ABSTRACT
Distal radius reconstruction in children should meet two requests: restoration of some joint function and preservation of the physiologic growth of the segment. None of the conventional options is likely to successfully achieve both goals. Conversely, a vascularized transfer of the proximal fibula including the growth plate provides enough bone stock for diaphyseal reconstruction, an articular surface for joint function, and the potential for longitudinal growth. From 1992 to 2006, eight children ranging in age between 2 and 10 years underwent a vascularized transfer of the proximal fibula for distal radius reconstruction after bone sarcoma resection. The follow-up ranges were between 1 year and 15 years. All the grafts were harvested based on the anterior tibial artery. Seven cases with a follow-up longer than 2 years have been evaluated both clinically and radiographically. All the grafts survived and had a satisfactory growth after the transplant. The functional outcome has been satisfactory, and the range of motion of the reconstructed wrist has been nearly normal in all cases but one. Proximal fibular epiphyseal transfer was an effective procedure for distal radius reconstruction in children who underwent tumor resection. Refinements in the operative technique have increased the reliability of this reconstructive option, which might be safely used also in congenital and posttraumatic disorders.
Keywords: Epiphyseal transfer, distal radius reconstruction, bone reconstruction, pediatric microsurgery
Osteoarticular wrist reconstruction in children should simultaneously restore the joint function and the growth potential. The non–microsurgical options are limited to allografts and prosthesis. Both these alternatives are not likely to be successful in the pediatric age: a frozen allograft of the proper size is difficult to obtain, is prone to resorption at medium term, and cannot provide any growth. Wrist prostheses have some indications in adults, but their endurance is unpredictable, their size is not suitable for children, and, of course, they cannot provide growth. In addition, wrist prosthesis cannot reconstruct a concomitant diaphyseal defect. In a two-bone region such as the forearm, maintenance of symmetrical growth between the reconstructed radius and the ipsilateral ulna is a crucial issue in planning the reconstruction. The growth of the ulna alone leads to an unavoidable deformity of the wrist and develops a radial club hand with subsequent functional and cosmetic impairment.
A vascularized transfer of the proximal fibula may be a very effective alternative.1 The proximal fibular epiphysis is actually quite similar in shape and size to the distal radius and contains an active growth plate that may provide the potential for growth in the new heterotopic location. In addition, the graft provides excellent tubular bone for diaphyseal reconstruction.
MATERIALS AND METHODS
In a period of time ranging between 1992 and 2006, 8 children aged between 2 and 11 years (average, 7.8 years) underwent distal radius resection because of bone sarcoma. Wide-margin resection was achieved in all cases. In five cases, the salvage of a sufficiently long proximal segment of the radius was allowed by the distal location of the tumor; in two cases, the radius was totally removed; and in one case, the distal ulna was also included in the resection. Six children were affected by osteosarcoma and two by Ewing sarcoma.
All the patients underwent reconstruction by means of a vascularized autologous graft of the proximal fibula based on the anterior tibial artery. The harvesting technique has been described elsewhere2,3 and consists of isolating the proximal fibula through an anterolateral approach taking care in preserving the anterior tibial vessels and their tiny vascular connections both to the diaphysis and the epiphysis. In five cases, the transferred fibula was fixed to the proximal radial stump, preferably by metal plate. In two cases of total resection, a surgical radioulnar synostosis was achieved and fixed by compression screws. In one case of resection including the distal ulna, a one-bone forearm was obtained after fixing the graft to the proximal ulnar stump (Fig. 1). A reverse-flow anastomosis was performed between the distal stump of the anterior tibial vessels and either the proximal interosseous artery or the radial artery. The recipient vein was the cephalic vein in all cases.
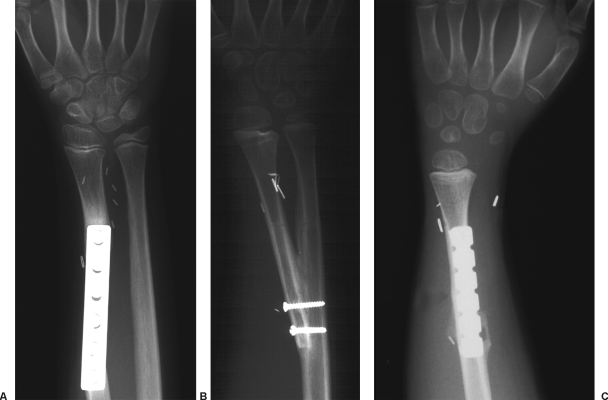
Figure 1.
According to the amount of the resection, three possible options may be chosen for bone fixation, as demonstrated in the following radiographs. (A) When only the distal part of the radius is resected, a stable osteosynthesis may be achieved with compression plate. (B) When the whole radius is resected, a surgical radioulnar synostosis is recommended. (C) When the distal ulna is also involved in the resection, a one-bone forearm is the only possible alternative.
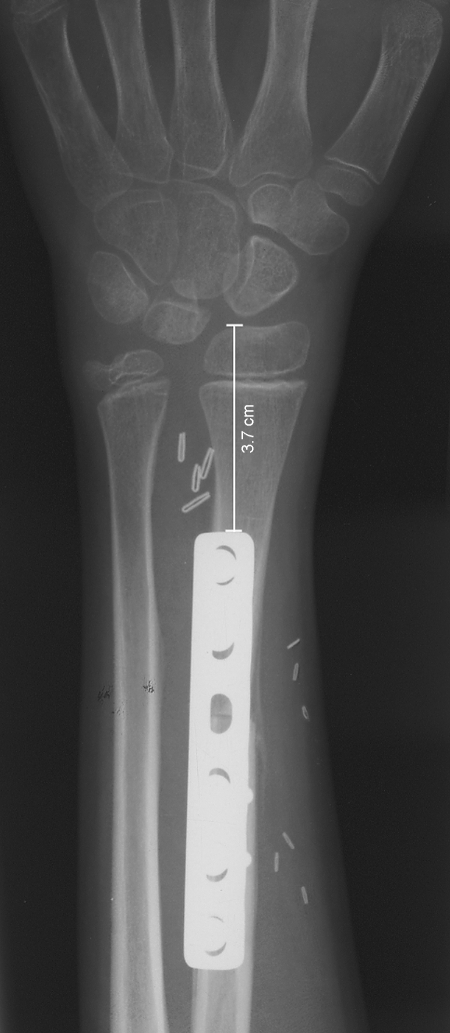
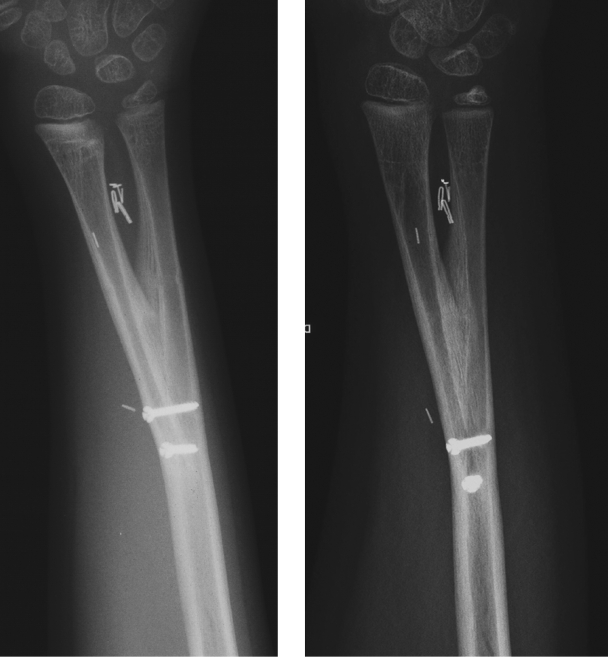
The patients have been followed for a period of time ranging between 1 year and 15 years by means of periodic clinical and radiographic controls. The longitudinal growth of the transplanted bones has been assessed by measuring the distance between the tip of the fibular head and the metal plate (Fig. 2).
Figure 2.
As demonstrated on the radiograph, the assessment of the distance between the tip of the epiphysis and the metal plate is a simple and reliable method to evaluate the growth.
RESULTS
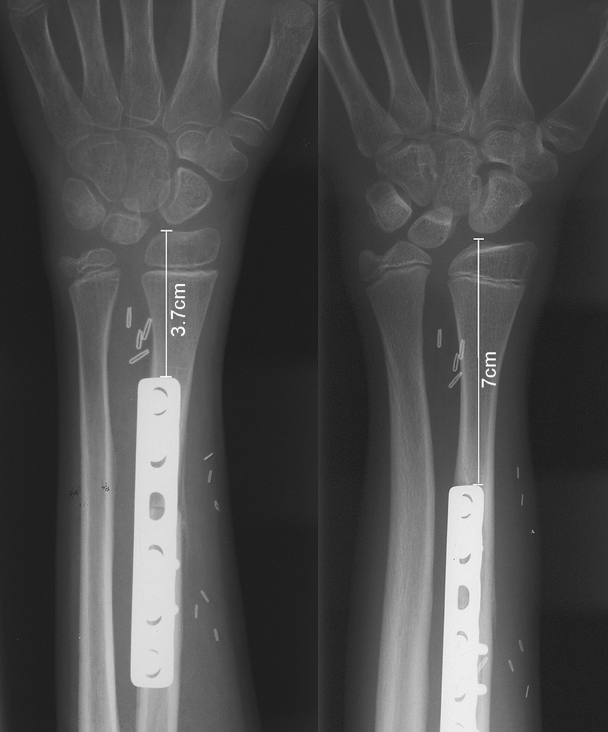
One patient died from lung metastasis 4 years after surgery, and the remaining seven patients are alive and free of disease. The analysis of the results refers to the seven patients with a follow-up of 3 years or longer. All the grafts survived and united with the recipient bone in a period of time ranging between 1 and 5 months. The amount of growth varied according to the age of the patient, being higher in younger patients. On average, the growth of the transferred fibula ranged between 0.7 and 1.1 cm per year providing effective prevention to limb-length discrepancy (Fig. 3). In almost all the cases, no growth was observed during the chemotherapy period. In the five cases where a two-bone forearm was preserved, the growth of the neoradius was at matching rate with the ipsilateral ulna. Therefore, neither radial club hand nor ulnocarpal impingement occurred in these patients.
Figure 3.
As observed on the radiographs, in this patient the longitudinal growth of the transferred fibula was 3.3 cm in a 4-year period with a growth rate per year of 0.8 cm.
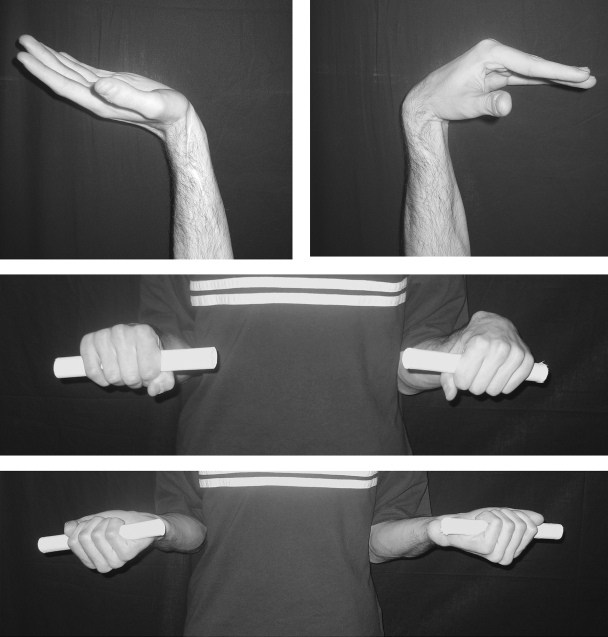
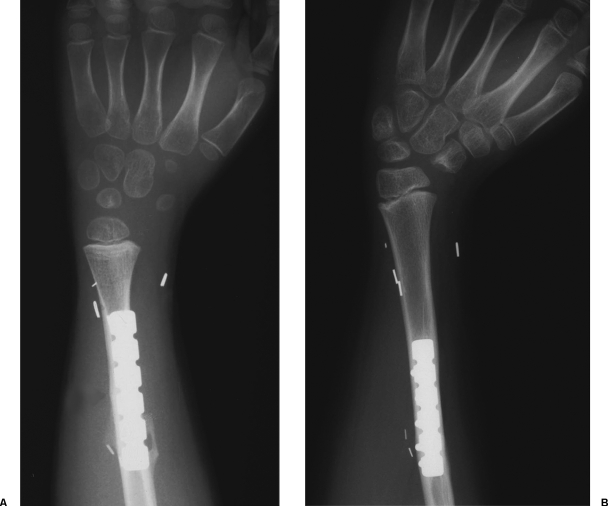
The functional outcome was excellent in the five cases with a two-bone forearm where flexion of the wrist ranged between 70 degrees and 80 degrees, extension between 60 degrees and 65 degrees, and almost full pronation and supination were achieved (Fig. 4). Acceptable flexion and extension were also recovered in the case of total resection of the radius where the fibula was fixed end-to-side to the ulna, and, surprisingly, some pronation and supination have been observed in spite of the surgical synostosis. The worst results have been achieved in the case where both radius and ulna were resected because of tumor extension. This patient developed some wrist instability and, although the growth has been satisfactory, the flexion and extension were limited to 30 degrees and 20 degrees, respectively.
Figure 4.
The functional outcome has been excellent in all cases where the ulna and the proximal part of the radius have been spared during tumor resection. At 9-year follow-up, this patient shows a nearly normal range of motion at the wrist joint.
The only complication observed was a fracture after an efficient trauma, which healed conservatively in a physiologic time. At the donor site, a palsy of the peroneal nerve occurred in four patients. All four patients had a division of motor branches of the peroneal nerve during the harvest of the proximal fibula that has been repaired using microsurgical techniques. Three completely recovered within 1 year postoperatively, and one patient suffered a permanent paralysis of the tibialis anterior muscle.
DISCUSSION
The first reports of free nonvascularized epiphyseal transplant go back to the middle of the past century. All the attempts in the premicrosurgery era had had discouraging results in terms of graft survival and growth, and a failure in early revascularization of the growth plate was identified as a crucial condition in determining success.4,5 Efforts were therefore oriented toward tackling the problem related to revascularization; reducing the thickness of the bony portion of the graft to improve the possibility to get some blood supply from the host bone. The results, however, did not improve significantly.
Advances in microvascular surgery gave a new impulse to experimental research, and many experiments were carried on with the aim to check the feasibility of vascularized epiphyseal transfer in an animal model. Restoring the metaphyseal and epiphyseal blood supply, Bowen, Boyer, Nettelblad, and others demonstrated the survival and the growth of the transplanted epiphysis.6,7,8,9,10,11
The procedure was then applied in clinical practice in the 1980s, when a few surgeons pioneered this innovative technique in the human being,12,13,14 achieving some encouraging results that convinced the scientific community to refine the technique and expand the indications.
Failure of axial growth and premature fusion of the growth plate are some of the potential complications that may be related to the choice of the pedicle.
The upper fibular epiphysis is supplied by three small arteries: the inferior genicular artery, a recurrent branch of the anterior tibial artery, and a not-constant unnamed artery directly rising from the popliteal artery. Much of the early published experiences report the use of the peroneal artery either alone or combined with an epiphyseal artery.12,13,14 The grafts based on the peroneal artery alone had unpredictable outcome, and the majority of them failed to achieve an acceptable growth. On the other hand, the double pedicled grafts, where besides the peroneal artery, either the descending genicular artery or the recurrent branch of the anterior tibial artery is harvested, are technically difficult because they need multiple anastomoses and vein grafts. In addition, there is an increased length of operating time and of the risk of thrombosis.
According to our personal experience and to a review of the literature, the anterior tibial vascular bundle seems to be the best choice to provide blood supply both to the epiphysis and diaphysis with a single pedicle.15,16,17,18,19 This simplifies the surgical procedure and minimizes the ischemia time thus increasing the success rate.
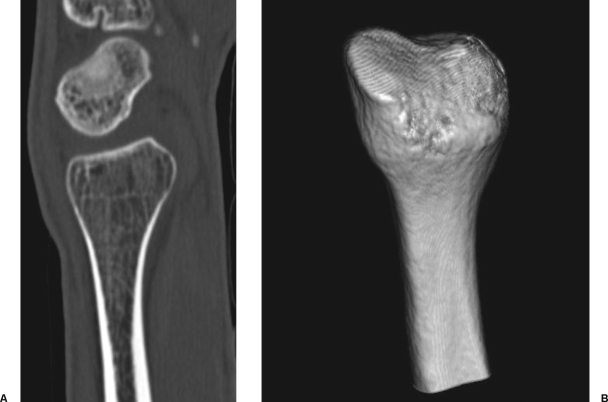
Although the proximal fibula has been used to reconstruct different bones such as the proximal humerus, the distal and proximal femur, the mandible, and the ulna, the distal radius is the ideal segment to be reconstructed according to the described procedure. A perfect correspondence exists between the diaphyseal segments of the two bones, and also the epiphyseal, articular portions are quite similar. In addition, the biomechanical environment at the wrist joint facilitates adaptive changes of the morphology of the transferred bone (Fig. 5), which gradually improves the functional outcome.
Figure 5.
Morphologic remodeling of the transferred epiphysis has been observed in all cases (panel A, CT scan; panel B, three-dimensional CT reconstruction). Under the influence of loading, the articular surface became concave, thus improving the articular congruity with the first carpal row.
In case of wrist reconstruction, where even a small amount of length discrepancy with the ulna may lead to significant functional impairment, this procedure may be recommended also in patients approaching the end of the growth with the aim to maintain a well-balanced joint and prevent further surgery.
In all the five cases where the resection of the radius was partial and the ulna was spared, excellent functional results were achieved. The remodeling potential in children is a powerful tool that gradually optimizes the reconstruction. Morphologic remodeling at the joint level has been described previously, but a new finding is the longitudinal plasticity of the transplanted fibula observed in the case of surgical radioulnar synostosis with a follow-up of more than 3 years. In fact, in that case the total resection of the radius and of the interosseous membrane suggested a bone fixation of the fibula end-to-side to the ulna with an angle of approximately 35 degrees. Because of the orientation of the neoradius, it might have been reasonable to suspect that at the distal radioulnar joint level, the two bones could diverge as long as the growth proceeded. Conversely, after 3 years, the space between the fibular and ulnar epiphysis did not increase due to progressive bowing of the fibula ulnarward (Fig. 6). This adaptive behavior provided a stable wrist joint with a very acceptable range of motion of 40 degrees in extension and 50 degrees in flexion and even preservation of some degree of pronation and supination.
Figure 6.
Although a progressive divergence of the neoradius and ulna might be expected as long as longitudinal growth occurs, the plasticity of the immature bone, governed by the biomechanical input, allowed for longitudinal remodeling. A progressive bowing ulnarward of the neoradius prevented an excessive opening of the space between the two bones and following possible functional impairment. Instead of diverging during growth, the slight ulnarward bowing of the neoradius provided a more stable joint closing the space between the radius and ulna.
The worst functional and aesthetic outcome occurred in the case where both radius and ulna were involved in the tumor resection. In this case, the fibula was fixed by metal plate to the proximal residual stump of the ulna with the aim to provide a one-bone forearm. Although the growth of the neoradius has been satisfactory, a progressive wrist instability and radial deviation has been observed a few years after surgery (Fig. 7). Without the adjacent ulna, the attempt to stabilize the joint with the residual capsule and a strip of biceps femoris tendon was not enough, and this patient is a candidate for radiolunate arthrodesis, which should correct the ulnar deviation without interfering with the midcarpal joint motion.
Figure 7.
(A) In case of resection of both radius and ulna, (B) the wrist joint is not stable enough to prevent a radial deviation, which in this patient gradually occurred at medium term as seen on the radiograph. This patient is a candidate for radiolunate arthrodesis.
CONCLUSION
A vascularized transfer of the proximal epiphysis of the fibula, along with a variable amount of the adjoining diaphysis, is probably the most effective surgical option in dealing with upper-limb bone defects that involve the distal radius in children. The key feature of such a procedure is the ability to reconstruct the bone loss while simultaneously restoring the growth potential. The feasibility of the technique depends both on adequate blood supply to the growth plate and to the diaphysis: A failure in revascularization actually leads to premature fusion of the growth plate and possible nonunion with the host bone.
Current indications for vascularized epiphyseal transfer include trauma, tumor, and congenital disorders involving the growth plate of a long bone in children. The proximal humerus and the distal radius may be optimally reconstructed according to such a procedure, but the technique has been occasionally used also in cases of custom-made reconstruction of lower-limb joints such as the hip and the knee. Appropriate selection of the patients and a meticulous surgical technique are the prerequisites for a successful outcome.
REFERENCES
- Innocenti M, Ceruso M, Manfrini M, et al. Free vascularized growth-plate transfer after bone tumor resection in children. J Reconstr Microsurg. 1998;14:137–143. doi: 10.1055/s-2007-1000157. [DOI] [PubMed] [Google Scholar]
- Innocenti M, Delcroix L, Romano G F. Epiphyseal transplant: harvesting technique of the proximal fibula based on the anterior tibial artery. Microsurgery. 2005;25:284–292. doi: 10.1002/micr.20130. [DOI] [PubMed] [Google Scholar]
- Innocenti M, Delcroix L, Manfrini M, et al. Vascularized proximal fibular epiphyseal transfer for distal radial reconstruction. J Bone Joint Surg Am. 2005;87(Suppl 1 Pt 2):237–246. doi: 10.2106/JBJS.E.00295. [DOI] [PubMed] [Google Scholar]
- Wenger H L. Transplantation of epiphyseal cartilage. Arch Surg. 1954;50:148–151. [Google Scholar]
- Wilson J N. Epiphyseal transplantation. A clinical study. J Bone Joint Surg Am. 1966;48:245–256. [PubMed] [Google Scholar]
- Bowen V, O'Brien B M. Experimental study of the microsurgical transfer of growth plates. Can J Surg. 1984;27:446. [Google Scholar]
- Bowen V. Experimental free vascularized epiphyseal transplants. Orthopedics. 1986;9:893–898. doi: 10.3928/0147-7447-19860601-16. [DOI] [PubMed] [Google Scholar]
- Donski P K, Carwell G R, Sharzer L A. Growth in revascularized bone grafts in young puppies. Plast Reconstr Surg. 1979;64:239–243. doi: 10.1097/00006534-197908000-00017. [DOI] [PubMed] [Google Scholar]
- Donski P K, O'Brien B. Free microvascular epiphyseal transplantation: an experimental study in dogs. Br J Plast Surg. 1980;33:169–178. doi: 10.1016/0007-1226(80)90007-7. [DOI] [PubMed] [Google Scholar]
- Boyer M I, Bray P W, Bowen C V. Epiphyseal plate transplantation: an historical review. Br J Plast Surg. 1994;47:563–569. doi: 10.1016/0007-1226(94)90141-4. [DOI] [PubMed] [Google Scholar]
- Nettelblad H, Randolph M A, Weiland A J. Physiologic isolation of the canine proximal fibular epiphysis on a vascular pedicle. Microsurgery. 1984;5:98–101. doi: 10.1002/micr.1920050210. [DOI] [PubMed] [Google Scholar]
- Tsai T M, Ludwig L, Tonkin M. Vascularized fibular epiphyseal transfer. A clinical study. Clin Orthop Relat Res. 1986;210:228–234. [PubMed] [Google Scholar]
- Pho R W, Patterson M H, Kour A K, et al. Free vascularised epiphyseal transplantation in upper extremity reconstruction. J Hand Surg [Br] 1988;13:440–447. doi: 10.1016/0266-7681_88_90175-1. [DOI] [PubMed] [Google Scholar]
- Zhong-Wei C, Guang-Jian Z. In: Pho RW, editor. Microsurgical Technique in Orthopaedics. London, UK: Butterworths; 1988. Epiphyseal transplantation. pp. 121–127.
- Bonnel F, Lesire M, Gomis R, et al. Arterial vascularization of the fibula microsurgical transplant techniques. Anat Clin. 1981;3:13–23. [Google Scholar]
- Restrepo J, Katz D, Gilbert A. Arterial vascularization of the proximal epiphysis and the diaphysis of the fibula. Int J Microsurg. 1980;2:48–51. [Google Scholar]
- Taylor G I, Wilson K R, Rees M D, et al. The anterior tibial vessels and their role in epiphyseal and diaphyseal transfer of the fibula: experimental study and clinical applications. Br J Plast Surg. 1988;41:451–469. doi: 10.1016/0007-1226(88)90001-x. [DOI] [PubMed] [Google Scholar]
- Innocenti M, Ceruso M, Delcroix L. In: F. Schuind, editor. Advances in Upper and Lower Extremity Microvascular Reconstructions. Singapore: World Scientific; 2002. Vascularized epiphyseal transfer in upper limb skeletal reconstruction in children. Indications and operative technique. pp. 90–101.
- Innocenti M, Delcroix L, Manfrini M, et al. Vascularized proximal fibular epiphyseal transfer for distal radial reconstruction. J Bone Joint Surg Am. 2004;86:1504–1511. doi: 10.2106/00004623-200407000-00021. [DOI] [PubMed] [Google Scholar]