ABSTRACT
Limb salvage has now replaced amputation as the standard treatment for primary bone tumors and can usually be offered to more than 85% of patients. Recently, a novel approach to limb salvage has been described by Capanna and colleagues whereby a massive bone allograft and intramedullary vascularized fibula are combined to reconstruct large, segmental bone defects. This produces a structurally competent reconstruction with enhanced vascular and osteogenic capabilities and the potential to achieve lower rates of infection, fracture, and nonunion. The Capanna technique has become a well-established means of long bone reconstruction and limb salvage in cases of large bony resection. The operative technique and reported outcomes of the Capanna technique are reviewed, and cases are presented.
Keywords: Intramedullary vascularized fibula, segmental bone reconstruction, massive allograft, Capanna technique, limb salvage
Significant advances in neoadjuvant chemotherapy protocols have led to major improvements in the treatment of primary bone tumors over the past three decades. Long-term survival rates have improved from 20% to more than 60% for osteosarcoma, and simultaneously surgical techniques for lower-limb salvage have evolved allowing most patients to avoid amputation without compromising oncological clearance.1
Massive allografts are widely used in long bone reconstruction often achieving good results but are associated with a significant set of complications including infection, nonunion, and fracture.2,3 The vascularized fibula graft is the most popular vascularized bone graft used in intercalary reconstructions of the femur and tibia, providing a well-perfused and osteogenic alternative but lacking the structural properties of allografts. Although hypertrophy of the transferred fibula gradually improves its strength, this reconstruction is prone to early fracture and often requires prolonged periods of immobilization of the affected limb.
Recently, a novel approach to limb salvage has been described by Capanna and colleagues4 who have also since reported their medium- and long-term results.5,6 This innovative technique combines a vascularized fibula graft with a conventional massive allograft to reconstruct large defects of the femur and tibia after oncologic resection. Combining the advantages of the separate components produces a structurally competent reconstruction with enhanced vascular and osteogenic capabilities with the potential to achieve lower rates of infection, fracture, and nonunion.
SURGICAL TECHNIQUE
We have found the Capanna technique to work well for femoral, humeral, or tibial reconstructions. After tumor resection, allograft is cut to match the resection defect. The fibula is then harvested from the contralateral limb in the standard fashion through a lateral approach. The fibula's vascular pedicle is based on the peroneal artery with its venae comitantes, providing both an endosteal nutrient artery to the medullary canal of the fibula as well as periosteal branches along its course that supply the cortical surface. Several authors have described the surgical technique of free fibula harvest in detail.7,8
In adults, a 24- to 26-cm segment of fibula can be harvested, leaving the fibula head and 6 cm of the distal part of the bone intact to ensure knee and ankle stability. The length of the fibular flap to be harvested should be kept at least 4 to 6 cm longer than the allograft, and the separate components are then combined either by intramedullary placement of the fibula or by bridging the allograft reconstruction externally in a parallel fashion.
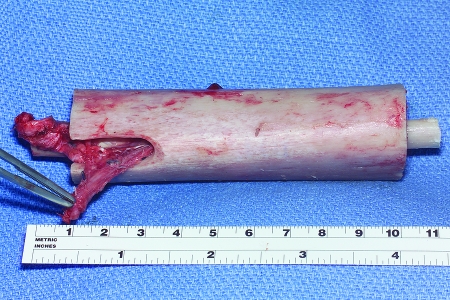
For intramedullary placement, the medullary canal of the allograft is enlarged with a reamer to allow sufficient room for the fibula. The free fibular flap is then passed through the intramedullary canal, and the fibular vessels are brought through a window created in the allograft with a burr (Fig. 1). A temporary K-wire is used to transfix the fibula to the allograft to prevent rotation during placement of the graft. The allograft and fibular construct are then slotted into both ends of the host bones, and the fibula acts as an intramedullary rod with a 1- to 2-cm portion extending into the proximal and distal host bone. The construct is secured with either plates and screws or with screws alone, and the microvascular anastomoses are performed after bony fixation, usually in an end-to-side fashion to the femoral vessels when reconstruction is performed in the thigh or to the posterior or anterior vessels when performing tibial reconstruction (Fig. 2).
Figure 1.
Figure illustrates the typical Capanna construct. The free fibular graft has been placed within the intramedullary canal of the allograft. The fibula is allowed to extend 1 to 2 cm proximal and distal to the end of the allograft, allowing the fibula to function as a vascularized intramedullary rod, adding to stability of the final construct. A trough is cut into the allograft through which the vessels are passed for microanastomosis.
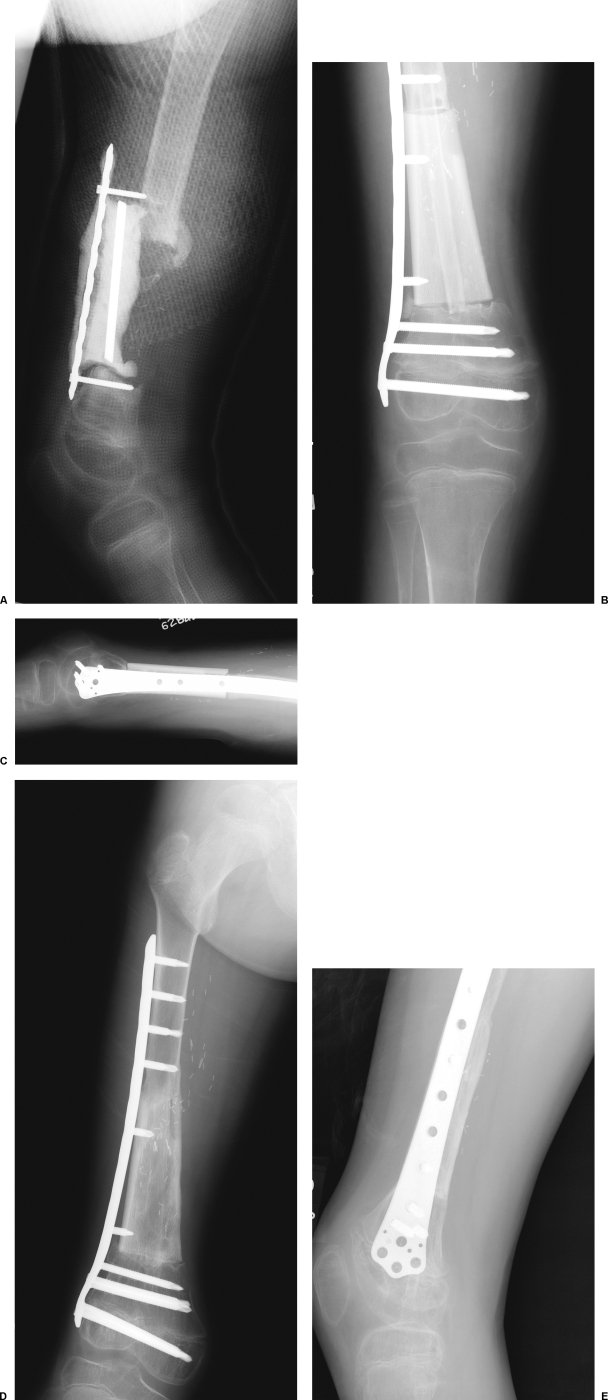
Figure 2.
A case example of a 6-year-old boy who presented after Ewing's tumor resection and reconstruction at an outside institution using femoral allograft. The allograft reconstruction became infected and was replaced with an antibiotic spacer. (A) A lateral radiograph of leg after failed initial treatment. The antibiotic spacer has migrated due to hardware failure. (B) The spacer is removed and reconstructed by Capanna technique with the construct shown in Fig. 1. Fixation is achieved with the use of a laterally placed plate. (D, E) Excellent incorporation of both the fibula and allograft are seen 4 months postoperatively.
This approach has been described for reconstruction of both femoral and tibial defects but is particularly suitable in tibial reconstructions where there is little room in the skin envelope for anything more than an anatomic reconstruction. In cases where there is also a skin defect, a skin paddle can be harvested with the fibula to allow for simultaneous soft tissue reconstruction (Fig. 3). The perforators for the skin paddle are brought through the trough created in the allograft.
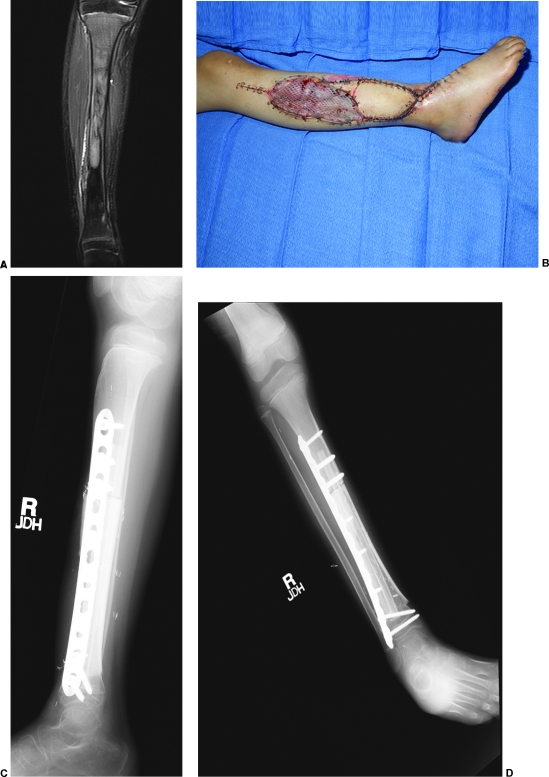
Figure 3.
(A) A case example of a 7-year-old girl with a Ewing's sarcoma identified on magnetic resonance imaging. The patient had undergone previous biopsy at an outside institution. Tumor resection required large skin excision necessitating need for additional soft tissue coverage in addition to Capanna reconstruction. The fibula graft was harvested with a large skin paddle to allow for additional soft tissue coverage. The fibula was placed within the allograft, and a trough was created to allow for the peroneal vessels and skin perforating vessels to exit the allograft. (B) Appearance of the leg after reconstruction. (C, D) Anteroposterior and lateral radiographs demonstrating bony reconstruction.
Extramedullary placement of the fibula is another option for reconstruction in these difficult cases; however, it is usually reserved for femoral diaphyseal or metadiaphyseal reconstructions where the surrounding soft tissue envelope is capable of hosting a more bulky bony reconstruction. The allograft is left intact and used to fill the femoral defect, and the fibula is used to bridge the allograft and both osteotomies on their medial surface. The reconstruction is completed by fixation with a laterally placed plate or intramedullary rod placement and tension banding of the fibula. Extramedullary placement has some potential benefits over the intramedullary approach. It avoids any possible weakening that may occur from creating a trough within the allograft. It also allows the use of a locked intramedullary nail for femoral fixation (Fig. 4). In addition, the medial positioning of the fibula places it within the mechanical axis of the femur and closer to branches of the profunda femoris, which can be used as recipient vessels.
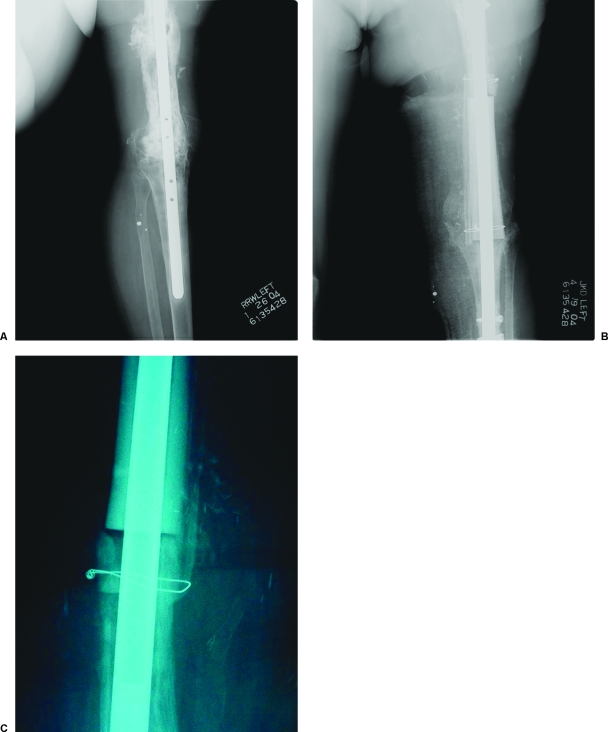
Figure 4.
A 54-year-old woman presented with an infection of her allograft after femoral reconstruction with knee fusion. After 6 weeks of antibiotic therapy, the allograft was removed and replaced. The new allograft was augmented with use of a vascularized fibular graft applied along the medial aspect of the femur. Good incorporation of the fibula can be seen at the distal osteosynthesis site 4 months after surgery.
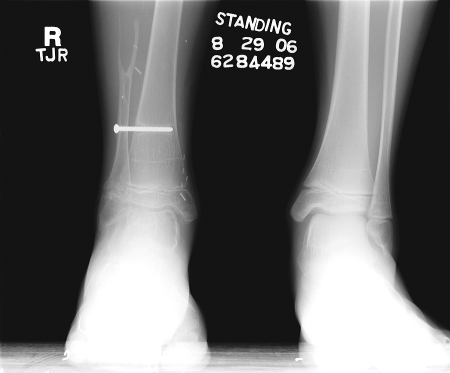
The fibula donor site is closed primarily, and in children or in patients where the distal fibular osteotomy is very close to the ankle joint, a distal tibiofibular syndesmosis should be performed to prevent a valgus deformity of the ankle (Fig. 5).
Figure 5.
Radiograph of the late appearance of a fibular donor site showing syndesmosis screw. Syndesmosis of the ankle after harvest of the fibula prevents the development of ankle instability in the pediatric population.
OUTCOMES
The scarcity of outcomes studies relating to this procedure reflects the originality of this innovative approach; however, all reports to date indicate that favorable outcomes are likely when these two commonly used reconstructive techniques are combined. The procedure of combining a massive allograft with a vascularized fibula was first presented by Capanna et al at international meetings, and the group subsequently published the technique and their midterm results.5 Most recently, they have also reported their long-term outcomes.6 The report describes the treatment of 90 patients over a 14-year period who underwent reconstruction of large segmental femoral or tibial defects, with an average follow-up period of 9 years. The overall success rate of the reconstruction was 93.5%. Twenty-eight percent of patients had a complication requiring an average of one further operation. Complications included infection (7.5%), nonunion (8.8%), and fracture (13.3%), and only one patient underwent an amputation secondary to a failed reconstruction. Functional outcomes of the successful reconstructions were evaluated according to the Musculoskeletal Tumor Society (MSTS) system: 72% achieved an excellent result, 20% a good result, 5% a fair result, and 3% a poor result. In an earlier study of 52 cases by the same group, Ceruso et al found that the mean time to union was 2.7 months for the fibular component and 8 months for the allograft component of the reconstruction. Average time to full weight-bearing was 13.7 months.5
Chang and Weber studied their results in 14 cases: 6 procedures for immediate reconstruction of intercalary defects, and 8 procedures performed for revision of allograft nonunions.9 All patients who underwent immediate reconstructions achieved bony union and full unrestricted use of the limb at an average time of 6 months postoperatively (range, 3 to 8 months). Similarly, 86% of procedures done for allograft nonunion resulted in bone healing at an average time of 10 months; however, the time taken to achieve full unrestricted use of these limbs was significantly longer (average 28 months; range, 13 to 45 months).
Moran et al have also reported their use of this technique in 7 children with an average age of 10.5 years.10 All patients achieved excellent or good functional outcomes and were able to return to strenuous physical activity such as karate and basketball. There were no cases of infection, average time to primary union was 9 months, and although there were two reported late allograft fractures, both of these healed and the patients returned to baseline functional status. All children reconstructed had good to excellent Mankin functional outcome scores.
The fate of the transferred fibula within the allograft shell is currently under investigation. Vascularized fibula grafts are known to undergo hypertrophy after transfer. The degree of hypertrophy is variable, and it is a gradual process that takes several years, slowly improving the graft's mechanical properties (Fig. 2). Manfrini et al15 performed serial radiographs and computed tomography scans on 24 patients who had undergone reconstruction of intercalary tibial or femoral defects by Capanna's technique and found three patterns of behavior. In 13 patients, the allograft maintained its architecture without fracturing and the fibula gradually enlarged and integrated into the allograft. In eight patients, fracture or nonunion of the allograft occurred, and the fibula reacted with rapid dense hypertrophy inducing bony bridges to the allograft. In the final three patients in the study, the fibula did not undergo any hypertrophic changes following which fractures with no evidence of callus formation were seen. The latter was interpreted as unsuccessful vascularization of the autograft. Bony fusion between the allograft and autograft was evident within 2 years for at least 75% of the cases.
DISCUSSION
Limb salvage has now replaced amputation as the standard treatment for primary bone tumors and can usually be offered to more than 85% of patients. Relative contraindications to limb salvage include involvement of the major neurovascular bundles, poor response to neoadjuvant chemotherapy, extensive soft tissue involvement, the presence of severe infection, and pathologic fracture causing a hematoma which violates the oncological compartment.11 The two most important principles that must be adhered to when considering limb salvage are that (1) survival rates should be no worse than those associated with amputation, and (2) the reconstructed limb must provide satisfactory function.
The first of these conditions has been the subject of many comparative studies, and several authors have been able to show that the oncologic outcome of limb-sparing surgery rivals or sometimes even surpasses that of amputation, especially when patients with poor prognostic features are excluded. Rougraff et al reviewed 227 patients who underwent treatment for osteosarcoma of the distal femur and found no statistically significant difference in local recurrence rates, the duration of postoperative disease-free period, or survival rates between limb salvage procedures and amputation.12 Picci et al also reported on a series of 355 patients treated for high-grade osteosarcoma and found that 8% of patients who underwent amputation and only 3% who had limb-sparing surgery with wide margins developed local recurrence. The rate of recurrence in patients with marginal margins was 29%.13 The same group has recently described its 27 year experience of 1148 patients treated for osteosarcoma.1 Paradoxically, the 5-year event-free survival for patients undergoing limb-sparing procedures was significantly higher than for those who had amputations (61% vs. 53%, p < 0.001), and there was no statistically significant difference in local recurrence between the two groups; multivariate analysis confirmed that the only factors with any prognostic significance were type of chemotherapy, histologic response to chemotherapy, tumor volume, and serum alkaline phosphatase levels.
Studies such as these have shown that limb-sparing surgery can certainly be an oncologically sound alternative to radical surgery such as amputation, hip disarticulation, and rotationplasty. The responsibility of the reconstructive surgeon is thus to fulfill the second of the principles previously mentioned—to provide a maximally functional limb reconstruction. As the majority of extirpations do not necessitate resection of the major neurovascular structures and the most of the muscular compartments are also usually spared, restoration of bone mass and articular surface is the critical determinant of limb function in these cases. The ideal reconstruction would be a good anatomic match to the defect, mechanically competent to prevent fractures, uniting quickly into the host bone (and not loosen), resisting infection, and providing a long-term solution that does not degenerate with time and use. In children, continued limb growth to avoid future limb-length discrepancies is also highly desirable.
Common approaches to long bone reconstruction include use of massive bone allografts, endoprostheses, vascularized fibula grafting, and distraction osteogenesis, each having a different complication profile. Allograft use has been studied extensively and can be complicated by infection, nonunion, and fracture, all of which are related to their avascular nature. Mankin et al reviewed 818 massive cadaveric allografts procedures involving the extremity.2 They primarily studied graft survival and long-term functional outcomes and as such only reported the complications of the 718 patients who had greater than 2-year follow-up. Nonunion occurred in 17% of cases, fracture in 19%, and infection in 11%, and these complications had a significant effect on long-term functional outcome. Furthermore, 16% of osteoarticular grafts required knee arthroplasty after an average time of 5 years. Endoprostheses can also carry a significant rate of infection, and the added problems of implant loosening and failure, which is often encountered in this functionally demanding patient population.
The biological profiles of a vascularized fibula and a structural allograft complement each other, with immediate structural strength provided by the allograft and the potential for osteogenesis provided by the vascularized fibula. Despite this, some of the usual complications of bone reconstruction will clearly still arise and are apparent from the reported series. Although most studies are not large enough to consider analysis of statistical significance, some apparently intuitive trends can be identified. Capanna's large series recognized that infections were more prevalent in tibial reconstructions (8.5%) than in femoral reconstructions (6%), and the same was also the case for nonunion rates (10.5% vs. 6%). The opposite was true for allograft fractures, which occurred in 18% of femoral reconstructions and 10.5% of tibias. In Moran's series of pediatric patients, the only cases of allograft nonunion both occurred in patients who underwent postoperative chemotherapy, despite the presence of well-vascularized intramedullary fibulas grafts in both patients. In Chang's series, an allograft nonunion occurred when the intramedullary fibula was too short to span both osteotomies. As may be expected, one osteotomy healed well, and the other required placement of a second vascularized fibula at a later date. Despite these problems, limb salvage rates have been excellent.
The Capanna technique has become a well-established means of long bone reconstruction and limb salvage in cases of large bony resection. Further prospective comparative studies will be required to further delineate the role of the Capanna technique in defects of less than 5 cm. This method of reconstruction should be considered in any patient undergoing bony tumor extirpation within the extremities
REFERENCES
- Bacci G, Longhi A, Fagioli F, Briccoli A, Versari M, Picci P. Adjuvant and neoadjuvant chemotherapy for osteosarcoma of the extremities: 27 year experience at Rizzoli Institute, Italy. Eur J Cancer. 2005;41:2836–2845. doi: 10.1016/j.ejca.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Mankin H J, Gebhardt M C, Jennings L C, Springfield D S, Tomford W W. Long-term results of allograft replacement in the management of bone tumors. Clin Orthop Relat Res. 1996;324:86–97. doi: 10.1097/00003086-199603000-00011. [DOI] [PubMed] [Google Scholar]
- Ortiz-Cruz E, Gebhardt M C, Jennings L C, Springfield D S, Mankin J H. The results of transplantation of intercalary allografts after resection of tumors. A long-term follow-up study. J Bone Joint Surg Am. 1997;79:97–106. doi: 10.2106/00004623-199701000-00010. [DOI] [PubMed] [Google Scholar]
- Capanna R, Bufalini C, Campanacci C. A new technique for reconstructions of large metadiaphyseal bone defects: a combined graft (allograft shell plus vascularized fibula) Orthop Traumatol. 1993;2:159–177. [Google Scholar]
- Ceruso M, Falcone C, Innocenti M, Delcroix L, Capanna R, Manfrini M. Skeletal reconstruction with a free vascularized fibular graft associated to bone allograft after resection of malignant bone tumors of limbs. Handchir Mikrochir Plast Chir. 2001;33:277–282. doi: 10.1055/s-2001-16597. [DOI] [PubMed] [Google Scholar]
- Capanna R, Campanacci D A, Belot N, et al. A new reconstructive technique for intercalary defects of long bones: the association of massive allograft with vascularized fibular autograft. Long-term results and comparison with alternative techniques. Orthop Clin North Am. 2007;38:51–60. doi: 10.1016/j.ocl.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Stevanovic M, Gutow A P, Sharpe F. The management of bone defects of the forearm after trauma. Hand Clin. 1999;15:299–318. [PubMed] [Google Scholar]
- Bishop A T. In: Green DP, Hotchkiss RN, Pederson WC, editor. Green's Operative Hand Surgery. Philadelphia, PA: Churchill Livingstone; 1999. Vascularized bone grafts.
- Chang D W, Weber K L. Use of a vascularized fibula bone flap and intercalary allograft for diaphyseal reconstruction after resection of primary extremity bone sarcomas. Plast Reconstr Surg. 2005;116:1918–1925. doi: 10.1097/01.prs.0000189203.38204.d5. [DOI] [PubMed] [Google Scholar]
- Moran S L, Shin A Y, Bishop A T. The use of massive bone allograft with intramedullary free fibular flap for limb salvage in a pediatric and adolescent population. Plast Reconstr Surg. 2006;118:413–419. doi: 10.1097/01.prs.0000227682.71527.2b. [DOI] [PubMed] [Google Scholar]
- DiCaprio M R, Friedlaender G E. Malignant bone tumors: limb sparing versus amputation. J Am Acad Orthop Surg. 2003;11:25–37. [PubMed] [Google Scholar]
- Rougraff B T, Simon M A, Kneisl J S, Greenberg D B, Mankin J H. Limb salvage compared with amputation for osteosarcoma of the distal end of the femur. A long-term oncological, functional, and quality-of-life study. J Bone Joint Surg Am. 1994;76:649–656. doi: 10.2106/00004623-199405000-00004. [DOI] [PubMed] [Google Scholar]
- Picci P, Sangiorgi L, Rougraff B T, Neff J R, Casadei R, Campanacci M. Relationship of chemotherapy-induced necrosis and surgical margins to local recurrence in osteosarcoma. J Clin Oncol. 1994;12:2699–2705. doi: 10.1200/JCO.1994.12.12.2699. [DOI] [PubMed] [Google Scholar]
- Enneking W F, Spanier S S, Goodman M A. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980;153:106–120. [PubMed] [Google Scholar]
- Manfrini M, Vanel D, De Paolis M, Malaguti C, Innocenti M, Ceruso M, Capanna R, Mercuri M. Imaging of vascularized fibula autograft placed inside a massive allograft in reconstruction of lower limb bone tumors. AJR Am J. Roentgenol. 2004;182:963–970. doi: 10.2214/ajr.182.4.1820963. [DOI] [PubMed] [Google Scholar]