ABSTRACT
Although osteomyelitis is a difficult problem, certain conditions make it even more difficult to address. Diabetes, peripheral vascular disease, and radiation are all comorbidities that interfere with wound healing and therefore make the treatment of osteomyelitis challenging. In this article, we discuss these conditions, their pathophysiology, and highlight the special considerations in treating osteomyelitis in patients with these comorbidities.
Keywords: Osteomyelitis, diabetes, peripheral vascular disease, radiation, wound healing
DIABETES
The Scope of the Problem
Diabetes has been estimated to affect 11 million Americans, and of these, ~25% will develop foot problems.1 It has been shown that at least 15% of all hospital admissions and 23% of all hospital days for diabetic patients are directly related to foot infections.2 The end result of diabetic foot infections by and large has been amputation. It has been estimated that 1 in 15 diabetic persons required an amputation in their lifetime.3 Two thirds of all nontraumatic amputations occur in diabetics. Ultimately, ~50% of diabetic patients with a below-knee amputation require a contralateral amputation within 2 years. Finally, patients with bilateral leg amputations have a nearly 100% mortality rate at 5 years. It is clear, therefore, that whereas progressively higher amputations may cure the osteomyelitis, its impact on long-term survival can be devastating.
Risk Factors for Ulceration and Infection in the Diabetic Patient
IMPAIRED PROTECTIVE SENSATION/SENSORY NEUROPATHY
The chronic hyperglycemia associated with diabetes contributes to accumulations of sorbitol and other metabolites, and nerves ultimately lose the ability to conduct electrical impulses. Endoneural hypoxia results in nerve fiber loss.4,5 This ultimately leads to sensory loss of the extremities in a “glove and stocking” fashion, motor function loss, and dysfunction of autonomic nerve regulation of the microvasculature.
Autonomic neuropathy inhibits the ability of the skin to sweat, which leads to excessively dry skin. The skin of the foot is prone to breakdown because the underlubricated skin forms fissures or cracks, providing a portal of entry for bacteria potentially to infect the foot.6
The neuropathy of the diabetic also affects the motor nerves of the intrinsic muscles of the foot, resulting in alterations in pressure distribution and changes in gait. The intrinsic and the extrinsic muscles of the foot are most often affected, and the resulting muscle imbalance leads to weak dorsiflexion and claw toes.7 Furthermore, as has been demonstrated in diabetic mice, the collagen fibrils of soft tissue such as the Achilles' tendon become glycosylated and thickened.8 Because of the loss of Achilles' tendon flexibility, the foot gradually loses its ability to dorsiflex during gait, exaggerating pressure on the forefoot, specifically the metatarsal heads. The loss of the rolling motion of the ankle creates a longer lever arm than normal and places abnormal forces on the midfoot. These forces cause a “nutcracker effect” at the ankle joint, and the deformity from a neuropathic joint can lead to Charcot collapse of the arch or cause localized soft tissue breakdown (i.e., skin and deeper soft tissue necrosis).9 Most commonly, these ulcers are seen under the metatarsal heads, specifically the first metatarsal; however, they can occur anywhere a bony prominence may be found. This “contiguous ulcer” becomes one source of infection that may lead to osteomyelitis.
CHARCOT ARTHROPATHY
The long-term effects of neuropathy in the diabetic foot warrant a discussion of Charcot arthropathy, as many an astute clinician has been fooled by its presentation, which often mimics a severely infected foot. Most commonly seen with diabetic neuropathy, the disorder results in progressive destruction of bone and soft tissues at weight-bearing joints; in its most severe form, it may cause significant disruption of the bony architecture. The exact cause of the arthropathy is unknown, but two theories exist, which are related to the above previously described neuropathic changes. In the neurotraumatic theory, the arthropathy is believed to be caused by an unperceived trauma or injury to an insensate foot. This trauma is compounded by the tight Achilles' tendon incurred, as previously described, as a result of glycosylation of the collagen fibrils. The sensory neuropathy renders the patient unaware of the osseous destruction that occurs with ambulation. This microtrauma leads to progressive destruction and damage to bone and joints. In the neurovascular theory, it is suggested that the autonomic neuropathy increases local blood flow to the extremity because of decreased sympathetic tone. This in turn alters the balance between bone destruction and synthesis, leading to osteopenia.10 In reality, Charcot arthropathy is probably related to a combination of mechanisms. The autonomic neuropathy leads to abnormal bone formation, the sensory neuropathy leads to an insensate joint that is susceptible to trauma, and the tight Achilles' tendon places extra stress on the skeletal framework. The development of abnormal bone with no ability to protect the joint results in gradual bone fracture and in subluxation and collapse of the joint.
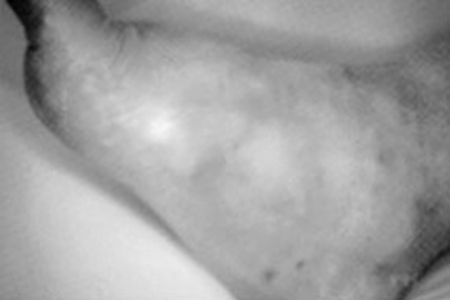
The clinical presentation of Charcot arthropathy can vary widely depending on the stage of the disease. Thus, symptoms can range from mild swelling and no deformity to moderate deformity with significant swelling. Acute Charcot arthropathy almost always presents with signs of inflammation. Profound unilateral swelling, an increase in local skin temperature compared with the unaffected foot, erythema, joint effusion, and bone resorption in an insensate foot are commonly present. These characteristics, in the presence of intact skin and a loss of protective sensation, are often pathognomonic of acute Charcot arthropathy. The distinction between an acute Charcot collapse and acute deep abscess or infection can be confusing, but with the latter, the patient will usually have a history of preceding ulcer. The key is to determine whether the presenting Charcot collapse is sterile or has superimposed infection (Figs. 1–3).
Figure 1.
Acute Charcot collapse with characteristic erythema, rubor, and swelling.1 Of note, there is no ulceration on this foot. However, with a history of breakdown or wound, a deep abscess cannot be excluded.
Figure 2.
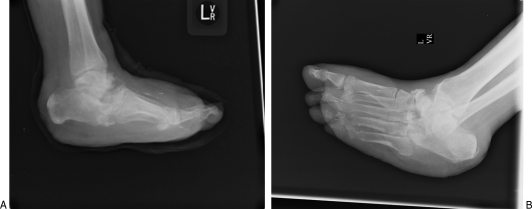
(A, B) Charcot arthropathy of the midfoot including the calcaneus and talus. The patient does not have underlying plantar ulceration. Note the progressive bony destruction with collapse of the midfoot, the dorsiflexed calcaneus due to a tight Achilles' tendon, and the lateral subluxation of the metatarsals relative to the tarsal bones.
Figure 3.
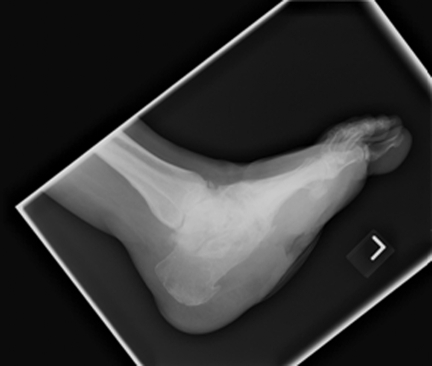
This patient has obvious Charcot collapse with contiguous soft tissue ulceration. This has led to the development of chronic midfoot osteomyelitis.
ALTERED IMMUNE RESPONSE
Once skin ulceration has occurred, the protective cutaneous barrier is lost, allowing a portal of entry for infection. The diabetic patient then has an altered immune response to infection, as hyperglycemia allows bacteria to replicate at an increased rate and causes defects in leukocyte function. These defects in infection control consist of defective chemotaxis and decreased bactericidal function. Other abnormalities in the diabetic that can affect the immune defenses are defective antibody synthesis and decreased complement levels.11 The persistent infection in turn causes hyperglycemia, leading to a vicious cycle of perpetuated infection and attenuated immune response. For all of the preceding reasons, many diabetics who present with a foot infection have underlying osteomyelitis, which can present as a persistent draining sinus or, in the presence of significant vascular disease, tissue necrosis with spreading cellulitis.
From the microbiology standpoint, aerobic gram-positive cocci are the predominant microorganisms that colonize and acutely infect breaks in the skin. Staphylococcus aureus and the β-hemolytic streptococci (groups A, C, and G, but especially group B) are the most commonly isolated pathogens in diabetic wounds. Chronic wounds develop a more complex colonizing flora, including enterococci, various Enterobacteriaceae, obligate anaerobes, Pseudomonas aeruginosa, and occasionally other nonfermentative gram-negative rods. Hospitalization, surgical procedures, and prolonged or broad-spectrum antibiotic therapy may predispose patients to colonization and/or infection with antibiotic-resistant organisms (e.g., methicillin-resistant Staphylococcus aureus [MRSA] or vancomycin-resistant enterococci [VRE]).12 Peculiarities about Staphylococcus species make it not surprising, and even expected, that a diabetic patient would develop osteomyelitis. It has been shown that S. aureus adheres to bone by expressing receptors (adhesions) for components of bone matrix (fibronectin, laminin, collagen, and bone sialoglycoprotein). S. aureus can also be internalized by osteoblasts and survive intracellularly, which may explain the difficulty of getting rid of chronic bone infection.13
Diagnosis of Osteomyelitis in the Diabetic Patient
In general, the diagnosis of osteomyelitis in the diabetic patient is made on the basis of physical findings, with the gold standard being a bone specimen and tissue diagnosis. On physical exam, patients will present with a chronic draining wound, possibly with exposed bone. The presence of exposed bone or probing to bone in a diabetic foot wound had always been thought to be representative of osteomyelitis,14 but newer literature suggests that this cannot necessarily be relied upon as an accurate diagnostic test. Shone et al15 in 2006 suggest that instead, one can more precisely say that the diagnosis of osteomyelitis is unlikely in any wound that cannot be probed to bone.
As was previously stated, the immune response in the diabetic patient may be altered or diminished. Therefore, a patient with chronic osteomyelitis may not have an elevated white blood cell (WBC) count or present with a fever. Armstrong et al16 reviewed the hospital admission data of 28 patients with type II diabetes with acute osteomyelitis secondary to neuropathic ulceration. The mean WBC count of all patients was 11.9, with 54% of those patients having a normal count. Erythrocyte sedimentation rate (ESR) was elevated in 96%, but this value will also be elevated in a patient with Charcot changes and no infection. Finally, the oral temperature was normal in 82% of the patients. Therefore, the authors conclude that a normal WBC count should not deter one from taking appropriate action to mitigate the propagation of a potentially limb-threatening foot infection.
Finally, as previously discussed, elevated serum glucose can be a marker of systemic and less often local infection in the diabetic patient. In their history, the compliant patient will often complain of long-standing difficulty with elevated blood sugars roughly corresponding with the time frame of the presence of their ulcer. Often, recalcitrant hyperglycemia is the only systemic manifestation of osteomyelitis in these patients.
The imaging modalities for osteomyelitis in the diabetic patient are generally the same as those for other patients with osteomyelitis. Plain radiographs are the most simple and inexpensive of the diagnostic tools. The sensitivity and specificity of plain films for the diagnosis of osteomyelitis in the diabetic foot are 60% and 66%, respectively.17 Unfortunately, osteomyelitis usually must be present for at least 10 days to 3 weeks before the infection becomes detectable on plain films.18 It also is difficult to diagnose in patients with severe neuropathic bone disease, because the radiographic findings are very similar.
Bone scans have a sensitivity of 86% and specificity of 45% in diagnosing foot osteomyelitis in diabetic patients and rank second among the least expensive tests for osteomyelitis.17 The main drawback of this test is a high false-positive rate in patients with diabetes, which is due to the presence of neuropathic osteoarthropathy and or a wound overlying the suspected site. Other causes of false-positive results in triple-phase bone scans include fractures and previous surgery.19
Computed tomography (CT) remains a diagnostic option when other studies are not available. However, the main limitation of this study is its inability to differentiate between soft tissue changes secondary to suppurative infection, fibrosis, chronic ischemia, neuropathic changes, or osteomyelitis.19 Recently, magnetic resonance imaging (MRI) has become widely available and provides an excellent means for differentiation between infections of soft tissue and bone. MRI has performed better than plain films, bone scans, and tagged leukocyte scans in diagnosing osteomyelitis in diabetic patients with soft tissue infections of the foot. Its sensitivity and specificity were 99% and 83%, respectively, in one study.17 MRI, however, can be prohibitively expensive and its results interpreter-dependent. Ultimately, bone biopsy is the best diagnostic option in the diabetic patient with accessible tissue; the reported sensitivity is 94%.17
Special Considerations in the Treatment of Osteomyelitis in the Diabetic Patient
At our institution, we employ a multidisciplinary team approach to the patient with a diabetic foot ulcer. The team consists of a plastic surgeon, podiatrist, infectious disease specialist, orthopedic surgeon, clinical wound care nurse practitioners, vascular surgeon, endocrinologists, hospitalist, nephrologists, nutritionist, pedorthist, and physical therapist.
The principles for osteomyelitis include aggressive debridement to healthy bleeding soft tissue and bone. Often, this means opening up the ulcer and filleting the tissue widely down to the nearest bone, if not already exposed and contiguous. All infected bone should be removed, and cultures of the infected bone and the margin of the residual bone should be sent. The wound is serially debrided until the bacterial count is down to “few or none.” This may take up to 10 debridements depending on the type of bacteria and the medical condition of the patient. If the infected bone is completely removed, as in the case of a phalanx or metatarsal, 1 week of antibiotic coverage after closure of the wound is appropriate. If only a part of the bone has been removed (i.e., calcaneus or tibia), then a full 6- to 8-week course of antibiotics should be administered.12
The Role of Biofilms in Perpetuation of Osteomyelitis
Many authors have described the role of “biofilm” in the perpetuation of infection. A biofilm colony is a complex, structured, interdependent community of microorganisms enclosed in a self-produced polymeric matrix (the biofilm, frequently referred to as glycocalyx or slime). Biofilm is adherent to inert and living surfaces that have sufficient moisture and/or nutrients to sustain its survival.20 It frequently forms on environmental surfaces, medical devices, and traumatized or compromised living and nonviable necrotic tissues such as wounds. The Centers for Disease Control and Prevention (CDC) has suggested that biofilms account for 80% of human infections. In one study, cultures of pus, exudate, joint aspirate, and blood were obtained aseptically from cases of osteomyelitis and septic arthritis. Glycocalyx was found in 76.3% of isolates of S. aureus, 57.1% of Staphylococcus epidermidis, 50% of P. aeruginosa, and 75% of Escherichia coli. In another study, tissues from biomaterials and prosthesis-related infection were examined in 25 surgical patients in a general hospital setting, and it was found that 76% of the causative bacteria grew in biofilms; 17 of these infections were associated with orthopedic prostheses, 59% of which were in biofilms. It is therefore understandable that the avascular and necrotic nature of osteomyelitic bone would be an excellent substrate for the adherence of biofilm.
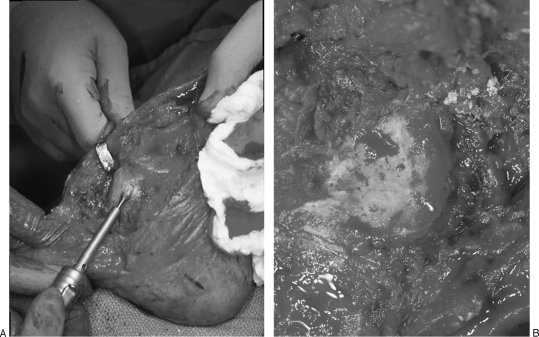
Laboratory research is ongoing into the molecular and chemical treatment of biofilms; however, there is no clear answer on how to eradicate it. It is the opinion of the senior author, however, that aggressive surgical debridement to only healthy, bleeding, viable tissue followed by copious irrigation with at least 3 L sterile irrigation can best attempt to eliminate biofilm. The Versajet (Smith & Nephew, London, UK) hydrosurgery device uses a high-pressure jet of sterile saline that travels parallel to the wound surface. This high-speed jet creates a Venturi effect that enables the surgeon to simultaneously hold, cut, and remove tissue while irrigating and aspirating the wound. This new technology allows the surgeon to differentiate between tissue types through technique as well as by varying power settings, therefore sparing viable tissue while precisely targeting and removing biofilm, debris, and damaged tissue. In the treatment of osteomyelitis, a nitrogen-driven or electrical sagittal saw is useful for serially sawing off bone slices until normal cortex and marrow is reached. Cutting burrs and rasps are likewise useful in the fine debridement of the bone surface to reach the telltale punctate bleeding at the freshened bone surface (paprika sign) (Fig. 4).
Figure 4.
(A, B) Cutting burrs and rasps are used in the fine debridement of the bone surface to reach the telltale punctate bleeding at the freshened bone surface (paprika sign).
Finally, whereas the orthopedic literature often advocates the ability to salvage infected hardware if it is important to bony union, we have not generally found it possible to eradicate infection around implanted hardware, especially in the diabetic limb. As previously stated, hardware is an excellent medium for biofilm and hence difficult to completely eradicate. We therefore advocate removal of all internal hardware in the setting of osteomyelitis and replacement with external fixation (i.e., Ilizarov frame).
After the blood flow is determined to be adequate, the wound has been adequately debrided, and the appropriate antibiotics are on board, attention can then be turned to the reconstruction of the bone deficit and options for soft tissue coverage. The orthopedic surgeon is instrumental in determining whether the extremity, after excision of the affected bone, can be functional. If traumatic fractures are within the field of the debrided wound bed, the external fixation, or Ilizarov approach, should be employed. This allows for clearance of infection from the debrided bone while employing the principles of rigid fixation and possible fusion of the affected bone ends.
In concert with the orthopedics team, bone cement impregnated with antibiotics (gentamicin or vancomycin) is often employed to help in local clearance of infection. This is a temporary solution, however, and is usually removed at a later reconstructive stage, when the wound has healed. If removed, the resulting defect is filled with bone graft. If there is a significant bony deficit (> 6 cm) in a load-bearing extremity, or significant soft tissue loss associated with the infection, free tissue transfer, such as a free fibula osteocutaneous flap, may be considered. However, these procedures are not without significant risk in the diabetic patient because of the patient's overall medical condition.
Lastly, whereas aggressive limb salvage should be considered early in any diabetic patient presenting with an ulcer or osteomyelitis, the risks of repeated operations is only justified if the patient can tolerate the procedures and the process will lead to a functional limb. The option of amputation should always be discussed with the patient.
PERIPHERAL VASCULAR DISEASE
Vascular disease and diabetes are inextricably linked, as diabetes is a risk factor for vascular disease. In addition to the microvascular insufficiency evidenced by neuropathy, nephropathy, and retinopathy, diabetes predisposes to macrovascular disease, specifically cerebrovascular occlusion, coronary artery disease, and, lastly, peripheral vascular disease. The most frequently affected vessels are at the popliteal bifurcation and involve the anterior tibia and the tibio-peroneal trunk. The vessels of the foot and ankle are usually spared.
The pathophysiology of osteomyelitis in the dysvascular patient is similar to that previously described for diabetes. It usually starts insidiously in an area of previously traumatized skin in a patient with claudication or rest pain. The trauma may be the tipping point in tissue that may have only marginal blood flow in the baseline, uninjured state. Cellulitis may be minimal as the infection progressively burrows its way to the underlying bone (i.e., phalanges, metatarsal heads, and tarsal bones). The infection and the ischemic bed become a perpetual chronic cycle. The relatively avascular and ischemic nature of the infected soft tissue and bone produces an area of lowered oxygen tension as well as an area that antibiotics cannot penetrate. The lowered oxygen tension effectively reduces the bactericidal activities of polymorphic nucleocytes (PMNs) and also favors the conversion of a previously aerobic infection to one that is anaerobic. The diffusion rate of antibiotics into dead bone is so low that frequently it is impossible to reach the organisms regardless of the external concentration. This may lead to ineffective antibiotic concentrations at the site of infection despite therapeutic serum levels.21
It has been theorized that in osteomyelitis in a diabetic patient with normal blood flow, the adequate vascular supply will allow the patient to contain the infection, and the result will be a draining sinus probing to bone. However, the dysvascular patient will develop gangrene when challenged with an infectious process.2
Diagnosis of Peripheral Vascular Disease
Any diabetic foot wound or chronic, nonhealing wound mandates a thorough vascular exam. This is important to determine not only the potential for healing or lack thereof but also the exact vascular status of the limb if the resultant wound will ultimately require microvascular free tissue transfer. The survey begins with a detailed history looking at claudication or rest pain, smoking history, coronary artery disease, cerebrovascular disease or stroke, hypertension, hypercholesterolemia, or kidney disease. The physical exam begins with an inspection of skin color and quality, temperature, as well as loss of hair on the extremity. With severe peripheral artery disease, dependent rubor is seen and may be mistaken for cellulitis. Next, the pedal pulses, including the dorsalis pedis and posterior tibial artery, are palpated and compared with the radial or carotid pulse. Palpable pulses can be misleading in patients with edema or in patients with severe atherosclerotic disease and calcified noncompressible vessels. The next tool in the examination of the extremity is the ultrasonic Doppler. A biphasic or triphasic signal with an ultrasonic Doppler usually indicates adequate flow. On ultrasound, average velocities through the popliteal and tibial segments should run around 60 to 70 cm/s to ensure adequate perfusion.22
Segmental pressures or ankle-brachial indices (ABIs) can be useful in the diagnosis of arterial occlusion. Segmental systolic blood pressure measurements are made by placing blood pressure cuffs and measuring pressures around the high thigh, above and below the knee, and at the ankle. Pressure decreases of ≥ 20 mm Hg between levels indicate obstruction. The ankle pressure is compared with the higher of the two brachial artery pressures, resulting in the ABI. An ABI of < 0.9 is diagnostic of vascular disease. Calcified vessels, a hallmark of diabetic occlusive disease, reduce the sensitivity of the test of the ABI. This should be suspected if the ankle pressure is > 50 mm Hg above brachial pressure. Arteries that do not occlude with cuffs inflated at ≥ 250 mm Hg are said to be noncompressible. Both of these circumstances will produce artificially elevated ABIs. These limitations can be overcome by measuring the toe-brachial index (TBI), because digital calcification is rarely a problem. A normal TBI is > 0.65.22
Segmental pressures give information about macrocirculation; however, tests assessing microcirculation and skin perfusion may be more meaningful in patients with diabetic foot ulcers, especially those whose ABIs are unreliable because of calcified tibial arteries. Transcutaneous oxygen tension (TcPO2) is a noninvasive measure of local oxygen supply to the tissue and has been used as a predictor of potential for wound healing.22 A surface electrode is affixed to the skin near the lesion of interest. Several measurements should be taken at three or four sites around the ulcer. Oxygen diffusing to the skin is reduced at the cathode to produce a current. The strength of the current is proportional to the amount of oxygen reduced. This test may be unreliable in the presence of cellulitis, smoking before examination, cool body temperature, or increased sympathetic tone. Thickened skin, such as the plantar aspect of the foot, may be unsuitable for this type of examination. Nevertheless, a measurement of partial pressure of oxygen (PO2) < 20 mm Hg is considered to be incompatible with healing.22
All these noninvasive studies, while posing the patient no risk, are unfortunately indirect measurements of perfusion of the limb. If there is any question, the patient should be referred to a vascular surgeon. Various imaging possibilities exist including MRI angiography, CT angiography, and contrast angiography. Contrast angiography remains the gold standard for diagnosis of peripheral vascular occlusion.
Treatment of Peripheral Vascular Disease in the Setting of Osteomyelitis
The presence of vascular disease must be considered in the patient with osteomyelitis. If the associated wound is stable, revascularization should occur after a deep culture has been obtained. If the wound is unstable, it should be initially debrided and then revascularization should proceed urgently. Once the necrotic tissue is removed, vascularity becomes of paramount importance and will determine if the infection can be eradicated and if the wound will go on to heal. As previously mentioned, the vascular disease in diabetics is usually in the tibial-peroneal trunk and calf vessels, often sparing pedal vessels. Percutaneous angioplasty and stenting are becoming more successful in treating these difficult lesions. Angioplasty at that level can be difficult, and the vascular surgeon may have to resort to a vein bypass graft or Gore-Tex (W.L. Gore & Associates, Inc., Elkson, MD) bypass graft with vein patch. Autogenous vein has been shown to have superior outcomes to both prosthetic grafts and noninvasive, percutaneous interventions. It is with adequate revascularization of the foot that diabetic patients can combat the osteomyelitis and emerge with a healed extremity. The vascular surgeon plays a critical role in the multidisciplinary approach to limb salvage in the diabetic patient.
IRRADIATION
Radiation has become a mainstay in the treatment for local-regional control of many neoplasms, specifically head and neck cancers. As effective a tool as it has proved to be, irradiation may cause the 3 “H” status—hypoxia, hypovascularity, hypocellularity—and impair normal collagen synthesis and cell production, leading to tissue breakdown and a chronic nonhealing wound.23,24 The hypothesized mechanism is as follows. The layer of endothelium supplying the irradiated area starts to proliferate, resulting in a proliferative endarteritis. This proliferation, most often noted in the capillaries, continues and interferes with normal tissue perfusion. The tissue begins to manifest ischemic changes and may become frankly necrotic. In irradiated areas, ischemia and necrosis can occur, and this tissue may survive without adequate blood supply for a long period of time, until a traumatic or infectious incident triggers the events leading to extensive tissue death.25 Additionally, fibroblasts are believed to suffer a “direct hit” from the radiation beam itself, as free radicals lead to fibroblast dysfunction and decreased collagen deposition in soft tissues.26
The most commonly described and devastating direct bony consequence of irradiation is osteoradionecrosis (ORN). It is traditionally described as exposed irradiated bone that fails to heal over a period of 3 months.24 Doses of radiation above 50 Gy usually are required to cause ORN, and the mandible is the most commonly involved bone. Other bones also affected include the frontal bone, cervical spine, maxilla, temporal bones, skull, and nasal bones.21 Despite improvements in radiotherapy technique, the risk of osteoradionecrosis is not totally eliminated. The avascular and possibly dead bone eventually presents an excellent medium for bacterial contamination, suprainfection, and then chronic infection and osteomyelitis.
Osteoradionecrosis with osteomyelitis will often present with a draining tract, chronic deep bone pain, erythema, fluctuating abscesses, and deformity or instability. It can also present with the more serious complications of pathologic fracture and over several years progress to possible malignant transformation and carcinoma.
The same devastating consequences of irradiation apply to the soft tissue envelope surrounding the osteomyelitic bone. Often, the field of damage is even wider and more severe than that of the bony skeleton and causes difficulties in the treatment and reconstruction of osteomyelitis. The inadvertent effects of radiotherapy are such that small blood vessels within “nontarget” tissues are frequently damaged to the point where they lose their ability to adequately perfuse. In addition to direct tissue necrosis and wounds caused by the radiation damage, the surrounding tissue also suffers ischemia. It often becomes erythematous and darkened, as evidenced by the “radiation shadow,” with telangiectasias representing clotted capillaries. The tissue is indurated and firm, caused by the destroyed subdermal lymphatics. Finally, the tissue is friable and easily traumatized, leading to recurrent ulceration.
Diagnosis of osteomyelitis in the setting of radiation is guided by the principles that apply to all of osteomyelitis. The diagnosis is usually clinical, with a draining wound usually probing to exposed bone. Radiographs may be helpful and show cortical destruction or lytic and hazy areas. A bone scan, CT scan, or MRI scan will all potentially show medullary destruction.
The general principles for treatment of osteomyelitis in the setting of radiation are similar to those for osteomyelitis in the diabetic or dysvascular patient. The chronic osteomyelitic bone sequestra must be aggressively debrided to healthy bleeding bone. A nitrogen-driven or electrical sagittal saw is useful for serially sawing off bone slices until normal cortex and marrow is reached. Cutting burrs and rasps are likewise useful in the fine debridement of the bone surface to reach the telltale punctate bleeding at the freshened bone surface (paprika sign). Furthermore, the surrounding radiated soft tissue must be debrided to healthy bleeding tissue if there is any evidence of infection or necrosis.
In terms of reconstruction of the bony defects caused by the osteomyelitis and its resection, radiation presents a challenging problem. In the rare case of irradiation to a long bone or extremity, an orthopedic surgeon should be involved to determine if weight-bearing will be affected. For bony defects, reconstruction with external fixation (such as the Ilizarov technique) may be necessary. Finally, free vascularized bone grafts, such as a free fibula osteocutaneous flap, may be necessary to reconstruct bony deficits of greater than 6 cm. This is especially true in the case of irradiation, as local bony distraction or local tissue rearrangement may not be an option due to local radiation damage. More often, as described earlier, the osteomyelitis and/or osteoradionecrosis will occur in the bones of the head and neck, often the mandible, frontal bones, maxilla, skull, or cervical spine. Here, local control of osteomyelitis and its resection will often have to be more conservative, because of the anatomic region and also because of aesthetic considerations. Still, the guiding principles of adequate debridement to healthy bleeding tissue and bone should be used. Sometimes, in the mandible, for instance, this will lead to radical sequestrectomy and even hemi-mandibulectomy. Oral maxillofacial surgery and/or head and neck surgery will usually be involved in these cases and can help determine whether the defect will need structural reconstruction or, more simply, soft tissue coverage. Generally, vascularized flap reconstruction is recommended in cases of severe, extensive osteoradionecrosis, such as coexistent pathologic fracture, multiple discharging fistulae, and a large area of exposed bone.
The mainstay of therapy for chronic osteomyelitis includes a combination of adequate surgical debridement and coverage with vascular soft tissue, using varied tissue types. However, as previously discussed, the field of damage in the setting of radiation often extends beyond the obvious wound. Therefore, local and rotational flaps are generally not successful as free tissue transfer, because their vascularity may also have been impaired by the radiation. Conservative therapy is attempted first. The Wound Vac (KCI, San Antonio, TX) can be used in the healing of radiated wounds. In addition to clearing the wound of edema, the negative pressure is thought to increase local tissue perfusion. It may also stimulate granulation tissue over the exposed bone, provided that it is again adequately debrided and free of infection (less than 100,000 cfu organisms). Again, in the setting of radiation, these more conservative therapies may fail and necessitate more radical therapies, including consideration of amputation or, in appropriate patients, free tissue transfer.
Finally, both osteomyelitis and osteoradionecrosis are indications for the adjunctive therapy of hyperbaric oxygen (HBO). As discussed in the article by Fang and Galiano in this issue of the journal, the aim of HBO is to increase local tissue oxygenation and thus enhance neovascularization and wound healing. It has been shown to elevate tissue oxygen tension and may stimulate collagen synthesis and fibroblastic proliferation. HBO alone, however, has been unsuccessful in treating osteoradionecrosis, as it cannot revitalize necrotic bone. Therefore, the dead sequestra must be surgically debrided. HBO therapy can theoretically minimize the extent of surgery. Like appropriate antibiotics, HBO must be considered an adjunct to effective surgical therapy.
CONCLUSION
Diabetes, peripheral vascular disease, and radiation all pose challenges in the pathogenesis, diagnosis, and treatment of osteomyelitis. The key is to diagnose the extent of the osteomyelitis and the type of bacteria involved. In addition, the blood flow to the area has to be optimized. It may require revascularization for large vessels or HBO for smaller vessels in ischemic tissue. Once the medical condition of the patient, the blood flow, and the antibiotic coverage has been optimized and the wound and underlying bone has been debrided to healthy clean tissue, the wound is ready for closure. The close involvement of orthopedists and/or podiatric surgeons is critical to ensure optimal skeletal alignment and muscular function. Standard wound closure techniques are used to close the wounds and include delayed primary closure, skin grafting, local flaps, pedicled flaps, and free flaps.
REFERENCES
- Frykberg R G. Epidemiology of the diabetic foot: ulcerations and amputations. Adv Wound Care. 1999;12:139–141. [PubMed] [Google Scholar]
- Hill S L, Holtzman G I, Buse R. Effects of peripheral vascular disease with osteomyelitis in the diabetic foot. Am J Surg. 1999;177:282–286. doi: 10.1016/s0002-9610(99)00050-1. [DOI] [PubMed] [Google Scholar]
- Cohen M, Roman A, Malcolm W G. Panmetatarsal head resection and transmetatarsal amputation vs. solitary partial ray resection in the neuropathic foot. J Foot Surg. 1991;30:29–33. [PubMed] [Google Scholar]
- Levine M E, Lawrence W O, Bowker J G. The Diabetic Foot. St. Louis, MO: Mosby; 1993.
- Wiersema-Bryant L A, Kraemer B A. In: Bryant RA, editor. Acute and Chronic Wounds: Nursing Management. 3rd ed. St. Louis, MO: Mosby; 2007. Vascular and neuropathic wounds: the diabetic wound. pp. 305–335.
- Nishimoto G S, Attinger C E, Cooper P S. Lengthening the Achilles tendon for the treatment of diabetic plantar forefoot ulceration. Surg Clin North Am. 2003;83:707–726. doi: 10.1016/S0039-6109(02)00191-3. [DOI] [PubMed] [Google Scholar]
- Caputo G M, Cavanagh P R, Ulbrecht J S, Gibbons G W, Karchmer A W. Assessment and management of foot disease in patients with diabetes. N Engl J Med. 1994;331:854–860. doi: 10.1056/NEJM199409293311307. [DOI] [PubMed] [Google Scholar]
- Reddy G K. Glucose-mediated in vitro glycation modulates biomechanical integrity of the soft tissues but not hard tissues. J Orthop Res. 2003;21:738–743. doi: 10.1016/S0736-0266(03)00006-8. [DOI] [PubMed] [Google Scholar]
- Milgram J W. Osteomyelitis in the foot and ankle associated with diabetes mellitus. Clin Orthop Relat Res. 1993;296:50–57. [PubMed] [Google Scholar]
- Schon L C, Easley M E, Weinfeld S B. Charcot neuroarthropathy of the foot and ankle. Clin Orthop Relat Res. 1998;349:116–131. doi: 10.1097/00003086-199804000-00015. [DOI] [PubMed] [Google Scholar]
- Lipsky B A. Foot ulceration and infections in elderly diabetics. Clin Geriatr Med. 1990;6:747–769. [PubMed] [Google Scholar]
- Lipsky B A, Berendt A R, Deery H G, et al. Diagnosis and treatment of diabetic foot infections. Plast Reconstr Surg. 2006;117(Suppl):212S–238S. doi: 10.1097/01.prs.0000222737.09322.77. [DOI] [PubMed] [Google Scholar]
- Lew D P, Waldvogel F A. Osteomyelitis. N Engl J Med. 1997;336:999–1007. doi: 10.1056/NEJM199704033361406. [DOI] [PubMed] [Google Scholar]
- Grayson M L, Gibbons G W, Balogh K, et al. Probing to bone in infected pedal ulcers: a clinical sign of underlying osteomyelitis in diabetic patients. JAMA. 1995;273:721–723. [PubMed] [Google Scholar]
- Shone A, Burnside J, Chipchase S, Game F, Jeffcoate W. Probing the validity of the probe-to-bone test in the diagnosis of osteomyelitis of the foot in diabetes. Diabetes Care. 2006;29:945. doi: 10.2337/diacare.29.04.06.dc05-2450. [DOI] [PubMed] [Google Scholar]
- Armstrong D G, Lavery L A, Sariaya M, Ashry H. Leukocytosis is a poor indicator of acute osteomyelitis of the foot in diabetes mellitus. J Foot Ankle Surg. 1996;35:280–283. doi: 10.1016/s1067-2516(96)80075-5. [DOI] [PubMed] [Google Scholar]
- Lipsky B A. Osteomyelitis of the foot in diabetic patients. Clin Infect Dis. 1997;25:1318–1326. doi: 10.1086/516148. [DOI] [PubMed] [Google Scholar]
- Newman L G. Imaging techniques in the diabetic foot. Clin Podiatr Med Surg. 1995;12:75–86. [PubMed] [Google Scholar]
- Schinabeck M K, Johnson J L. Osteomyelitis in diabetic foot ulcers. Prompt diagnosis can avert amputation. Postgrad Med. 2005;118:11–15. doi: 10.3810/pgm.2005.07.1678. [DOI] [PubMed] [Google Scholar]
- Saye D E. Recurring and antimicrobial-resistant infections: considering the potential role of biofilms in clinical practice. Ostomy Wound Manage. 2007;53:46–48. [PubMed] [Google Scholar]
- Prasad K C. Osteomyelitis in the head and neck. Acta Otolaryngol. 2007;127:194–205. doi: 10.1080/00016480600818054. [DOI] [PubMed] [Google Scholar]
- Teodorescu V J, Chen C, Morrissey N, et al. Detailed protocol of ischemia and the use of noninvasive vascular laboratory testing in diabetic foot ulcers. Am J Surg. 2004;187:S75–S80. doi: 10.1016/S0002-9610(03)00308-8. [DOI] [PubMed] [Google Scholar]
- Hao S P, Chen H C, Wei F C, et al. Systemic management of osteoradionecrosis in the head and neck. Laryngoscope. 1999;109:1324–1327. doi: 10.1097/00005537-199908000-00027. [DOI] [PubMed] [Google Scholar]
- Marx R E. Osteoradionecrosis: a new concept of its pathophysiology. J Oral Maxillofac Surg. 1983;41:283–288. doi: 10.1016/0278-2391(83)90294-x. [DOI] [PubMed] [Google Scholar]
- Heimbach R D. In: Davis CD, Hunt TK, editor. Problem Wounds: The Role of Oxygen. New York, NY: Elsevier; 1988. Radiation effect on tissues. pp. 53–63.
- Ariyan S. In: Thorne CT, Bartlett SP, Beasely RW, et al, editor. Radiation injury. Grabb and Smith's Plastic Surgery. 6th ed. 2006. pp. 835–853.