong C. H., Kampman M. T., Deutz N. E., Soeters P. B. Cerebral cortex ammonia and glutamine metabolism during liver insufficiency-induced hyperammonemia in the rat. J Neurochem. 1992 Sep;59(3):1071–1079. doi: 10.1111/j.1471-4159.1992.tb08349.x. [DOI] [PubMed] [Google Scholar]
- DuBose T. D., Jr, Good D. W. Chronic hyperkalemia impairs ammonium transport and accumulation in the inner medulla of the rat. J Clin Invest. 1992 Oct;90(4):1443–1449. doi: 10.1172/JCI116011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imler M., Chabrier G., Marescaux C., Warter J. M. Effects of 2,4-dinitrophenol on renal ammoniagenesis in the rat. Eur J Pharmacol. 1986 Apr 16;123(2):175–179. doi: 10.1016/0014-2999(86)90657-6. [DOI] [PubMed] [Google Scholar]
- Imler M., Schlienger J. L., Chabrier G., Simon C. Arterial ammonemia changes of renal origin induced in the rat by acid and alkaline diets. Res Exp Med (Berl) 1986;186(5):353–363. doi: 10.1007/BF01852101. [DOI] [PubMed] [Google Scholar]
- Kikeri D., Sun A., Zeidel M. L., Hebert S. C. Cellular NH4+/K+ transport pathways in mouse medullary thick limb of Henle. Regulation by intracellular pH. J Gen Physiol. 1992 Mar;99(3):435–461. doi: 10.1085/jgp.99.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen K. D., McCullough A. J. Problems with animal models of chronic liver disease: suggestions for improvement in standardization. Hepatology. 1989 Mar;9(3):500–503. doi: 10.1002/hep.1840090326. [DOI] [PubMed] [Google Scholar]
- Nagami G. T. Effect of angiotensin II on ammonia production and secretion by mouse proximal tubules perfused in vitro. J Clin Invest. 1992 Mar;89(3):925–931. doi: 10.1172/JCI115673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OWEN E. E., JOHNSON J. H., TYOR M. P. The effect of induced hyperammonemia on renal ammonia metabolism. J Clin Invest. 1961 Feb;40:215–221. doi: 10.1172/JCI104247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OWEN E. E., ROBINSON R. R. Amino acid extraction and ammonia metabolism by the human kidney during the prolonged administration of ammonium chloride. J Clin Invest. 1963 Feb;42:263–276. doi: 10.1172/JCI104713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phromphetcharat V., Welbourne T. C. Renal glutamine extraction and gut/liver interaction in glutamine homeostasis. Contrib Nephrol. 1985;47:9–14. doi: 10.1159/000411202. [DOI] [PubMed] [Google Scholar]
- Schröck H., Goldstein L. Interorgan relationships for glutamine metabolism in normal and acidotic rats. Am J Physiol. 1981 May;240(5):E519–E525. doi: 10.1152/ajpendo.1981.240.5.E519. [DOI] [PubMed] [Google Scholar]
- Swain M., Butterworth R. F., Blei A. T. Ammonia and related amino acids in the pathogenesis of brain edema in acute ischemic liver failure in rats. Hepatology. 1992 Mar;15(3):449–453. doi: 10.1002/hep.1840150316. [DOI] [PubMed] [Google Scholar]
- TYOR M. P., OWEN E. E., BERRY J. N., FLANAGAN J. F. The relative role of extremity, liver, and kidney as ammonia receivers and donors in patients with liver disease. Gastroenterology. 1960 Oct;39:420–424. [PubMed] [Google Scholar]
- Tannen R. L. Ammonia metabolism. Am J Physiol. 1978 Oct;235(4):F265–F277. doi: 10.1152/ajprenal.1978.235.4.F265. [DOI] [PubMed] [Google Scholar]
- Welbourne T. C., Dass P. D. Gamma glutamyltransferase contribution to renal ammoniagenesis in vivo. Pflugers Arch. 1988 May;411(5):573–578. doi: 10.1007/BF00582380. [DOI] [PubMed] [Google Scholar]
- Welbourne T. C. Effect of metabolic acidosis on hindquarter glutamine and alanine release. Metabolism. 1986 Jul;35(7):614–618. doi: 10.1016/0026-0495(86)90166-6. [DOI] [PubMed] [Google Scholar]
- Welbourne T. C. Glucocorticoid control of ammoniagenesis in the proximal tubule. Semin Nephrol. 1990 Jul;10(4):339–349. [PubMed] [Google Scholar]
- Welbourne T. C., Phromphetcharat V., Givens G., Joshi S. Regulation of interorganal glutamine flow in metabolic acidosis. Am J Physiol. 1986 Apr;250(4 Pt 1):E457–E463. doi: 10.1152/ajpendo.1986.250.4.E457. [DOI] [PubMed] [Google Scholar]
- Welbourne T. C. Role of the lung in glutamine homeostasis. Contrib Nephrol. 1988;63:178–182. doi: 10.1159/000415718. [DOI] [PubMed] [Google Scholar]
- Welbourne T., Weber M., Bank N. The effect of glutamine administration on urinary ammonium excretion in normal subjects and patients with renal disease. J Clin Invest. 1972 Jul;51(7):1852–1860. doi: 10.1172/JCI106987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieve L. Pathogenesis of hepatic encephalopathy. Metab Brain Dis. 1987 Sep;2(3):147–165. doi: 10.1007/BF00999607. [DOI] [PubMed] [Google Scholar]