Abstract
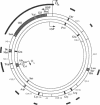
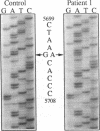
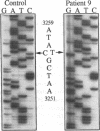
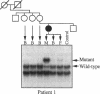
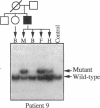
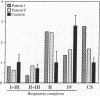
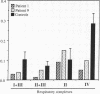
We identified two patients with pathogenic single nucleotide changes in two different mitochondrial tRNA genes: the first mutation in the tRNA(Asn) gene, and the ninth known mutation in the tRNA(Leu(UUR)) gene. The mutation in tRNA(Asn) was associated with isolated ophthalmoplegia, whereas the mutation in tRNA(Leu(UUR)) caused a neurological syndrome resembling MERRF (myoclonus epilepsy and ragged-red fibers) plus optic neuropathy, retinopathy, and diabetes. Both mutations were heteroplasmic, with higher percentages of mutant mtDNA in affected tissues, and undetectable levels in maternal relatives. Analysis of single muscle fibers indicated that morphological and biochemical alterations appeared only when the proportions of mutant mtDNA exceeded 90% of the total cellular mtDNA pool. The high incidence of mutations in the tRNA(Leu(UUR)) gene suggests that this region is an "etiologic hot spot" in mitochondrial disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Attardi G., Schatz G. Biogenesis of mitochondria. Annu Rev Cell Biol. 1988;4:289–333. doi: 10.1146/annurev.cb.04.110188.001445. [DOI] [PubMed] [Google Scholar]
- Ballinger S. W., Schurr T. G., Torroni A., Gan Y. Y., Hodge J. A., Hassan K., Chen K. H., Wallace D. C. Southeast Asian mitochondrial DNA analysis reveals genetic continuity of ancient mongoloid migrations. Genetics. 1992 Jan;130(1):139–152. doi: 10.1093/genetics/130.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulet L., Karpati G., Shoubridge E. A. Distribution and threshold expression of the tRNA(Lys) mutation in skeletal muscle of patients with myoclonic epilepsy and ragged-red fibers (MERRF). Am J Hum Genet. 1992 Dec;51(6):1187–1200. [PMC free article] [PubMed] [Google Scholar]
- Brown M. D., Voljavec A. S., Lott M. T., Torroni A., Yang C. C., Wallace D. C. Mitochondrial DNA complex I and III mutations associated with Leber's hereditary optic neuropathy. Genetics. 1992 Jan;130(1):163–173. doi: 10.1093/genetics/130.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cann R. L., Stoneking M., Wilson A. C. Mitochondrial DNA and human evolution. Nature. 1987 Jan 1;325(6099):31–36. doi: 10.1038/325031a0. [DOI] [PubMed] [Google Scholar]
- Chomyn A., Martinuzzi A., Yoneda M., Daga A., Hurko O., Johns D., Lai S. T., Nonaka I., Angelini C., Attardi G. MELAS mutation in mtDNA binding site for transcription termination factor causes defects in protein synthesis and in respiration but no change in levels of upstream and downstream mature transcripts. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4221–4225. doi: 10.1073/pnas.89.10.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomyn A., Meola G., Bresolin N., Lai S. T., Scarlato G., Attardi G. In vitro genetic transfer of protein synthesis and respiration defects to mitochondrial DNA-less cells with myopathy-patient mitochondria. Mol Cell Biol. 1991 Apr;11(4):2236–2244. doi: 10.1128/mcb.11.4.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. A. Replication and transcription of vertebrate mitochondrial DNA. Annu Rev Cell Biol. 1991;7:453–478. doi: 10.1146/annurev.cb.07.110191.002321. [DOI] [PubMed] [Google Scholar]
- Cortopassi G. A., Arnheim N. Detection of a specific mitochondrial DNA deletion in tissues of older humans. Nucleic Acids Res. 1990 Dec 11;18(23):6927–6933. doi: 10.1093/nar/18.23.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMauro S., Servidei S., Zeviani M., DiRocco M., DeVivo D. C., DiDonato S., Uziel G., Berry K., Hoganson G., Johnsen S. D. Cytochrome c oxidase deficiency in Leigh syndrome. Ann Neurol. 1987 Oct;22(4):498–506. doi: 10.1002/ana.410220409. [DOI] [PubMed] [Google Scholar]
- Edqvist J., Stråby K. B., Grosjean H. Pleiotrophic effects of point mutations in yeast tRNA(Asp) on the base modification pattern. Nucleic Acids Res. 1993 Feb 11;21(3):413–417. doi: 10.1093/nar/21.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y., Nonaka I., Horai S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990 Dec 13;348(6302):651–653. doi: 10.1038/348651a0. [DOI] [PubMed] [Google Scholar]
- Goto Y., Nonaka I., Horai S. A new mtDNA mutation associated with mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes (MELAS). Biochim Biophys Acta. 1991 Oct 21;1097(3):238–240. doi: 10.1016/0925-4439(91)90042-8. [DOI] [PubMed] [Google Scholar]
- Goto Y., Tojo M., Tohyama J., Horai S., Nonaka I. A novel point mutation in the mitochondrial tRNA(Leu)(UUR) gene in a family with mitochondrial myopathy. Ann Neurol. 1992 Jun;31(6):672–675. doi: 10.1002/ana.410310617. [DOI] [PubMed] [Google Scholar]
- Hammans S. R., Sweeney M. G., Brockington M., Morgan-Hughes J. A., Harding A. E. Mitochondrial encephalopathies: molecular genetic diagnosis from blood samples. Lancet. 1991 Jun 1;337(8753):1311–1313. doi: 10.1016/0140-6736(91)92981-7. [DOI] [PubMed] [Google Scholar]
- Hammans S. R., Sweeney M. G., Wicks D. A., Morgan-Hughes J. A., Harding A. E. A molecular genetic study of focal histochemical defects in mitochondrial encephalomyopathies. Brain. 1992 Apr;115(Pt 2):343–365. doi: 10.1093/brain/115.2.343. [DOI] [PubMed] [Google Scholar]
- Hayashi J., Ohta S., Kikuchi A., Takemitsu M., Goto Y., Nonaka I. Introduction of disease-related mitochondrial DNA deletions into HeLa cells lacking mitochondrial DNA results in mitochondrial dysfunction. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10614–10618. doi: 10.1073/pnas.88.23.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess J. F., Parisi M. A., Bennett J. L., Clayton D. A. Impairment of mitochondrial transcription termination by a point mutation associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1991 May 16;351(6323):236–239. doi: 10.1038/351236a0. [DOI] [PubMed] [Google Scholar]
- Holt I. J., Harding A. E., Cooper J. M., Schapira A. H., Toscano A., Clark J. B., Morgan-Hughes J. A. Mitochondrial myopathies: clinical and biochemical features of 30 patients with major deletions of muscle mitochondrial DNA. Ann Neurol. 1989 Dec;26(6):699–708. doi: 10.1002/ana.410260603. [DOI] [PubMed] [Google Scholar]
- Holt I. J., Harding A. E., Petty R. K., Morgan-Hughes J. A. A new mitochondrial disease associated with mitochondrial DNA heteroplasmy. Am J Hum Genet. 1990 Mar;46(3):428–433. [PMC free article] [PubMed] [Google Scholar]
- King M. P., Koga Y., Davidson M., Schon E. A. Defects in mitochondrial protein synthesis and respiratory chain activity segregate with the tRNA(Leu(UUR)) mutation associated with mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes. Mol Cell Biol. 1992 Feb;12(2):480–490. doi: 10.1128/mcb.12.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse B., Narasimhan N., Attardi G. Termination of transcription in human mitochondria: identification and purification of a DNA binding protein factor that promotes termination. Cell. 1989 Jul 28;58(2):391–397. doi: 10.1016/0092-8674(89)90853-2. [DOI] [PubMed] [Google Scholar]
- Larsson N. G., Andersen O., Holme E., Oldfors A., Wahlström J. Leber's hereditary optic neuropathy and complex I deficiency in muscle. Ann Neurol. 1991 Nov;30(5):701–708. doi: 10.1002/ana.410300511. [DOI] [PubMed] [Google Scholar]
- Lertrit P., Noer A. S., Jean-Francois M. J., Kapsa R., Dennett X., Thyagarajan D., Lethlean K., Byrne E., Marzuki S. A new disease-related mutation for mitochondrial encephalopathy lactic acidosis and strokelike episodes (MELAS) syndrome affects the ND4 subunit of the respiratory complex I. Am J Hum Genet. 1992 Sep;51(3):457–468. [PMC free article] [PubMed] [Google Scholar]
- Marzuki S., Noer A. S., Lertrit P., Thyagarajan D., Kapsa R., Utthanaphol P., Byrne E. Normal variants of human mitochondrial DNA and translation products: the building of a reference data base. Hum Genet. 1991 Dec;88(2):139–145. doi: 10.1007/BF00206061. [DOI] [PubMed] [Google Scholar]
- McClain W. H., Foss K. Nucleotides that contribute to the identity of Escherichia coli tRNA(Phe). J Mol Biol. 1988 Aug 20;202(4):697–709. doi: 10.1016/0022-2836(88)90551-7. [DOI] [PubMed] [Google Scholar]
- Moraes C. T., Ciacci F., Bonilla E., Ionasescu V., Schon E. A., DiMauro S. A mitochondrial tRNA anticodon swap associated with a muscle disease. Nat Genet. 1993 Jul;4(3):284–288. doi: 10.1038/ng0793-284. [DOI] [PubMed] [Google Scholar]
- Moraes C. T., Ciacci F., Silvestri G., Shanske S., Sciacco M., Hirano M., Schon E. A., Bonilla E., DiMauro S. Atypical clinical presentations associated with the MELAS mutation at position 3243 of human mitochondrial DNA. Neuromuscul Disord. 1993 Jan;3(1):43–50. doi: 10.1016/0960-8966(93)90040-q. [DOI] [PubMed] [Google Scholar]
- Moraes C. T., DiMauro S., Zeviani M., Lombes A., Shanske S., Miranda A. F., Nakase H., Bonilla E., Werneck L. C., Servidei S. Mitochondrial DNA deletions in progressive external ophthalmoplegia and Kearns-Sayre syndrome. N Engl J Med. 1989 May 18;320(20):1293–1299. doi: 10.1056/NEJM198905183202001. [DOI] [PubMed] [Google Scholar]
- Moraes C. T., Ricci E., Bonilla E., DiMauro S., Schon E. A. The mitochondrial tRNA(Leu(UUR)) mutation in mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes (MELAS): genetic, biochemical, and morphological correlations in skeletal muscle. Am J Hum Genet. 1992 May;50(5):934–949. [PMC free article] [PubMed] [Google Scholar]
- Moraes C. T., Shanske S., Tritschler H. J., Aprille J. R., Andreetta F., Bonilla E., Schon E. A., DiMauro S. mtDNA depletion with variable tissue expression: a novel genetic abnormality in mitochondrial diseases. Am J Hum Genet. 1991 Mar;48(3):492–501. [PMC free article] [PubMed] [Google Scholar]
- Nakase H., Moraes C. T., Rizzuto R., Lombes A., DiMauro S., Schon E. A. Transcription and translation of deleted mitochondrial genomes in Kearns-Sayre syndrome: implications for pathogenesis. Am J Hum Genet. 1990 Mar;46(3):418–427. [PMC free article] [PubMed] [Google Scholar]
- Oldfors A., Larsson N. G., Lindberg C., Holme E. Mitochondrial DNA deletions in inclusion body myositis. Brain. 1993 Apr;116(Pt 2):325–336. doi: 10.1093/brain/116.2.325. [DOI] [PubMed] [Google Scholar]
- Ozawa T., Tanaka M., Ino H., Ohno K., Sano T., Wada Y., Yoneda M., Tanno Y., Miyatake T., Tanaka T. Distinct clustering of point mutations in mitochondrial DNA among patients with mitochondrial encephalomyopathies and with Parkinson's disease. Biochem Biophys Res Commun. 1991 Apr 30;176(2):938–946. doi: 10.1016/s0006-291x(05)80276-1. [DOI] [PubMed] [Google Scholar]
- Puglisi J. D., Pütz J., Florentz C., Giegé R. Influence of tRNA tertiary structure and stability on aminoacylation by yeast aspartyl-tRNA synthetase. Nucleic Acids Res. 1993 Jan 11;21(1):41–49. doi: 10.1093/nar/21.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci E., Moraes C. T., Servidei S., Tonali P., Bonilla E., DiMauro S. Disorders associated with depletion of mitochondrial DNA. Brain Pathol. 1992 Apr;2(2):141–147. doi: 10.1111/j.1750-3639.1992.tb00682.x. [DOI] [PubMed] [Google Scholar]
- Rowland L. P., Blake D. M., Hirano M., Di Mauro S., Schon E. A., Hays A. P., Devivo D. C. Clinical syndromes associated with ragged red fibers. Rev Neurol (Paris) 1991;147(6-7):467–473. [PubMed] [Google Scholar]
- Rötig A., Cormier V., Blanche S., Bonnefont J. P., Ledeist F., Romero N., Schmitz J., Rustin P., Fischer A., Saudubray J. M. Pearson's marrow-pancreas syndrome. A multisystem mitochondrial disorder in infancy. J Clin Invest. 1990 Nov;86(5):1601–1608. doi: 10.1172/JCI114881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon E. A., Koga Y., Davidson M., Moraes C. T., King M. P. The mitochondrial tRNA(Leu)(UUR)) mutation in MELAS: a model for pathogenesis. Biochim Biophys Acta. 1992 Jul 17;1101(2):206–209. [PubMed] [Google Scholar]
- Shoffner J. M., Lott M. T., Lezza A. M., Seibel P., Ballinger S. W., Wallace D. C. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNA(Lys) mutation. Cell. 1990 Jun 15;61(6):931–937. doi: 10.1016/0092-8674(90)90059-n. [DOI] [PubMed] [Google Scholar]
- Silvestri G., Moraes C. T., Shanske S., Oh S. J., DiMauro S. A new mtDNA mutation in the tRNA(Lys) gene associated with myoclonic epilepsy and ragged-red fibers (MERRF). Am J Hum Genet. 1992 Dec;51(6):1213–1217. [PMC free article] [PubMed] [Google Scholar]
- Sprinzl M., Hartmann T., Weber J., Blank J., Zeidler R. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1989;17 (Suppl):r1–172. doi: 10.1093/nar/17.suppl.r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoneking M., Jorde L. B., Bhatia K., Wilson A. C. Geographic variation in human mitochondrial DNA from Papua New Guinea. Genetics. 1990 Mar;124(3):717–733. doi: 10.1093/genetics/124.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniike M., Fukushima H., Yanagihara I., Tsukamoto H., Tanaka J., Fujimura H., Nagai T., Sano T., Yamaoka K., Inui K. Mitochondrial tRNA(Ile) mutation in fatal cardiomyopathy. Biochem Biophys Res Commun. 1992 Jul 15;186(1):47–53. doi: 10.1016/s0006-291x(05)80773-9. [DOI] [PubMed] [Google Scholar]
- Torroni A., Schurr T. G., Yang C. C., Szathmary E. J., Williams R. C., Schanfield M. S., Troup G. A., Knowler W. C., Lawrence D. N., Weiss K. M. Native American mitochondrial DNA analysis indicates that the Amerind and the Nadene populations were founded by two independent migrations. Genetics. 1992 Jan;130(1):153–162. doi: 10.1093/genetics/130.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritschler H. J., Andreetta F., Moraes C. T., Bonilla E., Arnaudo E., Danon M. J., Glass S., Zelaya B. M., Vamos E., Telerman-Toppet N. Mitochondrial myopathy of childhood associated with depletion of mitochondrial DNA. Neurology. 1992 Jan;42(1):209–217. doi: 10.1212/wnl.42.1.209. [DOI] [PubMed] [Google Scholar]
- Wallace D. C. Diseases of the mitochondrial DNA. Annu Rev Biochem. 1992;61:1175–1212. doi: 10.1146/annurev.bi.61.070192.005523. [DOI] [PubMed] [Google Scholar]
- Yoneda M., Tanno Y., Horai S., Ozawa T., Miyatake T., Tsuji S. A common mitochondrial DNA mutation in the t-RNA(Lys) of patients with myoclonus epilepsy associated with ragged-red fibers. Biochem Int. 1990 Aug;21(5):789–796. [PubMed] [Google Scholar]
- Yoon K. L., Aprille J. R., Ernst S. G. Mitochondrial tRNA(thr) mutation in fatal infantile respiratory enzyme deficiency. Biochem Biophys Res Commun. 1991 May 15;176(3):1112–1115. doi: 10.1016/0006-291x(91)90399-r. [DOI] [PubMed] [Google Scholar]
- Zeviani M., Gellera C., Antozzi C., Rimoldi M., Morandi L., Villani F., Tiranti V., DiDonato S. Maternally inherited myopathy and cardiomyopathy: association with mutation in mitochondrial DNA tRNA(Leu)(UUR). Lancet. 1991 Jul 20;338(8760):143–147. doi: 10.1016/0140-6736(91)90136-d. [DOI] [PubMed] [Google Scholar]
- van den Ouweland J. M., Lemkes H. H., Ruitenbeek W., Sandkuijl L. A., de Vijlder M. F., Struyvenberg P. A., van de Kamp J. J., Maassen J. A. Mutation in mitochondrial tRNA(Leu)(UUR) gene in a large pedigree with maternally transmitted type II diabetes mellitus and deafness. Nat Genet. 1992 Aug;1(5):368–371. doi: 10.1038/ng0892-368. [DOI] [PubMed] [Google Scholar]