ABSTRACT
The diagnostic imaging of osteomyelitis can require the combination of diverse imaging techniques for an accurate diagnosis. Conventional radiography should always be the first imaging modality to start with, as it provides an overview of the anatomy and the pathologic conditions of the bone and soft tissues of the region of interest. Sonography is most useful in the diagnosis of fluid collections, periosteal involvement, and surrounding soft tissue abnormalities and may provide guidance for diagnostic or therapeutic aspiration, drainage, or tissue biopsy. Computed tomography scan can be a useful method to detect early osseous erosion and to document the presence of sequestrum, foreign body, or gas formation but generally is less sensitive than other modalities for the detection of bone infection. Magnetic resonance imaging is the most sensitive and most specific imaging modality for the detection of osteomyelitis and provides superb anatomic detail and more accurate information of the extent of the infectious process and soft tissues involved. Nuclear medicine imaging is particularly useful in identifying multifocal osseous involvement.
Keywords: Osteomyelitis, computed tomography, magnetic resonance imaging, ultrasound, nuclear medicine
Bone is normally resistant to infection, but trauma, bacteremia, surgery, or foreign bodies may disrupt and lead to the onset of osteomyelitis. It is a difficult-to-treat condition characterized by progressive inflammatory destruction and new apposition of bone.1 It is most commonly caused by pyogenic bacteria and mycobacteria. Its manifestations are heterogeneous, depending on the age of the patient, specific causative microorganism, anatomic area of involvement, segment of affected bone, route of contamination, systemic and local host factors, as well as the presence of underlying comorbidities. One of the greatest challenges of osteomyelitis is to make an opportune diagnosis to provide adequate treatment. Imaging techniques play a key role in the early diagnosis and follow-up.2 Global epidemiologic data regarding community-acquired bone infections in adults varies significantly, with a higher incidence in developing countries. Bone infections show a bimodal age distribution, occurring most commonly in people younger than 20 or older than 50 years of age.3 The disease generates substantial health costs and disability, although the estimated annual incidence in the United States is less than 2%.4
CLASSIFICATION SYSTEMS
There are different classification systems to categorize osteomyelitis. Historically, it has been labeled as acute, subacute, or chronic depending on its clinical course, histologic findings, and disease duration,5 but there is no consensual agreement on the temporal scale used or specific findings. As a result, some researchers have proposed more detailed classification systems for osteomyelitis.
Waldvogel et al6,7,8 proposed a staging system based on the infection's pathogenesis, dividing the disease into three separate groups: hematogenous, secondary to a contiguous focus of infection, and associated with vascular insufficiency.
An additional classification system was proposed by Cierny and colleagues9 (Table 1). This descriptive system takes into account the anatomic area of osseous involvement and the host's physiologic status, irrespective of the disease's etiology, skeletal location, or duration of infection, providing a useful framework for evaluation of a patient and treatment planning.
Table 1.
Cierny-Mader Staging for Osteomyelitis
| Anatomic type | |
| Stage 1 | Medullary osteomyelitis |
| Stage 2 | Superficial osteomyelitis |
| Stage 3 | Localized osteomyelitis |
| Stage 4 | Diffuse osteomyelitis |
| Physiologic class | |
| A host | Normal |
| B host | Systemic compromise (Bs) |
| B host | Local compromise (Bl) |
| B host | Systemic and local compromise (Bls) |
| C host | Treatment worse than disease |
| Systemic or local factors that affect immune surveillance, metabolism, and local vascularity | |
| Systemic (Bs) | Local (Bl) |
| Malnutrition | Chronic lymphedema |
| Renal or hepatic failure | Major-vessel compromise |
| Diabetes mellitus | Small-vessel compromise |
| Chronic hypoxia | Vasculitis |
| Immune disease | Venous stasis |
| Malignancy | Extensive scarring |
| Extremes of age | Radiation fibrosis |
| Immunosuppression or immune deficiency | Neuropathy |
| Tobacco abuse | |
PATHOGENESIS
Microorganisms can enter bone by the hematogenous route, by direct introduction from a contiguous focus of infection, or by a penetrating wound. Trauma, ischemia, and foreign bodies enhance the susceptibility of bone to microbial invasion. The initial changes in bone after the inoculation of bacteria are basically alterations in pH and capillary permeability that contribute to regional edema, cytokine release, tissue breakdown, leukocyte recruitment, decreased oxygen tension, increased local pressure, small-vessel thrombosis, and bone deterioration.10 As the infection spreads into the medullary cavity, increased pressure causes its extension into the cortex by Haversian and Volkmann canals with subsequent spread into the subperiosteal space and finally to the periosteum and adjacent soft tissues.
TERMINOLOGY
Descriptive terms have been applied to certain radiographic and pathologic characteristics that are encountered during the course of osteomyelitis. Infective osteitis indicates contamination of the bony cortex. Infective periostitis implies contamination of the periosteal cloak that surrounds the bone. A sequestrum represents a segment of necrotic bone that is separated from living bone by granulation tissue. An involucrum denotes a layer of living bone that has formed about dead bone; it can become perforated by tracts. An opening in the involucrum is termed cloaca. Tracts reaching the skin surface from the bone are termed sinuses, although they sometimes are described as fistulae. A bone abscess (Brodie's abscess) is a sharply delineated focus of infection. It is lined by granulation tissue and frequently is surrounded by eburnated bone.11
DIAGNOSIS
Early diagnosis of acute osteomyelitis is critical because prompt antibiotic therapy may prevent necrosis of bone. Osteomyelitis is primarily a clinical diagnosis, although the clinical picture may be confusing. An inadequate or late diagnosis significantly diminishes the cure rate and increases the degree of complications and morbidity; for these reasons, imaging modalities are essential to confirm the presumed clinical diagnosis and to provide information regarding the exact site and extent of the infectious process. Imaging information can be extremely helpful to the clinician planning medical or surgical treatment.12
IMAGING TECHNIQUES
Several imaging modalities have been used in the evaluation of suspected osteomyelitis, but no one can definitively confirm the presence or absence of infection.13 Cross-sectional imaging modalities such as computed tomography (CT) scanning and magnetic resonance imaging (MRI) are now considered standard in the diagnosis of osteomyelitis. Although expensive, they are sensitive and specific. These modalities give excellent anatomic delineation of the infected area and the surrounding soft tissues. Nuclear medicine techniques, although highly sensitive, are sometimes nonspecific. Confirmation of the presence of osteomyelitis usually entails a combination of imaging techniques. The main features of osteomyelitis on individual imaging techniques are summarized in Table 2.
Table 2.
Osteomyelitis Findings in Different Imaging Techniques
| Technique | Advantages | Disadvantages | Sensitivity/Specificity | Main Findings |
|---|---|---|---|---|
| Conventional X-ray | Inexpensive | Late diagnosis | 43 to 75%/75 to 83% | Lytic lesions, osteopenia, periosteal thickening, loss of trabecular architecture, new bone apposition |
| Reproducible | Confusing | |||
| Accessible | Radiation | |||
| Computed tomography | Excellent spatial resolution | Cost | 67%/50% (Chronic osteomyelitis) | Blurring of fat planes |
| Availability | Increased density of fatty marrow | |||
| Radiation exposure | Periosteal reaction | |||
| Cortical erosion or destruction | ||||
| Sequestra, involucra, intraosseous gas | ||||
| Ultrasound | Accessibility, inexpensive, real-time evaluation | Operator dependent | To be determined | Elevated periosteum |
| Guided aspiration- biopsy | US beam cannot cross cortical bone | Soft tissue abscess | ||
| Fluid collection | ||||
| Magnetic resonance imaging | Excellent spatial resolution | Cost | 82 to 100%/75 to 96% | Acute |
| Early detection | Availability | T1-weighted: low-signal-intensity medullary space | ||
| Assessment of the extent of tissue affected | Time requested | T2-weighted: high signal intensity surrounding inflammatory processes, edema | ||
| Gadolinium: enhances areas of necrosis | ||||
| Subacute | ||||
| Evidence of Brodie's abscess, single or multiple radiolucent abscesses | ||||
| T1-weighted: central abscess cavity with low signal intensity | ||||
| T2-weighted: high signal intensity of granulation tissue surrounded by low-signal-intensity band of bone sclerosis (double-line effect) | ||||
| Chronic | ||||
| T1- and T2-weighted: low-signal-intensity areas of devascularized fibrotic scarring in the marrow | ||||
| Three-phase bone scintigraphy | Sensitive | Nonspecific | ~85%/~25% | Focal hyperperfusion |
| Availability | Further imaging evaluation required | Focal hyperemia | ||
| Relatively inexpensive | Focal bone uptake | |||
| Early detection | ||||
| Combined bone andgallium scintigraphy | Reliable when clearly positive or negative | Need for two isotopes with multiple imaging sessions over several days | ~60%/~80% | Localized area of increased uptake |
| High radiation exposure | ||||
| Large number of equivocal results. | ||||
| Long examination time |
CONVENTIONAL RADIOGRAPHY
The evaluation usually begins with plain radiographs in all patients suspected of having osteomyelitis; plain radiographs may suggest the correct diagnosis, exclude other diagnostic possibilities, or provide clues for underlying pathologic conditions. Plain radiographs initially show soft tissue changes, muscle swelling, and blurring of the soft tissue planes. In pyogenic infections, the first change in bone indicates that the infectious process has been present for 2 to 3 weeks or more. In general, osteomyelitis must extend at least 1 cm and compromise 30 to 50% of bone mineral content to produce noticeable changes in plain radiographs. Early findings may be subtle, and changes may not be obvious until 5 to 7 days in children and 10 to 14 days in adults. Typical early bony changes include: periosteal thickening, lytic lesions, endosteal scalloping, osteopenia, loss of trabecular architecture, and new bone apposition.14 The specificity of plain radiographs for the detection of osteomyelitis is higher than its sensitivity, and because of this, use of alternative imaging methods such as scintigraphic modalities and MRI has been prompted. Single or multiple radiolucent abscesses can be evident during subacute or chronic stages of osteomyelitis. These abscesses now are defined as circumscribed lesions showing predilection for (but not confinement to) the extremes of tubular bones; they are characteristically found in subacute pyogenic osteomyelitis, usually of staphylococcal origin. Brodie's abscesses are especially common in children, more typically boys. In this age group, they appear in the metaphyses, particularly that of the distal or proximal portions of the tibia.
The distinguishing feature of chronic osteomyelitis is necrotic bone, which is formed in an average of 10 days, nevertheless plain radiographs are unable to detect sequestra or sclerotic bone for many weeks. Periostitis, involucrum formation, and sinus tracts are due to subperiosteal abscess with lifting of the periosteum, new bone formation, and soft tissue fistulas. All of these findings are indicative of the protracted nature of the infection process. Table 3 shows a radiographic-pathologic correlation in osteomyelitis.
Table 3.
Radiographic Correlation with Pathologic Changes
| Radiographic Abnormality | Pathologic Abnormality |
|---|---|
| Adapted from Resnick D, Nawayama G. Osteomyelitis, septic arthritis and soft tissue infection: mechanisms and situations. In: Resnick D, ed. Diagnosis of Bone and Joint Disorders. 3rd ed. Philadelphia, PA: WB Saunders; 1995:2335. | |
| Soft tissue swelling with obliteration of tissue planes and mass formation | Vascular changes, edema of soft tissues, and infectious penetration of periosteum |
| Periostitis and involucrum | Subperiosteal abscess formation with lifting of the periosteum and bone formation |
| Increasing lysis, cortical lucency | Infection in Haversian and Volkmann canals of cortex |
| Osteoporosis, bone lysis, and cortical lucency | Infection in medullary space, Haversian and Volkmann canals with abscess formation and trabecular destruction |
| Single or multiple radiolucent cortical or medullary lesions with surrounding sclerosis | Localized cortical and medullary abscess |
| Sequestration | Thrombosis of metaphyseal vessels and interruption of periosteal vessels with cortical necrosis |
| Sinus tracts | External migration of dead pieces of cortex with breakdown of skin and subcutaneous tissue |
SINOGRAPHY
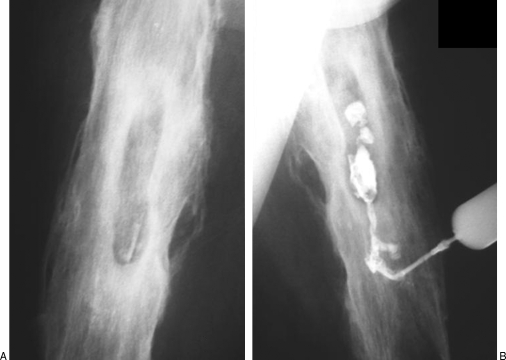
Opacification of a sinus tract can produce important information that influences the choice of therapy. In this technique, a small flexible catheter is placed within a cutaneous opening. Retrograde injection of contrast material defines the course and extent of the sinus tract and its possible communications with neighboring structures. Sinography may be combined with CT for better delineation of the sinus tracts. Figure 1 shows sinography in a patient with chronic osteomyelitis.
Figure 1.
Chronic osteomyelitis: role of sinography. (A) Anteroposterior view of the right femur demonstrates several radiodense, sharply marginated foci within lucent cavities suggestive of sequestration. (B) Oblique view showing retrograde opacification of a sinus tract defining the course and extent of the fistula and confirming the communication with an abscess in the bone.
COMPUTED TOMOGRAPHY
CT provides excellent multiplanar reconstructions of the axial images allowing delineation of even the most subtle osseous changes. In chronic osteomyelitis, CT demonstrates abnormal thickening of the affected cortical bone, with sclerotic changes, encroachment of the medullary cavity, and chronic draining sinus. Although CT may show these changes earlier than do plain radiographs, CT is less desirable than MRI because of decreased soft tissue contrast as well as exposure to ionizing radiation.
The major role of this technique in osteomyelitis is the detection of sequestra in cases of chronic osteomyelitis, as these pieces of necrotic bone can be masked by the surrounding osseous abnormalities on conventional radiography. The presence of pieces of sequestered bone suggests activity of the infectious process, and their detection is helpful to guide the therapeutic options. CT is superior to MRI for the detection of sequestra, cloacas, involucra, or intraosseous gas and can help in the guidance of needle biopsies and joint aspiration; furthermore, it is also valuable in cases of vertebral osteomyelitis.15
In a systematic review and meta-analysis assessing the accuracy of different imaging techniques for the evaluation of chronic osteomyelitis, CT yielded a sensitivity of 0.67 with a 95% confidence interval (0.24 to 0.94), and specificity of 0.50 (0.03 to 0.97).16
It is important to mention that when metal is present in or near the area of osteomyelitis, there is substantial loss of image resolution17 due to a beam-hardening artifact.9
ULTRASOUND
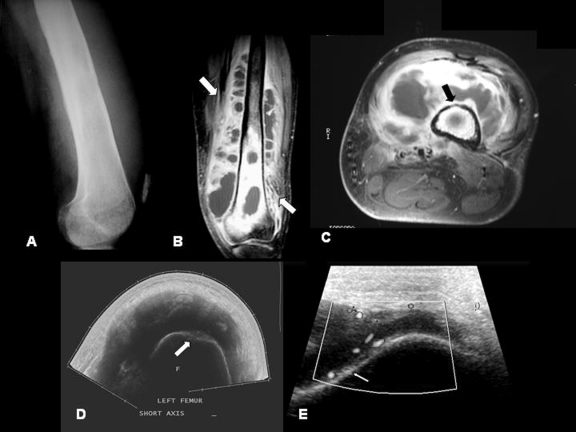
Ultrasound (US) has multiple advantages: it is readily accessible, can be performed quickly without delay and with minimal discomfort to the patient, it is useful in regions that are complicated by orthopedic instrumentation and therefore might not be well seen with MRI or CT, is useful in patients in who MRI is contraindicated, has a lower cost, does not use ionizing radiation, and offers real time imaging. For these reasons, US is a useful tool in the evaluation of musculoskeletal infections, particularly helpful in differentiating acute or chronic infections from tumors or noninfective conditions. It is also able to localize the site and extent of infection, identify precipitating factors such as foreign bodies or fistulae, and provides guidance for diagnostic or therapeutic aspiration or biopsy.18 US can detect features of osteomyelitis several days earlier than can conventional radiographs (predominately in children). Acute osteomyelitis is recognized by elevation of the periosteum by a hypoechoic layer of purulent material. In chronic osteomyelitis, US can also be used to assess involvement of the adjacent soft tissues. Soft tissue abscesses related to chronic osteomyelitis are identified as hypoechoic or anechoic fluid collections, which may extend around the bony contours. Finally, cortical erosions can become apparent on US2 (Fig. 2).
Figure 2.
Osteomyelitis due to direct implantation in a young patient. (A) Lateral radiograph of the left femur showing cortical irregularities and soft tissue swelling with increased density and obliteration of tissue planes. (B, C) Coronal and axial T1-weighted MRI scans show extensive soft tissue abscesses (arrows) with associated cortical irregularities (black arrow) and abnormal areas of high and low signal within the medullary cavity indicative of chronic osteomyelitis. (D) Transverse US panoramic scan of the thigh showing displacement of the soft tissues due to a huge staphylococcal abscess adjacent to an irregular femoral cortex (arrow). (E) Long axis view of the femur displaying periosteal lifting (arrow). Power Doppler highlights hyperemia around the periosteum.
In pediatric patients, US is able to identify joint effusion or subperiosteal fluid associated with septic arthritis or osteomyelitis even before any apparent findings on plain radiographs19 and does not require sedation of small children. Power Doppler sonography is useful to highlight hyperemia around the periosteum and surrounding soft tissue abscesses.
MAGNETIC RESONANCE IMAGING
MRI allows early detection of osteomyelitis and assessment of the extent of involvement and the activity of the disease in cases of chronic bone infection. It is considered the most useful imaging technique to evaluate suspected osteomyelitis because of its ability to demonstrate changes in the water content of bone marrow with an excellent structural definition and spatial resolution.20 MRI is highly sensitive for detecting osteomyelitis as early as 3 to 5 days after the onset of infection.21 MRI advantages go far beyond diagnosis only, helping the surgeon to plan the optimal surgical management22,23 and to assess the extent of devitalized tissue, which contributes to the definition of the critical adjacent structures involved that would require modified management to avoid morbidity and complications24 (Fig. 3). Metallic implants, however, may produce local artifacts that decrease image quality.
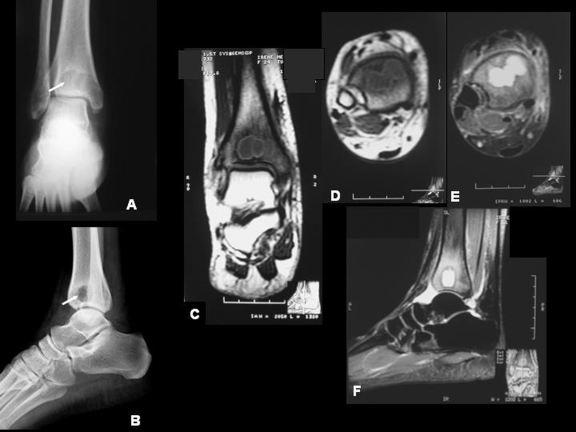
Figure 3.
Hematogenous osteomyelitis: Brodie's abscess. (A, B) Anteroposterior and lateral radiographs of the distal tibia outline a typical appearance of an abscess. Observe the well-circumscribed, oval, and radiolucent lesion with surrounding sclerosis extending to the closing joint (arrows). (C) Axial T1-weighted MRI scan showing an intramedullary hypointense, lobulated lesion, with a well-defined outline. (D, E) Coronal T1-weighted and T2 fat-suppressed MRI scans showing marrow involvement. (F) Sagittal T2 fat-suppressed MRI scan displaying hyperintense circular and well-defined lesion. The appearance is that of a Brodie's abscess.
The MRI findings are different depending on the pulse sequences used (T1-weighted or T2-weighted) and on the disease stage. Initial MRI screening usually includes T1-weighted and T2-weighted spin-echo pulse sequences. Different pulse sequences and imaging protocols can be used in the evaluation of the musculoskeletal system. Depending on the pulse sequences used, major differences can be noted on the signal intensity and appearance of normal and abnormal tissues. The combination of short-tau inversion-recovery (STIR) and T1 spin echo sequences shows a high sensitivity and specificity for the detection of osteomyelitis, thus obviating the need for any additional examinations.25 The earliest finding of acute osteomyelitis on MRI is an alteration of the normal marrow signal intensity, which can be appreciated as early as 1 to 2 days after the onset of infection; the edema and exudates within the medullary space produce an ill-defined low-signal intensity on the T1-weighted images and a high signal on T2-weighted and STIR or fat-suppressed sequences.
On MRI, a sequestrum is seen as a low signal intensity structure on T1-weighted and STIR sequences, whereas the surrounding granulation tissue is intermediate to low signal intensity on T1-weighted images and high signal intensity with STIR or T2-weighted sequences. With use of intravenous contrast (gadolinium), the granulation tissue is enhanced, whereas the sequestrum remains low signal intensity. The ossified periosteal shell and the dead tubular cortical bone of an involucrum have low signal intensity on all pulse sequences; periosteal reaction and cortical bone are separated by linear intermediate to high signal intensity on T2-weighted or STIR images. A cloaca is perceived by a linear low signal intensity periosteum that is elevated from the cortical bone or the thickened cortex that is interrupted by a high signal intensity gap on T2-weighted images.17 This high signal intensity can be seen extending into the soft tissues from the cloaca and may form a sinus tract or abscess.26 Demonstration of increased signal intensity of the bone marrow on T2-weighted images may represent postsurgical or postinfectious granulation tissue and not necessarily persistent infection. However, serial magnetic resonance studies showing progression of this process in the marrow indicates the presence of active osteomyelitis. Disadvantages of MRI are its occasional inability to distinguish infectious from reactive inflammation and its difficulty imaging sites with metallic implants, such as joint prostheses or fixation devices.
NUCLEAR MEDICINE IMAGING
Nuclear medicine imaging can detect osteomyelitis 10 to 14 days before changes are visible on plain radiographs. Several agents have been studied, including technetium-99m–labeled methylene diphosphonate (99mTc-MDP), gallium-67 citrate, and indium-111–labeled white blood cells. These are highly sensitive but have the inconvenience of low specificity.27 Consequently, it is difficult to differentiate osteomyelitis from other conditions such as crystal arthropathies, arthritis, fractures, neoplasia, or cellulites. Nuclear medicine scans may be a useful adjunctive study when x-rays are altered by pathologic or postsurgical changes.
Three-Phase Bone Scintigraphy
Polyphosphates and phosphates have revolutionized the field of nuclear bone imaging since their introduction in the 1970s. Radiopharmaceutical retention depends on blood flow, capillary permeability, and adsorption into the hydroxyapatite crystals. Some binding to collagen may also occur. Polyphosphates are subjected to enzymatic degradation in vivo and have been replaced by phosphonates, which are not subjected to enzymatic degradation in vivo. In clinical practice, bone scintigraphy is performed with 99mTc-labeled diphosphonates, usually methylene diphosphonate (99mTc-MDP). After intravenous injection, it has an initial blood clearance half-time of a few minutes. After leaving the capillaries, 99mTc-MDP accumulates first in the perivascular space, then in the bone fluid space, and finally in the bone.28 Depending on the clinical setting, one, three, or four phases are performed. The first phase (flow study or angiogram) consists of a dynamic study of the region of interest. One- to 3-second frames are acquired during the 60 seconds after injection. The second phase (blood pool) consists of static images performed a few minutes after injection and represents intravascular and extravascular activity allowing better spatial resolution between bone, joint, and soft tissues. The third phase is performed 2 to 4 hours after injection and demonstrates the bony structures. Whole body scan or multiple spot views are obtained. Finally, the delayed bone scan shows intense increased activity in the affected bone. Some soft tissue or surrounding bone uptake may also be present but is less intense. When used for suspected osteomyelitis, a three-phase bone scan should be performed. Nuclear medicine can image patients who have prostheses without interference from artifact.
Gallium Scintigraphy
Radiogallium attaches to transferrin, which leaks from the bloodstream into areas of inflammation showing increased isotope uptake in infection, sterile inflammatory conditions, and malignancy. This imaging technique is usually performed 18 to 72 hours after injection and is often performed in conjunction with radionuclide bone imaging. One difficulty with the gallium scan is that it does not show bone detail particularly well and may not distinguish well between bone and nearby soft tissue inflammation.
Gallium scans may reveal abnormal accumulation in patients who have active osteomyelitis when technetium scans reveal decreased activity (“cold” lesions) or perhaps normal activity. Furthermore, gallium accumulation seems to correlate more closely with activity in cases of osteomyelitis than does technetium uptake.29
In Vitro–Labeled and In Vivo–Labeled Leukocyte Imaging
Another nuclear medicine method is the white blood cell scan (WBCS) done with indium-111 tagged leukocytes and more recently with 99mTc-hexamethyl-propyleneamine oxime (HMPAO)-labeled white cells. Like gallium-67, indium-111 scans do not show bony detail or distinguish osteomyelitis from soft tissue infections. The main advantage of the WBCS, however, is the marked improvement in specificity compared with that of bone scans, particularly when complicating conditions are superimposed.17 Labeled leukocyte imaging is the radionuclide procedure of choice for diagnosing so-called complicating osteomyelitis.30 There are significant limitations to the in vitro–labeled leukocyte procedure. It is labor-intensive, not always available, and involves direct handling of blood products. For these reasons, considerable effort has been devoted to the search for in vivo methods of labeling leukocytes, including peptides and antigranulocyte antibodies. BW 250/183 (Granuloscint; CISBio International, Gif sur Yvette, France) is a murine monoclonal IgGI immunoglobulin that binds to the non-specific cross-reacting antigen-95 (NCA-95) antigen present on leukocytes. Sensitivity for osteomyelitis ranges from 69% in the hips to 100% for the lower leg and ankle, probably reflecting easier detection with decreasing marrow distally.
Sulesomab is a 50-kDa fragment antigen-binding (Fab) portion of a murine monoclonal antibody of the IgGI class that binds to the NCA-90 antigen on leukocytes.31 Clinical results have been variable. Reported sensitivity, specificity, and accuracy of the test were 90 to 93%, 85 to 89%, and 88 to 90%, respectively. 99mTc-fanolesumab is a murine M-class immunoglobulin that binds to the CD15 antigen expressed on human neutrophils, eosinophils, and lymphocytes. Binding increases proportionately with increasing numbers of circulating neutrophils and is upregulated with neutrophil activation. Sensitivity reaches 91% with a lower specificity of 69%; nevertheless in 2004, it was withdrawn from the U.S. market as a result of serious reports of cardiopulmonary events.32
Fluorine-18 Fluorodeoxyglucose–Positron Emission Tomography
Fluorine-18 fluorodeoxyglucose (FDG) is transported into cells via glucose transporters. Activated inflammatory cells demonstrate increased expression of glucose transporters. This technique has several potential advantages: results are available within 30 to 60 minutes of tracer administration, imaging is not affected by metallic implant artifacts, and it has a distinctly higher spatial resolution than do images obtained with single photon emitting tracers. Lastly, it is less expensive than other nuclear medicine techniques.33
Positron emission tomography (PET) systems are relatively novel techniques that are being applied in several medical fields. It has been demonstrated that FDG-PET has the highest diagnostic accuracy for confirming or excluding the diagnosis of chronic osteomyelitis in comparison with bone scintigraphy, MRI, or leukocyte scintigraphy; FDG-PET also is superior to leukocyte scintigraphy for detecting chronic osteomyelitis in the axial skeleton.26
Radiolabeled Antibiotics
Use of radiolabeled antibiotics is fast emerging as a promising diagnostic test for the detection of infective lesions because of their specific binding to the bacterial component. The main advantage of these agents may be the differentiation between infection and sterile inflammatory lesions34; they localize in foci of infection; and they may be taken up and metabolized by microorganisms. The compound most extensively studied is 99mTc-ciprofloxacin. Published results have been variable and at this time are available only on an investigational basis.
Radiolabeled Immunoglobulins
A 99mTc-labeled murine immunoglobulin M monoclonal antigranulocyte antibody that binds to human polymorphonuclear leukocyte CD15 antigens has been evaluated. The sensitivity is ~91% with a specificity of 70%. The initial report is promising; however, further studies are needed to evaluate its role in bone infections.35
REFERENCES
- Lew D P, Waldvogel F A. Osteomyelitis. N Engl J Med. 1997;336:999–1007. doi: 10.1056/NEJM199704033361406. [DOI] [PubMed] [Google Scholar]
- Pineda C, Vargas A, Vargas-Rodríguez A. Imaging of osteomyelitis: current concepts. Infect Dis Clin North Am. 2006;20:789–825. doi: 10.1016/j.idc.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Zink B. In: Marx et al, editor. Rosen's Emergency Medicine: Concepts and Clinical Practice. New York, NY: Mosby; 2006. Bone and joint infections.
- David R, Barron B J, Madewell J E. Osteomyelitis, acute and chronic. Radiol Clin North Am. 1987;25:1171–1201. [PubMed] [Google Scholar]
- Carek P J, Dickerson L M, Sack J L. Diagnosis and management of osteomyelitis. Am Fam Physician. 2001;63:2413–2420. [PubMed] [Google Scholar]
- Waldvogel F A, Medoff G, Swart M N. Osteomyelitis: a review of clinical features, therapeutic considerations, and unusual aspects (first of three parts) N Engl J Med. 1970;282:198–206. doi: 10.1056/NEJM197001222820406. [DOI] [PubMed] [Google Scholar]
- Waldvogel F A, Medoff G, Swartz M N. Osteomyelitis: a review of clinical features, therapeutic considerations, and unusual aspects (second of three parts) N Engl J Med. 1970;282:260–266. doi: 10.1056/NEJM197001292820507. [DOI] [PubMed] [Google Scholar]
- Waldvogel F A, Medoff G, Swartz M N. Osteomyelitis: a review of clinical features, therapeutic considerations, and unusual aspects (third of three parts) N Engl J Med. 1970;282:316–322. doi: 10.1056/NEJM197001222820406. [DOI] [PubMed] [Google Scholar]
- Cierny G, III, Mader J T, Pennick J J. A clinical staging system for adult osteomyelitis. Clin Orthop Relat Res. 2003;414:7–24. doi: 10.1097/01.blo.0000088564.81746.62. [DOI] [PubMed] [Google Scholar]
- Tsukayama D T. Pathophysiology of posttraumatic osteomyelitis. Clin Orthop Relat Res. 1999;360:22–29. doi: 10.1097/00003086-199903000-00005. [DOI] [PubMed] [Google Scholar]
- Resnick D, Niwayama G. In: Resnick D, editor. Diagnosis of Bone and Joint Disorders. 3rd ed. Philadelphia, PA: WB Saunders; 1995. Osteomyelitis, septic arthritis and soft tissue infection: mechanisms and situations. pp. 2325–2418.
- Sia I G, Berbari E F. Osteomyelitis. Best Pract Res Clin Rheumatol. 2006;20:1065–1081. doi: 10.1016/j.berh.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Haas D W, McAndrew M P. Bacterial osteomyelitis in adults: evolving considerations in diagnosis and treatment. Am J Med. 1996;101:550–561. doi: 10.1016/s0002-9343(96)00260-4. [DOI] [PubMed] [Google Scholar]
- Kothari N A, Pelchovitz D P, Meyer P J. Imaging of musculoskeletal infections. Radiol Clin North Am. 2001;39:653–671. doi: 10.1016/s0033-8389(05)70304-3. [DOI] [PubMed] [Google Scholar]
- Gold R H, Hawkins R A, Katz R D. Bacterial osteomyelitis: findings on plain radiography CT, MR and scintigraphy. AJR Am J Roentgenol. 1991;157:365–370. doi: 10.2214/ajr.157.2.1853823. [DOI] [PubMed] [Google Scholar]
- Termaat M F, Raijmakers P G, Scholtein H J, et al. The accuracy of diagnostic imaging for the assessment of chronic osteomyelitis: a systematic review and meta-analysis. J Bone Joint Surg Am. 2005;87:2464–2471. doi: 10.2106/JBJS.D.02691. [DOI] [PubMed] [Google Scholar]
- Bohndorf K. Infection of the appendicular skeleton. Eur Radiol. 2004;14(Suppl 3):E53–E63. doi: 10.1007/s00330-003-2039-9. [DOI] [PubMed] [Google Scholar]
- Cardinal E, Bureau N, Aubin B. Role of the ultrasound in musculoskeletal infections. Radiol Clin North Am. 2001;39:191–201. doi: 10.1016/s0033-8389(05)70272-4. [DOI] [PubMed] [Google Scholar]
- Kaiser S, Rosenborg M. Early detection of subperiosteal abscesses by ultrasonography: a means for further successful treatment in pediatric osteomyelitis. Pediatr Radiol. 1994;24:336–339. doi: 10.1007/BF02012120. [DOI] [PubMed] [Google Scholar]
- Meyers S P, Wiener S N. Diagnosis of hematogenous pyogenic vertebral osteomyelitis by magnetic resonance imaging. Arch Intern Med. 1991;151:683–687. [PubMed] [Google Scholar]
- Kocher M S, Lee B, Dolan M, Weinberg J, Shulman S T. Pediatric orthopedic infections; early detection and treatment. Pediatr Ann. 2006;35:112–122. doi: 10.3928/0090-4481-20060201-11. [DOI] [PubMed] [Google Scholar]
- Jevtic V. Vertebral infection. Eur Radiol. 2004;14(Suppl 3):E43–E52. doi: 10.1007/s00330-003-2046-x. [DOI] [PubMed] [Google Scholar]
- Flemming D, Murphey M, McCarthy K. Imaging of the foot and ankle: summary and update. Curr Opin Orthop. 2005;16:54–59. [Google Scholar]
- Towers J D. The use of intravenous contrast in MRI of extremity infection. Semin Ultrasound CT MR. 1997;18:269–275. doi: 10.1016/s0887-2171(97)80017-0. [DOI] [PubMed] [Google Scholar]
- Mahnken A H, Bücker A, Adam G, Günther R W. MRI of osteomyelitis: sensitivity and specificity of STIR sequences in comparison with contrast-enhaned T1 spin echo sequences. Rofo. 2000;172:1016–1019. doi: 10.1055/s-2000-9226. [DOI] [PubMed] [Google Scholar]
- Santiago-Restrepo C, Giménez C R, McCarthy K. Imaging of osteomyelitis and musculoskeletal soft tissue infections: current concepts. Rheum Dis Clin North Am. 2003;29:89–109. doi: 10.1016/s0889-857x(02)00078-9. [DOI] [PubMed] [Google Scholar]
- Littenberg B, Mushlin A I. Technetium bone scanning in the diagnosis of osteomyelitis: a meta-analysis of test performance. J Gen Intern Med. 1992;7:158–163. doi: 10.1007/BF02598005. [DOI] [PubMed] [Google Scholar]
- Schauwecker D S. The scintigraphic diagnosis of osteomyelitis. AJR Am J Roentgenol. 1992;158:9–18. doi: 10.2214/ajr.158.1.1727365. [DOI] [PubMed] [Google Scholar]
- Lewin J S, Rosenfield N S, Hoffer P B, Downing D. Acute osteomyelitis in children: combined Tc-99m and Ga-67 imaging. Radiology. 1986;158:795–804. doi: 10.1148/radiology.158.3.3945755. [DOI] [PubMed] [Google Scholar]
- Palestro C J, Love C, Miller T T. Imaging of musculoskeletal infections. Best Pract Res Clin Rheumatol. 2006;20:1197–1218. doi: 10.1016/j.berh.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Becker W, Blair J, Behr T, et al. Detection of soft tissue infections and osteomyelitis using a technetium-99m-labeled anti-granulocyte monoclonal antibody fragment. J Nucl Med. 1994;35:1436–1443. [PubMed] [Google Scholar]
- Love C, Tronco G, Palestro C. Imaging of infection and inflammation with 99m Tc-fanolesumab. Q J Nucl Med Mol Imaging. 2006;50:113–120. [PubMed] [Google Scholar]
- Stumpe K D, Strobel K. 18 F FDG-PET imaging in musculoskeletal infection. Q J Nucl Med Mol Imaging. 2006;50:131–142. [PubMed] [Google Scholar]
- Britton K E, Wareham D W, Das S S, et al. Imaging bacterial infection with 99mTc ciprofloxacin. J Clin Pathol. 2002;55:817–823. doi: 10.1136/jcp.55.11.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubello D, Casara D, Maran A, Avogaro A, Tiengo A, Muzzio P. Role of antigranulocyte Fab́ fragment antibody scintigraphy (Leukoscan) in evaluating bone infection: acquisition protocol, interpretation criteria and clinical results. Nucl Med Commun. 2004;25:39–47. doi: 10.1097/00006231-200401000-00006. [DOI] [PubMed] [Google Scholar]