ABSTRACT
Osteomyelitis of the craniofacial skeleton closely resembles osteomyelitis elsewhere in the body in its pathophysiology and medical management; subsequent reconstruction after debridement remains distinctly challenging. The goals of reconstruction must include the restoration of the complex and readily visible morphology of the cranium and face, as well as the adequate return of vital sensory, expressive, and digestive functions. In this article, the various reconstructive modalities will be discussed including pedicled and nonpedicled flaps with or without an osseous component, nonvascularized bone grafts, alloplastic implants, and bone regeneration using protein therapy. Although reconstruction of craniofacial defects after osteomyelitis commonly proves formidable, the satisfactory return of form and function remains a plausible reconstructive goal.
Keywords: Osteomyelitis, reconstruction, craniofacial, skull, mandible, midface, head and neck
Osteomyelitis denotes an infection of the bone and marrow.1 Although relatively rare by comparison, osteomyelitis of the craniofacial skeleton is physiologically similar to osteomyelitis elsewhere in the body. The major differences stem from the close proximity of critical neurovascular structures, which makes obtaining an adequate debridement problematic at best. Extensive reactive granulation tissue and inflammation significantly increase the risk of damage to these structures, often immediately subjacent to the infected site. Nonetheless, the complete removal of all infected and devitalized soft tissue and bone remains the obligatory first step in treatment, often requiring the creation of capacious defects. Moreover, even laymen are keenly aware of very subtle abnormalities of the cranium and face. Accurately re-creating the intricate and readily visible morphology of the craniofacial skeleton, while also providing durable protection of the underlying structures, remains a formidable task.
RECONSTRUCTION OF THE CRANIOFACIAL SKELETON
Calvarium
Infection of the calvarium most commonly results after surgical manipulation, occurring in up to 2.5 to 6.5% of calvarial reconstructions.2,3 This is consistent with the neurosurgical data, as a meta-analysis of more than 2000 post-craniotomy patients estimated the collective infection rate to be 2% with antibiotics and 8% without antibiotics.4 Although relatively low, this infection rate is significant in light of the sheer volume of patients who undergo calvarial surgery. Even so, the close postoperative surveillance after these procedures in conjunction with current antibiotic efficacy in the acute setting allows the majority of these calvarial surgical site infections to be successfully eradicated. In contrast with these superficial surgical site infections, true osteomyelitis of the frontal bone most commonly occurs via contiguous spread from the adjacent sinuses, especially the frontal sinus.5,6,7
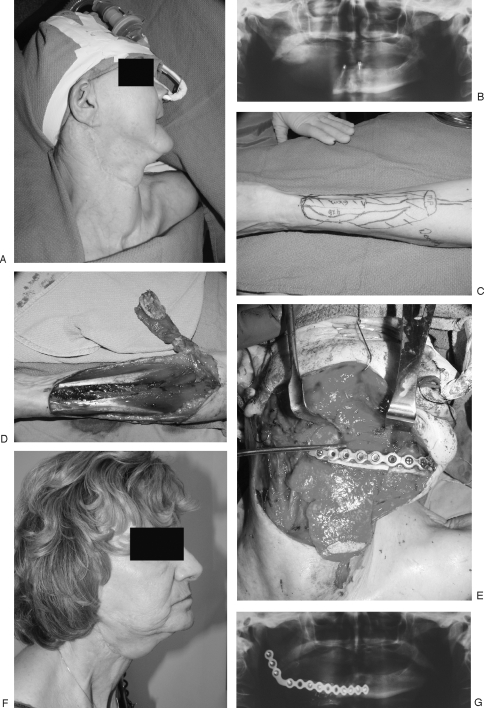
In reconstructing these defects, split calvarial bone grafts (CBGs) most closely obey the reconstructive maxim of replacing like with like. Since the initial description of large nonvascularized outer table calvarial bone grafts in the early 20th century, their use has become considerably more common and remains today the “gold standard” of skull reconstruction. The calvarial donor site provides a dependable source of bone graft of acceptable malleability and strength. Although useful for reconstruction of many craniofacial defect sites, restoring the gently curving contour of calvarial bone is readily facilitated through use of inner or outer table grafts from unaffected calvarium, adjacent to the defect site. In a recent review of 9650 cases employing split calvarial bone grafts, the reported complication rate was 0.25%; the authors concluded that the calvarial vault is the preferred source of bone graft for use in cranial and facial skeletal reconstruction8 (Fig. 1).
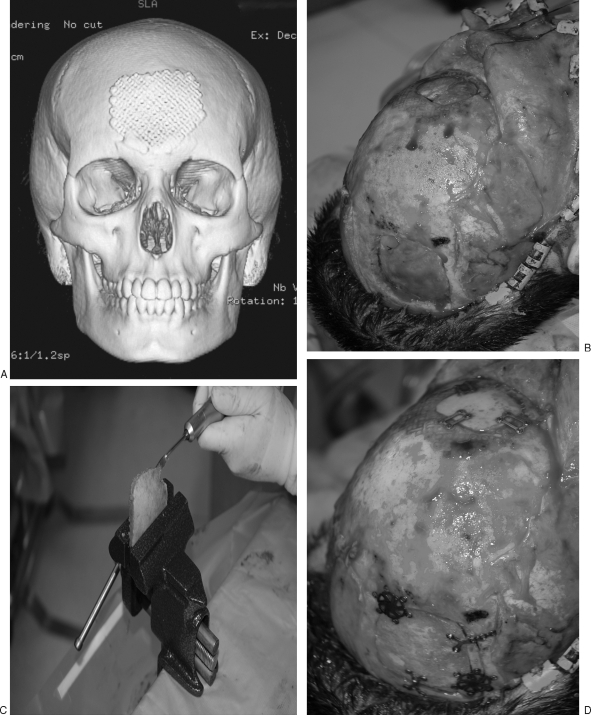
Figure 1.
(A) CT scan of frontal bone defect from Pott's puffy tumor, temporarily repaired with titanium mesh. (B) Frontal bone defect (top of image); harvested full-thickness calvarial graft from parietal bone (bottom of image). (C) Full-thickness calvarial graft in process of being split with saw. (D) Outer table graft repairing frontal defect (top of image); inner table graft replaced orthotopically (bottom of image).
Unfortunately, autogenous bone is sometimes unavailable. Such instances include the pediatric patient who has not yet developed an adequate diploic space amenable to split grafts, and the patient who may have failed primary reconstruction of large defects. In such cases, alloplastic implants provide a viable alternative, though more so in adults. They include hydroxyapatite, methylmethacrylate, porous polyethylene (Medpor; Porex Surgical, College Park, GA), bioactive glass, demineralized bone, and titanium mesh, among others.9 The major drawback to most alloplastic implants results from poor tissue compatibility and a lack of growth potential, especially problematic in children. Although alloplastic materials initially bring a welcomed rigidity to the reconstruction and provide lasting protection to the brain against external loads, the static implants may eventually become a significant restriction to craniofacial growth and development for the same reasons.
One of the first alloplastic materials to become widely available was the acrylic, methylmethacrylate. It is of low cost, readily available, and is rapidly resistant to compressive forces after hardening. Its major drawbacks stem from a near-complete lack of postoperative resorption or osseous integration. This may increase the risks of extrusion and infection, although several studies have reported a relatively low infection rate, ranging from no instances in a 42-patient series10 up to 5%.9 When used in the treatment of osteomyelitis, it also permits the addition of various antibiotic powders to the mix as it is being made. Vancomycin and aminoglycosides such as gentamicin and tobramycin are most commonly chosen because of their wide spectrum and heat stability (polymethylmethacrylate polymerization is a highly exothermic process); penicillins, cephalosporins, and erythromycin have also been used with success.11 Hydroxyapatite and bioactive glass have both been posited to allow tissue in-growth, replacement by living bone and to provide a scaffold that is at least osteoconductive, if not osteoinductive, though current data are inconclusive.9
Titanium mesh was originally developed to treat the broad range of maxillofacial injuries incurred during the Vietnam War. It proved to be a simple and readily adaptable method of semirigid fixation (Fig. 1A). Initial concerns that titanium mesh might increase the risks of infectious complications seem to be unfounded in light of several reports where removal was required in less than 2% of cases.12 In a retrospective review of 66 patients who underwent cranioplasty using a titanium prosthesis, no titanium plates had to be removed due to infection, even though 65% of the defects were greater than 100 cm2 and 45% of plates were used to replace infected bone flaps.13 Some studies have reported much higher infection rates, though, evidenced by a review of 88 patients undergoing craniofacial reconstruction using titanium mesh after oncologic resection where the overall incidence of exposure and infection was 6% and 1%, respectively.14 Although the authors believed these rates may have been inflated due to the majority of their patients having received chemotherapy and/or radiation, other studies have found similar infection rates to report. In a much larger report of more than 2659 instances of cranial reconstructions using metal plates, including titanium, the overall infection rate was 7.4% and the exposure rate was 3.3%.15 Titanium mesh also avails itself to the concomitant use of other alloplastic implants such as demineralized bone and methylmethacrylate, among others, to add bulk and improve contour.14,16 It may even be preoperatively fashioned using radiographic imaging to fit the specific defect of the patient.17,18 Use of metallics in children has been reported to be potentially problematic as the centrifugal growth of the outer calvarial table, in conjunction with resorption occurring along the deep aspect of the inner table, may result in an relative migration of the plate inwardly.19,20
Porous polyethylene (Medpor) has been used frequently for customized reconstruction of cranial defects and facial augmentations in patients in whom no further skull growth is anticipated9 (Fig. 2). It is a relative newcomer to craniofacial reconstruction and has several properties that differentiate it from other alloplastic materials. It may be individually prefabricated based on computed tomography scans, it is durable though somewhat flexible, and it may be carved intraoperatively to better tailor it to the desired dimension without decreasing its structural integrity. Its greatest attribute is that it is highly compatible with native tissue, as it is carbon based and possesses a specific porosity that allows significant bone and soft tissue ingrowth.9 This characteristic is not always beneficial though, as the tissue ingrowth thought to decrease infection, migration, and extrusion also makes these implants very difficult to remove.
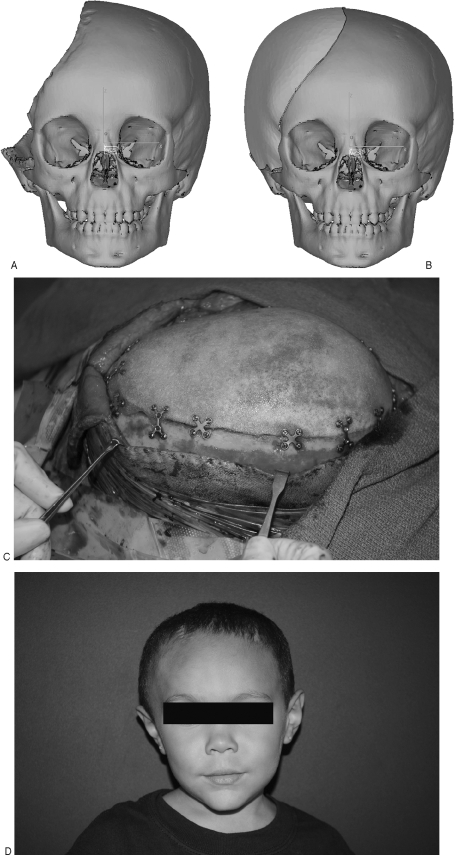
Figure 2.
(A) Near hemi-calvarial defect in young child. (B) Custom-made porous polyethylene implant. (C) Calvarial implant rigidly fixed in place. (D) Postoperative result.
Lateral Skull Base and Temporal Bone
Osteomyelitis of the temporal bone and lateral skull base may certainly occur as a complication of surgical manipulation; however, it most frequently results secondarily to the unchecked progression of malignant otitis externa or chronic suppurative otitis media,21,22 especially when severe enough to cause mastoiditis.23 Accumulating purulence and inflammation cause pressure necrosis of the adjacent bone and soft tissue, eventually propagating diffusely along subbasilar tissue planes and facilitating the initial spread of infection. Treatment is determinedly medical due to the myriad neurovascular structures, usually requiring a protracted courses of broad-spectrum antibiotics. The role of surgery is not very well defined, except for biopsy, drainage of purulence, and local debridement of necrotic tissue. Concern has been expressed that surgical dissection might further open tissue planes, allowing further spread of the infection into the uninvolved surrounding tissue.24 Even so, if the infection proves recalcitrant to medical therapy and local debridement, formal tympanomastoid procedures such as modified radical or radical mastoidectomy with partial petrous apicectomy and embolectomy for jugular vein thrombosis may be considered.24
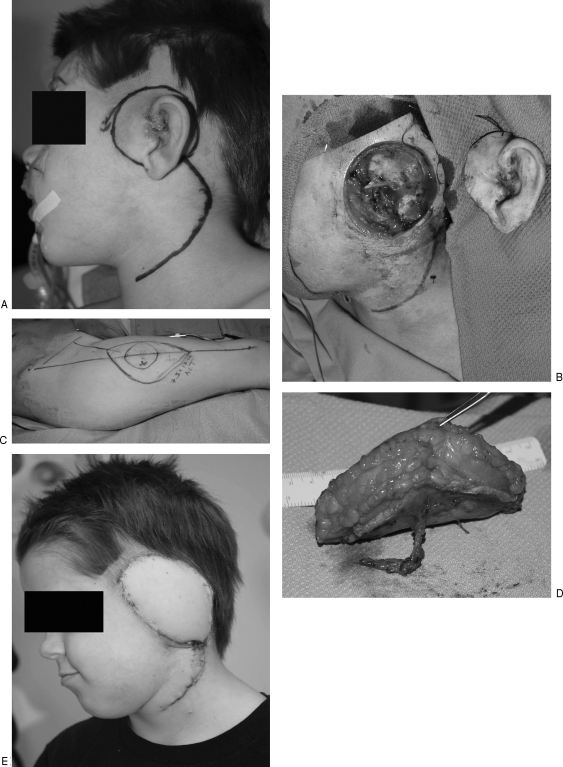
When reconstructing temporal bone and lateral skull base defects, pericranial and temporalis flaps are the workhorses for smaller basicranial defects and may commonly be used in combination with free bone grafts and alloplastic materials.25,26 In a single institution's 16-year review of 182 consecutive cases of craniofacial reconstruction with temporalis muscle flaps (including 12 cranial base reconstructions), a total flap viability rate of 98.4% was reported (Fig. 3). However, 35 cases (19.2%) of transient paresis and 5 cases (2.7%) of permanent facial paralysis of the forehead branch were recorded. In addition, in 19% of all cases, the temporal fossa donor site was obvious enough to warrant reconstruction. In these 35 patients, temporal fossa reconstruction with alloplastic implants was believed to be necessary; and, ultimately, 17% of these patients (6 patients) required an additional procedure to remove the alloplast secondary to persistent infection.27 In another study reviewing cranial base reconstruction in 77 patients, 40 with lateral cranial base defects, the authors report complication rates of 29% and 31% for temporalis flaps and free flaps, respectively. Their data demonstrated that neither the type of reconstruction nor the location of the defect had any significant association with the incidence of complications.28 These data must be interpreted in light of the fact that free tissue transfer is more likely to be used in larger defects, thereby biasing the comparison (Fig. 4).
Figure 3.
(A) Left orbital/skull base defect. (B) Temporalis muscle flap raised. (C) Temporalis muscle flap mobilized into skull base defect. (D) Left orbital/skull base defect filled with temporalis muscle flap.
Figure 4.
(A) Planned resection of temporal skull base lesion. (B) Temporal skull base defect. (C) Anterolateral thigh flap donor site marked. (D) Anterolateral thigh free flap. (E) Postoperative temporal skull defect with anterolateral thigh free flap reconstruction.
Extensive skull base defects often exceed the applicability of regional musculocutaneous flaps, as the distal tip of the pedicled flap with the most tenuous blood is used to fill the defect.29 For large defects involving the skull base, many authors feel that free tissue transfer is the best method of reconstruction25,30,31 (Fig. 4). In one of the larger reviews of purely lateral cranial base reconstruction, 18 patients received free tissue transfer reconstruction. The authors reported a 100% flap survival rate, although the complication rate was 33%, including three minor wound dehiscences, two hematomas, and one successfully treated venous thrombosis. In this study, postoperative adjuvant therapy was not delayed by the occurrence of complication in any patient.29 The authors report the majority of reconstructions used the rectus abdominis free flap. This is because the donor site eliminates the need for repositioning, possesses a long pedicle of reasonable diameter, and allows for a very large skin paddle to be incorporated into the flap. In this study, the mean skin island size was 161 cm2 in the rectus abdominis free flap group.29 Although use of radial forearm free flaps, serratus anterior free flaps, anterolateral thigh free flaps, and latissimus dorsi free flaps has been extensively reported,25,31 the authors concluded that the rectus abdominis is their workhorse free flap for lateral cranial base reconstruction. Although these reports followed oncologic resection, free tissue transfer may be ideal for reconstruction of lateral cranial base defects after osteomyelitis as well because of the benefits of having well-vascularized tissue in a previously infected surgical site.
ORBITS/MIDFACE/MAXILLARY SINUS
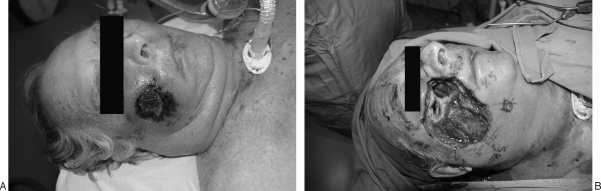
The central location of the orbits and midface permits infectious spread from any of the multiple surrounding structures, especially the adjacent ethmoid and maxillary sinuses. Central craniofacial infections and resulting osteomyelitis may occur secondary to surgery or primarily as sequelae of chronic sinusitis or hematogenous spread.32 Numerous pathogens have been described, including methicillin-resistant Staphylococcus aureus,32 Pseudomonas species,32 Aspergillus species,33 herpes zoster,33 and Mycobacterium tuberculosis.34 One of the most notoriously fatal causes of periorbital and/or midfacial osteomyelitis is caused by fungi belonging to the order Mucorales (Fig. 5). These fungi grow ubiquitously in soil and decaying matter and release large numbers of spores, such that all humans are exposed during daily life.35 The fungi responsible for mucormycosis thrive in acidic, glucose-rich environments with reduced oxygen tension, circumstances typical of diabetic ketoacidosis.33,35 For this reason, mucormycosis generally occurs in the diabetic or immunologically compromised patient, though it has been reported in healthy patients. If diagnosed and treated early, survival is now thought to exceed 80%.33 Often though, treatment is delayed as nonspecific clinical findings are misdiagnosed, resulting in extensive invasion; intracerebral involvement is almost uniformly fatal.32
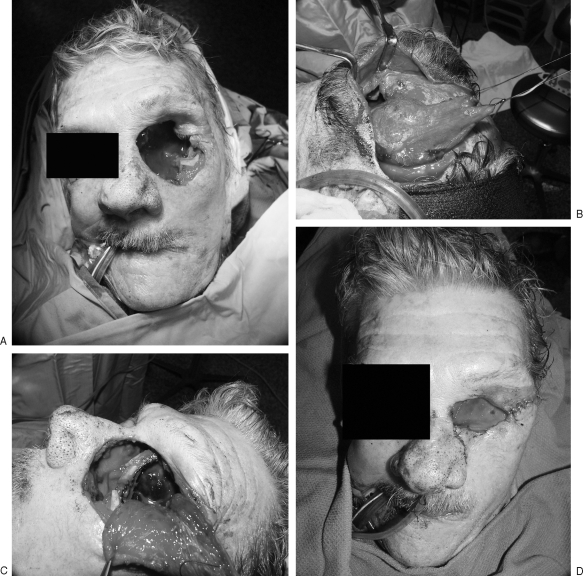
Figure 5.
(A) Mucormycosis infection of the facial soft tissues and maxillary sinus. (B) Staged resection of facial and maxillary sinus mucormycosis. The patient expired secondary to systemic disease prior to reconstruction.
The general goals of reconstruction include obliteration of the defect cavity, restoration of midfacial contours and aesthetics, and restoration of functional mastication, deglutition, and phonation.36 In classifying these defects, multiple systems have been suggested though none is universally accepted.36,37 The common concerns of these proposed classification schemes involve several watershed characteristics of the defect. Defects that involve the inferior maxilla must be evaluated for sufficient viable dentition to support a palatal prosthesis, with their absence making vascularized bone flaps more appealing. In the case of complete maxillectomy defects, the presence of a viable globe as well as adequate infraorbital and zygomatic projection becomes important. Most importantly, great care must be given to the specific proportions of soft tissue, bone, and skin defects, with respect to each other, as different flaps have very different benefits and drawbacks, and no flap is universally applicable36,38,39
A review of complications and toxicities of implantable biomaterials used in facial reconstructive and aesthetic surgery examined more than 200 studies, included 52 cases where porous polyethylene implants were used to reconstruct the orbit; the infection rate was 1.9% with no reported exposures or extrusions.15 In another review of 100 patients where titanium mesh and/or porous polyethylene was used to reconstruct craniofacial defects, the authors report a 7% complication rate, although 4 patients exhibited acute exposure consistent with vascular problems of the flap used for coverage, and 1 with malignant recurrence.14 In another report of 106 orbital reconstructions after primarily fracture or oncologic resection where porous polyethylene with embedded titanium was used, there were no cases of infection or implant exposure.40 One of the largest consecutive series of craniofacial augmentations and/or reconstructions using porous polyethylene included 370 implants in 162 patients, with the vast majority involving the orbit and/or midface. Over an average follow-up of 27 months, no extrusion and a 3% infection rate was reported.41
Proponents of periorbital reconstruction with titanium and/or porous polyethylene after oncologic resection tout the lack of donor-site morbidity, reduced operative time, and better approximation of presurgical craniofacial form. In contrast, the main concern of alloplastic reconstruction after osteomyelitis is that the implant may itself become a nidus of infection if the defect site is inadequately treated. Performing the reconstruction in a separate procedure after infectious resolution would theoretically allow mitigation of this risk, though there have been many case reports where it is difficult to determine if multiple bouts of sinusitis were truly recurrent acute processes or the repeated manifestation of a lingering chronic infection, which was never fully eradicated.32 In the case of the latter, the presence of an alloplastic implant such as porous polyethylene, which is notoriously arduous to remove once tissue ingrowth has occurred, would be genuinely dreadful.
As the central location of the orbits and midface permits infectious spread from any of the multiple surrounding structures, use of alloplastic implants in reconstruction of this region is troublesome. The purported benefits of well-vascularized tissue in previously or concomitantly infected defect sites make a completely autogenous reconstruction with free tissue transfer preferable to use of alloplasts. One of the most straightforward types of reconstruction is to simply fill the defect site with a thick musculocutaneous flap to obtain palliation and coverage (Fig. 3). Without rigid support, however, gradual sag of the imported tissue results in significant decrements in functional and aesthetic outcome. This is especially true when the globe remains viable in the absence of the orbital floor and infraorbital rim. In these cases, soft tissue reconstruction alone will result in dependent shift of the orbital contents, resulting in vertical dystopia, enophthalmos, diplopia, and facial deformity.42 Because of this, the reconstructive objectives in these cases are numerous. The orbital contents must have rigid support, the infraorbital rim must be re-created to restore one of the horizontal buttresses of the face, any communication between the nasopharynx and oropharynx must be interrupted to prevent reinfection, and the end result must be symmetric and aesthetically appealing.43 Bone grafts have been used to reconstruct midface support, though they may undergo significant resorption.
One study suggested that rigid fixation could be used to diminish graft resorption while not overly prolonging operative times. A notch created in the zygoma allowed an autogenous bone graft from the ilium or calvarium to be secured to the zygoma with a single transfixing screw in a “tongue-in-groove” fashion; a single small plate provided medial fixation. A free rectus abdominis flap was then used to fill in the remaining midfacial soft tissue deficit. Although this specific review involved only 9 patients, the authors report extensive experience in orbital and midfacial reconstructions and excellent outcomes in the majority of patients using this technique.37,39,43,44
As in the above study, free rectus abdominis flaps are commonly used once orbital support has been achieved or in maxillary defects with orbital sparing. If so designed, they may supply significant bulk and permit a very large skin paddle to be used to reconstruct large mucosal and skin defects. Although performed commonly, one of the drawbacks of only reconstructing the palate with soft tissue is that most patients must use a palatal prosthesis to have significant return of masticatory function. In many of these cases, the expensive prostheses are considered a dental concern and may not be covered by the patient's insurance carrier, often making their use cost-preclusive. The prostheses also require significant routine care and maintenance, which may further discourage their use. In light of these considerations, a primary reconstruction of the palatal defect using an osteocutaneous free flap from the fibula or iliac crest may be preferable, as the bone is substantial enough to permit placement of osseointegrated dental implants.38,45 These implants allow for a return of functional dentition without the need for a palatal prosthesis.
MANDIBLE
The development of acute mandibular osteomyelitis may result from hematogenous dissemination and intraoperative contamination, though contiguous spread from a neglected odontogenic source is most common.24 Intravenous drug abusers, patients with sickle cell anemia, and those patients with compromised mandibular perfusion are all at higher risk for this development.46
Reconstruction of osteomyelitis defects in the craniofacial skeleton is typically performed only after complete debridement of all devitalized and infected tissue. Alloplasts are generally avoided in reconstructing infected sites if vascularized tissue may be used, because of the fear that the alloplast may itself become infected. The mandible may be a unique situation though, because of its inherent mobility. There has been a great deal of data supporting the importance of rigid fixation in the healing of mandibular fractures, some even advocating the primary treatment of infected mandibular fractures with rigid internal fixation.47 Rigid internal fixation of the mandible with various metals has been reported for several decades.48,49 Success rates have generally been good after oncologic resection, from 75 to 100%.50 Even so, complication rates remain significant; up to 50% in one study of 19 patients.50 The benefits of immediate plate reconstruction of mandibular defects include lack of donor-site morbidity, cost-effectiveness, ability to contour the plate to the mandible before resection, the ability to reconstruct the condyle when necessary, and the ability to avoid multiple stages of reconstruction.50 One of the additional benefits of use of alloplastic plates or trays is that they can support demineralized bone matrix and/or autogenous bone graft.51,52 Since 1944, the osteoinductive capacity of autogenous cancellous bone graft has been well reported.53 For these reasons, alloplasts such as titanium plating and demineralized bone matrix remain a viable reconstructive alternative, especially when used in conjunction with autogenous bone graft and vascularized soft tissue flaps.
In the past couple of decades, free vascularized bone grafts have been increasingly used with great success, especially in cases where the defect site is irradiated, poorly vascularized, and devitalized. They have become a reliable and efficacious reconstructive modality for large mandibular defects. This is evidenced by a report of 150 consecutive mandibular reconstructions with vascularized osseous free flaps with a 100% flap survival rate.54
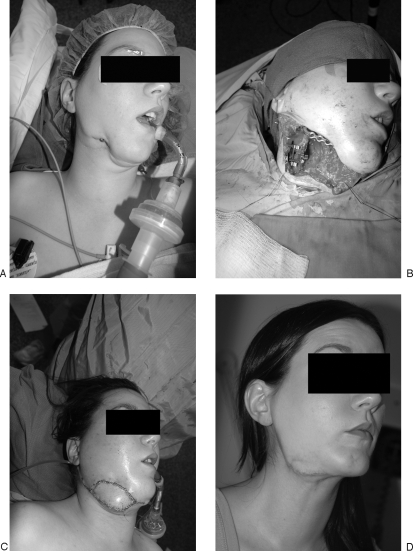
The most commonly used free osteocutaneous flaps for mandibular reconstruction are from the ilium, radius (Fig. 6), and fibula (Fig. 7), with each having specific benefits and drawbacks. The radial forearm flap provides very reliable, well-vascularized tissue with a thin, pliable skin paddle. Defects that require a large amount of intraoral lining are therefore better suited for reconstruction with the forearm flap.54 The major drawbacks to this donor site are the lower density of limited bone graft as well as subsequent radius fracture in 15% of patients.55 Because the radius supplies less substantial, unicortical bone, osseointegrated implants may not be placed after recovery. Large defects reconstructed with this flap force patients to use palatal prosthetics, which are often not covered by insurance and therefore cost-prohibitive. For these reasons, the radius has seemingly been relegated to use in defects that require a large amount of intraoral lining as well as small, lateral mandible and full-thickness cheek defects, especially where large amounts of tonsillar and pharyngeal mucosa is missing54,55
Figure 6.
(A) Preoperative right-sided mandibular defect. (B) Panorex of right-sided mandibular defect. (C) Markings for radial forearm osteocutaneous free flap. (D) Elevation of radial forearm osteocutaneous free flap. (E) Intraoperative inset of radial forearm osteocutaneous free flap and reconstruction plate. (F) Postoperative result: lateral view. (G) Postoperative Panorex.
Figure 7.
(A) Patient after dental infection, debridement, and segmental mandibular defect initially treated with reconstruction plate now exposed. (B) Intraoperative surgical defect. (C) Intraoperative inset: skin paddle of the fibular free flap used for cutaneous reconstruction. (D) Six-month follow-up: postoperative new after partial skin paddle removal.
The iliac crest free flap has become the mainstay of oromandibular reconstruction at some centers, with reported success rates reaching 96%.56 This flap affords a reasonable vascular pedicle and appropriately shaped bone, skin, and soft tissue, enabling it to be precisely tailored to fit the defect.56 Some reported limitations have included excessive soft tissue bulk, unreliability of the skin, and an inability to sustain multiple osteotomies without compromising the blood supply, thereby restricting the ability to shape the anterior segment of the mandible.54
In contrast, the fibula provides a very large amount of strong bicortical bone graft, allowing placement of dental implants. It can also sustain multiple osteotomies while still remaining well vascularized, allowing a great deal of precision in shaping the reconstructed mandible.54 The accompanying skin paddle is quite reliable, though it is generally much smaller and less supple than that with free forearm flap. It also obviates the risks of donor-site fracture that may be seen in the case of the radius. For these reasons, in a consecutive series of 150 vascularized bone grafts to mandibular defect sites, the free fibula was used in 90% of reconstructions and was the authors' flap of choice in these cases54 (Fig. 7).
A recent change in osseous reconstructive modalities has occurred with the development of bone morphogenetic protein (BMP) therapy. The BMPs are a group of extracellular signaling proteins belonging to the transforming growth factor β superfamily and were first discovered in demineralized bone matrix by Marshal Urist in 1965.57 They have been shown to be potent inducers of osteogenesis, and their efficacy and safety is very well reported in the orthopedic literature.57,58 Recently, they have been approved by the U.S. Food and Drug Administration (FDA) for use in maxillary sinus floor augmentation where they were shown to significantly increase the ability to place endosseous dental implants in patients requiring staged maxillary sinus floor augmentation.59 Recent use in the mandible has shown similar results, allowing increases in the successful healing of mandibular bone defects, with improved return of masticatory function.60,61 Although BMP treatment is very costly and its use in reconstructing the mandible is not FDA approved, it may provide the ideal combination of superior efficacy without the donor-site complications and morbidity of autologous bone harvest.
REFERENCES
- Nelaton A. Elements De Pathologie Chirurgicale. Paris, France: Germer-Bailliere; pp. 1844–1859.
- David D J, Cooter R D. Craniofacial infection in 10 years of transcranial surgery. Plast Reconstr Surg. 1987;80:213–225. doi: 10.1097/00006534-198708000-00008. [DOI] [PubMed] [Google Scholar]
- Fearon J A, Yu J, Bartlett S P, Munro I R, Chir B, Whitaker L. Infections in craniofacial surgery: a combined report of 567 procedures from two centers. Plast Reconstr Surg. 1997;100:862–868. doi: 10.1097/00006534-199709001-00006. [DOI] [PubMed] [Google Scholar]
- Barker F G., II Efficacy of prophylactic antibiotics for craniotomy: a meta-analysis. Neurosurgery. 1994;35:484–490. discussion 491–482. doi: 10.1227/00006123-199409000-00017. [DOI] [PubMed] [Google Scholar]
- Verbon A, Husni R N, Gordon S M, Lavertu P, Keys T F. Pott's puffy tumor due to Haemophilus influenzae: case report and review. Clin Infect Dis. 1996;23:1305–1307. doi: 10.1093/clinids/23.6.1305. [DOI] [PubMed] [Google Scholar]
- Babu R P, Todor R, Kasoff S S. Pott's puffy tumor: the forgotten entity. Case report. J Neurosurg. 1996;84:110–112. doi: 10.3171/jns.1996.84.1.0110. [DOI] [PubMed] [Google Scholar]
- Guillen A, Brell M, Cardona E, Claramunt E, Costa J M. Pott's puffy tumour: still not an eradicated entity. Childs Nerv Syst. 2001;17:359–362. doi: 10.1007/s003810000420. [DOI] [PubMed] [Google Scholar]
- Tessier P, Kawamoto H, Posnick J, Raulo Y, Tulasne J F, Wolfe S A. Taking calvarial grafts, either split in situ or splitting of the parietal bone flap ex vivo: tools and techniques. V: a 9650-case experience in craniofacial and maxillofacial surgery. Plast Reconstr Surg. 2005;116:54S–71S. discussion 92S–94S. doi: 10.1097/01.prs.0000173949.51391.d4. [DOI] [PubMed] [Google Scholar]
- Gosain A K. Biomaterials for reconstruction of the cranial vault. Plast Reconstr Surg. 2005;116:663–666. doi: 10.1097/01.prs.0000176289.05374.5b. [DOI] [PubMed] [Google Scholar]
- Manson P N, Crawley W A, Hoopes J E. Frontal cranioplasty: risk factors and choice of cranial vault reconstructive material. Plast Reconstr Surg. 1986;77:888–904. [PubMed] [Google Scholar]
- Hanssen A D, Spangehl M J. Practical applications of antibiotic-loaded bone cement for treatment of infected joint replacements. Clin Orthop Relat Res. 2004:79–85. doi: 10.1097/01.blo.0000143806.72379.7d. [DOI] [PubMed] [Google Scholar]
- Patel M F, Langdon J D. Titanium mesh (Timesh) osteosynthesis: a fast and adaptable method of semi-rigid fixation. Br J Oral Maxillofac Surg. 1991;29:316–324. doi: 10.1016/0266-4356(91)90118-o. [DOI] [PubMed] [Google Scholar]
- Joffe J M, Aghabeigi B, Davies E H, Harris M. A retrospective study of 66 titanium cranioplasties. Br J Oral Maxillofac Surg. 1993;31:144–148. doi: 10.1016/0266-4356(93)90112-a. [DOI] [PubMed] [Google Scholar]
- Janecka I P. New reconstructive technologies in skull base surgery: role of titanium mesh and porous polyethylene. Arch Otolaryngol Head Neck Surg. 2000;126:396–401. doi: 10.1001/archotol.126.3.396. [DOI] [PubMed] [Google Scholar]
- Rubin J P, Yaremchuk M J. Complications and toxicities of implantable biomaterials used in facial reconstructive and aesthetic surgery: a comprehensive review of the literature. Plast Reconstr Surg. 1997;100:1336–1353. doi: 10.1097/00006534-199710000-00043. [DOI] [PubMed] [Google Scholar]
- Malis L I. Titanium mesh and acrylic cranioplasty. Neurosurgery. 1989;25:351–355. doi: 10.1097/00006123-198909000-00005. [DOI] [PubMed] [Google Scholar]
- Mottaran R, Guarda-Nardini L, Fusetti S, Ferroneto G, Salar G. Reconstruction of a large post-traumatic cranial defect with a customized titanium plaque. J Neurosurg Sci. 2004;48:143–147. [PubMed] [Google Scholar]
- Wu C T, Lee S T, Chen J F, Lin K L, Yen S H. Computer-aided design for three-dimensional titanium mesh used for repairing skull base bone defect in pediatric neurofibromatosis type 1. A novel approach combining biomodeling and neuronavigation. Pediatr Neurosurg. 2008;44:133–139. doi: 10.1159/000113116. [DOI] [PubMed] [Google Scholar]
- Eppley B L, Morales L, Wood R, et al. Resorbable Plla-Pga plate and screw fixation in pediatric craniofacial surgery: clinical experience in 1883 patients. Plast Reconstr Surg. 2004;114:850–856. discussion 857. doi: 10.1097/01.prs.0000132856.69391.43. [DOI] [PubMed] [Google Scholar]
- Fearon J A, Munro I R, Bruce D A. Observations on the use of rigid fixation for craniofacial deformities in infants and young children. Plast Reconstr Surg. 1995;95:634–637. discussion 638. [PubMed] [Google Scholar]
- Nadol J B., Jr Histopathology of Pseudomonas osteomyelitis of the temporal bone starting as malignant external otitis. Am J Otolaryngol. 1980;1:359–371. doi: 10.1016/s0196-0709(80)80016-0. [DOI] [PubMed] [Google Scholar]
- Rowlands R G, Lekakis G K, Hinton A E. Masked pseudomonal skull base osteomyelitis presenting with a bilateral Xth cranial nerve palsy. J Laryngol Otol. 2002;116:556–558. doi: 10.1258/002221502760132700. [DOI] [PubMed] [Google Scholar]
- Dudkiewicz M, Livni G, Kornreich L, Nageris B, Ulanovski D, Raveh E. Acute mastoiditis and osteomyelitis of the temporal bone. Int J Pediatr Otorhinolaryngol. 2005;69:1399–1405. doi: 10.1016/j.ijporl.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Prasad K C, Prasad S C, Mouli N, Agarwal S. Osteomyelitis in the head and neck. Acta Otolaryngol. 2007;127:194–205. doi: 10.1080/00016480600818054. [DOI] [PubMed] [Google Scholar]
- Newman J, O'Malley B W, Jr, Chalian A, Brown M T. Microvascular reconstruction of cranial base defects: an evaluation of complication and survival rates to justify the use of this repair. Arch Otolaryngol Head Neck Surg. 2006;132:381–384. doi: 10.1001/archotol.132.4.381. [DOI] [PubMed] [Google Scholar]
- Hanasono M M, Utley D S, Goode R L. The temporalis muscle flap for reconstruction after head and neck oncologic surgery. Laryngoscope. 2001;111:1719–1725. doi: 10.1097/00005537-200110000-00009. [DOI] [PubMed] [Google Scholar]
- Clauser L, Curioni C, Spanio S. The use of the temporalis muscle flap in facial and craniofacial reconstructive surgery: a review of 182 cases. J Craniomaxillofac Surg. 1995;23:203–214. doi: 10.1016/s1010-5182(05)80209-4. [DOI] [PubMed] [Google Scholar]
- Chang D W, Langstein H N, Gupta A, et al. Reconstructive management of cranial base defects after tumor ablation. Plast Reconstr Surg. 2001;107:1346–1355. discussion 1356–1347. doi: 10.1097/00006534-200105000-00003. [DOI] [PubMed] [Google Scholar]
- Disa J J, Rodriguez V M, Cordeiro P G. Reconstruction of lateral skull base oncological defects: the role of free tissue transfer. Ann Plast Surg. 1998;41:633–639. doi: 10.1097/00000637-199812000-00009. [DOI] [PubMed] [Google Scholar]
- McGuckin M, Goldman R, Bolton L, Salcido R. The clinical relevance of microbiology in acute and chronic wounds. Adv Skin Wound Care. 2003;16:12–23. doi: 10.1097/00129334-200301000-00011. [DOI] [PubMed] [Google Scholar]
- Pusic A L, Chen C M, Patel S, Cordeiro P G, Shah J P. Microvascular reconstruction of the skull base: a clinical approach to surgical defect classification and flap selection. Skull Base. 2007;17:5–15. doi: 10.1055/s-2006-959331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Toukhy E, Szal M, Levine M R, Levine H L. Osteomyelitis of the orbit. Ophthal Plast Reconstr Surg. 1997;13:68–71. doi: 10.1097/00002341-199703000-00012. [DOI] [PubMed] [Google Scholar]
- Auluck A. Maxillary necrosis by mucormycosis. a case report and literature review. Med Oral Patol Oral Cir Bucal. 2007;12:E360–E364. [PubMed] [Google Scholar]
- Kaur A, Kant S, Bhasker S K. Periorbital tuberculosis. Orbit. 2007;26:39–42. doi: 10.1080/01676830600671482. [DOI] [PubMed] [Google Scholar]
- Munir N, Jones N S. Rhinocerebral mucormycosis with orbital and intracranial extension: a case report and review of optimum management. J Laryngol Otol. 2007;121:192–195. doi: 10.1017/S0022215106003409. [DOI] [PubMed] [Google Scholar]
- Futran N D, Mendez E. Developments in reconstruction of midface and maxilla. Lancet Oncol. 2006;7:249–258. doi: 10.1016/S1470-2045(06)70616-7. [DOI] [PubMed] [Google Scholar]
- Cordeiro P G, Santamaria E. A classification system and algorithm for reconstruction of maxillectomy and midfacial defects. Plast Reconstr Surg. 2000;105:2331–2346. discussion 2347–2338. doi: 10.1097/00006534-200006000-00004. [DOI] [PubMed] [Google Scholar]
- Futran N D. Primary reconstruction of the maxilla following maxillectomy with or without sacrifice of the orbit. J Oral Maxillofac Surg. 2005;63:1765–1769. doi: 10.1016/j.joms.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Cordeiro P G, Disa J J. Challenges in midface reconstruction. Semin Surg Oncol. 2000;19:218–225. doi: 10.1002/1098-2388(200010/11)19:3<218::aid-ssu3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Garibaldi D C, Iliff N T, Grant M P, Merbs S L. Use of porous polyethylene with embedded titanium in orbital reconstruction: a review of 106 patients. Ophthal Plast Reconstr Surg. 2007;23:439–444. doi: 10.1097/IOP.0b013e31815a1235. [DOI] [PubMed] [Google Scholar]
- Yaremchuk M J. Facial skeletal reconstruction using porous polyethylene implants. Plast Reconstr Surg. 2003;111:1818–1827. doi: 10.1097/01.PRS.0000056866.80665.7A. [DOI] [PubMed] [Google Scholar]
- Lee H B, Hong J P, Kim K T, Chung Y K, Tark K C, Bong J P. Orbital floor and infraorbital rim reconstruction after total maxillectomy using a vascularized calvarial bone flap. Plast Reconstr Surg. 1999;104:646–653. doi: 10.1097/00006534-199909030-00005. [DOI] [PubMed] [Google Scholar]
- Chen C M, Cordeiro P G. The tongue-in-groove technique for orbital floor reconstruction after maxillectomy. Plast Reconstr Surg. 2008;121:225–232. doi: 10.1097/01.prs.0000293865.28595.75. [DOI] [PubMed] [Google Scholar]
- Cordeiro P G, Santamaria E, Kraus D H, Strong E W, Shah J P. Reconstruction of total maxillectomy defects with preservation of the orbital contents. Plast Reconstr Surg. 1998;102:1874–1884. discussion 1885–1877. doi: 10.1097/00006534-199811000-00011. [DOI] [PubMed] [Google Scholar]
- Santamaria E, Cordeiro P G. Reconstruction of maxillectomy and midfacial defects with free tissue transfer. J Surg Oncol. 2006;94:522–531. doi: 10.1002/jso.20490. [DOI] [PubMed] [Google Scholar]
- Wald E R. Risk factors for osteomyelitis. Am J Med. 1985;78:206–212. doi: 10.1016/0002-9343(85)90386-9. [DOI] [PubMed] [Google Scholar]
- Koury M, Ellis E., III Rigid internal fixation for the treatment of infected mandibular fractures. J Oral Maxillofac Surg. 1992;50:434–443. discussion 443–434. doi: 10.1016/s0278-2391(10)80310-6. [DOI] [PubMed] [Google Scholar]
- Klotch D W, Prein J. Mandibular reconstruction using AO plates. Am J Surg. 1987;154:384–388. doi: 10.1016/0002-9610(89)90009-3. [DOI] [PubMed] [Google Scholar]
- Freitag V, Hell B, Fischer H. Experience with AO reconstruction plates after partial mandibular resection involving its continuity. J Craniomaxillofac Surg. 1991;19:191–198. doi: 10.1016/s1010-5182(05)80546-3. [DOI] [PubMed] [Google Scholar]
- Shockley W W, Weissler M C, Pillsbury H C. Immediate mandibular replacement using reconstruction plates. Arch Otolaryngol Head Neck Surg. 1991;117:745–749. discussion 750. doi: 10.1001/archotol.1991.01870190057011. [DOI] [PubMed] [Google Scholar]
- Zhe C, Tingchun W. Reconstruction of mandibular defects with composite autologous iliac bone and freeze-treated allogeneic rib grafts. J Oral Maxillofac Surg. 1982;40:29–33. doi: 10.1016/s0278-2391(82)80012-8. [DOI] [PubMed] [Google Scholar]
- deFries H O. Reconstruction of the mandible: use of combined homologous mandible and autologous bone. Otolaryngol Head Neck Surg. 1981;89:694–697. doi: 10.1177/019459988108900433. [DOI] [PubMed] [Google Scholar]
- Marx R E. Mandibular reconstruction. J Oral Maxillofac Surg. 1993;51:466–479. doi: 10.1016/s0278-2391(10)80501-4. [DOI] [PubMed] [Google Scholar]
- Cordeiro P G, Disa J J, Hidalgo D A, Hu Q Y. Reconstruction of the mandible with osseous free flaps: a 10-year experience with 150 consecutive patients. Plast Reconstr Surg. 1999;104:1314–1320. doi: 10.1097/00006534-199910000-00011. [DOI] [PubMed] [Google Scholar]
- Thoma A, Khadaroo R, Grigenas O, et al. Oromandibular reconstruction with the radial-forearm osteocutaneous flap: experience with 60 consecutive cases. Plast Reconstr Surg. 1999;104:368–378. discussion 379–380. doi: 10.1097/00006534-199908000-00007. [DOI] [PubMed] [Google Scholar]
- Shenaq S M, Klebuc M J. The iliac crest microsurgical free flap in mandibular reconstruction. Clin Plast Surg. 1994;21:37–44. [PubMed] [Google Scholar]
- Einhorn T A. Clinical applications of recombinant human BMPs: early experience and future development. J Bone Joint Surg Am. 2003;85(Suppl 3):82–88. doi: 10.2106/00004623-200300003-00014. [DOI] [PubMed] [Google Scholar]
- Baskin D S, Ryan P, Sonntag V, Westmark R, Widmayer M A. A prospective, randomized, controlled cervical fusion study using recombinant human bone morphogenetic protein-2 with the cornerstone-Sr allograft ring and the Atlantis anterior cervical plate. Spine. 2003;28:1219–1225. discussion 1225. doi: 10.1097/01.BRS.0000065486.22141.CA. [DOI] [PubMed] [Google Scholar]
- Boyne P J, Lilly L C, Marx R E, et al. De novo bone induction by recombinant human bone morphogenetic protein-2 (rhBMP-2) in maxillary sinus floor augmentation. J Oral Maxillofac Surg. 2005;63:1693–1707. doi: 10.1016/j.joms.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Herford A S, Boyne P J. Reconstruction of mandibular continuity defects with bone morphogenetic protein-2 (rhBMP-2) J Oral Maxillofac Surg. 2008;66:616–624. doi: 10.1016/j.joms.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Boyne P J, Nakamura A, Shabahang S. Evaluation of the long-term effect of function on rhBMP-2 regenerated hemimandibulectomy defects. Br J Oral Maxillofac Surg. 1999;37:344–352. doi: 10.1054/bjom.1999.0205. [DOI] [PubMed] [Google Scholar]