ABSTRACT
Over the past several decades, an improved understanding of the blood supply of local flaps, increased experience with tissue expansion, and the development of techniques for microsurgical transfer of distant flaps have greatly contributed to the ability of plastic surgeons to repair scalp defects. This article will review basic anatomy, principles, and pearls of reconstruction for simple to complex scalp defects. Included will be anatomic considerations, indications and contraindications for reconstruction, and an overview of reconstructive options.
Keywords: Scalp reconstruction, microsurgery, calvarium reconstruction, tissue expansion, scalp anatomy
Reconstruction in patients with scalp and/or calvarial defects can be simple or complex. Over the past several decades, an improved understanding of the blood supply of local flaps, increased experience with tissue expansion, and the development of techniques for microsurgical transfer of distant flaps have greatly contributed to the ability of plastic surgeons to repair these defects.
As with reconstructions at other locations, the reconstructive “ladder” applies to scalp and calvarial reconstruction. Primary closure is the first choice when feasible. Other methods of reconstruction, in ascending order of complexity, are skin grafts, local flaps with or without tissue expansion, occasionally regional flaps, and free flaps. This article will review basic anatomy, principles, and pearls of reconstruction for simple to complex scalp defects.
ANATOMY
A comprehensive knowledge of the anatomy of the scalp and calvaria is essential in planning reconstructions. The scalp is composed of five layers, which can be remembered using the well-known mnemonic SCALP: (1) skin, (2) subcutaneous fat, (3) galea aponeurotica, (4) loose areolar tissue, and (5) pericranium. The scalp integument is the thickest skin in the body (3 to 8 mm). The hair-bearing quality of the scalp in most patients makes aesthetically pleasing reconstruction a challenge.1 The galea aponeurotica continues as the frontal muscle anteriorly and the occipital muscle posteriorly. Laterally, the galea aponeurotica is continuous with the temporoparietal fascia. The pericranium fuses with the deep temporal fascia laterally.
In addition, the scalp is inelastic, with the galea aponeurotica being poorly elastic and the pericranium being nearly nondistensible. The parietal regions, located over the temporoparietal fascia, are the areas of the scalp with the greatest mobility.
A well-vascularized subcutaneous layer lies superficial to the galea aponeurotica. A robust choke vessel system allows relatively long local flaps to survive without distal necrosis. The vascular supply of the scalp includes the paired supratrochlear, supraorbital, superficial temporal, posterior auricular, and occipital arteries and their accompanying veins. The supratrochlear and supraorbital arteries arise from the ophthalmic artery, which is the first branch of the internal carotid artery. The remainder of the arterial vasculature supplying the scalp arises from the external carotid artery.
Paired sensory nerves of the scalp include the supratrochlear (V1), supraorbital (V1), zygomaticotemporal (V2), auriculotemporal (V3), lesser occipital (C2-C3), and greater occipital (C2) nerves. The supraorbital and supratrochlear nerves supply the forehead and frontoparietal scalp. The zygomaticotemporal nerve supplies the region lateral to the brow and the temporal scalp up to the temporal line. The auriculotemporal nerve supplies the lateral scalp, and the greater and lesser occipital nerves supply the occipital region. The temporal branches of the facial nerve are found on the deep surface of the temporoparietal fascia within the loose areolar tissue along an imaginary line that connects the ear lobe and a point 1.5 cm above the lateral eyebrow (Pitanguy's line). This branch of the facial nerve crosses the middle third of the zygoma, and dissection is usually performed in a plane deep to the superficial layer of the deep temporal fascia to prevent injury.
The calvaria is composed of bones that are formed by intramembranous ossification (frontal, parietal, and temporal bones) and bones that are formed by endochondral ossification (occipital and sphenoid bones). The calvaria has three layers: an outer table, a diploic space, and an inner table. These layers develop during early childhood. Most calvarial growth is complete by the age of 7 years.2
INDICATIONS AND CONTRAINDICATIONS FOR RECONSTRUCTION
Scalp and calvarial defects may result from trauma, thermal or electrical burns, resection of benign or malignant tumors, infections, osteoradionecrosis, or congenital lesions. Not every patient with a scalp wound requires operative intervention. Limited partial-thickness defects of the scalp may be left to heal by secondary intention. However, significant contracture may result, and the scar may have limited or no hair growth. Larger partial-thickness defects, especially in aesthetically important areas such as the forehead, usually require repair.
Patients with full-thickness scalp wounds should undergo some type of reconstructive surgery. Wounds occurring over allografts, bone grafts, and hardware should be covered with well-vascularized local or free flaps. In situations in which healing may be compromised, microsurgical reconstruction may be the best option, particularly when the creation of broadly based, tension-free local flaps is not feasible.
With the exception of partial-thickness calvarial defects, the calvarium is routinely reconstructed with a prosthetic plate that may involve bone graft. Most calvarial reconstructions are performed immediately unless the wound is infected. If infection is present, the reconstruction should be delayed until the infection has been treated.
Prior radiation, surgery, or infection may result in unstable wound coverage when local tissues are used for scalp reconstruction. In such cases, local scalp flaps should be avoided or planned so that affected areas are not included as part of the reconstruction. Smoking, diabetes, or corticosteroid use may delay wound healing and thus are relative contraindications to using local flaps. In addition, the potential need for postoperative radiotherapy may influence reconstructive strategy. In a patient who has a large complex scalp wound and multiple factors affecting wound healing, microvascular free-tissue transfer may be useful in bringing nonirradiated, well-vascularized tissue to the region to minimize subsequent wound complications.3,4,5 Additionally, it is important to realize that cerebrospinal fluid leaks may contribute to wound healing complications by adding fluid accumulation and potentially becoming infected.
SCALP RECONSTRUCTION
Primary Closure
Primary closure with adjacent hair-bearing tissue is a suitable choice for the closure of many small scalp defects. The scalp tissues have limited elasticity, so usually only defects less than 3 cm in diameter are amenable to primary closure.2 Even for small wounds, wide undermining of the scalp is usually required for primary closure. Excessive wound tension often results in wound breakdown and/or scar alopecia. The size of the defect is not the only criterion; the quality of the tissue being advanced is just as important. Stiff, irradiated scalp tissue will likely not be amenable to advancement and closure if there is even the slightest amount of tension.
Skin Grafts
Split-thickness or full-thickness skin grafts may be an option for scalp reconstruction when the wound bed is well vascularized. An intact pericranium or fascia is usually required for adequate skin graft “take.” A skin graft over bone devoid of pericranium usually has a low probability of survival and is susceptible to loss after only minimal trauma. When bone is exposed, the pericranium or fascia may first be advanced or rotated into the wound bed as a galeal, temporoparietal, or pericranial flap to improve skin graft survival. The survival of skin grafts on bone can also be improved by removing the outer table of bone using a burr to expose the vascularized diploic space. The drawbacks of this technique include potential skin graft slough and poor aesthetic outcome. Neoadjuvant or perioperative radiotherapy may result in increased wound complications when skin grafts are used alone.
Split-thickness skin grafts can also be used for temporary wound coverage while the scalp is being prepared for definitive reconstruction, such as with serial tissue expansion and advancement of local tissues, or while awaiting the pathologic results after oncologic resection. Skin grafting followed by staged, sequential excision of the graft and advancement or rotation of hair-bearing local tissues is also a useful strategy that can result in a good aesthetic outcome. However, there usually is less tissue elasticity after each excision, and areas of scar alopecia can be expected.
Tissue Expansion
In some patients, tissue expansion can be used to provide additional scalp tissue for rotation or advancement flap closure.6 Tissue expansion requires an adequate amount of expandable tissue, patient tolerance for staged reconstruction, and stable calvarial coverage during the expansion process.
A significant advantage of tissue expansion is the potential to repair the scalp defect with hair-bearing tissue; however, there is often loss of hair density with this technique. Expanded hair-bearing scalp tissue can also be used to replace non–hair-bearing tissue after the excision of burn scars, benign scalp neoplasms, and temporary skin grafts.
Tissue expansion is usually performed under elective conditions when immediate, permanent wound coverage is not essential. Tissue expansion is contraindicated when prompt resection and reconstruction of the scalp are required, such as in the setting of malignant scalp tumors. Tissue expansion also may not be appropriate when the scalp wound cannot be temporarily closed with a skin graft, such as in patients who have undergone cranial reconstruction with a bone graft or allograft. Tissue expansion of irradiated skin or skin adjacent to an infected or open wound is also not appropriate because of the increased complication rate.2
Patients with defects involving up to 50% of the scalp may have reconstruction with expanded scalp tissue.7 For scalp tissue expansion, one or more tissue expanders are placed in the subgaleal space. Multiple expanders are frequently used to gain the greatest amount of expanded scalp tissue prior to the reconstructive procedure. The expanded scalp tissue should be at least 20% larger than the size of the defect to allow for tissue recoil during rotation or advancement.8 Consideration to replacing hair in an anatomic pattern should be given when choosing the expander location.9
Local Flaps
Local scalp flaps may be used to cover moderate-sized scalp defects. Local scalp flaps have the advantage of providing hair-bearing tissue for potentially improved cosmesis. The nonirradiated scalp has an abundant blood supply from several regional sources. Local scalp flaps generally rely on extensive undermining and are based on the blood supply from the superficial temporal, occipital, postauricular, supratrochlear, or supraorbital arteries or a combination of these arteries. The Orticochea tripartite flap and its variants have been used for reconstruction of large central scalp defects.10,11
In certain situations, galeal scoring may be performed to gain flap length and reduce tension. Although Raposio et al12 found only modest improvements in flap length (1.67 mm per galeal incision) with galeal scoring, they found a 40% decrease in wound tension. Ideally, galeal scoring should be performed parallel to the subaponeurotic blood vessels, but even more importantly scoring must be perpendicular to the line of maximum tension, which is from the pivot point to the most distal tip of the defect. Care should be taken to avoid scoring too deeply, as this maneuver can result in blood vessel transection and vascular compromise of the flap.
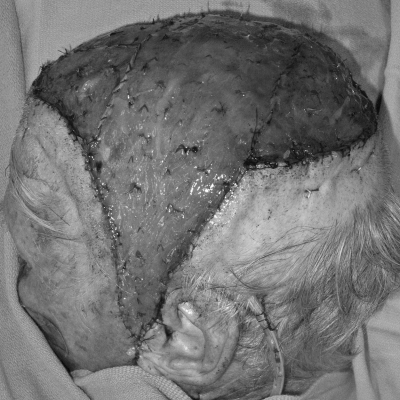
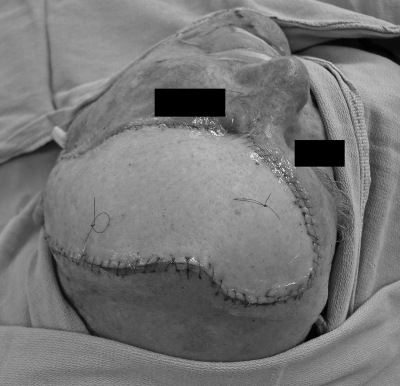
Local flaps with extensive undermining and galeal scoring may be adequate for closure of both the recipient and donor-site defects.13 In some patients, a more conservative technique, particularly for larger defects, is to create a local flap large enough to cover the recipient defect in its entirety and to place a skin graft over the donor site defect (Figs. 1 and 2). This concept is beneficial when the recipient site requires vascularized tissue coverage (e.g., in cases of exposed bone or the need for calvarial reconstruction) and the donor site contains intact periosteum that is suitable for skin graft coverage. The subgaleal fascia is well vascularized as a skin graft recipient site.1 Ideally, donor sites should be placed in less aesthetically sensitive areas when possible, taking advantage of adjacent hair to cover the skin-grafted area.
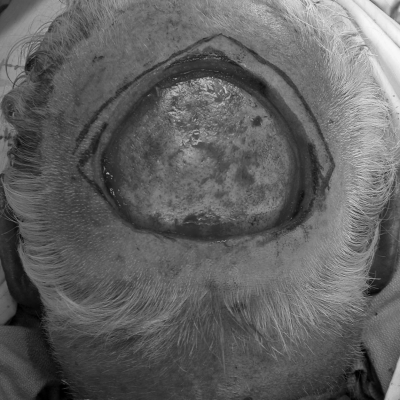
Figure 1.
A patient with large, posterior, full-thickness scalp defect with bone exposed.
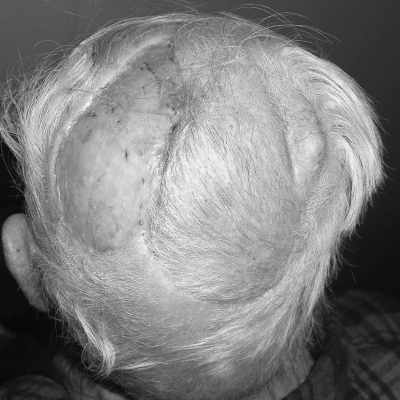
Figure 2.
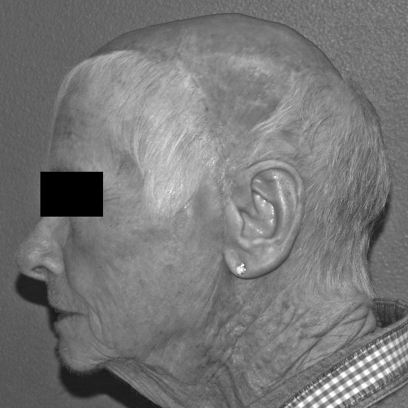
Postoperative photograph of the patient in Fig. 1 six months after reconstruction with an anteriorly based local scalp flap and split-thickness skin graft of the donor site.
In summary, there are several guiding principles to optimize outcomes in scalp reconstruction using nonmicrosurgical techniques. The design of local scalp flaps should incorporate at least one major scalp vessel. Burow's triangles should not be excised at the time of initial flap creation to maintain the vascular supply of the flap at its base. The parietal scalp has the greatest amount of laxity and, therefore, is a good region to incorporate into the design of local flaps.
Regional Flaps
In most patients, regional flaps are generally not available to repair central scalp defects due to the limited arc of transposition from neck and trunk muscles. However, the temporoparietal fascia flap and temporalis muscle flap are useful for scalp defects in the temporal region, and the trapezius muscle or myocutaneous flap as well as the latissimus dorsi muscle or myocutaneous flap may be useful for occipital wounds.
Free Flaps
Flap selection is based on multiple factors including defect size, vascular pedicle length, flap size and thickness, donor-site morbidity, and surgeon preference. The free latissimus dorsi muscle flap is considered by many to be the preferred option for repairing large scalp defects (Figs. 3–6). Pennington describes the use of latissimus dorsi reconstruction with calvarial defects.14 The latissimus dorsi is a relatively thin, broad, and well-vascularized muscle that is suitable for covering a cranial reconstruction performed with autologous bone or alloplastic materials. Atrophy of the muscle over time usually results in a soft tissue thickness that closely approximates that of the native scalp (Fig. 6). The pedicle length of this flap is ~10 cm if the subscapular vessels are included. The muscle is usually harvested without a skin paddle and may be covered with an unmeshed split-thickness skin graft for scalp reconstruction.
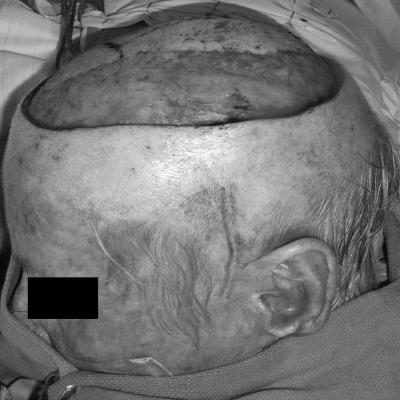
Figure 3.
Patient with a large, full-thickness scalp defect with bone exposure after resection of a large multifocal skin cancer.
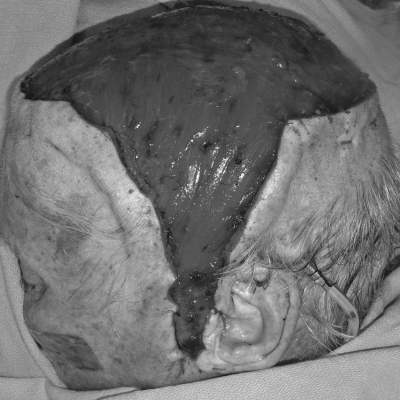
Figure 4.
Insetting of free latissimus dorsi muscle flap to neck vessels (same patient as shown in Fig. 3). Note that the preauricular skin has been divided and the flap partially advanced into this region to allow the pedicle to reach.
Figure 5.
Covering the latissimus dorsi muscle flap with an unmeshed skin graft is an aesthetic approach to scalp reconstruction (same patient as shown in Figs. 3 and 4).
Figure 6.
The same patient as shown in Figs. 3, 4 and 5 six months after surgery. The muscle atrophies significantly over time, and the unmeshed skin graft provides a reasonable result for reconstruction over a muscle flap.
The free serratus anterior muscle flap is an option for smaller scalp defects. The lower three or four muscle slips of the serratus anterior muscle can be harvested without significant scapular winging. The vascular pedicle is quite long (~15 cm) if the subscapular vessels are included with the serratus anterior branch and main trunk of the thoracodorsal blood vessels. The pedicle can therefore usually reach recipient vessels in the neck without the need for vein grafts. The surface area of the serratus anterior flap is usually limited to ~10 × 12 cm. If a large amount of tissue is needed for reconstruction, a chimeric muscle flap that includes the serratus anterior muscle and the latissimus dorsi muscle can be harvested based on the subscapular vessels. In addition, the sixth or seventh rib may be harvested with the serratus anterior muscle for select bone and soft tissue composite defects.
Other free flaps used for scalp reconstruction include the rectus abdominis muscle myofascial or myocutaneous flap; radial forearm fasciocutaneous flap; greater omental flap; and anterolateral thigh flap.15,16,17 The rectus abdominis myocutaneous flap can be used to reconstruct larger scalp defects. One advantage of the rectus abdominis muscle flap is the potential for a two-team approach to enable simultaneous tumor resection and flap harvest. The free radial forearm fasciocutaneous flap is particularly useful for reconstruction of the forehead or when a long pedicle is required for microsurgical anastomosis in the neck (Figs. 7 and 8). The free greater omental flap has been used with reasonable results but is usually reserved for situations in which a latissimus dorsi flap is contraindicated or not available; a major disadvantage of the greater omental flap is the requirement for a laparotomy. The anterolateral thigh free flap is a fasciocutaneous flap that is also used by some for scalp reconstruction; in certain patients, the anterolateral thigh flap can be a thin fasciocutaneous option for scalp reconstruction (Fig. 9).
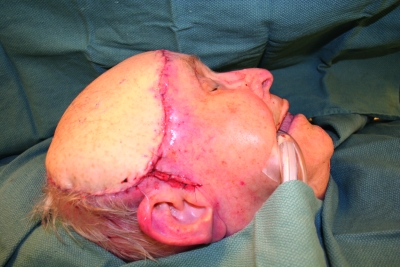
Figure 7.
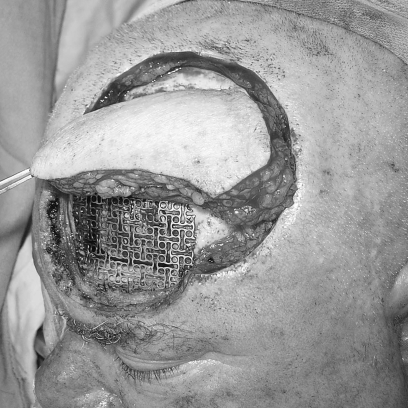
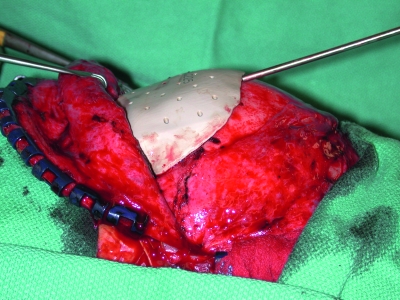
The scalp and forehead are reconstructed with a radial forearm fasciocutaneous free flap with anastomosis to the superficial temporal vessels. Titanium mesh is used frequently for calvarial reconstruction. In this patient, it was used for frontal bone reconstruction.
Figure 8.
Postoperative photograph of healed radial forearm free flap reconstruction of the scalp (same patient as shown in Fig. 7).
Figure 9.
In certain patients, the anterolateral thigh flap can be a thin fasciocutaneous flap for scalp reconstruction.
Placing an unmeshed split-thickness skin graft over muscle usually gives a superior aesthetic result with muscle flaps. In addition, the contour of muscle flaps usually improves significantly over time as the muscle atrophies, although revision procedures may sometimes be needed, particularly to address proximal muscle bulkiness.
The superficial temporal vessels are frequently used as recipient vessels in free-flap scalp reconstruction. These vessels are located in the preauricular area and are usually palpable just anterior to the root of the helix. At the level of the superior helix, the superficial temporal artery bifurcates into the frontal and parietal branches. Whenever possible, the superficial temporal vessels should be considered for recipient vessels due to their location. The preauricular region should be explored prior to division of the flap pedicle because the superficial temporal vessels vary in their size and anatomy. Use of the facial or external carotid blood vessels as recipient vessels usually requires that the flap be positioned at least partly in a preauricular or postauricular location. Vein grafts may be required when pedicle length is inadequate.
Scalp Replantation
Microvascular replantation of the scalp is preferred when faced with an avulsed full-thickness scalp defect. The avulsed scalp is cleansed, shaved, and debrided. A search for vessels is made under magnification. When possible, the temporal vessels are identified first, followed by the occipital vessels. To minimize ischemia time, a second team prepares the recipient site, isolating the temporal and occipital vessels. When possible, sensory nerves are also identified in the scalp and the head. Vein grafts are usually always necessary. They are harvested and anastomosed to the arteries of the head and the veins of the scalp (or vice versa), again to minimize ischemic time. The scalp is then reapproximated without tension over suction drains to prevent hematoma formation.
Having separate teams operate on the recipient head and the avulsed scalp minimizes warm ischemia time. When possible, a third team can be used to harvest vein grafts. By simultaneously performing vein graft anastomoses on the scalp and the head, a great deal of time is saved. Adequate debridement of injured vessels is also critical. When ischemic time is prolonged, the arterial anastomoses can be performed first and veins periodically allowed to drain. Cheng et al reported a series of 20 scalp replantations.18
The patient's head is elevated and the replanted scalp is closely monitored for adequate perfusion. Broad-spectrum antibiotics are also used for several days.
Scalp Reconstruction Postoperative Care
After nonmicrosurgical scalp reconstruction, elevation of the head to minimize edema is recommended. Bolster dressings may be helpful to immobilize skin grafts. Protecting skin grafts on the posterior scalp may be challenging because of the pressure and shear forces while the patient is in a supine position. Proper patient positioning, use of protective padding, and use of negative-pressure dressings can reduce skin graft loss. Drains are often used when wide undermining of the scalp has been performed for local flaps.
After microvascular free-flap scalp reconstruction, the patient's head should be maintained in a neutral position with the head of the bed elevated at least 45 degrees. The patient is also positioned to prevent pressure on the vascular pedicle or the flap. Low-pressure or negative-pressure dressings can be used over skin grafts.
Scalp Reconstruction Complications
Partial graft or flap loss after scalp reconstruction is not uncommon, particularly in cases of highly complex scalp wounds, such as those occurring in the settings of infection, irradiation, and prior surgery. If the pericranium is present at the base of the wound, the wound resulting from the loss may heal by secondary intention. If bone or alloplastic material is exposed, reoperation with readvancement of local flaps or reconstruction with free-tissue transfer is usually required. Exposure or infection of tissue expanders may occur in up to 25% of cases and usually requires explantation and reinsertion after infection resolution and healing have occurred.8
Potential adverse outcomes of free-flap scalp reconstruction include partial or total flap necrosis, wound dehiscence with bone or allograft exposure, donor-site seromas, and contour irregularities such as excessive bulk at the muscle origin. To prevent complications, it is important to widely undermine or even divide any tissue that lies over the flap pedicle. When possible, the proximal portion of the flap muscle should be placed over any exposed bone or allograft to prevent reexposure in the event of distal flap loss. Overlapping the remaining scalp over the muscle portion of the flap during inset by 1 to 2 cm can reduce contour deformities and dehiscences.19
CALVARIAL RECONSTRUCTION
Options
Calvarial reconstruction is performed to provide protective coverage of the intracranial contents and to restore cranial contour. Autologous bone is generally preferred when possible. The advantages of reconstruction with autologous bone include a lower incidence of graft loss than occurs with alloplastic material. Also, exposure and infection of autologous bone can sometimes be managed without complete graft loss, whereas when alloplastic materials become exposed or infected, often the only choice is removal of the foreign material.
Calvarial bone grafts have the benefit of being harvested from the same operative field as the defect. Split outer table grafts are taken from the parietal region of the skull, posterior to the coronal suture, where the skull is thickest. A trough is burred around the graft site, and a saw or osteotome is used to remove the outer table bone graft. The graft should not be harvested in the midline because of the risk of injuring the sagittal sinus. A disadvantage of calvarial grafts is the limited size of graft available, particularly when the defect is adjacent to the graft donor site. Another disadvantage of calvarial grafts is the risk of violating the inner table or dura during harvest.
Split-rib grafts may be used to repair larger calvarial defects. The grafts are harvested through an anterolateral chest wall incision centered over the seventh rib. To prevent chest wall instability, no more than two adjacent ribs should be harvested. Harvesting grafts from alternate ribs minimizes chest wall weakness and contour deformity. In addition, usually no more than three ribs are harvested per side. The ribs are harvested subperiosteally to protect the underlying pleura. The harvested ribs are then contoured with a rib bender, and the bone grafts are placed adjacent to each other in the calvarial defect. Splitting the rib grafts may increase surface coverage for reconstruction. Gaps can be filled with bone chips or bone paste. Disadvantages of rib grafts include the potential for an irregular, washboard-like contour after reconstruction and donor-site morbidity.20
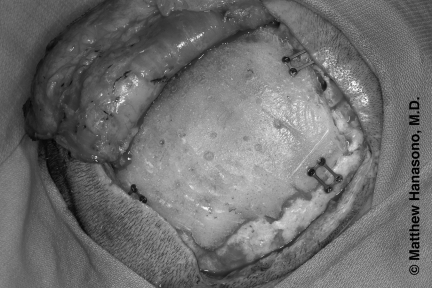
Alloplastic material is used to repair calvarial defects in conjunction with neurosurgical procedures.21 Titanium mesh has a long history of successful use (Fig. 7). Its advantages include the ability to cover very large defects and the production of minimal foreign body reaction. Its disadvantages include greater conductance of heat and cold and a higher rate of infection than occurs with bone grafts. Titanium mesh is also radiopaque and may cause scatter artifacts that limit the usefulness of subsequent radiographic studies.
Methyl methacrylate is also commonly used for calvarial reconstruction (Fig. 10). It is durable, can be easily contoured, and conducts heat and cold less than metal alloys do. The hardening process is exothermic, and cool irrigation is required during curing.
Figure 10.
Delayed methyl methacrylate cranioplasty placed beneath a latissimus dorsi muscle free flap covered with a split-thickness skin graft.
Calcium hydroxyapatite cements are also an option for alloplastic cranioplasty. They are usually used in combination with titanium mesh to provide a smooth contour to the reconstructed calvaria. The material allows some bone ingrowth, or osteoconductivity. The hardening process of calcium hydroxyapatite is isothermic.
To optimize outcomes in calvarial reconstruction, stable soft tissue coverage is critical to prevent exposure and infection of autologous or alloplastic cranioplasties. When possible, scalp incisions should be planned so that they do not expose graft materials or compromise the vascular base of a scalp flap. As a matter of surgical principle, tension-free closure aids in the wound-healing process.
Calvarial Reconstruction Complications
High rates of infection and graft removal occur if autologous grafts or alloplastic implants are placed into infected wounds. When infection is already present, cranioplasty should be delayed and performed as a secondary procedure after debridement and antibiotic treatment. If a cranioplasty site develops infection, the graft or implant should be removed and further reconstruction delayed until the infection has been treated. In certain cases, scalp reconstruction with a free flap may be performed at the time of debridement of infected calvarial wounds to provide both stable coverage and well-vascularized viable tissue in regions of prior surgery and/or radiotherapy. Careful neurologic assessment and vigilance for cerebrospinal fluid leaks are paramount after calvarial reconstruction. Intraoperative or postoperative leaks are usually managed with the assistance of a neurosurgeon.
CONCLUSION
Patients with previously irradiated wound beds, infection, or prior surgery may present with complex scalp defects. The scalp defect size and location, presence or absence of pericranium to accept skin grafts, and quality of the remaining scalp tissue influence the selection of reconstructive technique. Primary closure of scalp defects is generally restricted to wounds measuring less than 3 cm in diameter due to the limited elasticity of the scalp. In some situations, a skin graft can be used over bone if the pericranium is intact or the outer cortex is removed to expose the diploic space. Tissue expansion requires an adequate amount of expandable tissue, patient tolerance for staged reconstruction, and stable calvarial coverage during the expansion process.
Attention to the vascularity of the scalp is crucial in planning local scalp flaps. Proper design of local scalp flaps includes incorporation of major vascular pedicles within broadly based flaps and closure without excessive tension. Galeal scoring may be performed to increase flap length, but care should be taken not to disrupt blood vessels that lie superficial to the galea aponeurotica.
Microsurgical flap reconstruction may be the most reliable option for large scalp defects, particularly those occurring in the setting of compromised tissues. Numerous free flaps are available for scalp reconstruction, including the latissimus dorsi muscle flap, serratus anterior muscle flap, rectus abdominis muscle flap and its variants, radial forearm flap, greater omental flap, and anterolateral thigh flap.
Options for calvarial reconstruction include autologous split calvarial and rib grafts and alloplastic materials such as titanium mesh, methyl methacrylate, and calcium hydroxyapatite. Successful reconstruction of the calvaria and scalp may require multiple steps involving multiple rungs on the reconstructive “ladder.”
REFERENCES
References
- Tolhurst D E, Carstens M H, Greco R S, et al. The surgical anatomy of the scalp. Plast Reconstr Surg. 1991;87:603–612. [PubMed] [Google Scholar]
- Hoffman J F. Management of scalp defects. Otolaryngol Clin North Am. 2001;34:571–582. doi: 10.1016/s0030-6665(05)70006-2. [DOI] [PubMed] [Google Scholar]
- Ioannides C, Fossion E, McGrouther A D. Reconstruction for large defects of the scalp and cranium. J Craniomaxillofac Surg. 1999;27:145–152. doi: 10.1016/s1010-5182(99)80042-0. [DOI] [PubMed] [Google Scholar]
- Newman M I, Hanasono M M, Disa J J, et al. Scalp reconstruction: a 15-year experience. Ann Plast Surg. 2004;52:501–506. doi: 10.1097/01.sap.0000123346.58418.e6. [DOI] [PubMed] [Google Scholar]
- Hussussian C J, Reece G P. Microsurgical scalp reconstruction in the patient with cancer. Plast Reconstr Surg. 2002;109:1828–1834. doi: 10.1097/00006534-200205000-00008. [DOI] [PubMed] [Google Scholar]
- Baker S R, Swanson N A. Tissue expansion of the head and neck. Arch Otolaryngol Head Neck Surg. 1990;116:1147–1153. doi: 10.1001/archotol.1990.01870100041009. [DOI] [PubMed] [Google Scholar]
- Manders E K, Schenden M J, Furrey J A, et al. Skin expansion to eliminate large scalp defects. Plast Reconstr Surg. 1984;74:493–507. doi: 10.1097/00000637-198404000-00001. [DOI] [PubMed] [Google Scholar]
- Leedy J E, Janis J E, Rohrich R J. Reconstruction of acquired scalp defects: an algorithmic approach. Plast Reconstr Surg. 2005;116:54e–72e. doi: 10.1097/01.prs.0000179188.25019.6c. [DOI] [PubMed] [Google Scholar]
- Rappard J H Van, Molenaar J, Doorn D Van, et al. Surface-area increase in tissue expansion. Plast Reconstr Surg. 1988;82:833–839. doi: 10.1097/00006534-198811000-00016. [DOI] [PubMed] [Google Scholar]
- Orticochea M. New three-flap scalp reconstruction technique. Br J Plast Surg. 1971;24:184–188. doi: 10.1016/s0007-1226(71)80038-3. [DOI] [PubMed] [Google Scholar]
- Arnold P G, Rangarathnam C S. Multiple-flap scalp reconstruction: Orticochea revisited. Plast Reconstr Surg. 1982;69:605–613. doi: 10.1097/00006534-198204000-00003. [DOI] [PubMed] [Google Scholar]
- Raposio E, Nordstrom R E, Santi P L. Undermining of the scalp: quantitative effects. Plast Reconstr Surg. 1998;101:1218–1222. doi: 10.1097/00006534-199804050-00007. [DOI] [PubMed] [Google Scholar]
- Lesavoy M A, Dubrow T J, Schwartz R J, et al. Management of large scalp defects with local pedicle flaps. Plast Reconstr Surg. 1993;91:783–790. doi: 10.1097/00006534-199304001-00005. [DOI] [PubMed] [Google Scholar]
- Pennington D G, Stern H S, Lee K K. Free flap reconstruction of large defects of the scalp and calvarium. Plast Reconstr Surg. 1989;83:655–661. doi: 10.1097/00006534-198904000-00010. [DOI] [PubMed] [Google Scholar]
- Losken A, Carlson G W, Culbertson J H, et al. Omental free flap reconstruction in complex head and neck deformities. Head Neck. 2002;24:326–331. doi: 10.1002/hed.10082. [DOI] [PubMed] [Google Scholar]
- Lutz B S, Wei F C, Chen H C, et al. Reconstruction of scalp defects with free flaps in 30 cases. Br J Plast Surg. 1998;51:186–190. doi: 10.1054/bjps.1997.0182. [DOI] [PubMed] [Google Scholar]
- Ozkan O, Coskunfirat O K, Ozgentas H E, et al. Rationale for reconstruction of large scalp defects using the anterolateral thigh flap: structural and aesthetic outcomes. J Reconstr Microsurg. 2005;21:539–545. doi: 10.1055/s-2005-922433. [DOI] [PubMed] [Google Scholar]
- Cheng K, Zhou S, Jiang K, et al. Microsurgical replantation of the avulsed scalp: report of 20 cases. Plast Reconstr Surg. 1996;97:1099–1106. doi: 10.1097/00006534-199605000-00001. [DOI] [PubMed] [Google Scholar]
- Lipa J E, Butler C E. Enhancing the outcome of free latissimus dorsi muscle flap reconstruction of scalp defects. Head Neck. 2004;26:46–53. doi: 10.1002/hed.10338. [DOI] [PubMed] [Google Scholar]
- Earley M J, Green M F, Millang M A. A critical appraisal of the use of free flaps in primary reconstruction of combined scalp and calvarial cancer defects. Br J Plast Surg. 1990;43:283–289. doi: 10.1016/0007-1226(90)90073-9. [DOI] [PubMed] [Google Scholar]
- Manson P N, Crawley W A, Hoopes J E. Frontal cranioplasty: risk factors and choice of cranial vault reconstructive material. Plast Reconstr Surg. 1986;77:888–904. [PubMed] [Google Scholar]
Editor's Comments
Drs. Lin, Hanasono, and Skoracki have written an excellent overview of scalp and calvarial reconstruction, which highlights their extensive experience on these difficult reconstructions.
I would like to add several observations and comments from our own institutional experience. One is the use of outer-table calvarial burring with or without conjunction of the VAC therapy to provide a bed of granulation tissue for split thickness graft coverage. We do not consider this a permanent solution to scalp coverage as the wound is often unstable and unreliable. We will use this technique as bridge coverage for up to 3 to 6 months until suitable long-term coverage can be achieved (for example, to allow for adjacent tissue expansion).
The second point I would like to make is we agree completely that the latissimus dorsi muscle flap is the workhorse flap of scalp reconstruction; however, in the properly selected patient anterior lateral thigh flap (ALT), flaps can provide more durable coverage over a large area, particularly mesh or allopathic implants, and are more resistant to wound breakdown than muscle-only flaps.
In addressing calvarial reconstruction, we echo the authors' comments that alloplastic or autogenous material placed in an infected wound bed yields unacceptably high infection rates. In the cases of infected skull, we will remove all alloplastic or autogenous material and treat with antibiotics until there are no systemic signs of infection, including normalized sedimentation rates. At this point the patient will require a minimum interval of 6 months prior to consideration for reconstruction.
Finally, the use of prefabricated alloplastic implants has greatly simplified our reconstructive algorithm in the appropriate patient. PEEK or PMA implants can be premade from 3D CT scans to precisely match the calvarial defect, providing final cosmetic results superior to acrylic reproductions of the bone plate.
James F. Thornton, M.D.
Figure 1.
Near total scalp reconstruction with ALT free flap.
Figure 2.
PEEK custom premade calvarial implant.