ABSTRACT
Since the inception of Mohs micrographic surgery in the 1930s, this technique has proved its utility in the treatment of cutaneous tumors. This review describes the technique of Mohs micrographic surgery and the various indications for which it is used. We discuss the use of Mohs micrographic surgery for the following cutaneous tumors: basal cell carcinoma, squamous cell carcinoma, melanoma in situ, dermatofibrosarcoma protuberans, Merkel cell carcinoma, microcystic adnexal carcinoma, atypical fibroxanthoma, and sebaceous carcinoma. Mohs micrographic surgery is cost effective in the U.S. health care system because billing for the surgeon-pathologist and laboratory processing is bundled together. However, Mohs micrographic surgery may be more expensive in European systems because the Mohs technique surgeon, pathologist, and laboratory fees may be billed separately.
Keywords: Mohs micrographic surgery, cutaneous oncology, skin cancer
In the early 1930s, Dr. Frederic Mohs developed the procedure that bears his name while working as an assistant in a cancer research laboratory during medical school.1 While studying rats implanted with skin cancer, he noticed that the carcinomatous tissue, when fixed in 20% zinc chloride, maintained its histologic architecture after extirpation, thus aiding in microscopic examination.2 To ensure margin clearance, he formulated the technique of chemical fixation, excision by saucerization, and microscopic examination of horizontal sections.2 In 1936, he started using this technique in patients deemed incurable. He would apply the 20% zinc chloride paste on the skin cancer in vivo and leave it overnight to fixate the skin. While painful for the patient, this was quite effective in preserving the microscopic anatomy of the skin. Only one stage could be taken per day because the tissue was processed using paraffin sections. Subsequent stages could be taken depending on the microscopic results. The presence of zinc chloride on the skin forced Dr. Mohs to allow the wounds to heal by secondary intention as the inflammation left the wound bed inhospitable to reconstructive techniques. With this protocol, he was able to successfully treat most of his patients.
Because Dr. Mohs was formally trained as a general surgeon, he first reported his success in using this technique in 440 patients in the Archives of Surgery in 1941.3 However, the reception within the surgical community was modest at best. His technique diverged so dramatically from the accepted procedure that the presence of purulent-looking, open wounds and the associated pain served to confirm the suspicion of his colleagues that this technique was unacceptable.2 Over time, the impressive cure rate and acceptable cosmetic outcome of granulation slowly improved the medical community's impression of his technique.
In 1946, Dr. Mohs presented his technique at the American Academy of Dermatology annual meeting in Chicago piquing the interest of the dermatology community. During a conference given by the dermatology section of the California Medical Association, Dr. Mohs realized that his captive audience was dermatologists.4
In 1953, Dr. Mohs was forced to use frozen section without chemical fixation to excise a recurrent tumor on the eyelid while filming an educational clip on his technique.4 He had the film crew for 1 day only; therefore, he shortened the time required by using local anesthesia and color-coded the edges of the specimen, mapping the area in his usual manner. There was less inflammation in the surrounding tissue and he was still successful in clearing the malignancy. He then started using this modification on all eyelid tumors. Sixteen years later, he reported a 100% 5-year cure rate for this fresh tissue technique on eyelid malignancies.1,4
In the 1960s, Dr. Theodore Tromovitch first started using the fresh tissue technique on tumors located on other parts of the body.5 He noted less pain, discomfort, and anxiety in patients treated with this technique compared with those treated with chemical fixation.4 He presented his data at the American College of Chemosurgery, and, once again, the new procedure was met with skepticism. Dr. Tromovitch surmised that the success of the fresh tissue technique lay not with the process of chemical fixation but with the microscopic margin control. The modifications by Dr. Tromovitch of Dr. Mohs' technique enabled the procedure to be done in a single day. The lack of inflammatory response permitted prompt, same-day reconstruction of the surgical defect.2 The fresh tissue technique, also known as the Mohs technique or Mohs micrographic surgery, is currently the standard method of microscopically controlled surgery. In a survey of Mohs surgery practices done in 1991, 72% of Mohs technique surgeons were using only the fresh tissue technique. The remaining surgeons reported use of the fixed tissue technique in less than 5% of their patients.6
TECHNIQUE
Traditional excisional specimens sent to the pathology laboratory for evaluation of margins are processed in a cross-sectional or bread-loaf manner. Representative tissue is sliced vertically at 2- to 4-mm intervals to check for tumor presence at the surgical margin.7 With bread-loaf sectioning, 2 to 4 mm of tissue is left unexamined at each interval so less than 1% of the excised margin is examined.8,9 This is particularly inadequate with tumor types that have irregular, finger-like projections. Residual tumor may be left behind if the tumor should extend to areas that are not examined, and recurrence is likely. Tumors with asymmetric growth patterns are at an increased risk for tumor persistence due to incomplete histologic examination of all surgical margins. In contrast, with Mohs surgery, visualization of the complete peripheral and deep margins is performed. The unique processing technique employed by Mohs histotechnicians allows 100% examination of the peripheral and deep margins. As with any destructive treatment modality, discontiguous tumors or those with skip lesions may still have a higher recurrence or persistence rate.10
The fresh tissue technique has been described in-depth in the literature and offers the following advantages: elimination of pain and discomfort caused by the fixative, ability to take multiple stages in a day, and prompt reconstruction of the surgical defect without waiting for the obligatory sloughing previously associated with the fixative used directly on the patient.7,11,12,13,14
In our practice, patients undergoing Mohs micrographic surgery are treated with the following protocol. After a thorough informed consent, the patient is asked to identify the exact location of the lesion and biopsy site. The clinically apparent tumor is marked with a surgical marker, and the patient is again asked to confirm the location. Photographs and diagrams sent by the referring physician can be useful particularly in patients with multiple biopsies and in those with memory problems or dementia. Family members can also help to identify and confirm the sites in difficult cases.
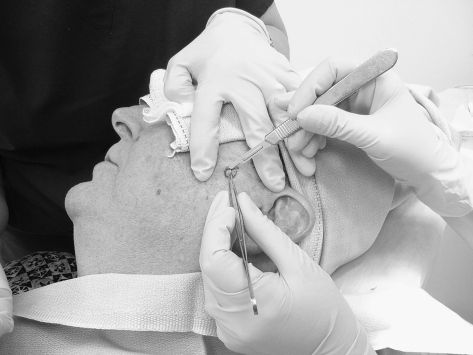
After infiltration of a local anesthetic, the clinically apparent tumor is debulked using either a scalpel or curette (Fig. 1). The utility of scalpel and curette debulking has been debated in the literature.15,16 The debulked tissue is usually discarded; however, we choose to send the debulked specimens for permanent histopathologic evaluation in the case of melanoma in situ because an invasive component may be missed due to sampling error during the initial biopsy.7 Frozen section processing of debulked tissue can also be helpful in unusual cases or to look for perineural invasion.
Figure 1.
Debulking of the gross tumor using the scalpel technique.
Taking a “stage” of Mohs micrographic surgery is the process of removing a specimen to attempt to clear the patient of the carcinoma. This specimen is subsequently processed with hematoxylin and eosin or toluidine blue staining. Multiple stages may be needed to completely remove the skin cancer.
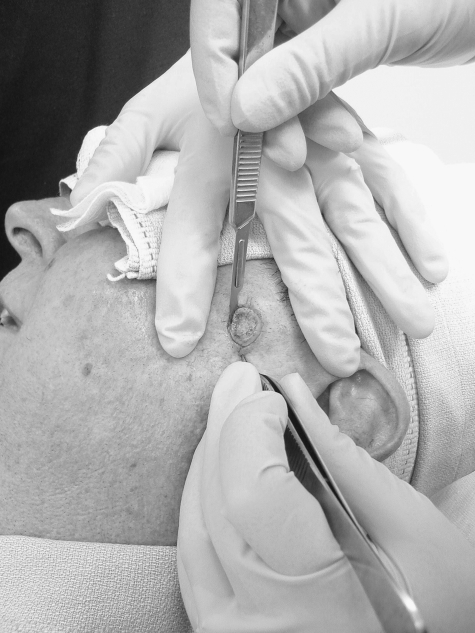
Prior to taking the first stage, any remaining gross tumor is debulked either with a curette or with a scalpel. Then, tissue surrounding the debulked site is surgically excised. A 2- to 3-mm margin of normal-appearing tissue is removed circumferentially and at the depth. Care is taken during removal of the specimen to ensure epidermis is present 360 degrees around the tissue and that no rents are present at the depth of the specimen (Fig. 2).
Figure 2.
Taking a stage for Mohs micrographic surgery. Care is taken to bevel the edges, keep epidermis intact around the entire specimen, and to avoid any defects at the depth of the specimen.
Orientation of the specimen during sectioning must be ensured; hash marks and inking the patient with surgical marker corresponding with marks on the specimen are two commonly employed techniques. Our technique is to score a set of subtle hash marks on the tissue and the patient. We place a subtle double hash mark at the 12 o'clock position to facilitate precise orientation, and single hash marks are similarly placed at the 3, 6, and 9 o'clock positions for fine refinement of possible residual tumor location. The area is then outlined in a saucerized manner by holding the blade at a 45-degree angle. This beveled removal facilitates flattening the lateral margins of the tissue by the histotechnologist allowing complete visualization of the epidermis.
Once the specimen is entirely removed, each hash mark is inked with a separate identifiable color (Fig. 3). A two-dimensional map of the lesion and corresponding area is drawn using four symbols to identify colors inked on each hash mark (Fig. 4). The four dyes used in our practice are red (merbromin), green (chromium), blue (ferrous cyanide), and black (India ink). Another commonly employed technique entails only one orienting hash mark or using only two colors to mark the specimen.
Figure 3.
Once the specimen is completely removed from the patient, the hash marks are inked with colored ink to allow for precise orientation.
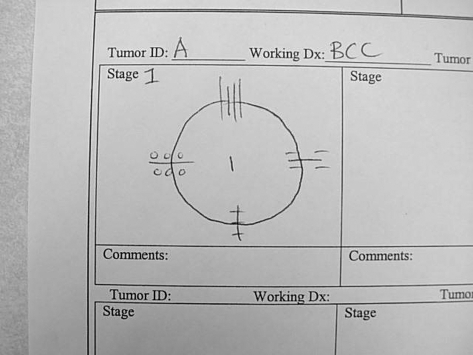
Figure 4.
An example of a map drawn for stage 1. Note symbols used to indicate ink colors at each of the four hash marks (at 12, 3, 6, and 9 o'clock positions).
The specimen may be too large for one histologic block requiring division of the tissue into smaller segments. Any cut edges are also completely inked so that the complete deep and peripheral margins of the specimen can be identified on the histologic slides (Fig. 5). When the slides are reviewed, completeness of the entire depth and circumference of the specimen are confirmed by the presence of ink at all appropriate margins.
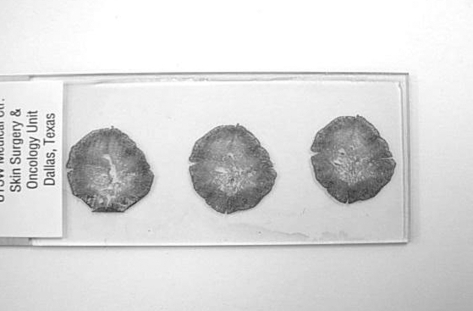
Figure 5.
An example of a histologic slide prepared from stage 1 of Mohs micrographic surgery. Note the intact epidermis surrounding the entire specimen and the complete nature of the central portion of each section. This ensures the complete surgical margin is evaluated.
Errors with frozen section interpretation are usually technical in nature. This includes specimen orientation, inking, and mapping; poor staining; and dermatopathologic artifacts and mimickers of carcinoma.17 Immediately inking the specimen after it is taken from the patient, placing hash marks, and completely inking the periphery of a specimen that has no skin edge reduce the number of orientation errors. Creating control slides daily ensures quality of staining. Experience with frozen section histopathology is critical to avoid interpretation errors. To ensure the best possible quality assurance, a 3-year dermatology residency with an appropriate dermatopathology component and a 1-year Mohs surgery fellowship program with extensive frozen section histopathology interpretation is recommended. Data presented at the American College of Mohs Surgery Annual Meeting in May 2007 demonstrate a minimum of 6 months is required before interpretation errors are avoided by the fellow-in-training.18 The dual role of the physician as both surgeon and pathologist allows clinical and histopathologic correlation with a higher degree of accuracy.19
If the specimen is positive during the first stage, the process is repeated. A 2- to 3-mm margin is again taken, however now only around the area noted to be “positive” for nonmelanoma skin cancer during histopathologic evaluation. It is during these subsequent stages that precise delineation of the tumor on the specimen using the hash technique is critical. Additional hashes may be placed at the edges of the new specimen and the wound bed to delineate the edges of the specimen taken. Established hashes may be reinforced or lengthened as well. If the specimen is positive only at the depth and not the periphery, the second stage can be taken of the depth only without enlarging the diameter of the defect. The border of the specimen is again inked depending on the orientation of the specimen with a new drawing precisely indicating the inking pattern.
The process is repeated until carcinoma is no longer seen in any of the histologic sections. Careful examination for perineural invasion and histologic mimickers is prudent.
INDICATIONS
Indications for Mohs micrographic surgery are varied and may be clinical or pathologic. Frequent indications include histologic and gross characteristics of the tumor, anatomic location, and recurrence (Table 1).
Table 1.
Indications for Mohs Micrographic Surgery
| 1. Tumors with ill-defined clinical borders |
| 2. Recurrent tumors |
| 3. Aggressive tumors |
| 4. Large tumors |
| 5. Tumors with perineural invasion |
| 6. Tumors located in embryonic fusion planes |
| 7. Tumors extending to bone and cartilage |
| 8. Tumor locations with a high risk for recurrence (lips, ears, etc.) |
| 9. Tissue conservation critical for functional/aesthetic outcome (Fig. 2) |
| 10. Tumors in immunocompromised patients |
| 11. Tumors arising in irradiated skin |
Tumor Characteristics
Mohs micrographic surgery is indicated for malignant cutaneous tumors that have irregular, asymmetric tumor conformation such as dermatofibrosarcoma protuberans and for certain histologic subtypes of basal cell carcinoma. Margins of tumors exhibiting irregular budding may be inadequately excised with seemingly appropriate gross excision margins. Primary basal cell carcinoma and squamous cell carcinomas with indistinct borders may also benefit from the Mohs technique, particularly if present on actinically damaged skin.
Mohs micrographic surgery is also indicated for tumors arising in certain anatomic locations with high recurrence rate such as the lips and ears.7 Other high-risk anatomic sites (present in embryonic fusion planes) include temples, the nose, nasolabial folds, periorbital lesions, and pre- and postauricular locations. Tumor size is also important and should be considered; tumors greater than 2 cm on any anatomic location may be appropriately treated with Mohs micrographic surgery.
Aggressive Tumors
Tumors with aggressive histologic subtypes including sclerosing (morpheaform), micronodular, and infiltrative basal cell carcinoma; basosquamous carcinoma; and poorly differentiated squamous cell carcinoma should be considered for Mohs micrographic surgery. Infiltrating or multicentric tumors are also at higher risk for recurrence without Mohs surgical intervention. Mohs micrographic surgery is also indicated for tumors that demonstrate aggressive qualities such as perineural nerve sheath invasion, cartilaginous extension and boney invasion or entrapment. Persistent, recurrent, or incompletely excised tumors should also be treated with Mohs surgery unless clinical factors indicate otherwise.
Tissue Conservation
Another advantage of Mohs micrographic surgery is tissue conservation (Fig. 4). If conventional surgical margins may leave functionally or esthetically unacceptable results, Mohs micrographic surgery may reduce the amount of normal tissue excised thus resulting in superior results.20 Periocular, perioral, and auricular tumors are a few examples of potentially challenging cosmetic locations. Tumors present in functionally or cosmetically sensitive areas such as nail units, acral locations such as the hand, foot, or digits, and genital and perianal skin should be considered for Mohs surgery as well.
Mohs micrographic surgery allows tissue conservation while achieving optimal margin control.21 Tissue conservation and therefore smaller postsurgical defects often permit more straightforward reconstruction procedures and aesthetically pleasing outcomes.
It should be noted that for certain tumor types (e.g., melanoma in situ, dermatofibrosarcoma protuberans), standard surgical margins are not routinely reduced when performing Mohs surgical excision. In these cases, Mohs surgery is employed for margin control, not for tissue conservation.
Patient Characteristics
Patients at high risk for recurrence, metastasis, and tumor persistence should undergo appropriate and definitive treatment for all cutaneous malignancies. Tumors in intrinsically (such as in HIV patients) or iatrogenically (often pharmaceutical in nature) immunosuppressed patients are often treated with Mohs surgery. Patients with genetic predispositions to skin cancer (such as basal cell nevus syndrome and xeroderma pigmentosa) or exposures to environmental agents (such as arsenic) should also undergo Mohs surgery for their cutaneous malignancies. Mohs surgery is also appropriate for tumors developing in high-risk sites such as areas of prior radiation therapy and chronic wounds.
EFFECTIVENESS FOR CUTANEOUS MALIGNANCIES
Basal cell carcinoma is the most common skin cancer in North America followed by squamous cell carcinoma. Melanoma accounts for ~4% of all skin cancers. Other cutaneous malignancies treated with Mohs micrographic surgery include, but are not limited to, dermatofibrosarcoma protuberans, Merkel cell carcinoma, microcystic adnexal carcinoma, atypical fibroxanthoma, and sebaceous carcinoma. The majority of Mohs micrographic surgery practices treat primarily basal cell carcinoma and squamous cell carcinoma.
Basal Cell Carcinoma
Basal cell carcinoma (BCC) is the most common cutaneous malignancy. It is a slow-growing, locally destructive tumor that rarely metastasizes. The cure rate for primary basal cell carcinoma after Mohs micrographic surgery is greater than 99%.7 Recurrent BCCs treated with Mohs micrographic surgery are cured ~96% of the time. In contrast, reported cure rates for primary and recurrent BCCs treated with traditional surgical excision are 89.9% and 82.9%, respectively; with electrodesiccation and curettage, 92.3% and 60.0%; and for therapeutic radiation, 91.3% and 90.2%.7 Size and location of the lesion are also important prognostic indicators. When treated with Mohs micrographic surgery, tumors less than 3 cm in diameter have a cure rate of 99%; for those more than 3 cm in diameter, cure rate drops to 93%. Periocular and perioral BCCs have an overall cure rate of 98%.22
In a literature review by Lane and Kent, recurrence rates for primary BCCs treated with standard excision and Mohs micrographic surgery were 10% and 1%, respectively.23 For recurrent BCCs, standard excision has a 5-year recurrence rate of 5 to 40%, whereas Mohs micrographic surgery has a 5-year recurrence rate of 3 to 8%. Another review of 10,000 cases of BCC treated with either standard surgical excision with traditional bread-loaf margin assessment or Mohs micrographic surgery showed a 5-year recurrence rate of 10.1% for excision versus 1.0% for Mohs surgery.24
In a randomized controlled study comparing 397 primary and 201 recurrent BCCs treated with either surgical excision or Mohs micrographic surgery, 3% of primary BCCs treated with standard excision recurred after 30 months, whereas 2% of primary BCCs treated with Mohs micrographic surgery recurred after the same period.25 Of the 201 recurrent tumors treated, 3% of those treated with standard surgical excision recurred after 18 months. In the same follow-up period, there were no recurrences among the recurrent tumors treated with Mohs micrographic surgery.
Squamous Cell Carcinoma
Squamous cell carcinoma (SCC) is the second most common type of skin cancer; in distinction with BCC, SCC has metastatic potential.26 Contributing factors for SCC include ionizing radiation, ultraviolet light, immunosuppression, scars, chronic nonhealing wounds, congenital diseases such as oculocutaneous albinism and xeroderma pigmentosum, and exposure to chemicals such as arsenic, aromatic hydrocarbons, anthracene, and creosote oil.27 The majority of patients, however, cite only sun exposure as a risk factor. High-risk SCC is characterized by the following characteristics27:
Poor cellular differentiation (grade 3 or 4 tumors are twice more likely to recur and 3 times more likely to metastasize).
Large size (tumors greater than 2 cm have a risk for metastasis of 9% or greater).
Auricular (11% rate of metastasis) or lip (10 to 14% rate of metastasis) location.
Perineural invasion (47% local recurrence rate and a 35% regional lymph node metastatic rate).
Local recurrence (25 to 45% metastatic rate).
Ninety-five percent of local recurrences and metastases are found within the first 5 years after surgery.28
Similar to BCC, lesion size is an important prognostic indicator for SCC. Lesions less than 2 cm in diameter have a 5-year cure rate of 99%; those between 2 and 3 cm have a 5-year cure rate of 82%; and SCCs greater than 3 cm have a cure rate of 59%.7
Careful interpretation of the histologic subtype is also pertinent to determining prognosis. The more differentiated grades 1 and 2 SCCs have a 5-year cure rate of 99% and 94%, respectively.7 Less differentiated grades 3 and 4 SCCs have cure rates of 74% and 45%, respectively. Higher grade SCCs have a greater tendency to metastasize and extend into vital structures.
McCombe et al reported a 10-year local recurrence rate of 3% and a 92.3% 10-year survival rate for 323 cases of SCC of the lower lip.29
Melanoma in Situ
Melanoma in situ (MIS) is a malignant neoplasm of melanocytes characterized by a prolonged growth phase and may exhibit significant subclinical extension. Neoplastic melanocytes are located at the dermoepidermal junction and do not invade the dermis. In a 2005 study by Mahoney et al using surgical excision with a modified Mohs technique in 23 patients, the final surgical defect was found to be 2 to 10 times the original clinical size.30 The subclinical extension of atypical junctional melanocytic hyperplasia often results in wider removed margins than what is recommended in a standard surgical excision, particularly when the neoplasm is located on actinically damaged skin.31 If unresected, atypical junctional melanocytic hyperplasia may result in local recurrence and future metastatic potential.
Traditional excisional surgery has been noted to have a recurrence rate ranging from 6 to 20% with a follow-up period of at least 3 years.32 When treated with Mohs micrographic surgery, recurrence rates ranged from 0 to 3.6% with a follow-up period of at least 18 months.32 Although Mohs micrographic surgery is not necessary for all cases of MIS, it is the treatment of choice for ill-defined lesions, particularly those in sun-exposed areas.
Treatment modalities for invasive melanoma include traditional excisional surgery and Mohs micrographic surgery. Less commonly, cryosurgery, laser surgery, electrodesiccation and curettage, and radiotherapy can be used in specific patient populations.33 The first study directly comparing the fresh tissue technique of Mohs surgery and the fixed tissue technique for invasive melanoma was done by Zitelli in 1989.34 Nagi et al reported comparable cure rates between the two techniques and excisional surgery.35 Despite these data, traditional surgical excision continues to be the most common treatment of choice for most cases of malignant melanoma. Mohs micrographic surgery can be of particular assistance when invasive melanoma is noted centrally with MIS at the margins or to further demarcate ill-defined margins.
Although conventional surgical excision margins depend on Breslow thickness, many MIS as well as invasive melanoma require wider than recommended margins to clear the entire tumor when all margins are examined.35,36 Zitelli et al showed that 6-mm margins cleared 83%, 9-mm margins cleared 95%, and 12-cm margins cleared 97%.37 In a study using fresh tissue Mohs micrographic surgery complemented by HMB-45 (Human Melanoma Black), MART-1 (Melanoma Antigen Recognized by T-Cells), S-100, and Mel-5 immunohistochemical stains, the average margin needed to clear patients with MIS was 8 mm. To clear 96% of patients, 15-mm margins were required.38 Invasive malignant melanoma required an average of 11 mm to clear patients, and 26 mm was needed to clear 95% of the lesions.38
Mohs micrographic surgery as an adequate treatment modality for MIS has been debated because of concerns regarding use of frozen sections to evaluate melanocytic lesions. Artifact from the frozen tissue technique can make the interpretation of melanocytic lesions more challenging. Immunohistochemical stains including MART-1, HMB-45, Mel-5, and S-100 have been used to aid in histologic evaluation. Zalla et al recommend MART-1 for frozen section confirmation of melanoma at tissue margins because it was the most consistently clear and easily interpretable immunohistochemical stain in their experience.39 Similarly, Albertini et al established that MART-1 is more sensitive when compared with HMB-45 and that the use of MART-1 improves the diagnostic accuracy of Mohs micrographic surgery.40 In 2005, Davis et al published a confirmatory study that MART-1 is more sensitive when compared with S-100 and HMB-45.41
Mohs micrographic surgery can be a powerful tool for resection of challenging melanocytic lesions such as MIS when performed in the hands of an experienced Mohs surgeon with an excellent histology laboratory staff.
Dermatofibrosarcoma Protuberans
Dermatofibrosarcoma protuberans (DFSP) is a locally aggressive, soft tissue sarcoma with a propensity for local recurrence and distant metastasis. Mohs surgeons may work in collaboration with plastic surgeons in the treatment of this challenging tumor.42 In a retrospective review of 28 cases of scalp DFSP treated at the Roswell Park Cancer Institute, the investigators found that there were no recurrences in lesions treated with Mohs micrographic surgery.43 DFSP treated with traditional surgical excision had a recurrence rate of 35%. Clinical tumor size is used to determine the appropriate surgical margin. Lesions less than 2 cm may be adequately excised with a 1.5-cm margin, whereas larger tumors require at least a 2.5-cm margin.44 Depth of the excision should be through the deep fascia for non–scalp cases and through the periosteum for scalp lesions.44
A review series done by Gloster et al found that Mohs micrographic surgery had the lowest recurrence rate at 1.6% compared with wide local excision and simple excision.45 Wide local excision had a recurrence rate of 20%, whereas 40% of those treated with simple excision had recurrence. To reduce the risk of recurrence, Mohs micrographic surgery should be used whenever possible as the treatment of choice.43
Merkel Cell Carcinoma
Merkel cell carcinoma is an aggressive cutaneous malignancy of neuroendocrine origin. It has a propensity to recur locally and metastasizes with a high rate of lethality. In a retrospective study done by Gollard et al, all patients with Merkel cell carcinoma treated with Mohs micrographic surgery (8 of 22) had no recurrence after a mean follow-up period of 37 months.46 Traditional surgical excision with margins up to 3 cm results in tumor persistence in 26 to 44% of cases.47
Microcystic Adnexal Carcinoma
Microcystic adnexal carcinoma (MAC) is a locally destructive, aggressive tumor of eccrine origin with a tendency for perineural invasion. Snow et al showed that treatment with Mohs micrographic surgery results in a 10% rate of recurrence.48 In contrast, wide excision with a margin up to 5 cm may result in recurrence rates greater than 50%.49 Mohs micrographic surgery has been the treatment of choice for this locally aggressive and recurrent tumor within the dermatologic community.50 Immunohistochemical stains and permanent paraffin sections of the final layer taken during Mohs surgery have been suggested by some authors.50
Atypical Fibroxanthoma
Atypical fibroxanthoma (AFX) is a rare, locally aggressive, and recurrent tumor, with an ability to metastasize distally. A retrospective study comparing Mohs micrographic surgery with wide excision demonstrated a 12% recurrence rate after wide excision (mean follow-up period of 73.6 months).51 Patients treated with Mohs micrographic surgery did not develop recurrence; however, the follow-up period was only 29.6 months. Other studies have similarly shown a low recurrence rate of 0 to 6% with Mohs micrographic surgery.51
Sebaceous Carcinoma
Sebaceous carcinoma is a locally aggressive cutaneous malignancy originating from sebaceous glands. The majority of sebaceous carcinoma occurs in the periocular region (75%).52 Traditional surgical excision with a 5- to 6-mm margin has a recurrence rate of 32%.53 A review of 18 patients treated with Mohs micrographic surgery revealed a recurrence rate of 11.1% after an average follow-up period of 37 months.54 In nine reviewed cases with pagetoid spread, a dramatic 59% mortality rate prevailed despite treatment with Mohs micrographic surgery.54 It has been recommended that in cases with histologic evidence of pagetoid spread, additional tissue should be removed to decrease local tumor recurrence.55
COST
In a cost analysis done by Cook and Zitelli, the cost of Mohs micrographic surgery ($1243) was comparable with that of traditional surgical excision (TSE; $1167) when using permanent paraffin sections and less expensive than TSE when using frozen sections whether the TSE was performed in an office setting ($1400) or an ambulatory care center ($1973).56
The cost of doing Mohs surgery outright on Mohs-appropriate lesions is comparable with that of TSE when Mohs surgery was subsequently performed for positive surgical margins after traditional excision (Mohs micrographic surgery $937 vs. TSE then Mohs micrographic surgery $1029; p = 0.16).57 Mohs micrographic surgery is also less expensive when the subsequent procedure after inadequate margins is a second surgical excision (Mohs micrographic surgery $937 vs. TSE $1399).
In the United States, Mohs micrographic surgery reimbursement consists of both excision as well as pathology processing and interpretation. Cost is similar to that of TSE when using paraffin section processing and histologic interpretation, which is billed separately. The caveat arises in other parts of the world where billing may be different.56
Other European studies have found Mohs micrographic surgery less cost effective when compared with standard surgical excision, however they used separate physicians (pathologists) for interpretation of the histologic sections thereby increasing the cost of the procedure.25,58 This is not billable as Mohs surgery in the United States and therefore is not a valid comparison here.
RECONSTRUCTION
Reconstruction of surgical defects after Mohs micrographic surgery is frequently done in the office setting by the dermatologic surgeon. As with the extirpation procedure, local anesthesia is used. Most Mohs surgeons are proficient in complex closures, including random and axial pattern skin flaps and full- and partial-thickness skin grafts and perform these procedures routinely in their offices.
However, several factors may necessitate referral to our surgical colleagues. If the size of the defect exceeds that for which the patient will be comfortable with local anesthesia or exceeds the limits of usage of local anesthesia, referral to a physician with access to anesthesiology is critical. Additionally, the health of the patient or simply patient preference may also be reason to refer. Dermatologic surgeons also have varied training regarding specific complex anatomic sites including periorbital, perioral, and nasal or auricular defects and may choose to refer to oculoplastic, plastic, or otolaryngology surgeons.
A defect greater than 50% of the cosmetic unit characterizes a complex skin defect.59 Many Mohs surgeons repair eyelid defects; however, many refer periocular lesions greater than 50% of the cosmetic unit to an oculoplastic or plastic surgeon. Full-thickness nasal lesions and intranasal lesions affecting the nasal sinuses may be best repaired by a plastic surgeon or otolaryngologist.59 Involvement of the parotid gland during Mohs extirpation requiring superficial or parotidectomy or periosteal involvement requiring boney resection or other intervention are several situations when we rely on interdisciplinary care to best manage individual patients.
A tumor board composed of multiple subspecialties can be an important venue for coordinating care for complex cases and can be an advantage academic practices offer.27 Plastic surgeons Dobeke and Miller concluded that familiarity with components of surgical defects after Mohs micrographic surgery and teamwork with the Mohs surgeon are important components of successful multidisciplinary patient care.60 Multiple surgical subspecialties can collaborate in the management of cases that cannot be completed in the office setting or whose repair is best done in the operating room.
CONCLUSION
Mohs micrographic surgery is well established as the standard of care in many cases of BCC and SCC, the most common cutaneous malignancies. These nonmelanoma skin cancers, if they are present on high-risk anatomic locations, demonstrate aggressive histologic or clinical behavior, are recurrent or incompletely excised, have indistinct clinical borders, or are present in a cosmetically or functionally sensitive location, should be treated with Mohs micrographic surgery. Other indications for Mohs surgery have been reviewed here. Additionally, Mohs micrographic surgery is warranted in ill-defined MIS, particularly in sun-exposed areas and in the treatment of less common cutaneous tumors including DFSP, Merkel cell carcinoma, MAC, AFX, and sebaceous carcinoma.
REFERENCES
- Mohs F E. Mohs micrographic surgery: a historical perspective. Dermatol Clin. 1989;7:609–611. [PubMed] [Google Scholar]
- Brodland D G, Amonette R, Hanke C W, Robins P. The history and evolution of Mohs micrographic surgery. Dermatol Surg. 2000;26:303–307. doi: 10.1046/j.1524-4725.2000.00504.x. [DOI] [PubMed] [Google Scholar]
- Mohs F E. Chemosurgery: a microscopically controlled method of cancer excision. Arch Surg. 1941;42:279. [Google Scholar]
- Mohs F E. In: Roenigk RK, Roenigk HH, editor. Dermatologic Surgery: Principles and Practice. New York, NY: Marcel Dekker; 1989. History of Mohs micrographic surgery. pp. 783–789.
- Tromovitch T A, Stegman S J. Microscopically controlled excision of skin tumors: chemosurgery (Mohs): fresh tissue technique. Arch Dermatol. 1974;110:231–232. [PubMed] [Google Scholar]
- McGillis S T, Wheeland R G, Sebben J E. Current issues in the performance of Mohs micrographic surgery. J Dermatol Surg Oncol. 1991;17:681–684. doi: 10.1111/j.1524-4725.1991.tb01320.x. [DOI] [PubMed] [Google Scholar]
- Shriner D L, McCoy D K, Goldberg D J, Wagner R F., Jr Mohs micrographic surgery: clinical review. J Am Acad Dermatol. 1998;39:79–97. doi: 10.1016/s0190-9622(98)70405-0. [DOI] [PubMed] [Google Scholar]
- Pennington B, Brent M R. Nonmelanoma of the skin and Mohs surgery. Plast Reconstr Surg. 2004;113:2233–2234. doi: 10.1097/01.prs.0000123625.76158.73. [DOI] [PubMed] [Google Scholar]
- Rigel DS, editor. Cancer of the Skin. Philadelphia, PA: Elsevier; 2005. pp. 167–173.
- Motley R, Kersey P, Lawrence C. Multiprofessional guidelines for the management of the patient with primary cutaneous squamous cell carcinoma. Br J Dermatol. 2002;146:18–25. doi: 10.1046/j.0007-0963.2001.04615.x. [DOI] [PubMed] [Google Scholar]
- Morman M R. Mohs micrographic surgery. N J Med. 1989;86:369–373. [PubMed] [Google Scholar]
- Dzubow L M. Mohs surgery. Lancet. 1994;343:433–434. doi: 10.1016/s0140-6736(94)92687-5. [DOI] [PubMed] [Google Scholar]
- Robinson J K. Mohs micrographic surgery. Clin Plast Surg. 1993;20:149–156. [PubMed] [Google Scholar]
- Clark D. Cutaneous micrographic surgery. Otolaryngol Clin North Am. 1993;26:185–202. [PubMed] [Google Scholar]
- Chung V Q, Bernardo L, Jiang S B. Presurgical curettage reduces the number of Mohs stages by better delineating the subclinical extensions of tumor margins. Dermatol Surg. 2005;31:1094–1099. doi: 10.1097/00042728-200509000-00002. [DOI] [PubMed] [Google Scholar]
- Jih M H, Friedman P M, Goldberg L H, Kimyai-Asadi A. Currettage prior to Mohs micrographic surgery for previously biopsied nonmelanoma skin cancers: what are we curetting? Retrospective, prospective, and comparative study. Dermatol Surg. 2005;31:10–15. doi: 10.1111/j.1524-4725.2005.31001. [DOI] [PubMed] [Google Scholar]
- Davis D A, Pellowski D M, Hanke C W. Preparation of frozen sections. Dermatol Surg. 2004;30:1479–1485. doi: 10.1111/j.1524-4725.2004.30506.x. [DOI] [PubMed] [Google Scholar]
- Murphy M, Brodland D, Zitelli J. The frequency of errors in reading Mohs pathology sections over a one-year fellowship. Naples, FL: Paper presented at: Annual Meeting of the Mohs College; May 3, 2007.
- Stein J M, Hrabovsky S, Schuller D E, Siegle R J. Mohs micrographic surgery and the otolaryngologist. Am J Otolaryngol. 2004;25:385–393. doi: 10.1016/j.amjoto.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Lang P G., Jr The role of Mohs micrographic surgery in the management of skin cancer and a perspective on the management of the surgical defect. Clin Plast Surg. 2004;31:5–31. doi: 10.1016/s0094-1298(03)00096-8. [DOI] [PubMed] [Google Scholar]
- Touma D J. Mohs' (sic) surgery to reduce the size of facial defects and necessity for complex repairs. Plast Reconstr Surg. 2002;110:1601. doi: 10.1097/00006534-200211000-00043. [DOI] [PubMed] [Google Scholar]
- Downes R N, Walker N P, Collin J R. Micrographic (Moh's) surgery in the management of periocular basal cell epitheliomas. Eye. 1990;4:160–168. doi: 10.1038/eye.1990.21. [DOI] [PubMed] [Google Scholar]
- Lane J E, Kent D E. Surgical margins in the treatment of nonmelanoma skin cancer and Mohs micrographic surgery. Curr Surg. 2005;62:518–526. doi: 10.1016/j.cursur.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Rowe D E, Carroll R J, Day C L. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol. 1989;15:315–328. doi: 10.1111/j.1524-4725.1989.tb03166.x. [DOI] [PubMed] [Google Scholar]
- Smeets N W, Krenkels G A, Essers B A, et al. Surgical excision vs. Mohs micrographic surgery for basal cell carcinoma of the face: randomized controlled trial. Lancet. 2004;364:1766–1772. doi: 10.1016/S0140-6736(04)17399-6. [DOI] [PubMed] [Google Scholar]
- Goldman G D. Squamous cell cancer: a practical approach. Semin Cutan Med Surg. 1998;17:80–95. doi: 10.1016/s1085-5629(98)80002-3. [DOI] [PubMed] [Google Scholar]
- Rudolph R, Zelac D E. Squamous cell carcinoma of the skin. Plast Reconstr Surg. 2004;114:82e–94e. doi: 10.1097/01.prs.0000138243.45735.8a. [DOI] [PubMed] [Google Scholar]
- Marks R. Squamous cell carcinoma. Lancet. 1996;347:735–738. doi: 10.1016/s0140-6736(96)90081-1. [DOI] [PubMed] [Google Scholar]
- McCombe D, MacGill K, Ainslie J, Beresford J, Matthews J. Squamous cell carcinoma of the lower lip: a retrospective review of the Peter MacCallum Cancer Institute experience 1979–1988. Aust N Z J Surg. 2000;70:358–361. doi: 10.1046/j.1440-1622.2000.01827.x. [DOI] [PubMed] [Google Scholar]
- Mahoney E J, Dolan R W, Choi E E, Olbricht S M. Surgical reconstruction of lentigo maligna defects. Arch Facial Plast Surg. 2005;7:342–346. doi: 10.1001/archfaci.7.5.342. [DOI] [PubMed] [Google Scholar]
- Cohen L M, McCall M W, Hodge S J, et al. Successful treatment of lentigo maligna and lentigo maligna melanoma with Mohs micrographic surgery aided by rush permanent sections. Cancer. 1994;73:2964–2970. doi: 10.1002/1097-0142(19940615)73:12<2964::aid-cncr2820731213>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Dawn M E, Dawn A G, Miller S J. Mohs surgery for the treatment of melanoma in situ: a review. Dermatol Surg. 2007;33:395–402. doi: 10.1111/j.1524-4725.2007.33085.x. [DOI] [PubMed] [Google Scholar]
- Anderson K W, Baker S R, Lowe L, et al. Treatment of head and neck melanoma, lentigo maligna subtype. Arch Facial Plast Surg. 2001;3:202–206. doi: 10.1001/archfaci.3.3.202. [DOI] [PubMed] [Google Scholar]
- Zitelli J A, Mohs F E, Larson P, Snow S. Mohs micrographic surgery for melanoma. Dermatol Clin. 1989;7:833–843. [PubMed] [Google Scholar]
- Nagi C, O'Grady T C, Izadpanah A. Mohs micrographically controlled surgery and the treatment of malignant melanoma. Semin Oncol. 2002;29:336–340. doi: 10.1053/sonc.2002.34111. [DOI] [PubMed] [Google Scholar]
- Harris T J, Hinckley D M. Melanoma of the head and neck in Queensland. Head Neck Surg. 1983;5:197–203. doi: 10.1002/hed.2890050303. [DOI] [PubMed] [Google Scholar]
- Zitelli J, Brown C D, Hanusa B H. Surgical margins for excision of primary cutaneous melanoma. J Am Acad Dermatol. 2001;45:579–586. doi: 10.1016/s0190-9622(97)70144-0. [DOI] [PubMed] [Google Scholar]
- Chan F M, O'Donell B A, Whitehead K, et al. Treatment and outcomes of malignant melanoma of the eyelid. A review of 29 cases in Australia. Ophthalmology. 2007;114:187–192. doi: 10.1016/j.ophtha.2006.08.043. [DOI] [PubMed] [Google Scholar]
- Zalla M J, Lim K K, DiCaudo D J, Gagnot M M. Mohs micrographic excision of melanoma using immunostains. Dermatol Surg. 2000;26:771–784. doi: 10.1046/j.1524-4725.2000.00081.x. [DOI] [PubMed] [Google Scholar]
- Albertini J G, Elston D M, Libow F F, Smith S B, Farley M F. Mohs micrographic surgery for melanoma: a case series, a comparative study of immunostains, an informative case report, and a unique mapping technique. Dermatol Surg. 2002;28:656–665. doi: 10.1046/j.1524-4725.2002.02024.x. [DOI] [PubMed] [Google Scholar]
- Davis D A, Kurtz K A, Robinson R A. Ultrarapid staining for cutaneous melanoma: study and protocol. Dermatol Surg. 2005;31(7 Pt 1):753–756. doi: 10.1097/00042728-200507000-00004. [DOI] [PubMed] [Google Scholar]
- Simman R, DeFranzo A, Sanger C, Thompson J. Dermatofibrosarcoma protuberans of the face: surgical management. J Craniofac Surg. 2005;16:439–444. doi: 10.1097/01.scs.0000148045.80745.fa. [DOI] [PubMed] [Google Scholar]
- Loss L, Zeitouni N C. Management of scalp dermatofibrosarcoma protuberans. Dermatol Surg. 2005;31:1428–1433. doi: 10.2310/6350.2005.31209. [DOI] [PubMed] [Google Scholar]
- Parker T L, Zitelli J A. Surgical margins for excision of dermatofibrosarcoma protuberans. J Am Acad Dermatol. 1995;32:233–236. doi: 10.1016/0190-9622(95)90132-9. [DOI] [PubMed] [Google Scholar]
- Gloster M W, Harris K R, Roenigk R K. A comparison between Mohs micrographic surgery and wide surgical excision for the treatment of dermatofibrosarcoma protuberans. J Am Acad Dermatol. 1996;35:82–87. [PubMed] [Google Scholar]
- Gollard R, Weber R, Kostky M P, et al. Merkel cell carcinoma: review of 22 cases with surgical, pathologic, and therapeutic considerations. Cancer. 2000;88:1842–1851. [PubMed] [Google Scholar]
- O'Connor W J, Roenigk R K, Brodland D G. Merkel cell carcinoma—comparison of Mohs micrographic surgery and wide excision in eighty-six patients. Dermatol Surg. 1997;23:929–933. [PubMed] [Google Scholar]
- Snow S, Madjar D D, Hardy S, et al. Microcystic adnexal carcinoma; report of 13 cases and review of the literature. Dermatol Surg. 2001;27:401–408. doi: 10.1046/j.1524-4725.2001.00208.x. [DOI] [PubMed] [Google Scholar]
- Sebastien T S, Nelson B R, Lowe L, et al. Microcystic adnexal carcinoma. J Am Acad Dermatol. 1993;29:840–845. doi: 10.1016/0190-9622(93)70251-n. [DOI] [PubMed] [Google Scholar]
- Clement C I, Genge J, O'Donnell B A, Lochhead A G. Orbital and periorbital microcystic adnexal carcinoma. Ophthal Plast Reconstr Surg. 2005;21:97–102. doi: 10.1097/01.iop.0000155508.52870.da. [DOI] [PubMed] [Google Scholar]
- Davis J L, Randle H W, Zalla M J, Roenigk R K, Brodland D G. A comparison of Mohs micrographic surgery and wide excision for the treatment of atypical fibroxanthoma. Dermatol Surg. 1997;23:105–110. doi: 10.1111/j.1524-4725.1997.tb00670.x. [DOI] [PubMed] [Google Scholar]
- Nelson B R, Hamlet K R, Gillard M, et al. Sebaceous carcinoma. J Am Acad Dermatol. 1995;33:1–15. doi: 10.1016/0190-9622(95)90001-2. [DOI] [PubMed] [Google Scholar]
- Ratz J L, Luu-Duong S, Kulwin D R. Sebaceous carcinoma of the eyelid treated with Mohs surgery. J Am Acad Dermatol. 1986;14:668–673. doi: 10.1016/s0190-9622(86)70084-4. [DOI] [PubMed] [Google Scholar]
- Spencer J M, Nossa R, Tse D T, Sequeira M. Sebaceous carcinoma of the eyelid treated with Mohs micrographic surgery. J Am Acad Dermatol. 2001;44:1004–1009. doi: 10.1067/mjd.2001.113692. [DOI] [PubMed] [Google Scholar]
- Snow S N, Larson P O, Lucarelli M J, et al. Sebaceous carcinoma of the eyelid treated by Mohs surgery: report of nine cases with review of literature. Dermatol Surg. 2002;28:623–631. doi: 10.1046/j.1524-4725.2002.01306.x. [DOI] [PubMed] [Google Scholar]
- Cook J, Zitelli J A. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39:698–703. doi: 10.1016/s0190-9622(98)70041-6. [DOI] [PubMed] [Google Scholar]
- Bialy T L, Whalen J, Veledar E, et al. Mohs micrographic surgery vs. traditional surgical excision: a cost comparison analysis. Arch Dermatol. 2004;140:736–742. doi: 10.1001/archderm.140.6.736. [DOI] [PubMed] [Google Scholar]
- Essers B A, Dirksen C D, Nieman F H, et al. Cost-effectiveness of Mohs micrographic surgery vs. surgical excision for basal cell carcinoma of the face. Arch Dermatol. 2006;142:187–194. doi: 10.1001/archderm.142.2.187. [DOI] [PubMed] [Google Scholar]
- Gladstone H B, Stewart D. Algorithm for the reconstruction of complex facial defects. Skin Therapy Lett. 2007;12:6–9. [PubMed] [Google Scholar]
- Dobke M K, Miller S H. Tissue repair after Mohs surgery: a plastic surgeon's view. Dermatol Surg. 1997;23:1061–1066. doi: 10.1111/j.1524-4725.1997.tb00448.x. [DOI] [PubMed] [Google Scholar]