Abstract
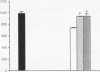
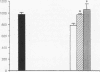
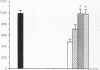
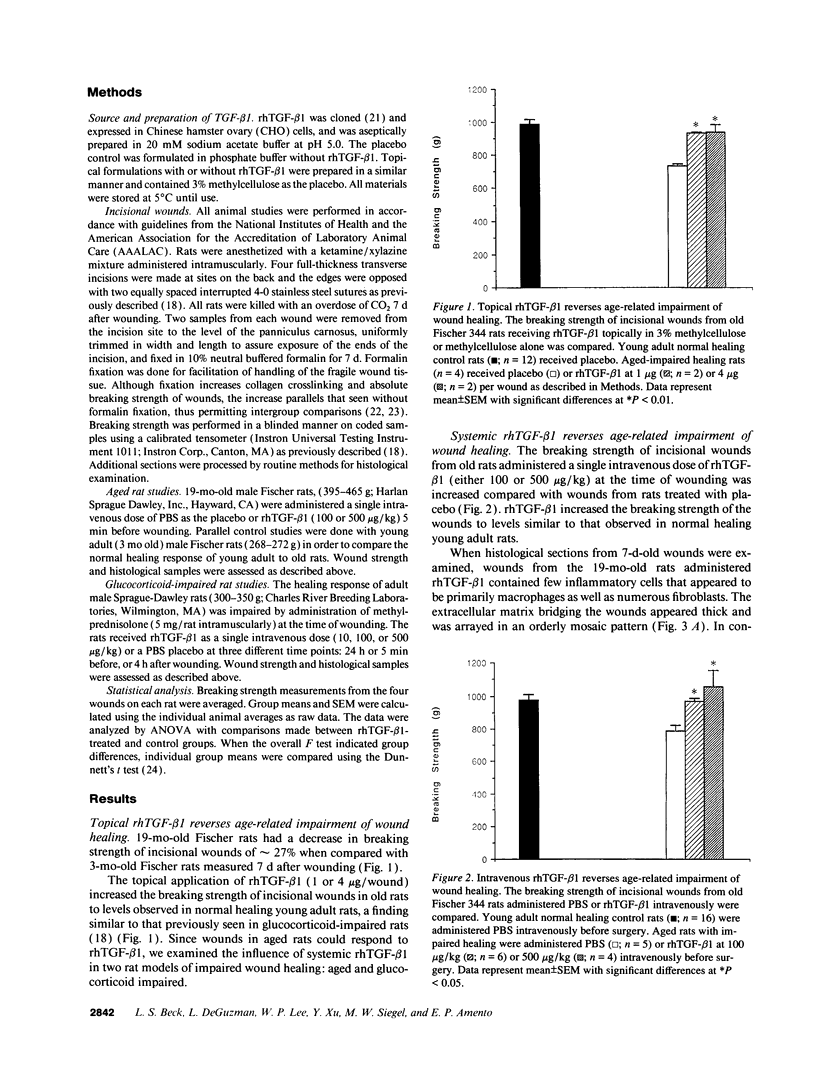
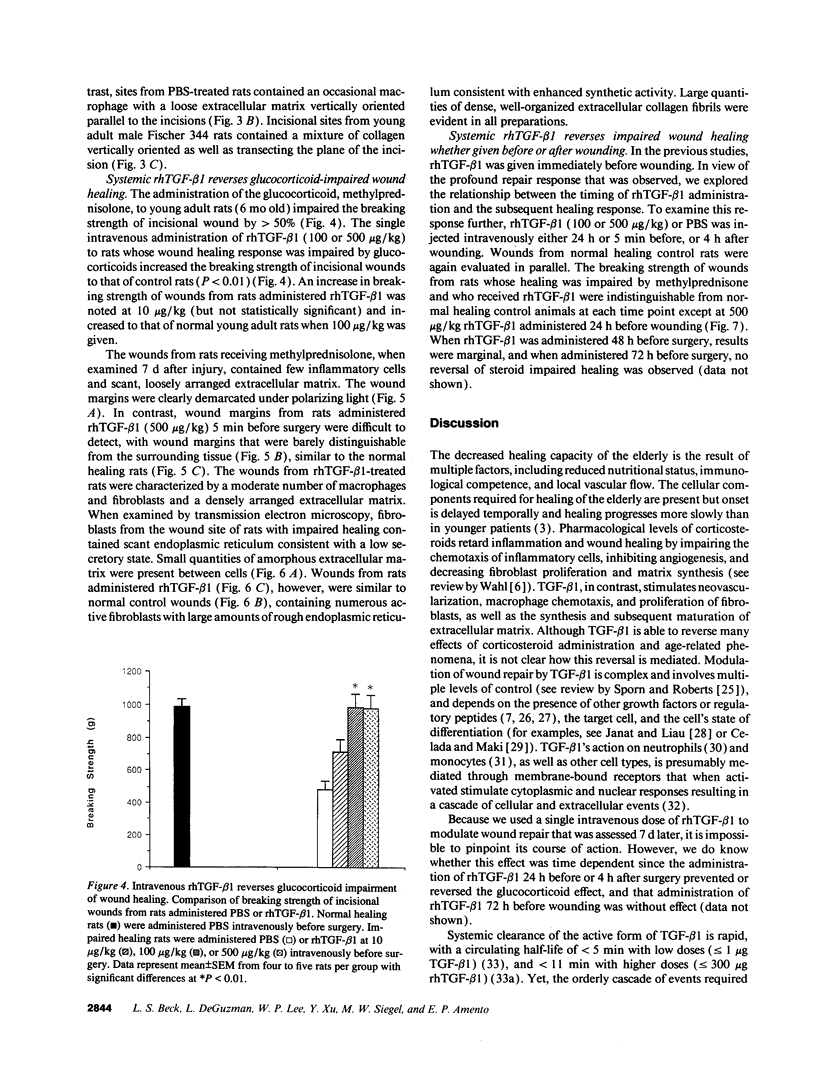
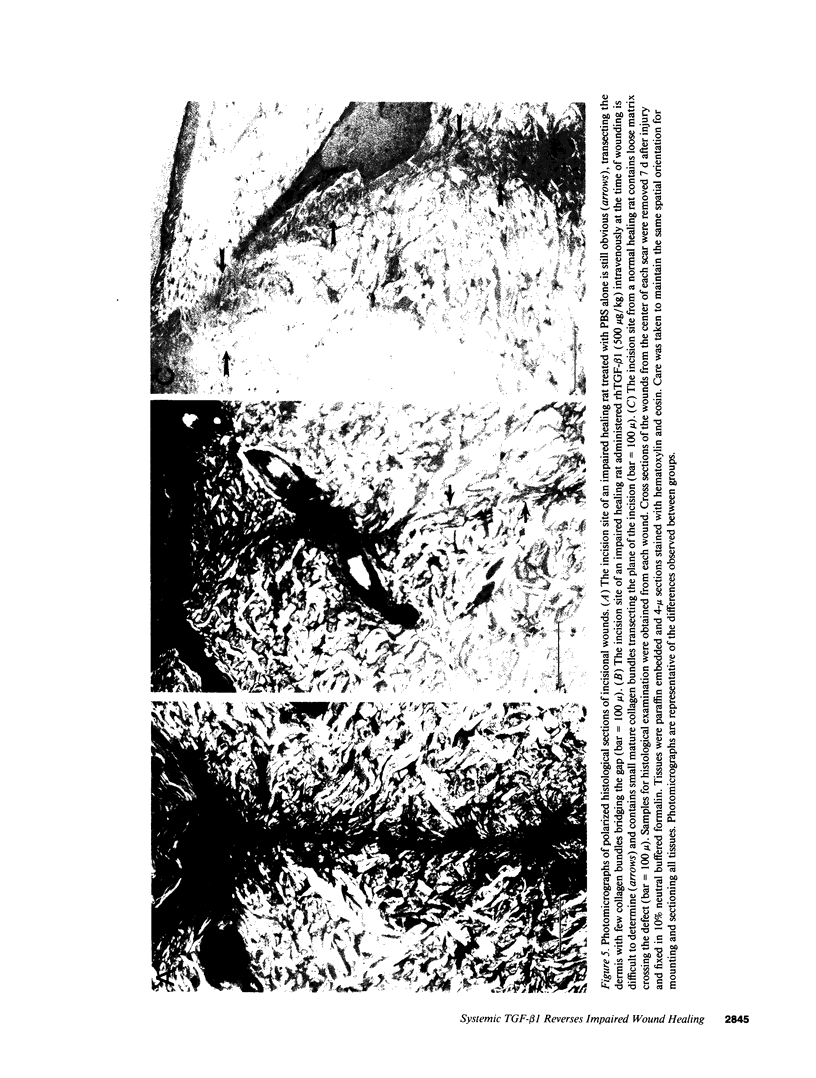
The role of intravenously administered recombinant human transforming growth factor-beta 1 (rhTGF-beta 1) on the healing of incisional wounds in rats with impaired healing due to age or glucocorticoid administration was investigated. The administration of methylprednisolone to young adult rats decreased wound breaking strength to 50% of normal control. Breaking strength of incisional wounds from 19-mo-old rats was decreased approximately 27% compared with wounds from normal healing young adult rats. A single intravenous administration of rhTGF-beta 1 (100 or 500 micrograms/kg) increased wound breaking strength from old rats or young adult rats with glucocorticoid-induced impaired healing to levels similar to normal healing control animals when determined 7 d after injury. Even though the circulating half-life of systemically administered rhTGF-beta 1 is < 5 min, a sustained stimulatory effect on extracellular matrix secretion was evident in glucocorticoid-impaired rats when rhTGF-beta 1 was administered at the time of wounding, 4 h after wounding, or even 24 h before wounding. These observations indicate a previously unrecognized potential for the active form of TGF-beta 1 to profoundly influence the wound healing cascade after brief systemic exposure.
Full text
PDF

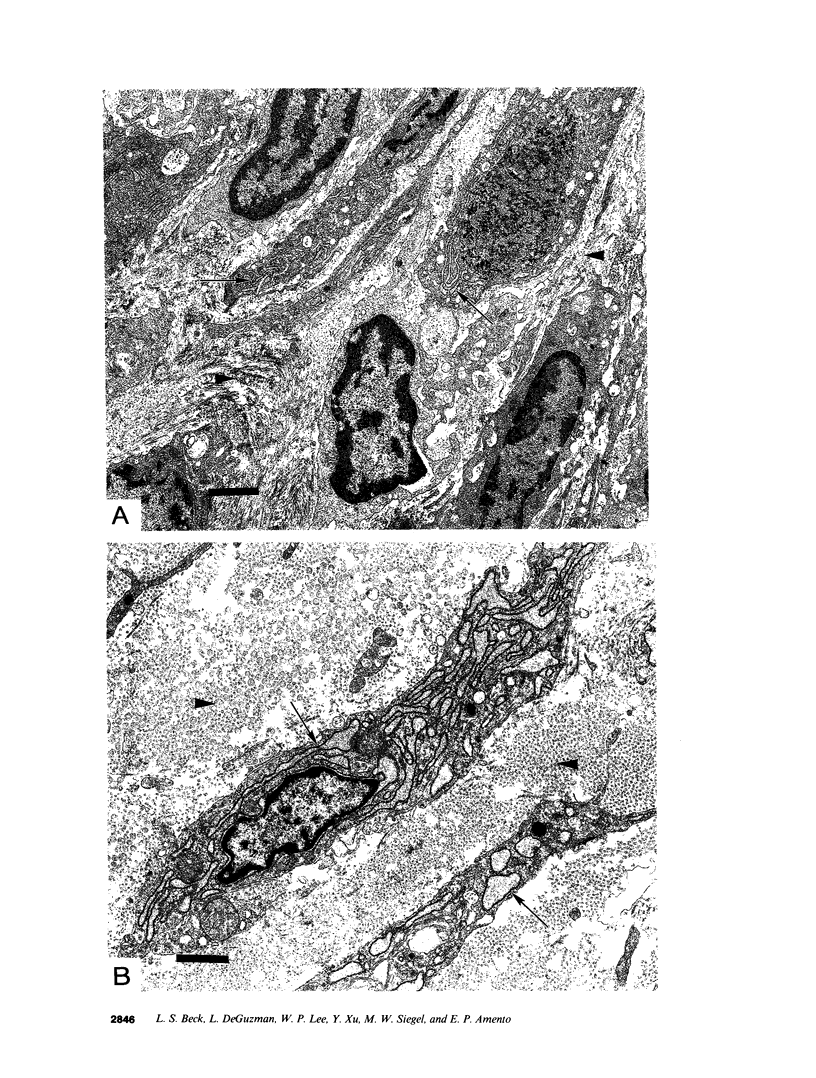



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck L. S., Chen T. L., Hirabayashi S. E., Deguzman L., Lee W. P., McFatridge L. L., Xu Y., Bates R. L., Ammann A. J. Accelerated healing of ulcer wounds in the rabbit ear by recombinant human transforming growth factor-beta 1. Growth Factors. 1990;2(4):273–282. doi: 10.3109/08977199009167022. [DOI] [PubMed] [Google Scholar]
- Beck L. S., Chen T. L., Mikalauski P., Ammann A. J. Recombinant human transforming growth factor-beta 1 (rhTGF-beta 1) enhances healing and strength of granulation skin wounds. Growth Factors. 1990;3(4):267–275. doi: 10.3109/08977199009003669. [DOI] [PubMed] [Google Scholar]
- Beck L. S., Deguzman L., Lee W. P., Xu Y., McFatridge L. A., Amento E. P. TGF-beta 1 accelerates wound healing: reversal of steroid-impaired healing in rats and rabbits. Growth Factors. 1991;5(4):295–304. doi: 10.3109/08977199109000293. [DOI] [PubMed] [Google Scholar]
- Brandes M. E., Mai U. E., Ohura K., Wahl S. M. Type I transforming growth factor-beta receptors on neutrophils mediate chemotaxis to transforming growth factor-beta. J Immunol. 1991 Sep 1;147(5):1600–1606. [PubMed] [Google Scholar]
- Brandes M. E., Wakefield L. M., Wahl S. M. Modulation of monocyte type I transforming growth factor-beta receptors by inflammatory stimuli. J Biol Chem. 1991 Oct 15;266(29):19697–19703. [PubMed] [Google Scholar]
- Broadley K. N., Aquino A. M., Hicks B., Ditesheim J. A., McGee G. S., Demetriou A. A., Woodward S. C., Davidson J. M. The diabetic rat as an impaired wound healing model: stimulatory effects of transforming growth factor-beta and basic fibroblast growth factor. Biotechnol Ther. 1989 1990;1(1):55–68. [PubMed] [Google Scholar]
- Bruce S. A., Deamond S. F. Longitudinal study of in vivo wound repair and in vitro cellular senescence of dermal fibroblasts. Exp Gerontol. 1991;26(1):17–27. doi: 10.1016/0531-5565(91)90058-t. [DOI] [PubMed] [Google Scholar]
- Celada A., Maki R. A. Transforming growth factor-beta enhances the M-CSF and GM-CSF-stimulated proliferation of macrophages. J Immunol. 1992 Feb 15;148(4):1102–1105. [PubMed] [Google Scholar]
- Coffey R. J., Jr, Kost L. J., Lyons R. M., Moses H. L., LaRusso N. F. Hepatic processing of transforming growth factor beta in the rat. Uptake, metabolism, and biliary excretion. J Clin Invest. 1987 Sep;80(3):750–757. doi: 10.1172/JCI113130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B. J., Danon D., Roth G. S. Wound repair in mice as influenced by age and antimacrophage serum. J Gerontol. 1987 May;42(3):295–301. doi: 10.1093/geronj/42.3.295. [DOI] [PubMed] [Google Scholar]
- Cox D. A., Kunz S., Cerletti N., McMaster G. K., Burk R. R. Wound healing in aged animals--effects of locally applied transforming growth factor beta 2 in different model systems. EXS. 1992;61:287–295. doi: 10.1007/978-3-0348-7001-6_46. [DOI] [PubMed] [Google Scholar]
- Curtsinger L. J., Pietsch J. D., Brown G. L., von Fraunhofer A., Ackerman D., Polk H. C., Jr, Schultz G. S. Reversal of Adriamycin-impaired wound healing by transforming growth factor-beta. Surg Gynecol Obstet. 1989 Jun;168(6):517–522. [PubMed] [Google Scholar]
- Danon D., Kowatch M. A., Roth G. S. Promotion of wound repair in old mice by local injection of macrophages. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2018–2020. doi: 10.1073/pnas.86.6.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R., Jarrett J. A., Chen E. Y., Eaton D. H., Bell J. R., Assoian R. K., Roberts A. B., Sporn M. B., Goeddel D. V. Human transforming growth factor-beta complementary DNA sequence and expression in normal and transformed cells. Nature. 1985 Aug 22;316(6030):701–705. doi: 10.1038/316701a0. [DOI] [PubMed] [Google Scholar]
- Eaglstein W. H. Wound healing and aging. Clin Geriatr Med. 1989 Feb;5(1):183–188. [PubMed] [Google Scholar]
- Ehrlich H. P., Hunt T. K. Effects of cortisone and vitamin A on wound healing. Ann Surg. 1968 Mar;167(3):324–328. doi: 10.1097/00000658-196803000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson W. H., 3rd, Hunt T. K. Wound healing and aging. J Invest Dermatol. 1979 Jul;73(1):88–91. doi: 10.1111/1523-1747.ep12532775. [DOI] [PubMed] [Google Scholar]
- Grinnell F. Wound repair, keratinocyte activation and integrin modulation. J Cell Sci. 1992 Jan;101(Pt 1):1–5. doi: 10.1242/jcs.101.1.1. [DOI] [PubMed] [Google Scholar]
- Ishikawa O., Yamakage A., LeRoy E. C., Trojanowska M. Persistent effect of TGF-beta 1 on extracellular matrix gene expression in human dermal fibroblasts. Biochem Biophys Res Commun. 1990 May 31;169(1):232–238. doi: 10.1016/0006-291x(90)91458-5. [DOI] [PubMed] [Google Scholar]
- Janat M. F., Liau G. Transforming growth factor beta 1 is a powerful modulator of platelet-derived growth factor action in vascular smooth muscle cells. J Cell Physiol. 1992 Feb;150(2):232–242. doi: 10.1002/jcp.1041500203. [DOI] [PubMed] [Google Scholar]
- Jones P. L., Millman A. Wound healing and the aged patient. Nurs Clin North Am. 1990 Mar;25(1):263–277. [PubMed] [Google Scholar]
- Kane C. J., Hebda P. A., Mansbridge J. N., Hanawalt P. C. Direct evidence for spatial and temporal regulation of transforming growth factor beta 1 expression during cutaneous wound healing. J Cell Physiol. 1991 Jul;148(1):157–173. doi: 10.1002/jcp.1041480119. [DOI] [PubMed] [Google Scholar]
- Karasawa A., Guo J. P., Ma X. L., Lefer A. M. Beneficial effects of transforming growth factor-beta and tissue plasminogen activator in splanchnic artery occlusion and reperfusion in cats. J Cardiovasc Pharmacol. 1991 Jul;18(1):95–105. doi: 10.1097/00005344-199107000-00013. [DOI] [PubMed] [Google Scholar]
- Kuruvilla A. P., Shah R., Hochwald G. M., Liggitt H. D., Palladino M. A., Thorbecke G. J. Protective effect of transforming growth factor beta 1 on experimental autoimmune diseases in mice. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2918–2921. doi: 10.1073/pnas.88.7.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVENSON S. M., GEEVER E. F., CROWLEY L. V., OATES J. F., 3rd, BERARD C. W., ROSEN H. THE HEALING OF RAT SKIN WOUNDS. Ann Surg. 1965 Feb;161:293–308. doi: 10.1097/00000658-196502000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefer A. M., Tsao P., Aoki N., Palladino M. A., Jr Mediation of cardioprotection by transforming growth factor-beta. Science. 1990 Jul 6;249(4964):61–64. doi: 10.1126/science.2164258. [DOI] [PubMed] [Google Scholar]
- Leibovich S. J., Danon D. Promotion of wound repair in mice by application of glucan. J Reticuloendothel Soc. 1980 Jan;27(1):1–11. [PubMed] [Google Scholar]
- Martin G. M., Sprague C. A., Epstein C. J. Replicative life-span of cultivated human cells. Effects of donor's age, tissue, and genotype. Lab Invest. 1970 Jul;23(1):86–92. [PubMed] [Google Scholar]
- McGee G. S., Broadley K. N., Buckley A., Aquino A., Woodward S. C., Demetriou A. A., Davidson J. M. Recombinant transforming growth factor beta accelerates incisional wound healing. Curr Surg. 1989 Mar-Apr;46(2):103–106. [PubMed] [Google Scholar]
- Mustoe T. A., Pierce G. F., Morishima C., Deuel T. F. Growth factor-induced acceleration of tissue repair through direct and inductive activities in a rabbit dermal ulcer model. J Clin Invest. 1991 Feb;87(2):694–703. doi: 10.1172/JCI115048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustoe T. A., Pierce G. F., Thomason A., Gramates P., Sporn M. B., Deuel T. F. Accelerated healing of incisional wounds in rats induced by transforming growth factor-beta. Science. 1987 Sep 11;237(4820):1333–1336. doi: 10.1126/science.2442813. [DOI] [PubMed] [Google Scholar]
- Pienta K. J., Coffey D. S. Characterization of the subtypes of cell motility in ageing human skin fibroblasts. Mech Ageing Dev. 1990 Nov;56(2):99–105. doi: 10.1016/0047-6374(90)90001-v. [DOI] [PubMed] [Google Scholar]
- Pierce G. F., Mustoe T. A., Lingelbach J., Masakowski V. R., Gramates P., Deuel T. F. Transforming growth factor beta reverses the glucocorticoid-induced wound-healing deficit in rats: possible regulation in macrophages by platelet-derived growth factor. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2229–2233. doi: 10.1073/pnas.86.7.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce G. F., Mustoe T. A., Lingelbach J., Masakowski V. R., Griffin G. L., Senior R. M., Deuel T. F. Platelet-derived growth factor and transforming growth factor-beta enhance tissue repair activities by unique mechanisms. J Cell Biol. 1989 Jul;109(1):429–440. doi: 10.1083/jcb.109.1.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaglino D., Jr, Nanney L. B., Kennedy R., Davidson J. M. Transforming growth factor-beta stimulates wound healing and modulates extracellular matrix gene expression in pig skin. I. Excisional wound model. Lab Invest. 1990 Sep;63(3):307–319. [PubMed] [Google Scholar]
- Quirinia A., Viidik A. The influence of age on the healing of normal and ischemic incisional skin wounds. Mech Ageing Dev. 1991 May;58(2-3):221–232. doi: 10.1016/0047-6374(91)90094-g. [DOI] [PubMed] [Google Scholar]
- Racke M. K., Dhib-Jalbut S., Cannella B., Albert P. S., Raine C. S., McFarlin D. E. Prevention and treatment of chronic relapsing experimental allergic encephalomyelitis by transforming growth factor-beta 1. J Immunol. 1991 May 1;146(9):3012–3017. [PubMed] [Google Scholar]
- Rodland K. D., Muldoon L. L., Magun B. E. Cellular mechanisms of TGF-beta action. J Invest Dermatol. 1990 Jun;94(6 Suppl):33S–40S. doi: 10.1111/1523-1747.ep12875031. [DOI] [PubMed] [Google Scholar]
- Rømer J., Lund L. R., Eriksen J., Ralfkiaer E., Zeheb R., Gelehrter T. D., Danø K., Kristensen P. Differential expression of urokinase-type plasminogen activator and its type-1 inhibitor during healing of mouse skin wounds. J Invest Dermatol. 1991 Nov;97(5):803–811. doi: 10.1111/1523-1747.ep12486833. [DOI] [PubMed] [Google Scholar]
- Sato Y., Tsuboi R., Lyons R., Moses H., Rifkin D. B. Characterization of the activation of latent TGF-beta by co-cultures of endothelial cells and pericytes or smooth muscle cells: a self-regulating system. J Cell Biol. 1990 Aug;111(2):757–763. doi: 10.1083/jcb.111.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri T., Campisi J. Repression of c-fos transcription and an altered genetic program in senescent human fibroblasts. Science. 1990 Jan 12;247(4939):205–209. doi: 10.1126/science.2104680. [DOI] [PubMed] [Google Scholar]
- Varga J., Rosenbloom J., Jimenez S. A. Transforming growth factor beta (TGF beta) causes a persistent increase in steady-state amounts of type I and type III collagen and fibronectin mRNAs in normal human dermal fibroblasts. Biochem J. 1987 Nov 1;247(3):597–604. doi: 10.1042/bj2470597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E., Gundersen D. Increased organization of cytoskeleton accompanying the aging of human fibroblasts in vitro. Exp Cell Res. 1984 Sep;154(1):191–202. doi: 10.1016/0014-4827(84)90679-7. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Mann D. M., Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990 Jul 19;346(6281):281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]