ABSTRACT
Long bone osteomyelitis presents a variety of challenges to the physician. The severity of the disease is staged depending upon the infection's particular features, including its etiology, pathogenesis, extent of bone involvement, duration, and host factors particular to the individual patient (infant, child, adult, or immunocompromised). Long bone osteomyelitis may be either hematogenous or caused by a contiguous spread of infection. A single pathogenic organism is almost always recovered from the bone in hematogenous osteomyelitis; Staphylococcus aureus is the most common organism isolated. A variety of multidrug-resistant organisms of bacteria continue to be a source of concern in arresting infection. The primary weapons to treat these infections are culture-specific antibiotics, aggressive debridement, muscle flaps, and bone grafts. This article offers a basic review of the classification, etiology, epidemiology, pathogenesis, and treatment of long bone osteomyelitis.
Keywords: Osteomyelitis, long bones, antibiotics, debridement
Osteomyelitis in long bones presents a variety of challenges depending on the infection's particular features (etiology, pathogenesis, extent of bone involvement, and duration) and the patient (infant, child, adult, or immunocompromised). Over the past four decades, we have made tremendous progress in treating this disease as the many factors that account for the occurrence and persistence of infection have been identified and a variety of antimicrobials with different spectrums of activity against specific pathogens have been developed. Also, new operative techniques, such as Ilizarov fixation and muscle flaps, and innovative delivery systems for antibiotics have been added to our arsenal. In spite of these advances, however, osteomyelitis remains difficult to treat, with considerable morbidity and costs. This article offers a basic review of the classification, etiology, epidemiology, pathogenesis, and treatment of long bone osteomyelitis.
CLASSIFICATION
Osteomyelitis can be classified by duration (acute or chronic), pathogenesis (trauma, contiguous spread, hematogenous, surgical), site, extent, or type of patient. Although several classifications of osteomyelitis have been described by different authors, the two most widely used in the medical literature and in clinical practice are the classification systems by Waldvogel et al.1 and Cierny et al.2 Under the Waldvogel system, osteomyelitis is first described according to duration, either acute or chronic. Second, the disease is classified according to source of infection, as hematogenous when it originates from a bacteremia or as contiguous focus when it originates from an infection in a nearby tissue. A final category of the classification is vascular insufficiency. One of the limitations of the Waldvogel classification system is that it does not consider infection originating from direct penetration of microorganisms into the bone, as may occur after trauma or surgery. In addition, it is an etiologic classification system that does not readily lend itself to guiding surgical or antibiotic therapy. Because of the wide variability in the etiology of osteomyelitis, a classification based on the pathogenesis of the disease, such as that of the Waldvogel system, has limited value in clinical practice.
The second system is known as the Cierny-Mader classification (Table 1). The Cierny-Mader classification is a clinical classification based on anatomic, clinical, and radiologic features. It characterizes osteomyelitis as being in one of four anatomic stages. In stage 1, or medullary, osteomyelitis is confined to the medullary cavity of the bone. Stage 2, or superficial, osteomyelitis involves only the cortical bone and most often originates from a direct inoculation or a contiguous focus infection. Stage 3, or localized, osteomyelitis usually involves both cortical and medullary bone. In this stage, the bone remains stable, and the infectious process does not involve the entire bone diameter. Stage 4, or diffuse, osteomyelitis involves the entire thickness of the bone, with loss of stability, as in infected nonunion. The Cierny-Mader system adds a second dimension, characterizing the host as either A, B, or C. The A hosts are patients without systemic or local compromising factors. B hosts are affected by one or more compromising factors. C hosts are patients so severely compromised that the radical treatment necessary would have an unacceptable risk-benefit ratio. One shortfall of this system is that by definition, the C host category is a subjective evaluation. Also not taken into account are other properties including the length of time that an infection has been able to persist, if indwelling medical devices are in the infected area, and whether it is a pediatric or teen/adult patient. Many cases of osteomyelitis may be treated with antimicrobial therapy alone. One example is seen in pediatric osteomyelitis cases where the ability of the younger patient to resorb dead bone thereby removes the devitalized surfaces where bacteria can persist in a biofilm mode of growth (discussed later). In addition, rapidly diagnosed infections in adults can first be treated with antimicrobial therapy because significant areas of devitalized bone have not yet formed. Generally, however, this classification seems to be of value in clinical practice, because it addresses the dynamic nature of the disease and adds the second dimension of the host's physiologic, metabolic, and immunologic capabilities.
Table 1.
Cierny-Mader Staging System
| Anatomic type | |
| Stage 1: Medullary osteomyelitis | |
| Stage 2: Superficial osteomyelitis | |
| Stage 3: Localized osteomyelitis | |
| Stage 4: Diffuse osteomyelitis | |
| Physiologic class | |
| A host: Normal host | |
| B host | |
| Systemic compromise (Bs)* | |
| Local compromise (Bl)* | |
| Systemic and local compromise (Bls)* | |
| C host: Treatment worse than the disease | |
| Systemic or local factors that affect immune surveillance, metabolism, and local vascularity | |
| Systemic (Bs) | Local (Bl) |
| Malnutrition | Chronic lymphedema |
| Renal or hepatic failure | Venous stasis |
| Diabetes mellitus | Major-vessel compromise |
| Chronic hypoxia | Arteritis |
| Immune disease | Extensive scarring |
| Malignancy | Radiation fibrosis |
| Extremes of age | Small-vessel disease |
| Immunosuppression or neuropathy | Complete loss of sensation |
| Immune deficiency | Tobacco abuse |
ETIOLOGY
A single pathogenic organism is almost always recovered from the bone in hematogenous osteomyelitis. In adults, Staphylococcus aureus is the most common organism isolated.3 However, other gram-positive cocci (including coagulase-negative staphylococci and Streptococcus spp.), gram-negative bacilli, and anaerobic organisms (listed in descending order of incidence) are also frequently isolated, and multiple organisms are usually isolated in contiguous focus osteomyelitis. In fact, with the advent of molecular biology in diagnosis, many other bacterial species that were viable but unculturable may also be present. However, the pathogenic importance of these species has yet to be determined. In infants, the pathogens most frequently isolated from blood or bone are Staphylococcus aureus, Streptococcus agalactiae, and Escherichia coli. However, in children more than 1 year of age, Staphylococcus aureus, Streptococcus pyogenes, and Haemophilus influenzae are most commonly isolated.4 After age 4, the incidence of Haemophilus influenzae infection decreases, and the overall incidence of H. influenzae as a cause of osteomyelitis is dramatically decreasing with the ever-increasing use of an improved H. influenzae vaccine.5,6
Skeletal tuberculosis is the result of hematogenous spread of Mycobacterium tuberculosis early in the course of a primary infection. On rare occasions, skeletal tuberculosis may be a contiguous infection from an adjacent caseating lymph node. Atypical mycobacteria, including Mycobacterium marinum, Mycobacterium avium-intracellulare, Mycobacterium fortuitum, and Mycobacterium gordonae, all have been associated with osteoarticular infections. In addition, infections may also be caused by a variety of fungal organisms, including coccidioidomycosis, blastomycosis, cryptococcus, and sporotrichosis.7
EPIDEMIOLOGY
Perhaps the most significant epidemiologic change regarding long bone osteomyelitis, and all osteoarticular infections, is the ongoing rise of methicillin-resistant Staphylococcus aureus (MRSA) and other multidrug-resistant organisms (MDROs). A 2006 study of pediatric patients in Memphis, Tennessee, demonstrated a significant increase in both the incidence and severity of community-acquired MRSA infections between 2000 and 2004.8 However, the community-acquired infections are usually not invasive and remain localized on the skin. In adults, physicians in North America may see more mycobacterial bone infections—once relatively rare before the arrival of acquired immune deficiency syndrome (AIDS) in the 1980s—as a result of increased travel, immigration, and use of immunosuppressive medications.9 Most recently, MDROs in extremity wounds suffered by soldiers returning from the wars in Iraq and Afghanistan have become a source of concern.10,11 A survey of 908 cultures from a military care facility in Iraq during 2003 and 2004 showed that the bacteria most commonly isolated from U.S. troops were coagulase-negative staphylococci, accounting for 34% of isolates, Staphylococcus aureus (26%), and streptococcal species (11%). Antimicrobial susceptibility testing demonstrated broad resistance among the gram-negative and gram-positive bacteria.12 Large-scale research efforts led by civilian and military physicians currently are under way to develop strategies to combat infection patterns seen in returning U.S. troops.13
PATHOGENESIS
Osteomyelitis may be caused from hematogenous spread, direct inoculation of microorganisms into bone, or from a contiguous focus of infection. A trivial skin infection may be the source of bacteremia or it may emerge as the result of a more serious infection such as acute or subacute bacterial endocarditis. Injection drug abuse has been linked to hematogenous osteomyelitis involving the long bones or vertebrae.14 Hematogenous osteomyelitis usually involves the metaphysis of long bones in children or the vertebral bodies in adults. With hematogenous osteomyelitis, the joint is usually spared from infection in children, unless the metaphysis is intracapsular, as is found at the proximal radius, humerus, or femur.15,16 The most common causes of direct inoculation osteomyelitis are penetrating injuries and surgical contamination. Contiguous focus osteomyelitis commonly occurs in patients with severe vascular disease.
Host Factors
Host factors are primarily involved with containment of the infection once it has been introduced adjacent to or into the bone, but on occasion, specific host factors may predispose individuals to develop osteomyelitis. Host deficiencies that favor bacteremia thus favor the development of hematogenous osteomyelitis. Host deficiencies involved with direct inoculation of organisms and/or contiguous spread of infection from an adjacent area of soft tissue infection are primarily involved with the lack of containment of the initial infection. Also, patients with sickle cell anemia, chronic granulomatous disease, and diabetes mellitus have a notable susceptibility to acute skeletal infections.17,18 Many systemic and local factors (Table 1) influence the ability of the host to elicit an effective response to infection and treatment.
Pathology
Acute osteomyelitis presents as a suppurative infection accompanied by edema, vascular congestion, and small vessel thrombosis. In early acute disease, the vascular supply to the bone is decreased by infection extending into the surrounding soft tissue. Large areas of dead bone (sequestra) may be formed when the medullary and periosteal blood supplies are compromised.19 Acute osteomyelitis can be arrested before dead bone develops if treated promptly and aggressively with antibiotics and surgery (if necessary). In an established infection, fibrous tissue and chronic inflammatory cells form around the granulation tissue and dead bone. After the infection is contained, there is a decrease in the vascular supply to it, inhibiting an effective inflammatory response. Chronic osteomyelitis is the result of the coexistence of infected, nonviable tissues and an ineffective host response.20
Bacteria have been shown to persist within glycocalyx-enclosed microcolonies adherent to the bone and to prosthetic devices in cases of osteomyelitis.21,22 Biofilms are typically composed of cells embedded in a highly hydrated polysaccharide matrix with nucleic acids and proteins throughout. These biofilms are associated with the refractory nature of chronic infections such as osteomyelitis.23 It has been reported that concentrations of antimicrobial agents required for the eradication of bacteria in biofilms are more than 50 to 1000 times higher than those needed for killing of the free-floating planktonic cells. Usually, that level of antibiotics is impossible to achieve because of patient toxicity. In the bones, this is further complicated by questionable penetration of antibiotics into infected and ischemic areas leading to subpotent antibiotic concentrations.23 The reason for the reduced ability of antimicrobial agents to eradicate these infections is due to the reduced antibiotic penetration and the very slow growth rate and differential upregulation of stress response genes by cells within the biofilm.
Pathologic features of chronic osteomyelitis are the presence of necrotic bone, the formation of new bone, and the exudation of polymorphonuclear leukocytes joined by large numbers of lymphocytes, histiocytes, and, occasionally, plasma cells. New bone forms from the surviving fragments of periosteum and endosteum in the region of the infection. An encasing sheath of live bone, an involucrum, surrounds the dead bone under the periosteum. The involucrum is irregular and is often perforated by openings through which purulence may track into the surrounding soft tissues and eventually drain to the skin surfaces, forming a chronic sinus. The involucrum may gradually increase in density and thickness to form part or all of a new diaphysis. New bone increases in amount and density for weeks or months, according to the size of the bone and extent and duration of infection. Endosteal new bone may proliferate and obstruct the medullary canal. After host defense or operative removal of the sequestrum, the remaining cavity may fill with new bone, especially in children. However, in adults, the cavity may persist or the space may be filled with fibrous tissue, which may connect with the skin surface via a sinus tract.20
DIAGNOSIS
Osteomyelitis is known as the “great pretender” because of the difficulties encountered in diagnosis. Several conditions may present symptoms similar to those of osteomyelitis, such as many malignant and benign tumors, cysts, noninfected nonunions, old trauma, bone infarcts secondary to sickle cell anemia, or other hemoglobinopathies.24 In these cases, results of radiographs may be consistent with osteomyelitis, but patients generally lack other symptoms, such as fever, erythema, and drainage. The correct diagnosis is usually based on culture results and histologic features.
Children with hematogenous osteomyelitis may present with acute signs of infection including fever, irritability, lethargy, and local signs of inflammation. However, not all of these signs may be present. A study of 86 children suffering from hematogenous osteomyelitis showed that half presented with vague complaints, including pain of the involved limb of 1 to 3 months in duration and minimal, if any, temperature elevation.15 Children usually are capable of an effective response to hematogenous osteomyelitis because of the presence of noninfected soft tissue enveloping the infected bone.15,16 Adults with primary or recurrent hematogenous osteomyelitis usually present with vague complaints consisting of nonspecific pain and low-grade fever of 1 to 3 months in duration. However, acute clinical presentations with fever, chills, swelling, and erythema over the involved bone or bones are occasionally seen.14
Patients with contiguous focus osteomyelitis may present with localized bone and joint pain, erythema, swelling, and drainage around the area of trauma, surgery, or wound infection. Signs of bacteremia such as fever, chills, and night sweats may be present in the acute phase of osteomyelitis, but not in the chronic phase. Both hematogenous and contiguous focus osteomyelitis can progress to a chronic condition. Local bone loss, sequestrum formation, and bony sclerosis are common. Persistent drainage and/or sinus tracts are often found adjacent to the area of infection. The patient usually presents with chronic pain and drainage, and a fever, if present, is usually low grade. The erythrocyte sedimentation rate is usually elevated, reflecting chronic inflammation, but the blood leukocyte count is usually normal. Diagnosis of long bone osteomyelitis rests on isolation of the pathogen from bone lesion or blood culture.
Cultures
In the case of hematogenous osteomyelitis, positive blood cultures often can eliminate the need for a bone biopsy, provided there is radiographic evidence of osteomyelitis. Otherwise, antibiotic treatment should be based on bone cultures taken at debridement or during bone biopsy. Whenever possible, cultures should be obtained before antimicrobial therapy has begun. Sinus tract cultures are reliable for confirming S. aureus, but they do not predict the presence or absence of gram-negative organisms that cause osteomyelitis.25,26
It must also be recognized that acute and more often chronic cases of osteomyelitis may often have negative cultures. The reasons for the inability of clinicians to obtain accurate microbiological identification are varied. One reason may be due to inappropriate culture conditions. In addition, sampling purulent discharge, sinus tracts, or areas of devitalized tissue during surgical procedures may be flawed because a localized biofilm may be on a location other than that sampled. Lastly, the infecting microbes may be viable but nonculturable. In this case, biofilm microbes are removed from their resident in vivo biofilm mode of growth and transferred to a planktonic environment where the microbe is not suited to grow. Therefore, the sample may often be incorrectly described as culture negative. Therefore, culture-independent methods such as polymerase chain reaction–based detection methods are much more sensitive and accurate.27,28,29
In the majority of cases, antibiotic treatment will be directed on the basis of cultures or deep bone biopsy and antibiotic sensitivity tests.30 Today, the laboratory must use a variety of susceptibility test methods, each tailored specifically to a particular pathogen or group of pathogens.31 Traditional methods include broth microdilution, disk diffusion, and antibiotic gradient (E-test). More recently, automated methods have gained significant attention with the promise of improved accuracy and faster results.32,33,34,35 A recent study of three automated systems (MicroScan WalkAway, [Dade Behring, Inc., West Sacramento, CA] VITEK, and VITEK 2 [bioMerieux, Durham, NC]), however, demonstrated that they generally failed to accurately detect piperacillin-tazobactam resistance among clinically significant isolates of Pseudomonas aeruginosa. All three systems showed high error rates, ranging from 19 to 27%.36 False-intermediate and false-resistant results by automated methods with cefepime and aztreonam also have been reported.37,38,39,40
Microbiologists now recognize that a single method, regardless of whether it is conventional or automatic, cannot test all antimicrobial agents against all microorganisms and detect all patterns of resistance.41 The strategy of using multiple susceptibility testing methods continues to evolve, particularly in regard to gram-negative organisms. A current issue is the susceptibility testing of the polymyxin class of antimicrobial agents (colistin or polymyxin B and polymyxin B) for the therapy of infections caused by multidrug-resistant isolates of Pseudomonas aeruginosa and Acinetobacter species.42 Guidelines for testing gram-negative control strains with polymyxin B and colistin have been established for broth microdilution.43 Currently, however, guidelines do not exist for disk-diffusion testing of polymyxins. Synergy testing represents a shift in the approach by microbiology laboratories driven by the growing challenge of multiresistant bacteria and the need to correlate in vitro tests with in vivo outcomes.
However, the current method of antibiotic sensitivity is wholly lacking with respect to biofilms. Clinical microbiologists test antimicrobial efficacy against planktonic and non-biofilm agar-based growth cultures. Therefore, the biofilm phenotype and the associated 50- to 500-fold increase in antibiotic resistance (compared with their planktonic counterparts) are often ignored. The result is that antimicrobial therapy is prescribed against a non-biofilm infection and what is effective against planktonic infections is not always the same as what is effective against a biofilm infection.
Imaging and Recent Advances
RADIOGRAPHS
In cases of hematogenous osteomyelitis, radiographic changes usually lag at least 2 weeks behind the infection's evolution. The earliest changes seen are swelling of soft tissue, periosteal thickening and/or elevation, and focal osteopenia. Radiographs will usually not indicate lytic changes until at least 50 to 75% of the bone matrix is destroyed. Infected areas typically appear dark (Figs. 1–3). The more diagnostic lytic changes are delayed and associated with subacute and chronic osteomyelitis. Similarly, radiographic evidence of improvements may lag behind clinical recovery.44 In contiguous focus osteomyelitis, radiographic changes are subtle and require careful clinical correlation to have diagnostic significance.
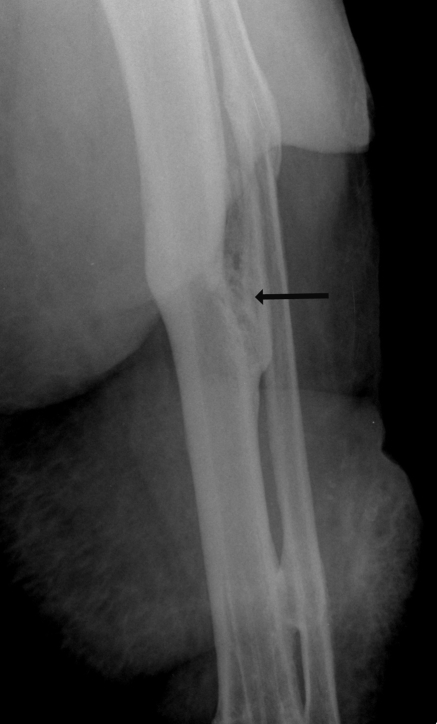
Figure 1.
Lateral Tibia, Fibula radiograph with the arrow pointing to the osteomyelitis of the tibia of a 46-year-old woman injured in a motor vehicle accident more than 10 years before this image was taken and who suffered recurrent bouts of infection since.
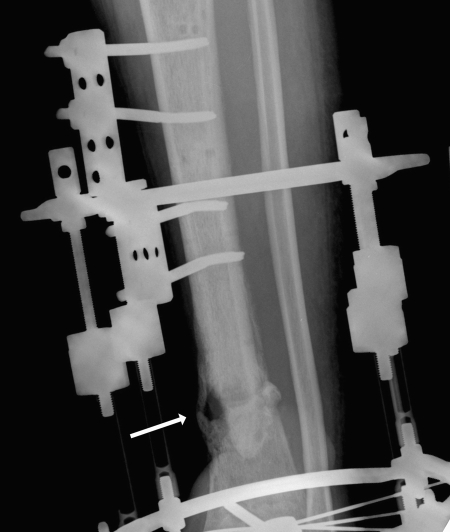
Figure 2.
A 32-year-old man injured in a fall suffered pilon fracture and a major infection (indicated by the arrow). He was treated with antibiotics and external fixation; however, amputation proved necessary to arrest the spread of infection.
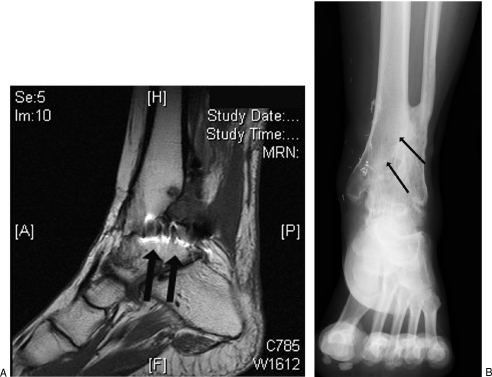
Figure 3.
(A) MRI scan of a 45-year-old woman who suffered a pilon fracture in an automobile accident and suffered from infection after the injury. The bright areas, a characteristic appearance of infection in MRI scans, are indicated by arrows. (B) In an x-ray image, the area of infection is the oval area pointed to by the arrows in the center of the distal tibia.
RADIONUCLIDE SCANS
When the diagnosis of osteomyelitis is ambiguous or it is necessary to gauge the extent of bone and/or soft tissue inflammation, radionuclide scans may be helpful. In general, however, they are not usually necessary for the diagnosis of long bone osteomyelitis. The technetium polyphosphate 99mTc scan demonstrates increased isotope accumulation in areas of increased blood flow and new bone formation reactive to the infection.45 In cases of hematogenous osteomyelitis that have been confirmed by biopsy, the finding is usually positive as early as 48 hours after the initiation of bone infection.25 A negative 99mTc scan effectively rules out the diagnosis of osteomyelitis.
MAGNETIC RESONANCE IMAGING AND COMPUTED AXIAL TOMOGRAPHY
Magnetic resonance imaging (MRI) has become an increasingly useful tool for the diagnosis of musculoskeletal sepsis. However, because of its expense, it should only be requested when the diagnosis of osteomyelitis is equivocal. The typical appearance of acute osteomyelitis in an MRI scan is a localized area of abnormal marrow with decreased signal intensity on T1-weighted images and increased signal intensity on T2-weighted images. Computed axial tomography (CAT) may play a role in diagnosis, as increased marrow density occurs early in the infection, and intramedullary gas has been reported in patients with hematogenous osteomyelitis.46,47 CAT scans may also help identify areas of necrotic bone and assess areas of soft tissue involvement.
White blood cell scans offer a marked improvement in specificity (80to 90%) compared with bone scans, particularly when complicated conditions are superimposed.48 The white blood cell scan was performed originally with indium-111 (111In)-labeled white blood cells and more recently with 99mTc hexamethylpropylene amine oxime (HMPAO)-labeled white cells.49 111In scans have sensitivity of more than 90% and specificity of 78%.50 By comparison, the sensitivity of leukocyte scintigraphy decreased from 84 to 21% for chronic osteomyelitis in the axial skeleton.51
Several other promising diagnostic scans are under evaluation:
The radiolabeled antibiotics scan is a fast-emerging diagnostic test for detection of infectious lesions because of their specific binding to bacterial components. These agents may prove to differentiate between infection and sterile inflammatory lesions.52,53
The Fluorodeoxyglucose-Positron emission tomography (FDG-PET) scan is a relatively novel technique that has demonstrated the highest diagnostic accuracy for confirming or excluding chronic osteomyelitis in comparison with bone scintigraphy, MRI, or leukocyte scintigraphy. PET and Single photon emission computed tomography (SPECT) are highly accurate techniques for the evaluation of chronic osteomyelitis, particularly of the axial skeleton, allowing differentiation from soft tissue infection.54 FDG-PET should be avoided in the first 3 to 6 months after debridement surgery or trauma because of high false-positive results in postsurgical and traumatic bone healing. It has limited utility in differentiating infection with a failed prosthesis or a tumor.54,55,56,57,58,59,60,61
Recent studies show that use of superparamagnetic iron oxide (SPIO) MRI contrast agent for bone marrow imaging appears promising for differentiation between inflammatory lesions and neoplastic metastasis compared with the standard gadolinium complex MRI scans.62
More on the radiographic evaluation of osteomyelitis is included in the article by Pineda et al in this issue of the journal.
TREATMENT
Therapy for long bone osteomyelitis includes adequate drainage, thorough debridement, obliteration of dead space, wound protection, and culture-directed antibiotic coverage. If the patient is a compromised host, efforts should be made to correct or improve host defenses, and particular attention should be paid to the patient's nutrition, cessation of smoking, and dealing with specific abnormalities such as diabetes.
Antibiotic Management
After cultures have been obtained, a parenteral course of antibiotics is begun to cover clinically suspected pathogens. When the organism is identified, a specific antibiotic or antibiotics are selected through sensitivity testing. If immediate debridement surgery is required before cultures can be obtained, empirical broad-spectrum antibiotics may be initiated and the regimen modified when the results of cultures and sensitivity tests are known. Initial antibiotic therapy for long bone osteomyelitis may consist of either nafcillin or clindamycin (or vancomycin when MRSA, methicillin-resistant Staphylococcus epidermidis, or Enterococcus spp. are suspected) and ciprofloxacin (except with children, where an aminoglycoside should be used). Levofloxacin has been used, but serum levels fall below minimum inhibitory concentrations, and at present dosing failed in both human and animal osteomyelitis studies.63,64 Bone requires 3 to 4 weeks to revascularize after debridement surgery, and thus antibiotics are used to treat live infected bone and to protect bone as it undergoes revascularization. After the last major debridement surgery, the patient is treated with 4 to 6 weeks of antimicrobial therapy, and outpatient intravenous therapy may be used.65,66
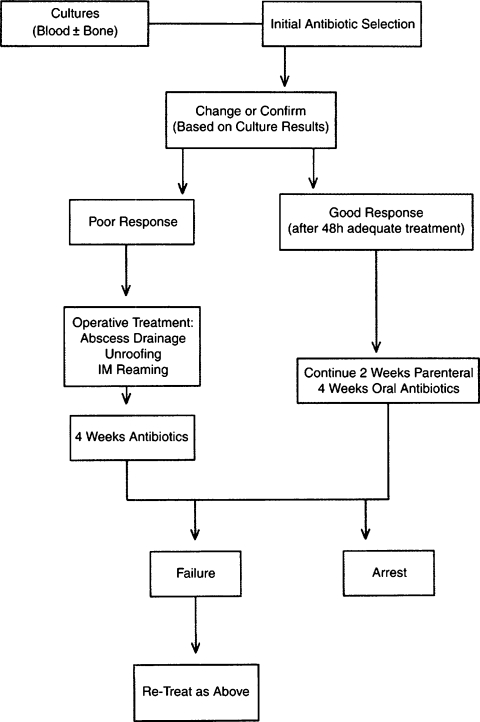
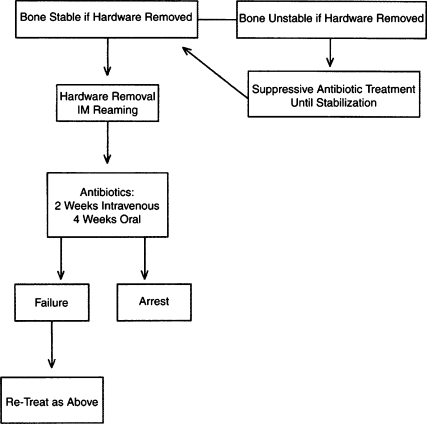
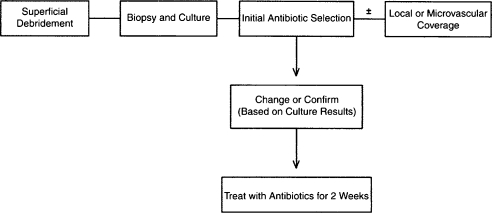
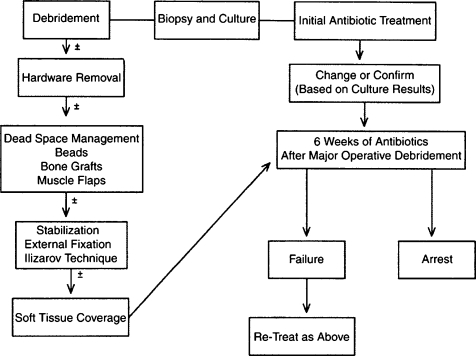
Antibiotic therapy may be directed with regard to the stage of the infection. With Cierny-Mader stage 1 osteomyelitis, children may be treated with antibiotic therapy alone, because their bones are highly vascular and respond effectively to infection. In adults, a stage 1 infection is more refractory to therapy and usually is treated with both antibiotics and surgery. With stage 2 infections, the patient may be treated with a 2-week course of antibiotics after superficial debridement and soft tissue coverage. In these cases, the arrest rate is ~80%. In stages 3 and 4, the patient is treated with 4 to 6 weeks of antimicrobial therapy dated from the last major debridement. At this stage of the disease, most antibiotic regimens will fail without adequate debridement regardless of the duration of therapy. Even after necrotic tissue has been debrided, the remaining bed of tissue must be considered contaminated. Treatment regimens, both antibiotic and surgical, for the various stages can be summarized in a series of algorithms represented in Figs. 4–7.
Figure 4.
Treatment algorithm of Cierny-Mader stage 1, or hematogenous, long bone osteomyelitis. (From Lazzarini L, Mader JT, Calhoun JH. Osteomyelitis in long bones. J Bone Joint Surg Am 2004;86:2305–2318. Reprinted with permission from The Journal of Bone and Joint Surgery, Inc.)
Figure 5.
Treatment algorithm of Cierny-Mader stage 1 long bone osteomyelitis associated with infection at the site of hardware. (From Lazzarini L, Mader JT, Calhoun JH. Osteomyelitis in long bones. J Bone Joint Surg Am 2004;86:2305–2318. Reprinted with permission from The Journal of Bone and Joint Surgery, Inc.)
Figure 6.
Treatment algorithm of Cierny-Mader stage 2 long bone osteomyelitis. (From Lazzarini L, Mader JT, Calhoun JH. Osteomyelitis in long bones. J Bone Joint Surg Am 2004;86:2305–2318. Reprinted with permission from The Journal of Bone and Joint Surgery, Inc.)
Figure 7.
Treatment algorithm of Cierny-Mader stages 3 and 4 long bone osteomyelitis. (From Lazzarini L, Mader JT, Calhoun JH. Osteomyelitis in long bones. J Bone Joint Surg Am 2004;86:2305–2318. Reprinted with permission from The Journal of Bone and Joint Surgery, Inc.)
Surgery
Surgical management of osteomyelitis is often challenging. Adequate surgical debridement decreases the bacterial load, removes dead necrotic tissues, and gives a chance for the host immune system and antibiotics to arrest infection (Fig. 8). Adequate debridement may leave a large bony defect, or dead space. Appropriate management of dead space is essential to arrest the disease and for maintenance of the bone's integrity. The objective of dead-space management is the replacement of dead bone and scar tissue with durable vascularized tissue.65,66 Complete wound closure should be attained whenever possible, and local tissue flaps or free flaps may be used to fill dead space.67,68 An alternative technique is to place cancellous bone grafts beneath local or transferred tissues until structural integrity has improved. This technique requires careful preoperative planning to conserve the patient's limited cancellous bone reserves. Open cancellous grafts without soft tissue coverage are useful when free tissue transfer is not an option and local tissue flaps are inadequate. Antibiotic-impregnated acrylic beads (Fig. 9) or antibiotic-loaded cement also may be used to sterilize and temporarily maintain dead space.68,69,70 The beads are usually removed after 2 to 4 weeks and replaced with a cancellous graft. The antibiotics used in the beads are most often vancomycin, tobramycin, and gentamicin.71 Chronic osteomyelitis of bone with nonunion or bone defects is traditionally treated by a two-stage procedure involving initial debridement and antibiotic delivery, with initial external fixation, and then definitive internal fixation. Antibiotic cement-coated interlocking intramedullary nails can help convert two-stage processes into a single-stage procedure and can be used in patients who are not ideal candidates for external fixation, as well as in patients who do not want to have an external fixator applied.72,73,74
Figure 8.
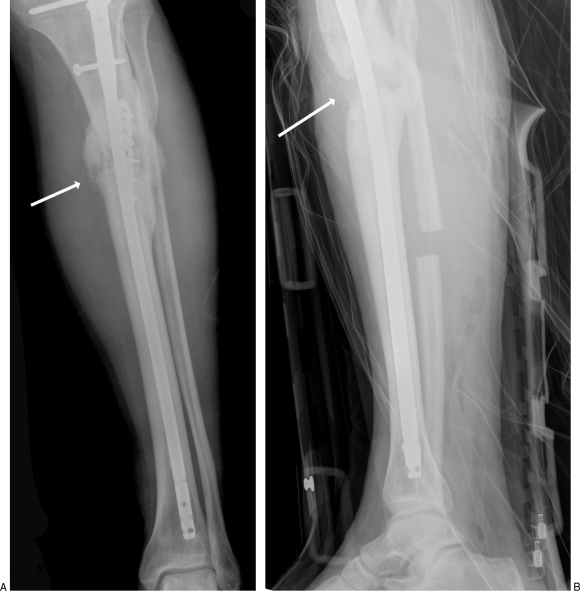
(A) A 29-year-old man suffered a proximal tibial diaphyseal fracture in a motor vehicle accident and developed infection as shown by the arrow. (B) A day after irrigation and debridement surgery, the area of infection is removed as indicated by the arrow.
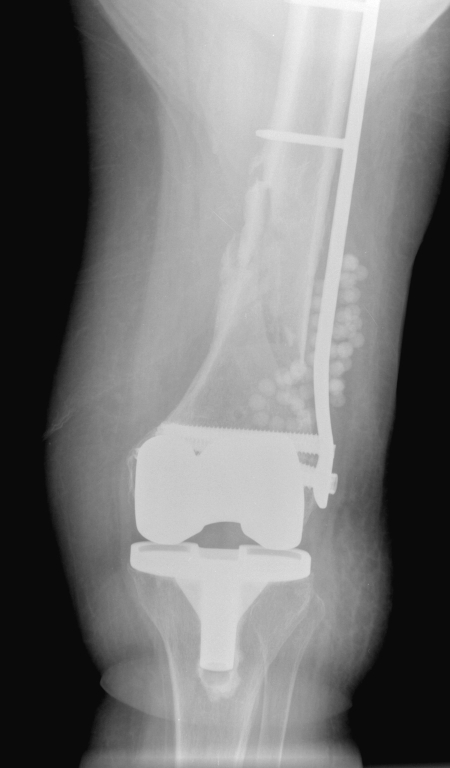
Figure 9.
Antibiotic-impregnated beads are used in the right knee of a 75-year-old woman with an infected fracture (stabilized by internal fixation) and a knee prosthesis.
As with antibiotic therapy, surgical management of long bone osteomyelitis can be guided by the Cierny-Mader classification system. In stage 1, the nidus of infection is limited to the medullary canal and usually caused by blood-borne bacteria or surgical hardware, such as an intermedullary nail. In pediatric patients without hardware, surgical therapy is usually not required. For adults with primary or secondary stage 1 osteomyelitis, a thorough intermedullary reaming and unroofing are usually performed, with bone grafting if necessary. Soft tissues are reapproximated, and the limb is protected by an external brace or cast until the bone has reestablished structural integrity. In stage 2 osteomyelitis, the bone's surface is exposed as the result of an overlying soft tissue defect. The superficial cortex is involved with the infection and becomes sequestered if treatment is delayed. The most important facet of treating this condition is soft tissue coverage to the bleeding cortex after debridement either through use of local tissue or with a free tissue transfer. Stage 3 treatment involves modalities applied to stages 1 and 2. Bone is sequestered, medullary extension of the infection is common, and major soft tissue defects may be present. Complex reconstruction of both bone and soft tissue is frequently required along with external fixation for structural support as the bone graft incorporates. Stage 4 treatment combines strategies used in the first three stages. Treatment often is focused on establishing structural stability and eliminating debridement gaps through the use of cancellous bone grafts or Ilizarov external fixation. Free flaps and vascularized bone grafts also are often used.
Together, debridement and immediate muscle-flap coverage are the primary surgical strategies to provide effective, single-stage treatment of chronic osteomyelitic wounds and allow the restriction of antibiotics to short-term use. Muscle flaps covered with skin grafts provide durable coverage while allowing subsequent ancillary procedures, such as bone grafts, to be performed.75 More on the reconstruction of osteomyelitis defects is presented in the article by Dinh et al in this issue of the journal.
PREVENTION OF OSTEOMYELITIS
Most cases of long bone osteomyelitis are posttraumatic or postoperative. With the increasing number of accidents and orthopedic procedures performed, it is unlikely that this infection rate will decrease. However, the clinician may reduce the chances that the chronic form of the infection will develop. Surgical debridement, wound irrigation, and muscle flap or vascularized tissue grafts play major roles in prevention and treatment by removing dead tissue, decreasing the bacterial load, and filling up the dead space with vascularized tissue. Early antibiotics and sensitivity-specific antibiotics also play a major role in decreasing the incidence of acute and chronic osteomyelitis. Also, internal fixation of contaminated dead bone inevitably leads to osteomyelitis so this should be avoided.76
Patients with diabetes mellitus can prevent osteomyelitis by minimizing foot trauma and by preventing foot ulcers.77 This includes education about proper foot care, including daily inspection of the feet. Daily foot washing and use of moisturizing creams are necessary to avoid breaking of the skin. Furthermore, patients should avoid activities that might cause unnecessary trauma to vasculitic neuropathic feet. This includes walking barefoot or wearing improperly fitting shoes.
RECENT ADVANCES
Intracellular persistence of the pathogen (Staphylococcus aureus) within the host osteoblast in chronic osteomyelitis cases has been identified. Although biofilm formation on devitalized tissue is the main cause of the clinical quiescence of chronic osteomyelitis, intracellular pathogens may also have a role at some point in the pathogenesis of this disease. This finding may lead to the development of new modalities to treat chronic osteomyelitis.78,79
CONCLUSION
Long bone osteomyelitis is difficult to treat and is responsible for significant morbidity and expense. The goal of treatment is to arrest its spread and repair the damage it has caused. Appropriate treatment includes culture-directed antibiotic therapy and operative debridement of all necrotic bone and soft tissue. Treatment is often staged and includes antibiotic therapy (including a combination of antibiotics and a variety of delivery systems), debridement (often multiple), dead-space management, soft tissue coverage, restoration of blood supply, and stabilization. It is essential that patients understand the importance of proper nutrition and the negative effects of smoking and other habits. Most of all, caregivers and patients must share a clear understanding of the goals of treatment and the difficulties that may persist after the initial course of therapy or surgical intervention. Over the past few decades, we have made significant advances in diagnosing and treating long bone infections, but the path to arresting the disease still requires judicious use of antibiotics, meticulous surgical intervention from the caregiver, and a patient who is actively involved in and informed of the course of treatment.
ACKNOWLEDGMENTS
The authors wish to acknowledge the assistance of Santaram Vallurupalli, M.D., in the preparation of the manuscript of this article.
REFERENCES
- Waldvogel F A, Medoff G, Swartz M N. Osteomyelitis—a review of clinical features, therapeutic considerations and unusual aspects. 3: osteomyelitis associated with vascular insufficiency. N Engl J Med. 1970;282:316–322. doi: 10.1056/NEJM197002052820606. [DOI] [PubMed] [Google Scholar]
- Cierny G, III, Mader J T, Penninck J J. A clinical staging system for adult osteomyelitis. Clin Orthop Relat Res. 2003;414:7–24. doi: 10.1097/01.blo.0000088564.81746.62. [DOI] [PubMed] [Google Scholar]
- Lew D P, Waldvogel F A. Osteomyelitis. N Engl J Med. 1997;336:999–1007. doi: 10.1056/NEJM199704033361406. [DOI] [PubMed] [Google Scholar]
- Song K M, Sloboda J F. Acute hematogenous osteomyelitis in children. J Am Acad Orthop Surg. 2001;9:166–175. doi: 10.5435/00124635-200105000-00003. [DOI] [PubMed] [Google Scholar]
- De Jonghe M, Glaesener G. Type B Haemophilus influenzae infections. Experience at the Pediatric Hospital of Luxembourg. Bull Soc Sci Med Grand Duche Luxemb. 1995;132:17–20. [PubMed] [Google Scholar]
- Blyth M J, Kincaid R, Craigen M A, Bennet G C. The changing epidemiology of acute and subacute haematogenous osteomyelitis in children. J Bone Joint Surg Br. 2001;83:99–102. doi: 10.1302/0301-620x.83b1.10699. [DOI] [PubMed] [Google Scholar]
- Meier J L, Beekmann S E. Mycobacterial and fungal infections of bone and joints. Curr Opin Rheumatol. 1995;7:329–336. doi: 10.1097/00002281-199507000-00011. [DOI] [PubMed] [Google Scholar]
- Arnold S R, Elias D, Buckingham S C, et al. Changing patterns of acute hematogenous osteomyelitis and septic arthritis: emergence of community-associated methicillin-resistant Staphylococcus aureus. J Pediatr Orthop. 2006;26:703–708. doi: 10.1097/01.bpo.0000242431.91489.b4. [DOI] [PubMed] [Google Scholar]
- Gardam M, Lim S. Mycobacterial osteomyelitis and arthritis. Infect Dis Clin North Am. 2005;19:819–830. doi: 10.1016/j.idc.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Murray C K, Roop S A, Hospenthal D R, et al. Bacteriology of war wounds at the time of injury. Mil Med. 2006;171:826–829. doi: 10.7205/milmed.171.9.826. [DOI] [PubMed] [Google Scholar]
- Hawley J S, Murray C K, Griffith M E, et al. Susceptibility of Acinetobacter strains isolated from deployed U.S. military personnel. Antimicrob Agents Chemother. 2007;51:376–378. doi: 10.1128/AAC.00858-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun H C, Murray C K, Roop S A, Hospenthal D R, Gourdine E, Dooley D P. Bacteria recovered from patients admitted to a deployed U.S. military hospital in Baghdad, Iraq. Mil Med. 2006;171:821–825. doi: 10.7205/milmed.171.9.821. [DOI] [PubMed] [Google Scholar]
- Pollak A N, Calhoun J H. Introduction. J Am Acad Orthop Surg. 2006;14(10, Suppl):viii–ix. [PubMed] [Google Scholar]
- Beronius M, Bergman B, Anderson R. Vertebral osteomyelitis in Goteborg, Sweden: a retrospective study of patients during 1990–95. Scand J Infect Dis. 2001;33:527–532. doi: 10.1080/00365540110026566. [DOI] [PubMed] [Google Scholar]
- Dahl L B, Hoyland A L, Dramsdahl H, Kaaresen P I. Acute osteomyelitis in children: a population-based retrospective study 1965 to 1994. Scand J Infect Dis. 1998;30:573–577. doi: 10.1080/00365549850161124. [DOI] [PubMed] [Google Scholar]
- Trobs R, Moritz R, Buhligen U, et al. Changing pattern of osteomyelitis in infants and children. Pediatr Surg Int. 1999;15:363–372. doi: 10.1007/s003830050600. [DOI] [PubMed] [Google Scholar]
- Epps C H, Jr, Bryant D D, III, Coles M J, Castro O. Osteomyelitis in patients who have sickle-cell disease. Diagnosis and management. J Bone Joint Surg Am. 1991;73:1281–1294. [PubMed] [Google Scholar]
- Moutschen M P, Scheen A J, Lefebvre P J. Impaired immune responses in diabetes mellitus: analysis of the factors and mechanisms involved. Relevance to the increased susceptibility of diabetic patients to specific infections. Diabete Metab. 1992;18:187–201. [PubMed] [Google Scholar]
- Emslie K R, Ozanne N R, Nade S M. Acute haematogenous osteomyelitis: an experimental model. J Pathol. 1983;141:157–167. doi: 10.1002/path.1711410206. [DOI] [PubMed] [Google Scholar]
- Ciampolini J, Harding K G. Pathophysiology of chronic bacterial osteomyelitis. Why do antibiotics fail so often? Postgrad Med J. 2000;76:479–483. doi: 10.1136/pmj.76.898.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gristina A G, Oga M, Webb L X, Hobgood C D. Adherent bacterial colonization in the pathogenesis of osteomyelitis. Science. 1985;228:990–993. doi: 10.1126/science.4001933. [DOI] [PubMed] [Google Scholar]
- Nickel J C, Ruseska I, Wright J B, Costerton J W. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob Agents Chemother. 1985;27:619–624. doi: 10.1128/aac.27.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar H, Dasgupta M K, Costerton J W. Testing the susceptibility of bacteria in biofilms to antibacterial agents. Antimicrob Agents Chemother. 1990;34:2043–2046. doi: 10.1128/aac.34.11.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A L, Sakamoto K M, Johnson E E. Differentiating osteomyelitis from bone infarction in sickle cell disease. Pediatr Emerg Care. 2001;17:60–63;. doi: 10.1097/00006565-200102000-00018. quiz 64. [DOI] [PubMed] [Google Scholar]
- Treves S, Khettry J, Broker F H, Wilkinson R H, Watts H. Osteomyelitis: early scintigraphic detection in children. Pediatrics. 1976;57:173–186. [PubMed] [Google Scholar]
- Russin L D, Staab E V. Unusual bone-scan findings in acute osteomyelitis: case report. J Nucl Med. 1976;17:617–619. [PubMed] [Google Scholar]
- Trainor V C, Udy R K, Bremer P J, Cook G M. Survival of Streptococcus pyogenes under stress and starvation. FEMS Microbiol Lett. 1999;176:421–428. doi: 10.1111/j.1574-6968.1999.tb13692.x. [DOI] [PubMed] [Google Scholar]
- Barer M R, Harwood C R. Bacterial viability and culturability. Adv Microb Physiol. 1999;41:93–137. doi: 10.1016/s0065-2911(08)60166-6. [DOI] [PubMed] [Google Scholar]
- Lleò Mdel M, Benedetti D, Tafi M C, Signoretto C, Canepari P. Inhibition of the resuscitation from the viable but non-culturable state in Enterococcus faecalis. Environ Microbiol. 2007;9:2313–2320. doi: 10.1111/j.1462-2920.2007.01345.x. [DOI] [PubMed] [Google Scholar]
- Ericsson H M, Sherris J C. Antibiotic sensitivity testing. Report of an international collaborative study. Acta Pathol Microbiol Scand [B] Microbiol Immunol. 1971;217(Suppl 217) [PubMed] [Google Scholar]
- Turnidge J D, Ferraro M J, Jorgensen J H. In: Murray PR, Baron EJ, Jorgensen JH, editor. Manual of Clinical Microbiology. 8th ed. Washington, DC: ASM Press; 2003. Susceptibility test methods: general considerations. pp. 1102–1107.
- Menozzi M G, Eigner U, Covan S, et al. Two-center collaborative evaluation of performance of the BD phoenix automated microbiology system for identification and antimicrobial susceptibility testing of gram-negative bacteria. J Clin Microbiol. 2006;44:4085–4094. doi: 10.1128/JCM.00614-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara C M. Evaluation of the Phoenix 100 ID/AST system and NID panel for identification of Enterobacteriaceae, Vibrionaceae, and commonly isolated nonenteric gram-negative bacilli. J Clin Microbiol. 2006;44:928–933. doi: 10.1128/JCM.44.3.928-933.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roveta S, Marchese A, Debbia E A. Antibiotic susceptibility tests directly on urine samples using Uro-Quick, a rapid automated system. J Chemother. 2006;18:12–19. doi: 10.1179/joc.2006.18.1.12. [DOI] [PubMed] [Google Scholar]
- Itani L Y, Cherry M A, Araj G F. Efficacy of BACTEC TB in the rapid confirmatory diagnosis of mycobacterial infections. A Lebanese tertiary care center experience. J Med Liban. 2005;53:208–212. [PubMed] [Google Scholar]
- Sader H S, Fritsche T R, Jones R N. Accuracy of three automated systems (MicroScan WalkAway, VITEK, and VITEK 2) for susceptibility testing of Pseudomonas aeruginosa against five broad-spectrum beta-lactam agents. J Clin Microbiol. 2006;44:1101–1104. doi: 10.1128/JCM.44.3.1101-1104.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedenbach D J, Jones R N. Interpretive errors using an automated system for the susceptibility testing of imipenem and aztreonam. Diagn Microbiol Infect Dis. 1995;21:57–60. doi: 10.1016/0732-8893(94)00069-9. [DOI] [PubMed] [Google Scholar]
- Biedenbach D J, Marshall S A, Jones R N. Accuracy of cefepime antimicrobial susceptibility testing results for Pseudomonas aeruginosa tested on the MicroScan WalkAway System. Diagn Microbiol Infect Dis. 1999;33:305–307. [PubMed] [Google Scholar]
- Jones R N, Biedenbach D J, Marshall S A, Pfaller M A, Doern G V. Evaluation of the Vitek system to accurately test the susceptibility of Pseudomonas aeruginosa clinical isolates against cefepime. Diagn Microbiol Infect Dis. 1998;32:107–110. doi: 10.1016/s0732-8893(98)00051-0. [DOI] [PubMed] [Google Scholar]
- Saegeman V, Huynen P, Colaert J, Melin P, Verhaegen J. Susceptibility testing of Pseudomonas aeruginosa by the Vitek 2 system: a comparison with Etest results. Acta Clin Belg. 2005;60:3–9. doi: 10.1179/acb.2005.002. [DOI] [PubMed] [Google Scholar]
- Reller L B, Stratton C W. Serum dilution test for bactericidal activity. II. Standardization and correlation with antimicrobial assays and susceptibility tests. J Infect Dis. 1977;136:196–204. doi: 10.1093/infdis/136.2.196. [DOI] [PubMed] [Google Scholar]
- Stein A, Raoult D. Colistin: an antimicrobial for the 21st century? Clin Infect Dis. 2002;35:901–902. doi: 10.1086/342570. [DOI] [PubMed] [Google Scholar]
- Jones R N, Anderegg T R, Swenson J M. Quality control guidelines for testing gram-negative control strains with polymyxin B and colistin (polymyxin E) by standardized methods. J Clin Microbiol. 2005;43:925–927. doi: 10.1128/JCM.43.2.925-927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt W P. The radiology of infection. Clin Orthop Relat Res. 1973;96:20–30. [PubMed] [Google Scholar]
- Jones A G, Francis M D, Davis M A. Bone scanning: radionuclidic reaction mechanisms. Semin Nucl Med. 1976;6:3–18. doi: 10.1016/s0001-2998(76)80032-3. [DOI] [PubMed] [Google Scholar]
- Kuhn J P, Berger P E. Computed tomographic diagnosis of osteomyelitis. Radiology. 1979;130:503–506. doi: 10.1148/130.2.503. [DOI] [PubMed] [Google Scholar]
- Propst-Proctor S L, Dillingham M F, McDougall I R, Goodwin D. The white blood cell scan in orthopedics. Clin Orthop Relat Res. 1982;168:157–165. [PubMed] [Google Scholar]
- Pineda C, Vargas A, Rodriguez A V. Imaging of osteomyelitis: current concepts. Infect Dis Clin North Am. 2006;20:789–825. doi: 10.1016/j.idc.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Merkel K D, Brown M L, Dewanjee M K, Fitzgerald R H., Jr Comparison of indium-labeled-leukocyte imaging with sequential technetium-gallium scanning in the diagnosis of low-grade musculoskeletal sepsis. A prospective study. J Bone Joint Surg Am. 1985;67:465–476. [PubMed] [Google Scholar]
- Al-Sheikh W, Sfakianakis G N, Mnaymneh W, et al. Subacute and chronic bone infections: diagnosis using In-111, Ga-67 and Tc-99m MDP bone scintigraphy, and radiography. Radiology. 1985;155:501–506. doi: 10.1148/radiology.155.2.3157204. [DOI] [PubMed] [Google Scholar]
- Termaat M F, Raijmakers P G, Scholten H J, Bakker F C, Patka P, Haarman H J. The accuracy of diagnostic imaging for the assessment of chronic osteomyelitis: a systematic review and meta-analysis. J Bone Joint Surg Am. 2005;87:2464–2471. doi: 10.2106/JBJS.D.02691. [DOI] [PubMed] [Google Scholar]
- Singh B, Mittal B R, Bhattacharya A, Aggarwal A, Nagi O N, Singh A K. Technetium-99m ciprofloxacin imaging in the diagnosis of postsurgical bony infection and evaluation of the response to antibiotic therapy: a case report. J Orthop Surg (Hong Kong) 2005;13:190–194. doi: 10.1177/230949900501300217. [DOI] [PubMed] [Google Scholar]
- Britton K E, Wareham D W, Das S S, et al. Imaging bacterial infection with (99m)Tc-ciprofloxacin (infecton) J Clin Pathol. 2002;55:817–823. doi: 10.1136/jcp.55.11.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang H, Duarte P S, Pourdehand M, Shnier D, Alavi A. Exclusion of chronic osteomyelitis with F-18 fluorodeoxyglucose positron emission tomographic imaging. Clin Nucl Med. 2000;25:281–284. doi: 10.1097/00003072-200004000-00009. [DOI] [PubMed] [Google Scholar]
- Kalicke T, Schmitz A, Risse J H, et al. Fluorine-18 fluorodeoxyglucose PET in infectious bone diseases: results of histologically confirmed cases. Eur J Nucl Med. 2000;27:524–528. doi: 10.1007/s002590050538. [DOI] [PubMed] [Google Scholar]
- Jones-Jackson L, Walker R, Purnell G, et al. Early detection of bone infection and differentiation from post-surgical inflammation using 2-deoxy-2-[18F]-fluoro-D-glucose positron emission tomography (FDG-PET) in an animal model. J Orthop Res. 2005;23:1484–1489. doi: 10.1016/j.orthres.2005.03.010.1100230635. [DOI] [PubMed] [Google Scholar]
- Meller J, Koster G, Liersch T, et al. Chronic bacterial osteomyelitis: prospective comparison of (18)F-FDG imaging with a dual-head coincidence camera and (111)In-labelled autologous leucocyte scintigraphy. Eur J Nucl Med Mol Imaging. 2002;29:53–60. doi: 10.1007/s00259-001-0661-9. [DOI] [PubMed] [Google Scholar]
- de Winter F, de Wiele C van, Vogelaers D, de Smet K, Verdonk R, Dierckx R A. Fluorine-18 fluorodeoxyglucose-position emission tomography: a highly accurate imaging modality for the diagnosis of chronic musculoskeletal infections. J Bone Joint Surg Am. 2001;83:651–660. doi: 10.2106/00004623-200105000-00002. [DOI] [PubMed] [Google Scholar]
- El-Haddad G, Zhuang H, Gupta N, Alavi A. Evolving role of positron emission tomography in the management of patients with inflammatory and other benign disorders. Semin Nucl Med. 2004;34:313–329. doi: 10.1053/j.semnuclmed.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Love C, Tomas M B, Tronco G G, Palestro C J. FDG PET of infection and inflammation. Radiographics. 2005;25:1357–1368. doi: 10.1148/rg.255045122. [DOI] [PubMed] [Google Scholar]
- Basu S, Chryssikos T, Houseni M, et al. Potential role of FDG PET in the setting of diabetic neuro-osteoarthropathy: can it differentiate uncomplicated Charcot's neuroarthropathy from osteomyelitis and soft-tissue infection? Nucl Med Commun. 2007;28:465–472. doi: 10.1097/MNM.0b013e328174447f. [DOI] [PubMed] [Google Scholar]
- Fukuda Y, Ando K, Ishikura R, et al. Superparamagnetic iron oxide (SPIO) MRI contrast agent for bone marrow imaging: differentiating bone metastasis and osteomyelitis. Magn Reson Med Sci. 2006;5:191–196. doi: 10.2463/mrms.5.191. [DOI] [PubMed] [Google Scholar]
- Greenberg R N, Newman M T, Shariaty S, Pectol R W. Ciprofloxacin, lomefloxacin, or levofloxacin as treatment for chronic osteomyelitis. Antimicrob Agents Chemother. 2000;44:164–166. doi: 10.1128/aac.44.1.164-166.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtliff M E, Calhoun J H, Mader J T. Comparative evaluation of oral levofloxacin and parenteral nafcillin in the treatment of experimental methicillin-susceptible Staphylococcus aureus osteomyelitis in rabbits. J Antimicrob Chemother. 2001;48:253–258. doi: 10.1093/jac/48.2.253. [DOI] [PubMed] [Google Scholar]
- Cierny G, III, Mader J T. In: Evarts CMC, editor. Surgery of the Musculoskeletal System. New York, NY: Churchill Livingstone; 1983. The surgical treatment of adult osteomyelitis. pp. 15–35.
- Mader J T, Ortiz M, Calhoun J H. Update on the diagnosis and management of osteomyelitis. Clin Podiatr Med Surg. 1996;13:701–724. [PubMed] [Google Scholar]
- May J W, Jr, Jupiter J B, Gallico G G, III, Rothkopf D M, Zingarelli P. Treatment of chronic traumatic bone wounds. Microvascular free tissue transfer: a 13-year experience in 96 patients. Ann Surg. 1991;214:241–250. doi: 10.1097/00000658-199109000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papagelopoulos P J, Mavrogenis A F, Tsiodras S, Vlastou C, Giamarellou H, Soucacos P N. Calcium sulphate delivery system with tobramycin for the treatment of chronic calcaneal osteomyelitis. J Int Med Res. 2006;34:704–712. doi: 10.1177/147323000603400618. [DOI] [PubMed] [Google Scholar]
- Kent M E, Rapp R P, Smith K M. Antibiotic beads and osteomyelitis: here today, what's coming tomorrow? Orthopedics. 2006;29:599–603. doi: 10.3928/01477447-20060701-02. [DOI] [PubMed] [Google Scholar]
- Diefenbeck M, Muckley T, Hofmann G O. Prophylaxis and treatment of implant-related infections by local application of antibiotics. Injury. 2006;37(Suppl 2):S95–S104. doi: 10.1016/j.injury.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Mader J T, Calhoun J H. In: Mader JT, Calhoun JH, editor. Musculoskeletal Infections. New York, NY: Marcel Dekker; 2003. Adult long bone osteomyelitis. pp. 149–182.
- Thonse R, Conway J. Antibiotic cement-coated interlocking nail for the treatment of infected nonunions and segmental bone defects. J Orthop Trauma. 2007;21:258–268. doi: 10.1097/BOT.0b013e31803ea9e6. [DOI] [PubMed] [Google Scholar]
- Ohtsuka H, Yokoyama K, Higashi K, et al. Use of antibiotic-impregnated bone cement nail to treat septic nonunion after open tibial fracture. J Trauma. 2002;52:364–366. doi: 10.1097/00005373-200202000-00025. [DOI] [PubMed] [Google Scholar]
- Paley D, Herzenberg J E. Intramedullary infections treated with antibiotic cement rods: preliminary results in nine cases. J Orthop Trauma. 2002;16:723–729. doi: 10.1097/00005131-200211000-00007. [DOI] [PubMed] [Google Scholar]
- Anthony J P, Mathes S J, Alpert B S. The muscle flap in the treatment of chronic lower extremity osteomyelitis: results in patients over 5 years after treatment. Plast Reconstr Surg. 1991;88:311–318. doi: 10.1097/00006534-199108000-00023. [DOI] [PubMed] [Google Scholar]
- Evans R P, Nelson C L, Harrison B H. The effect of wound environment on the incidence of acute osteomyelitis. Clin Orthop Relat Res. 1993;286:289–297. [PubMed] [Google Scholar]
- Calhoun J H, Cantrell J, Cobos J, et al. Treatment of diabetic foot infections: Wagner classification, therapy, and outcome. Foot Ankle. 1988;9:101–106. doi: 10.1177/107110078800900301. [DOI] [PubMed] [Google Scholar]
- Webb L X, Wagner W, Carroll D, Tyler H, Coldren F, Martin E. Osteomyelitis and intraosteoblastic Staphylococcus aureus. J Surg Orthop Adv. 2007;16:73–78. [PubMed] [Google Scholar]
- Ellington J K, Harris M, Hudson M C, Vishin S, Webb L X, Sherertz R. Intracellular Staphylococcus aureus and antibiotic resistance: implications for treatment of staphylococcal osteomyelitis. J Orthop Res. 2006;24:87–93. doi: 10.1002/jor.20003. [DOI] [PubMed] [Google Scholar]