ABSTRACT
Reconstruction of large skeletal defects secondary to osteomyelitis remains a challenging problem. Osteomyelitis can result from a variety of etiologies; most often, it is a consequence of trauma to a long bone. Despite advances in antibiotic therapy, treatment of chronic osteomyelitis requires adequate surgical debridement, which can often lead to large soft tissue and bone loss. Free vascularized bone can be used to reconstruct large skeletal defects greater than 6 cm or bone defects of smaller size that failed to heal with nonvascularized bone grafting. The length, cortical strength, and anatomic configuration of the free vascular fibular graft make it an ideal bone graft to bridge extremity defects, and it can be transferred with skin, fascia, and muscle to fill soft tissue defects in the recipient site.
Keywords: Osteomyelitis, vascularized fibular graft, skeletal defects, osteocutaneous flaps
Treatment for osteomyelitis is a difficult challenge for the reconstructive surgeon. Successful treatment of this disease involves adequate debridement of all involved soft tissue and bone until only viable bleeding tissue remains. Inadequate debridement to preserve structural integrity of the bone leads to recurrence of infection.1,2
Advances in microsurgery have made possible the reconstruction of soft tissue and bone defects using muscle flaps, fasciocutaneous flaps, and vascularized bone grafts. Vascularized bone grafts retain their intrinsic blood supply and enable the surgeon to achieve sound bony union irrespective of the length of the bone defect. Our intent is to present our method of reconstruction of skeletal defects primarily with vascularized bone grafts.
OSTEOMYELITIS
Osteomyelitis is a severe, progressive inflammatory process caused by an infecting microorganism leading to bony destruction, which can be limited to a single portion of the bone or can be multifocal. Three distinct mechanisms of osteomyelitis are described: hematogenous spread, local spread from a contiguous infectious process, and bone exposure and contamination secondary to a soft tissue defect.3
Among pathogenic microorganisms, Staphylococcus aureus is by far the most commonly involved. Various factors are responsible for the extensive bone destruction in osteomyelitis. The initial trauma itself may result in segmental loss of bone, and the radical surgical debridement may also lead to a large bony defect.4 In addition, it has been shown that cytokines, such as interleukin-1 and tumor necrosis factor, present at the site of infection, are potent osteolytic promoters.5,6 Within the infected regions, vascular channels are compressed and obliterated by the inflammatory process leading to bony ischemia. With time, segments of bone devoid of vascular supply become separated, form sequestra, and continue to harbor bacteria despite aggressive antibiotic treatment due to biofilm formation.
Biofilm is an aggregation of microbe colonies enclosed within an extracellular polysaccharide matrix (glycocalyx) that adheres to the surface of implants or devitalized bone. This biofilm microbial growth allows itself enhanced protection against natural host defenses and antibiotic therapy.7 Removal of biofilm by implant extraction and debridement is necessary for successful treatment of infection.3
The most widely used classification of osteomyelitis is the Cierny-Mader system for adult osteomyelitis.8 This system (discussed extensively in the article by Calhoun et al in this issue of the journal) stages osteomyelitis based on the combination of the anatomic type of involvement and host classification. Staging is correlated with treatment and prognosis. It is important to recognize and define the stage of a patient prior to treatment of osteomyelitis. Systemic and local compromising factors can lead to poor wound healing and difficulty controlling infection.
Local clinical signs of acute osteomyelitis often include redness, swelling, warmth, and tenderness. Fluctuance and discharge may be present at the wound site. Systemic clinical signs such as fever, chills, and malaise occur more frequently in hematogenous osteomyelitis and in children. Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are elevated. Radiographic findings are rare in acute osteomyelitis. Plain films often show soft tissue swelling at initial presentation, but bone destruction with narrowing of joint spaces and periosteal reaction may not be present until 10 to 21 days after an infection.9 Advanced imaging studies such as technetium-, gallium-, and indium-labeled white blood cell (WBC) scans are useful in identifying acute osteomyelitis when standard radiographs are normal.10,11 Both computed tomography (CT) and magnetic resonance imaging (MRI) detect periosteal reaction, cortical destruction, and soft tissue involvement. CT provides excellent definition of cortical bone but only fair assessment of the surrounding soft tissue. MRI is very useful in the early presentation of osteomyelitis given its ability to reveal bony edema.
Identification of the offending organism is essential for diagnosis, treatment, and prognosis of osteomyelitis. Cultures can be obtained from blood cultures, sinus swabs, or preferably by direct biopsy from the involved bone.12 Specimen obtained from swabs of open sinus tracts or fistulas are often misleading owning to the presence of nonpathogenic microorganisms colonizing the site.13
MANAGEMENT PROTOCOL FOR OSTEOMYELITIS
Management of patients with chronic osteomyelitis includes both adequate surgical debridement and systemic antibiotic therapy. This is a multidisciplinary effort involving the surgical team, infectious disease specialists, nutritionists, and, in some cases, psychologists. Host comorbidities need to be addressed, such as optimizing sugar levels in diabetic patients, and smoking should be stopped. Patients must be made aware of the potentially long treatment process.
Antibiotics should be started empirically after cultures have been obtained, usually at the time of debridement. Once culture results and sensitivities have been obtained, the antibiotic regimen should be tailored accordingly. Mader et al14 recommended for type 3 and 4 osteomyelitis ~4 weeks of intravenous antibiotics from the last surgical debridement. The general requirement of 4 to 6 weeks of antibiotics is based on the fact that it takes that long for debrided bone to be protected prior to revascularization.15 It should be emphasized that antibiotic regimens will fail without adequate debridement, soft tissue coverage, and bone vascularity.14
Different management protocols exist in the literature regarding timing of the vascularized bone transfer. Doi et al16 reported successful use of a one-stage protocol with debridement of all devitalized bone and immediate transfer of free vascularized osteocutaneous fibula or iliac crest in infected bone defects. In their series, 25 of 26 patients had control of the infection with no recurrence, adequate soft tissue coverage, and bony union. Their average follow-up, however, was less than 2 years.
We prefer a two-stage approach with the initial stage including removal of all implants, radical debridement, temporary stabilization of the fracture, and intravenous antibiotics. The extent of bone debridement is determined at the time of surgical exploration. Debridement is aimed at removing all infected or necrotic bone and soft tissue until bleeding healthy tissue is encountered.17,18,19 Meticulous soft tissue handling and knowledge of local vascularity to maintain blood supply and soft tissue coverage is crucial.20 Serial debridements are often necessary.
Dead space resulting from extensive debridement can be filled with polymethylmethacrylate (PMMA) beads impregnated with antibiotics such as tobramycin, vancomycin, or other microbial-specific antibiotics that are heat-stable and available in powder form.21 The beads can be inserted in the gap area, and the wound is temporarily sealed with a bio-occlusive dressing until soft tissue coverage is complete (bead-pouch technique).3 Alternatively, the defect can be filled with an antibiotic-impregnated block. Use of a block rather than beads can reduce soft tissue ingrowth, augment local stability, and maintain length and space for the later reconstruction stage of the treatment. The diameters of the antibiotic bead or block spacers should be consistently greater than the usual diameter of the intended bone graft to ensure adequate room and to preclude compression of the pedicle of the future vascularized graft.22,23
Stabilization of the bone during the first stage of the protocol aids in eradication of the infection and diminishing patient pain. To avoid implants at the site of infection, we generally prefer external fixation. However, antibiotic-impregnated intramedullary cement rods have been used successfully for infections after nailing.24 After 6 weeks of antibiotic treatment, laboratory tests including ESR and CRP with examination of the local skin area can help confirm the eradication of infection. If there is no evidence of persistence of infection, we commonly proceed with the vascularized fibular graft (VFG) procedure.
VASCULARIZED BONE GRAFTS
Defects less than 6 cm can be bridged with conventional autogenous bone grafts such as corticocancellous iliac crest graft.25,26,27,28 Defects greater than 6 cm will likely require distraction osteogenesis with Ilizarov frames29,30 or a vascularized bone graft. These grafts have become an important tool for the reconstructive surgeon dealing with the management of long bone defects or difficult nonunions. They are especially important in the setting of osteomyelitis treatment because they combine the advantages of viable cancellous autografts with the stability of cortical analogues while leaving the nutrient blood supply intact. Indications for their use include skeletal defects more than 6 cm in length,25,31,32,33 defects associated with a poor soft tissue envelope, and in cases where smaller sized nonvascularized bone grafts have failed to incorporate. Vascularized bone grafts incorporate into the recipient site through a different process than that of avascular grafts. The vascularized grafts bypass the process of creeping substitution, which involves necrosis of the graft, resorption, and new bone formation. They maintain their mass, architecture, and biomechanical strength. Furthermore, the transferred vascularized bone has the ability to hypertrophy,34 especially in the lower extremity because of increased mechanical loading. Available donor bones to be used for vascularized bone grafts include the fibula,35,36,37 ilium,38 scapula,39 and rib flaps.40 We will discuss the VFG because it is the most commonly used graft for reconstruction at our institution.
FIBULAR GRAFT
The fibula's length, straight configuration, cortical support, and composite tissue make it ideal for the reconstruction of segmental long bone defects. The VFG can be used to reconstruct defects of up to 26 cm.41 Its size and relatively straight configuration allow it to be used within the humerus, forearm, clavicle, as well as in the tibia and femur.
The peroneal artery is closely positioned to the fibula (Fig. 1). The artery arises from the tibioperoneal trunk, ~3 to 4 cm distal to the takeoff of the anterior tibial artery. The peroneal artery sends perforators laterally to the skin of the lower leg, sometimes in a septocutaneous fashion via the lateral intermuscular septum, but often with muscular perforators. The peroneal artery continues distally with its two peroneal veins, lying between the flexor hallucis longus and posterior tibialis muscle. The diameter of the artery is 1.5 to 2 mm and the diameter of the veins is 2.5 to 4 mm.
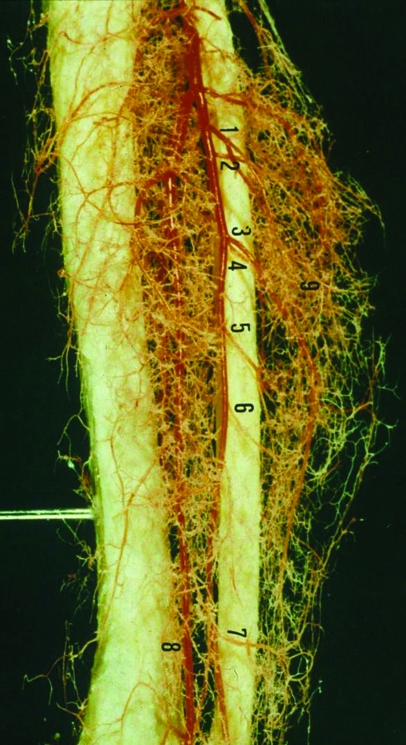
Figure 1.
Latex injection of the peroneal artery demonstrating a rich arterial plexus supplying the periosteum of the fibula and the numerous perforating branches to the skin and surrounding muscle. Numbers denote perforating arteries.
Blood supply to the VFG is both endosteal and periosteal. The endosteal blood supply is derived from the nutrient artery, which stems 6 to 14 cm from the peroneal artery bifurcation. It enters the middle third junction of the VFG and divides into an ascending and descending branch.42 Blood supply to the distal fibula is mainly periosteal, being derived from several periosteal branches. Abnormalities of the arterial supply of the leg may be congenital or acquired. Congenital absence of the peroneal artery is rare and would exclude use of the VFG. Peroneal arteria magna is a condition where the peroneal artery is the dominant artery that supplies the foot, and in such a case the harvesting of a fibular flap would have disastrous ischemic consequences for the foot (Fig. 2). Carroll and Esclamado43 reported the incidence of peroneal arteria magna to be between 5% and 7%. However, in the senior author's (M.V.S.) experience of more than 400 VFGs, we have only encountered two cases of peroneal arteria magna. It should be noted that patients with a dominant peroneal artery may still have normally palpable distal pulses.44 However, in the adult population, atherosclerotic disease is the main cause of acquired arterial abnormalities in the lower extremity.
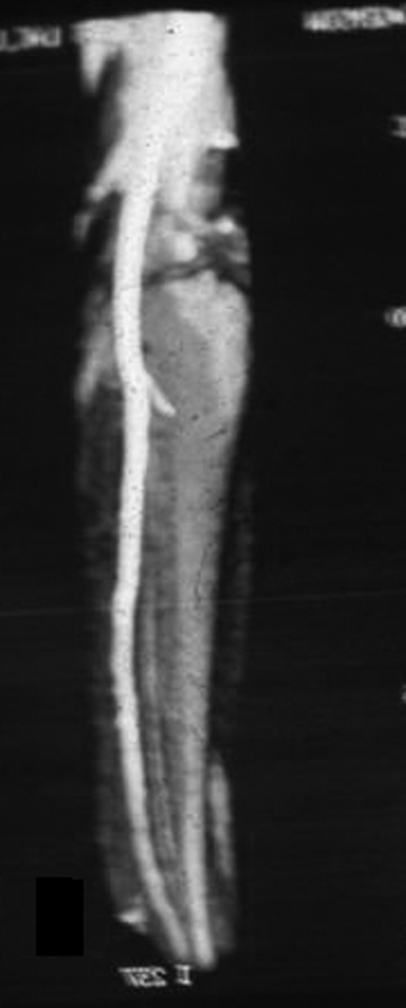
Figure 2.
Magnetic resonance angiography of lower extremity demonstrating peroneal arteria magna with hypoplastic anterior tibial and posterior tibial arteries.
A single-strut fibular graft is adequate for the tibia, radius, ulna, but less optimal for the femur with bone gaps greater than 6 cm due to its smaller cross-sectional area. In this situation, a double-barrel fibula vascular bone graft is preferable because of its superior mechanical support. In addition, the fibula flap may be used for craniofacial defects resulting from debridement after osteomyelitis. Other commonly used flaps include free radial forearm osteocutaneous flap, iliac crest osteocutaneous flap, and scapula osteocutaneous flap. These all provide a reliable means of obtaining good wound healing with acceptable aesthetic and functional results for advanced osteomyelitis of the craniofacial skeleton after radical resectional debridement.45
The VFG is versatile in its composition. The graft can be transferred with skin, fascia, muscle, or its growth plate, depending on the defect requirements. Based on perforating fasciocutaneous branches at the middle and distal thirds of the pedicle, a skin paddle of up to 24 × 12 cm can be transferred to facilitate coverage,46 as well as allow patency testing of the anastomosis.47 Additionally, sections of the soleus or flexor hallucis longus muscle can be included to reconstruct soft tissue defects and cover exposed bone.48
PATIENT EVALUATION
When considering use of a free osteocutaneous flap for reconstruction, it is important to evaluate carefully both the intended recipient site and the donor site for integrity and vascularity to ensure minimal morbidity and reconstructive success. Radiographs and three-dimensional imaging of the recipient site is useful in determining the length of the bony defect and the amount of bone stock necessary for transfer. Determination of thickness and length of bone required will help in determining what donor site to choose if preparing for vascularized bone transfer. Smaller defects may be managed with cadaveric bone or bone substitutes depending on the recipient bed and use of adjunctive therapy (i.e., hyperbaric oxygen therapy) preoperatively and postoperatively.
In planning reconstruction of the limb after osteomyelitis debridement, the length and alignment of the limb should be considered so that any deformities can be addressed. In addition, an adequate vascular assessment prior to fibula transfer is performed, including the presence of adequate pulses in both the posterior tibial and dorsalis pedis arteries along with Doppler ultrasound to confirm adequate blood flow in the donor site. Any abnormal pulses or the presence of major trauma to the donor site could imply variability in the vessels, and magnetic resonance angiography or conventional angiography is warranted. For the recipient limb, it is important to establish that the arteries and veins are of sufficient caliber. Consideration should also be given to the most appropriate method of fibular stabilization. Depending on the defect length and location within the recipient bone, bony fixation devices such as transcortical screws, compression plates, intramedullary nails, and external fixation can be used. Finally, it is important to document the preoperative status of the common peroneal nerve at the donor site as injury to this nerve can occur during the course of fibular harvesting.
SURGICAL TECHNIQUE
Our surgical technique involves fibular graft harvesting, recipient site preparation, and reconstruction of the defect. Ideally, two surgical teams can work simultaneously to facilitate the procedure and decrease the surgical time.
Technique of Fibula Harvest
The patient is usually supine or in the lateral decubitus position. A tourniquet should be used. The nutrient artery usually enters the fibula at a point between the junction of the proximal and middle third. Thus, the intended segment of fibula to be harvested should be centered over the proximal third of the bone. A length of 5 or 6 cm longer than the defect site should be harvested to account for intramedullary placement in cases of humeral, femoral, or tibial defects.49 The incision and skin paddle are marked on the skin. The incision parallels the fibula, lying slightly posterior to the midlateral line and curving posteriorly and anteriorly over the midportion of the fibula to create an elliptical skin paddle. Because the fibula is part of the ankle joint, the distal 6 cm of the fibula proximal to the syndesmosis must be preserved to avoid instability to the ankle joint.50 The fibula is approached between the superficial posterior compartment and the lateral compartment. The proximal portion of the incision is made prior to elevating the skin paddle, and the crural fascia between the peroneus longus and soleus is split longitudinally. The deep peroneal nerve is identified in the anterior compartment adjacent to the fibula. We have had no cases of peroneal nerve palsy because we use digital retraction, rather than instruments, to retract the anterior compartment musculature as well as the peroneal nerve during dissection. The interval between the peroneus longus and soleus is also developed distal to the skin paddle. The skin paddle can be elevated off the underlying muscle at the level of the muscle fascia. Stay sutures should be placed to hold the fascia and subcutaneous tissue to the skin edges to prevent shearing injury to the perforating branches. The skin paddle is initially elevated from its posterior aspect toward the anterior, with care taken to identify and protect the cutaneous perforators.
The fibula is then exposed through an extraperiosteal dissection. It is unnecessary to leave more than a 2-mm cuff of muscle on the periosteum, but care must be taken not to disturb the periosteum and the blood vessels running in it. The peroneal artery and vena comitantes lie on the posterior surface of the interosseous membrane (IOM) and should not be separated from it.
The peroneus longus is extraperiosteally elevated off the anterior aspect of the fibula, with care not to injure the deep peroneal nerve that was identified at the start of the dissection. The dissection is then continued anterior, medially elevating the extensor musculature off the fibula and the lateral aspect of the IOM. The IOM is perforated proximally and distally with a right-angle clamp at the level of the osteotomy. This should allow sufficient space to place malleable retractors to protect the peroneal vessels and nerve as the fibula is cut with an oscillating saw. At the proximal and distal osteotomy site, a 5- to 10-mm segment of fibula is removed to facilitate mobilization of the fibula during dissection of the vessels.25 For patients less than 10 years of age, the distal fibular stump is fixed to the tibia with a transfixing screw to prevent mortise widening and progressive valgus angulation of the ankle.22,50
The peroneal artery is ligated distally, allowing the fibula to be mobilized from its bed. The fibula is controlled with a Kocher clamp placed into the distal medullary canal, and the fibula is pulled laterally from its distal aspect as the IOM is carefully divided. Posteriorly, the tibialis posterior muscle is dissected from the IOM. To ensure retention of the pedicle with the fibula, the surgeon should alternate the dissection medial and lateral within the first 2 to 3 inches until the pedicle is clearly elevated with the fibula. Once the fibula has been fully mobilized, the peroneal artery and accompanying vena comitantes are traced back to their junction with the posterior tibial artery. The fibular graft is then placed back in the bed, the tourniquet is released, and the vascularity of both the foot and the graft are checked. The authors usually wait ~15 minutes to monitor perfusion prior to transecting the pedicle.
Preparation of the Recipient Site
The incision should be carefully planned to avoid compromised skin or damage to the recipient vessels. The recipient site vessels should be carefully assessed intraoperatively in the presence of a scarred soft tissue envelope. If the recipient vessels are deemed to be inadequate, anastomosis should be performed at a site more proximal, and vein grafts should be used if the pedicle is of insufficient length.37
For tibia defects, generally the contralateral fibula is transferred because the ipsilateral fibula is usually surrounded by scarred or damaged tissue. Depending on which recipient-site vessels are selected for anastomosis, the fibula may be positioned orthograde to be anastomosed with the anterior tibial vessels or retrograde to be anastomosed with the posterior tibial vessels. For tibia reconstruction, the fibular placement should be in direct line with the weight-bearing load. Thus, intramedullary placement of the fibular into the tibia is generally preferred (Fig. 3). The type of fixation can include compression plating, transcortical screws, or external fixation. External fixation is generally preferred in infected cases to avoid presence of hardware at the defect site. In addition, it does not require wound closure nor does it interfere with graft vascularity. Augmentation of the osteosynthesis site with cancellous autograft can be performed at this time.
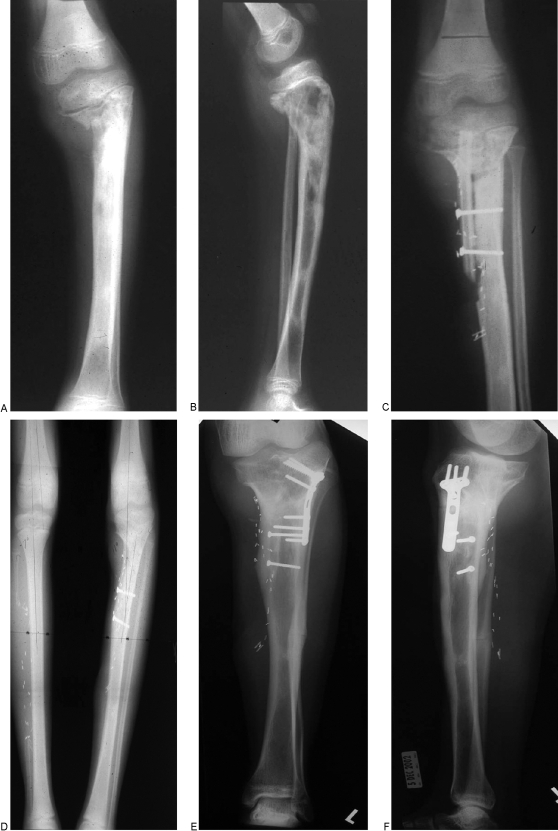
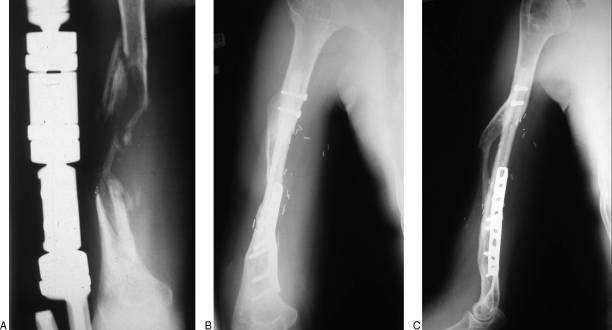
Figure 3.
(A, B) Anteroposterior and lateral radiographs of left tibial hematogenous osteomyelitis. (C) After multiple debridements, an intramedullary placement of a free VFG was performed. (D) Complete union and hypertrophy of graft at 10 months. (E, F) Seven years later, a corrective osteotomy was performed.
Reconstruction of the femur is more challenging than that of the tibia because of greater difficulties with bony stabilization and vascular access. Usually, the ipsilateral fibula is transferred. For a midfemoral defect, a medial incision is recommended for vascular access as well as bony stabilization. The superficial femoral artery with end-to-side anastomosis and end-to-end venous anastomosis to either the superficial femoral veins or greater saphenous veins can be used for a midfemoral site. A better choice, if possible, is to use a branch of the profunda femoral artery to limit the high flow velocity and pressure gradient. More proximally, the lateral circumflex femoral vessels are the best choice. For very distal femoral defects, the end-to-side anastomosis to the genicular vessels can be used. Depending on the exposure for vascular anastomosis, the fibula may be positioned orthograde or retrograde. The cross-sectional size of the femur allows for intramedullary placement of the fibula or an eccentric end-to-end within the segmental defect. Hou et al45 successfully used two-strut free VFGs to reconstruct femoral defects and noted an average fracture healing time of 7 months. Stabilization is generally with external fixation to avoid implants at the recipient site (Fig. 4).
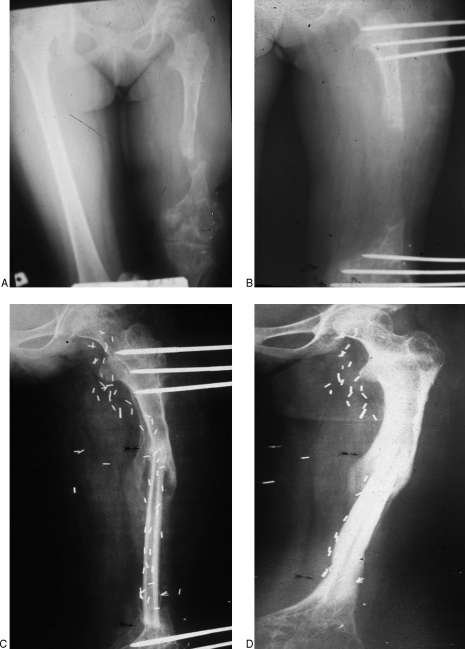
Figure 4.
(A) Anteroposterior radiograph of an 11-year-old child with left femur osteomyelitis with severe shortening. (B) Lengthening was performed with external fixation. (C) Subsequent transfer of 17-cm free VFG was performed. (D) Eighteen months postoperative with union at the junction sites (arrows).
For humeral defects, a single medial incision is generally used for vascular access as well as bony stabilization. An end-to-end anastomosis to the perforating branches of the brachial artery and end-to-end anastomosis to the deep comitante brachial vein can be used. We prefer two transfixing screws proximally and plates and screws distally (Fig. 5).
Figure 5.
(A) Anteroposterior radiograph of right humeral osteomyelitis after multiple debridements. (B, C) Anteroposterior and lateral radiographs of the same humerus after successful transfer of free VFG. Note proximal intramedullary placement with transfixing screw stabilization and distal fixation with plates and screws.
Reconstruction of the ulna or radius can be performed with either the right or left fibula. Bony placement is usually end-to-end because of the similar cross-sectional match of the fibula to the ulna or radius. Either the ulna or radial vessels can be used with end-to-end or end-to-side anastomosis. Plate and screw fixation is preferable.
POSTOPERATIVE MANAGEMENT
For the tibia and femur, immobilization and non–weight bearing is continued until there is radiographic evidence of union. Progressive weight-bearing is initiated once evidence of union is seen; however, full weight-bearing is not allowed until evidence of graft hypertrophy. For humeral defects, sufficient hypertrophy is less of an issue compared with the lower extremity. Postoperatively, weight-bearing activities can be initiated once radiographic union has occurred.
COMPLICATIONS
Recipient site complications are multiple and can include vascular compromise, infection, nonunion of the graft-host site, and fatigue fracture of the graft. Infection usually is related to inadequate debridement of the recipient site, retained hardware, or inappropriate antibiotic treatment for the osteomyelitis. Nonunion of the junction site can be due to persistent infection, failure of the anastomosis, or inadequate stabilization. Fatigue fractures occur more commonly in lower-extremity grafting than in upper-extremity grafting. de Boer and Hermans34 noted stress fractures in 35% of tibial and 32% of femoral reconstruction. Minami et al51 noted a similar incidence of stress fractures in 32% of tibial reconstructions. Fatigue fractures usually occur during the first postoperative year and are due to excessive weight-bearing of an unincorporated graft. This complication can be prevented by limiting weight-bearing until evidence of union as well as hypertrophy of the graft, by use of supportive devices (fracture brace), and by stable fixation to protect the graft postoperatively. Stress fractures can be managed with either case immobilization or open reduction with bone grafting.52
Vail and Urbaniak53 noted the most common donor-site complication is transient weakness of the flexor hallucis longus (FHL), extensor hallucis, or peroneal muscles. Motor weakness was present in 10% of lower extremities at 3 months postoperatively but resolved in most patients and was present in 3% at 5 years.53 We highly recommend using the technique of digital retraction around the peroneal nerve to prevent a traction injury. Contracture of the FHL after its detachment from the fibula can lead to great toe clawing. Prevention of this complication can be performed by attaching the FHL to the soleus with absorbable sutures and placing a short leg cast extending past the toes. Harvesting the fibula in skeletally immature patients can lead to a valgus deformity of the ankle joint, thus transfixation with cortical screws is recommended.22,51,54,55
CLINICAL OUTCOMES
Free VFGs have been used successfully to treat defects of infectious etiology. Han et al52 reported on a series of 160 patients treated with vascularized bone graft. Their indications for the procedure included skeletal defects from nonunion, tumor resection, osteomyelitis, traumatic bone loss, and congenital anomalies. For the entire series, they achieved a 61% primary union rate and an 81% union rate after secondary procedures. However, they did note the subset of patients with osteomyelitis had the worst primary union rates of 48% (29 of 60 patients) and overall union rate of 77% after secondary procedures. The authors recommended vascularized bone transfer as a viable option in the treatment of large skeletal defects associated with infection because amputation is often the only alternative.
Yajima et al41 reported successfully treating 30 of 33 patients with osteomyelitis or infected nonunions using free VFGs. Local recurrence of infection occurred in four patients. The same author also treated methicillin-resistant Staphylococcus aureus using free VFGs.56 In this series, 18 of 20 (90%) patients had successful union, but 6 of the 20 patients had a recurrence of local infection requiring additional procedures for final eradication. One fibular graft ultimately failed and required removal. Dell and Sheppard57 successfully used VFGs to treat infected nonunions of forearm fractures. Their series was limited to only four patients, but all bone junctures healed primarily with the exception of one that required supplemental cancellous grafting. Other studies reported similar results.2,16
Reconstruction of large skeletal defects secondary to osteomyelitis using free VFGs has also been successful in children. Zalavras et al22 reported a series of 8 children with an average age of 7 years and an average skeletal defect of 11.8 cm treated with free VFGs. Union occurred primarily in 7 of 8 patients with a mean time of 3.5 months, and after iliac crest bone grafting in the remaining patient. No recurrence of deep infection occurred in this series.
CONCLUSION
Although other vascularized bone grafts are available for long bone osteomyelitis reconstruction, the VFG has remained our most valuable reconstructive option for the treatment of these defects. The indications for vascularized bone grafting are skeletal defects greater than 6 cm or smaller defects that have failed to heal with nonvascular bone graft. The VFG can be transferred with skin, fascia, and muscle to fill soft tissue defects. Reported success rates using the VFG to bridge skeletal defects from osteomyelitis range from 77 to 90%. Complications include anastomosis failure, donor-site morbidity, and fracture of the grafted bone.
ACKNOWLEDGMENT
The authors have no commercial or proprietary interest in any of the materials noted in this article.
REFERENCES
- Lew D P, Waldvogel F A. Osteomyelitis. N Engl J Med. 1997;336:999–1007. doi: 10.1056/NEJM199704033361406. [DOI] [PubMed] [Google Scholar]
- Moore J R, Weiland A J. Vascularized tissue transfer in the treatment of osteomyelitis. Clin Plast Surg. 1986;13:657–662. [PubMed] [Google Scholar]
- Patzakis M J, Zalavras C G. Chronic posttraumatic osteomyelitis and infected nonunion of the tibia: current management concepts. J Am Acad Orthop Surg. 2005;13:417–427. doi: 10.5435/00124635-200510000-00006. [DOI] [PubMed] [Google Scholar]
- Tu Y K, Yen C Y, Yeh W L, Wang I C, Wang K C, Ueng W N. Reconstruction of posttraumatic long bone defect with free vascularized bone graft: good outcome in 48 patients with 6 years' follow-up. Acta Orthop Scand. 2001;72:359–364. doi: 10.1080/000164701753542014. [DOI] [PubMed] [Google Scholar]
- Manolagas S C. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- Klosterhalfen B, Peters K M, Tons C, Hauptmann S, Klein C L, Kirkpatrick C J. Local and systemic inflammatory mediator release in patients with acute and chronic posttraumatic osteomyelitis. J Trauma. 1996;40:372–378. doi: 10.1097/00005373-199603000-00008. [DOI] [PubMed] [Google Scholar]
- Gristina A G, Costerton J W. Bacterial adherence to biomaterials and tissue. The significance of its role in clinical sepsis. J Bone Joint Surg Am. 1985;67:264–273. [PubMed] [Google Scholar]
- Cierny G, III, Mader J T, Penninck J J. A clinical staging system for adult osteomyelitis. Clin Orthop Relat Res. 2003;Sep(414):7–24. doi: 10.1097/01.blo.0000088564.81746.62. [DOI] [PubMed] [Google Scholar]
- Kaim A H, Gross T, von Schulthess G K. Imaging of chronic posttraumatic osteomyelitis. Eur Radiol. 2002;12:1193–1202. doi: 10.1007/s00330-001-1141-0. [DOI] [PubMed] [Google Scholar]
- Mudun A, Unal S, Aktay R, Akmehmet S, Cantez S. Tc-99m nanocolloid and Tc-99m MDP three-phase bone imaging in osteomyelitis and septic arthritis. A comparative study. Clin Nucl Med. 1995;20:772–778. doi: 10.1097/00003072-199509000-00004. [DOI] [PubMed] [Google Scholar]
- Oyen W J, Horn J R van, Claessens R A, Slooff T J, der Meer J W van, Corstens F H. Diagnosis of bone, joint, and joint prosthesis infections with In-111-labeled nonspecific human immunoglobulin G scintigraphy. Radiology. 1992;182:195–199. doi: 10.1148/radiology.182.1.1727281. [DOI] [PubMed] [Google Scholar]
- Howard C B, Einhorn M, Dagan R, Yagupski P, Porat S. Fine-needle bone biopsy to diagnose osteomyelitis. J Bone Joint Surg Br. 1994;76:311–314. [PubMed] [Google Scholar]
- Mackowiak P A, Jones S R, Smith J W. Diagnostic value of sinus-tract cultures in chronic osteomyelitis. JAMA. 1978;239:2772–2775. doi: 10.1001/jama.239.26.2772. [DOI] [PubMed] [Google Scholar]
- Mader J T, Cripps M W, Calhoun J H. Adult posttraumatic osteomyelitis of the tibia. Clin Orthop Relat Res. 1999;Mar(360):14–21. doi: 10.1097/00003086-199903000-00004. [DOI] [PubMed] [Google Scholar]
- Waldvogel F A, Medoff G, Swartz M N. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects. 3. Osteomyelitis associated with vascular insufficiency. N Engl J Med. 1970;282:316–322. doi: 10.1056/NEJM197002052820606. [DOI] [PubMed] [Google Scholar]
- Doi K, Kawakami F, Hiura Y, Oda T, Sakai K, Kawai S. One-stage treatment of infected bone defects of the tibia with skin loss by free vascularized osteocutaneous grafts. Microsurgery. 1995;16:704–712. doi: 10.1002/micr.1920161009. [DOI] [PubMed] [Google Scholar]
- Duwelius P J, Schmidt A H. Assessment of bone viability in patients with osteomyelitis: preliminary clinical experience with laser Doppler flowmetry. J Orthop Trauma. 1992;6:327–332. doi: 10.1097/00005131-199209000-00010. [DOI] [PubMed] [Google Scholar]
- Swiontkowski M F. Surgical approaches in osteomyelitis. Use of laser Doppler flowmetry to determine nonviable bone. Infect Dis Clin North Am. 1990;4:501–512. [PubMed] [Google Scholar]
- Dahners L E, Bos G D. Fluorescent tetracycline labeling as an aid to debridement of necrotic bone in the treatment of chronic osteomyelitis. J Orthop Trauma. 2002;16:345–346. doi: 10.1097/00005131-200205000-00009. [DOI] [PubMed] [Google Scholar]
- McCraw J B, Dibbell D G, Carraway J H. Clinical definition of independent myocutaneous vascular territories. Plast Reconstr Surg. 1977;60:341–352. [PubMed] [Google Scholar]
- Holtom P D, Patzakis M J. Newer methods of antimicrobial delivery for bone and joint infections. Instr Course Lect. 2003;52:745–749. [PubMed] [Google Scholar]
- Zalavras C G, Femino D, Triche R, Zionts L, Stevanovic M. Reconstruction of large skeletal defects due to osteomyelitis with the vascularized fibular graft in children. J Bone Joint Surg Am. 2007;89:2233–2240. doi: 10.2106/JBJS.E.01319. [DOI] [PubMed] [Google Scholar]
- Salgado C J, Mardini S, Jamali A A, Ortiz J A, Gonzales R, Chen H C. Muscle versus non-muscle flaps in the reconstruction of chronic osteomyelitis defects. Plast Reconstr Surg. 2006;118:1401–1411. doi: 10.1097/01.prs.0000239579.37760.92. [DOI] [PubMed] [Google Scholar]
- Paley D, Herzenberg J E. Intramedullary infections treated with antibiotic cement rods: preliminary results in nine cases. J Orthop Trauma. 2002;16:723–729. doi: 10.1097/00005131-200211000-00007. [DOI] [PubMed] [Google Scholar]
- Stevanovic M, Gutow A P, Sharpe F. The management of bone defects of the forearm after trauma. Hand Clin. 1999;15:299–318. [PubMed] [Google Scholar]
- Chan Y S, Ueng S W, Wang C J, Lee S S, Chao E K, Shin C H. Management of small infected tibial defects with antibiotic-impregnated autogenic cancellous bone grafting. J Trauma. 1998;45:758–764. doi: 10.1097/00005373-199810000-00023. [DOI] [PubMed] [Google Scholar]
- Ueng S W, Wei F C, Shih C H. Management of femoral diaphyseal infected nonunion with antibiotic beads local therapy, external skeletal fixation, and staged bone grafting. J Trauma. 1999;46:97–103. doi: 10.1097/00005373-199901000-00016. [DOI] [PubMed] [Google Scholar]
- Chen C Y, Ueng S W, Shih C H. Staged management of infected humeral nonunion. J Trauma. 1997;43:793–798. doi: 10.1097/00005373-199711000-00010. [DOI] [PubMed] [Google Scholar]
- Green S A. Osteomyelitis. The Ilizarov perspective. Orthop Clin North Am. 1991;22:515–521. [PubMed] [Google Scholar]
- Green S A, Jackson J M, Wall D M, Marinow H, Ishkanian J. Management of segmental defects by the Ilizarov intercalary bone transport method. Clin Orthop Relat Res. 1992:136–142. [PubMed] [Google Scholar]
- Lin C H, Wei F C, Levin L S, et al. Free composite serratus anterior and rib flaps for tibial composite bone and soft-tissue defect. Plast Reconstr Surg. 1997;99:1656–1665. [PubMed] [Google Scholar]
- Rhomberg M, Frischhut B, Ninkovic M, Schwabegger A H. A single-stage operation in the treatment of chronic osteomyelitis of the lower extremity including reconstruction with free vascularized iliac bone graft and free-tissue transfer. Plast Reconstr Surg. 2003;111:2353–2361. discussion 2362–2353. doi: 10.1097/01.PRS.0000061006.99819.24. [DOI] [PubMed] [Google Scholar]
- Weiland A J, Phillips T W, Randolph M A. Bone grafts: a radiologic, histologic, and biomechanical model comparing autografts, allografts, and free vascularized bone grafts. Plast Reconstr Surg. 1984;74:368–379. [PubMed] [Google Scholar]
- de Boer H HWM, Hermans J. Reconstruction of large skeletal defects by vascularized fibula transfer: factors that influenced the outcome of union in 62 cases. Int Orthop. 1990;14:121–128. doi: 10.1007/BF00180115. [DOI] [PubMed] [Google Scholar]
- Malizos K N, Zalavras C G, Soucacos P N, Beris A E, Urbaniak J R. Free vascularized fibular grafts for reconstruction of skeletal defects. J Am Acad Orthop Surg. 2004;12:360–369. doi: 10.5435/00124635-200409000-00010. [DOI] [PubMed] [Google Scholar]
- Aldridge J M, III, Berend K R, Gunneson E E, Urbaniak J R. Free vascularized fibular grafting for the treatment of postcollapse osteonecrosis of the femoral head. Surgical technique. J Bone Joint Surg Am. 2004;86(Suppl 1):87–101. doi: 10.2106/00004623-200403001-00012. [DOI] [PubMed] [Google Scholar]
- Malizos K N, Nunley J A, Goldner R D, Urbaniak J R, Harrelson J M. Free vascularized fibula in traumatic long bone defects and in limb salvaging following tumor resection: comparative study. Microsurgery. 1993;14:368–374. doi: 10.1002/micr.1920140603. [DOI] [PubMed] [Google Scholar]
- Taylor G I, Townsend P, Corlett R. Superiority of the deep circumflex iliac vessels as the supply for free groin flaps. Plast Reconstr Surg. 1979;64:595–604. [PubMed] [Google Scholar]
- Teot L, Bosse J P, Tassin X. Scapular crest. Anatomical and harvesting technique. Ann Chir Plast Esthet. 1993;38:100–106. [PubMed] [Google Scholar]
- Yazar S, Lin C H, Wei F C. One-stage reconstruction of composite bone and soft-tissue defects in traumatic lower extremities. Plast Reconstr Surg. 2004;114:1457–1466. doi: 10.1097/01.prs.0000138811.88807.65. [DOI] [PubMed] [Google Scholar]
- Yajima H, Tamai S, Mizumoto S, Inada Y. Vascularized fibular grafts in the treatment of osteomyelitis and infected nonunion. Clin Orthop Relat Res. 1993:256–264. [PubMed] [Google Scholar]
- Manojlovic R. Anatomy of the Osteoseptocutaneous Flap of Fibulae and Clinical Applications [master's thesis] Belgrade, Serbia: University of Belgrade; 1989.
- Carroll W R, Esclamado R. Preoperative vascular imaging for the fibular osteocutaneous flap. Arch Otolaryngol Head Neck Surg. 1996;122:708–712. doi: 10.1001/archotol.1996.01890190006003. [DOI] [PubMed] [Google Scholar]
- Young D M, Trabulsy P P, Anthony J P. The need for preoperative leg angiography in fibula free flaps. J Reconstr Microsurg. 1994;10:283–287. discussion 287–289. doi: 10.1055/s-2007-1006596. [DOI] [PubMed] [Google Scholar]
- Chang D W, Oh H K, Robb G L, Miller M J. Management of advanced mandibular osteoradionecrosis with free flap reconstruction. Head Neck. 2001;23:830–835. doi: 10.1002/hed.1121. [DOI] [PubMed] [Google Scholar]
- Yajima H, Tamai S, Mizumoto S, Sugimura M, Horiuchi K. Vascularized fibular graft for reconstruction after resection of aggressive benign and malignant bone tumors. Microsurgery. 1992;13:227–233. doi: 10.1002/micr.1920130504. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Shimamura K, Iwai Y, Yamauchi S, Ueno T. Free vascularized fibular transplant. A new method for monitoring circulation of the grafted fibula. J Bone Joint Surg Am. 1983;65:1295–1301. [PubMed] [Google Scholar]
- Pelissier P, Casoli V, Demiri E, Martin D, Baudet J. Soleus-fibula free transfer in lower limb reconstruction. Plast Reconstr Surg. 2000;105:567–573. doi: 10.1097/00006534-200002000-00014. [DOI] [PubMed] [Google Scholar]
- Wood M B. In: Wood MD, editor. Atlas of Reconstructive Microsurgery. Rockville, MD: Aspen Publishers; 1990. Free osseous tissue transfers. pp. 65–91.
- Weiland A J. Vascularized bone transfers. Instr Course Lect. 1984;33:446–460. [PubMed] [Google Scholar]
- Minami A, Kasashima T, Iwasaki N, Kato H, Kaneda K. Vascularized fibular grafts. An experience of 102 patients. J Bone Joint Surg Br. 2000;82:1022–1025. doi: 10.1302/0301-620x.82b7.10332. [DOI] [PubMed] [Google Scholar]
- Han C S, Wood M B, Bishop A T, Cooney W P., III Vascularized bone transfer. J Bone Joint Surg Am. 1992;74:1441–1449. [PubMed] [Google Scholar]
- Vail T P, Urbaniak J R. Donor-site morbidity with use of vascularized autogenous fibular grafts. J Bone Joint Surg Am. 1996;78:204–211. doi: 10.2106/00004623-199602000-00006. [DOI] [PubMed] [Google Scholar]
- Dormans J P, Krajbich J I, Zuker R, Demuynk M. Congenital pseudarthrosis of the tibia: treatment with free vascularized fibular grafts. J Pediatr Orthop. 1990;10:623–628. doi: 10.1097/01241398-199009000-00010. [DOI] [PubMed] [Google Scholar]
- Simonis R B, Shirali H R, Mayou B. Free vascularized fibular grafts for congenital pseudarthrosis of the tibia. J Bone Joint Surg Br. 1991;73:211–215. doi: 10.1302/0301-620X.73B2.2005141. [DOI] [PubMed] [Google Scholar]
- Yajima H, Kobata Y, Shigematsu K, et al. Vascularized fibular grafting in the treatment of methicillin-resistant Staphylococcus aureus osteomyelitis and infected nonunion. J Reconstr Microsurg. 2004;20:13–20. doi: 10.1055/s-2004-818044. [DOI] [PubMed] [Google Scholar]
- Dell P C, Sheppard J E. Vascularized bone grafts in the treatment of infected forearm nonunions. J Hand Surg [Am] 1984;9:653–658. doi: 10.1016/s0363-5023(84)80006-4. [DOI] [PubMed] [Google Scholar]