Abstract
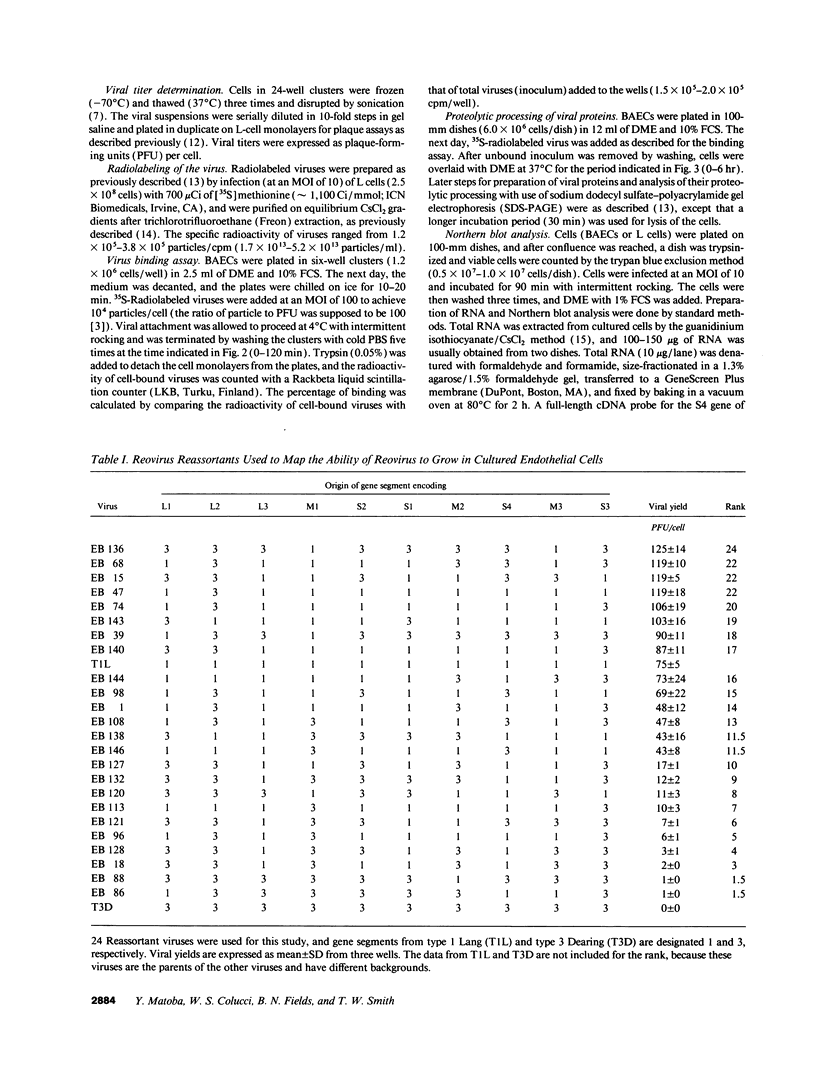
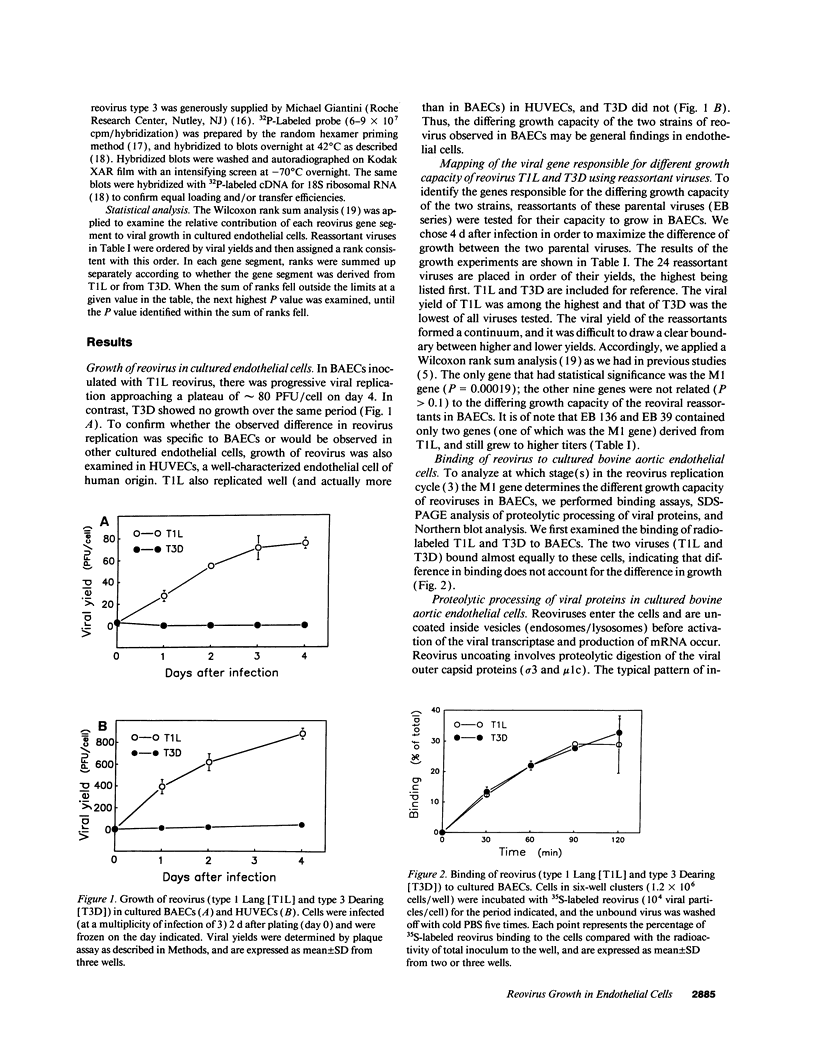
Since blood-borne viruses often interact with endothelial cells before tissue invasion, the interaction between viruses and endothelial cells is likely to be important in viral pathogenicity. Two reovirus isolates (type 1 Lang and type 3 Dearing) differ in their capacity to grow in cultured bovine aortic endothelial cells. The mammalian reoviruses have 10 double-stranded RNA gene segments in their genome. By using 24 reassortant viruses, observed differences in the capacity of different strains to grow in cultured endothelial cells were mapped to the M1 gene (P = 0.00019), which encodes the viral core protein mu 2. No differences were detected in binding or proteolytic processing of viral outer capsid proteins of parental virions between the two reovirus isolates. Northern blot analysis showed a decreased production of viral mRNA in endothelial cells infected with type 3 Dearing reovirus, but not type 1 Lang. Thus, we have identified a viral gene (the M1 gene) responsible for determining the difference in growth capacity of the two reovirus isolates in cultured endothelial cells. Reovirus is an attractive model in which to study the interaction of viruses with endothelial cells at a molecular genetic level.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews B. S., Theofilopoulos A. N., Peters C. J., Loskutoff D. J., Brandt W. E., Dixon F. J. Replication of dengue and junin viruses in cultured rabbit and human endothelial cells. Infect Immun. 1978 Jun;20(3):776–781. doi: 10.1128/iai.20.3.776-781.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwater J. A., Munemitsu S. M., Samuel C. E. Biosynthesis of reovirus-specified polypeptides. Molecular cDNA cloning and nucleotide sequence of the reovirus serotype 1 Lang strain s4 mRNA which encodes the major capsid surface polypeptide sigma 3. Biochem Biophys Res Commun. 1986 Apr 14;136(1):183–192. doi: 10.1016/0006-291x(86)90893-4. [DOI] [PubMed] [Google Scholar]
- Bodkin D. K., Fields B. N. Growth and survival of reovirus in intestinal tissue: role of the L2 and S1 genes. J Virol. 1989 Mar;63(3):1188–1193. doi: 10.1128/jvi.63.3.1188-1193.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cunningham M. J., Pasternak R. C. The potential role of viruses in the pathogenesis of atherosclerosis. Circulation. 1988 May;77(5):964–966. doi: 10.1161/01.cir.77.5.964. [DOI] [PubMed] [Google Scholar]
- Davies P. F., Rennke H. G., Cotran R. S. Influence of molecular charge upon the endocytosis and intracellular fate of peroxidase activity in cultured arterial endothelium. J Cell Sci. 1981 Jun;49:69–86. doi: 10.1242/jcs.49.1.69. [DOI] [PubMed] [Google Scholar]
- Dec G. W., Jr, Palacios I. F., Fallon J. T., Aretz H. T., Mills J., Lee D. C., Johnson R. A. Active myocarditis in the spectrum of acute dilated cardiomyopathies. Clinical features, histologic correlates, and clinical outcome. N Engl J Med. 1985 Apr 4;312(14):885–890. doi: 10.1056/NEJM198504043121404. [DOI] [PubMed] [Google Scholar]
- Dichter M. A., Weiner H. L. Infection of neuronal cell cultures with reovirus mimics in vitro patterns of neurotropism. Ann Neurol. 1984 Nov;16(5):603–610. doi: 10.1002/ana.410160512. [DOI] [PubMed] [Google Scholar]
- Drayna D., Fields B. N. Activation and characterization of the reovirus transcriptase: genetic analysis. J Virol. 1982 Jan;41(1):110–118. doi: 10.1128/jvi.41.1.110-118.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drayna D., Fields B. N. Genetic studies on the mechanism of chemical and physical inactivation of reovirus. J Gen Virol. 1982 Nov;63(Pt 1):149–159. doi: 10.1099/0022-1317-63-1-149. [DOI] [PubMed] [Google Scholar]
- Drayna D., Fields B. N. Genetic studies on the mechanism of chemical and physical inactivation of reovirus. J Gen Virol. 1982 Nov;63(Pt 1):149–159. doi: 10.1099/0022-1317-63-1-149. [DOI] [PubMed] [Google Scholar]
- Etingin O. R., Silverstein R. L., Friedman H. M., Hajjar D. P. Viral activation of the coagulation cascade: molecular interactions at the surface of infected endothelial cells. Cell. 1990 May 18;61(4):657–662. doi: 10.1016/0092-8674(90)90477-v. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Finkel M. S., Oddis C. V., Jacob T. D., Watkins S. C., Hattler B. G., Simmons R. L. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science. 1992 Jul 17;257(5068):387–389. doi: 10.1126/science.1631560. [DOI] [PubMed] [Google Scholar]
- Flamand A., Gagner J. P., Morrison L. A., Fields B. N. Penetration of the nervous systems of suckling mice by mammalian reoviruses. J Virol. 1991 Jan;65(1):123–131. doi: 10.1128/jvi.65.1.123-131.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerritsen M. E. Functional heterogeneity of vascular endothelial cells. Biochem Pharmacol. 1987 Sep 1;36(17):2701–2711. doi: 10.1016/0006-2952(87)90252-8. [DOI] [PubMed] [Google Scholar]
- Gerson S. L., Friedman H. M., Cines D. B. Viral infection of vascular endothelial cells alters production of colony-stimulating activity. J Clin Invest. 1985 Oct;76(4):1382–1390. doi: 10.1172/JCI112114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giantini M., Seliger L. S., Furuichi Y., Shatkin A. J. Reovirus type 3 genome segment S4: nucleotide sequence of the gene encoding a major virion surface protein. J Virol. 1984 Dec;52(3):984–987. doi: 10.1128/jvi.52.3.984-987.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr Culture of vascular endothelium. Prog Hemost Thromb. 1976;3:1–28. [PubMed] [Google Scholar]
- Hrdy D. B., Rubin D. H., Fields B. N. Molecular basis of reovirus neurovirulence: role of the M2 gene in avirulence. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1298–1302. doi: 10.1073/pnas.79.4.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., West D. C., Ager A. Heterogeneity in endothelial cells from large vessels and microvessels. Differentiation. 1987;36(1):57–70. doi: 10.1111/j.1432-0436.1987.tb00181.x. [DOI] [PubMed] [Google Scholar]
- Lee R. T., Bloch K. D., Pfeffer J. M., Pfeffer M. A., Neer E. J., Seidman C. E. Atrial natriuretic factor gene expression in ventricles of rats with spontaneous biventricular hypertrophy. J Clin Invest. 1988 Feb;81(2):431–434. doi: 10.1172/JCI113337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor R. R., Friedman H. M., Macarak E. J., Kefalides N. A. Virus infection of endothelial cells increases granulocyte adherence. J Clin Invest. 1980 Jun;65(6):1469–1477. doi: 10.1172/JCI109811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macarak E. J., Friedman H. M., Kefalides N. A. Herpes simplex virus type 1 infection of endothelium reduces collagen and fibronectin synthesis. Lab Invest. 1985 Sep;53(3):280–286. [PubMed] [Google Scholar]
- Matoba Y., Sherry B., Fields B. N., Smith T. W. Identification of the viral genes responsible for growth of strains of reovirus in cultured mouse heart cells. J Clin Invest. 1991 May;87(5):1628–1633. doi: 10.1172/JCI115177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera T., Toniolo A., Ray U. R., Jenson A. B., Knazek R. A., Notkins A. L. Virus-induced diabetes mellitus. XX. Polyendocrinopathy and autoimmunity. J Exp Med. 1981 Jun 1;153(6):1457–1473. doi: 10.1084/jem.153.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramig R. F., Cross R. K., Fields B. N. Genome RNAs and polypeptides of reovirus serotypes 1, 2, and 3. J Virol. 1977 Jun;22(3):726–733. doi: 10.1128/jvi.22.3.726-733.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re R. N. The cellular biology of angiotensin: paracrine, autocrine and intracrine actions in cardiovascular tissues. J Mol Cell Cardiol. 1989 Dec;21 (Suppl 5):63–69. doi: 10.1016/0022-2828(89)90772-4. [DOI] [PubMed] [Google Scholar]
- Sharpe A. H., Ramig R. F., Mustoe T. A., Fields B. N. A genetic map of reovirus. 1. Correlation of genome RNAs between serotypes 1, 2, and 3. Virology. 1978 Jan;84(1):63–74. doi: 10.1016/0042-6822(78)90218-0. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J., LaFiandra A. J. Transcription by infectious subviral particles of reovirus. J Virol. 1972 Oct;10(4):698–706. doi: 10.1128/jvi.10.4.698-706.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry B., Fields B. N. The reovirus M1 gene, encoding a viral core protein, is associated with the myocarditic phenotype of a reovirus variant. J Virol. 1989 Nov;63(11):4850–4856. doi: 10.1128/jvi.63.11.4850-4856.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry B., Schoen F. J., Wenske E., Fields B. N. Derivation and characterization of an efficiently myocarditic reovirus variant. J Virol. 1989 Nov;63(11):4840–4849. doi: 10.1128/jvi.63.11.4840-4849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shubeita H. E., McDonough P. M., Harris A. N., Knowlton K. U., Glembotski C. C., Brown J. H., Chien K. R. Endothelin induction of inositol phospholipid hydrolysis, sarcomere assembly, and cardiac gene expression in ventricular myocytes. A paracrine mechanism for myocardial cell hypertrophy. J Biol Chem. 1990 Nov 25;265(33):20555–20562. [PubMed] [Google Scholar]
- Sturzenbecker L. J., Nibert M., Furlong D., Fields B. N. Intracellular digestion of reovirus particles requires a low pH and is an essential step in the viral infectious cycle. J Virol. 1987 Aug;61(8):2351–2361. doi: 10.1128/jvi.61.8.2351-2361.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdin E. M., King G. L., Maratos-Flier E. Characterization of a common high-affinity receptor for reovirus serotypes 1 and 3 on endothelial cells. J Virol. 1989 Mar;63(3):1318–1325. doi: 10.1128/jvi.63.3.1318-1325.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin H. W., 4th, Bassel-Duby R., Fields B. N., Tyler K. L. Antibody protects against lethal infection with the neurally spreading reovirus type 3 (Dearing). J Virol. 1988 Dec;62(12):4594–4604. doi: 10.1128/jvi.62.12.4594-4604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Millward S., Graham A. F. Regulation of transcription of the Reovirus genome. J Mol Biol. 1968 Aug 28;36(1):107–123. doi: 10.1016/0022-2836(68)90223-4. [DOI] [PubMed] [Google Scholar]
- Weiner H. L., Ault K. A., Fields B. N. Interaction of reovirus with cell surface receptors. I. Murine and human lymphocytes have a receptor for the hemagglutinin of reovirus type 3. J Immunol. 1980 May;124(5):2143–2148. [PubMed] [Google Scholar]
- Weiner H. L., Powers M. L., Fields B. N. Absolute linkage of virulence and central nervous system cell tropism of reoviruses to viral hemagglutinin. J Infect Dis. 1980 May;141(5):609–616. doi: 10.1093/infdis/141.5.609. [DOI] [PubMed] [Google Scholar]