1 Introduction
Aromatic polyketides comprise an important and structurally diverse group of natural products synthesized from microbes and plants.1 This family includes a number of highly successful pharmaceutical compounds, including tetracycline, daunorubicin and mithramycin. On the other hand, some human or plant diseases have been linked to fungal mycotoxins,2 many of which are aromatic polyketides. The rich biological activities, both beneficial and harmful, and structural complexity of aromatic polyketides have attracted research interests into the discovery and biosynthesis of these compounds.
Most aromatic polyketides are highly substituted, fused-ring polyphenols. Each compound in this family contains an aromatic aglycon derived from a poly-β-ketone backbone. Each aglycon is characterized by three structural features, which are: i) number of rings as determined by the overall length of the carbon backbone; ii) cyclization patterns as dictated by the regioselectivities of individual ring-fusing carbon–carbon bonds; and iii) ring topology as a result of different oxidative rearrangement reactions. The aglycon can be further decorated with tailoring reactions such as methylation, amination, oxidation and glycosylation to afford the bioactive natural products.3
Despite the structural similarity shared between bacterial and fungal aromatic polyketides, numerous feeding experiments using isotopically labeled precursors revealed fundamental differences in cyclization strategies (Fig. 1). In a comprehensive review by Thomas, the cyclization modes of fused-ring aromatic polyketides were classified into F-mode for those of fungal origin, or S-mode for those from a filamentous bacteria source, with some exceptions.4 For the F-mode, the first cyclized phenol ring contains two intact acetate building blocks with the other two carbons from two different acetate units, while in the S-mode three intact acetate units are incorporated to assemble the first ring. Using the polyketide carbon numbering system in which the thioester α-carbon is designated C1 and the remaining carbons are numbered consecutively, these F- and S-modes can be designated numerically. In the F-mode, C2–C7, C4–C9 and C6–C11 first ring cyclizations are commonly observed, while in the S-mode, the most commonly found patterns are C7–C12 and C9–C14.
Fig. 1.
Comparison between bacterial and fungal aromatic PKSs and the respective cyclization modes.
The apparent orthogonal strategies employed by bacteria and fungi to cyclize reactive poly-β-ketone backbones are attributed to the different regioselectivities of the cyclization enzymes associated with the respective aromatic polyketide synthases (PKSs). Aromatic PKSs represent a collection of enzymes required to synthesize the carbon backbone from acetate building blocks and modify the backbone into the aromatic polyketides. The poly-β-ketone backbone of bacterial and fungal polyketides are produced by the minimal PKS, which consists of the ketosynthase (KS), acyl-carrier protein (ACP) and the malonyl-CoA:ACP transacylase (MAT).5 Polymerization proceeds through the iterative reactions of: i) selection of malonyl-CoA by MAT as a building block; ii) malonylation of ACP by MAT; iii) decarboxylative condensation catalyzed by the KS; and iv) transfer of the extended polyketide product to the KS and departure of the unacylated holo-ACP.
Following the landmark discovery of the actinorhodin (act) gene cluster from Streptomyces coelicolor A3(2),6,7 a large number of aromatic polyketide biosynthetic pathways have been elucidated from bacterial hosts. The bacterial aromatic PKS, also known as type II PKS, comprises a collection of dissociated enzymes (Fig. 1). The minimal PKS includes the KS (KSα)-Chain Length Factor (CLF or KSβ) heterodimer and a dissociated ACP.1 The minimal PKS shares bacterial fatty acid synthase FabD as the MAT.8 The chain length, or the number of Claisen-like chain extension steps, is largely determined by the CLF.9 In bacteria, the observed S-mode cyclization regioselectivity of the backbone is predominantly controlled by dissociated tailoring enzymes such as cyclases. Each biosynthetic gene cluster contains 2–4 cyclases, depending on the number of rings in the final product. In the absence of cyclases, aberrant intramolecular cyclization occurs readily and leads to formation of shunt products. The programming rules of different families of cyclases have been determined through various mix-and-match experiments in both native10 and heterologous hosts.11
In contrast to the dissociated nature of the bacterial enzymes, fungal polyketides are mostly synthesized by megasynthases in which catalytic components are present on a single polypeptide.12 The individual domains are linearly juxtaposed to resemble an enzymatic assembly line (Fig. 1). Unlike bacterial type I modular PKSs,13 the single set of domains found in a fungal megasynthase is employed iteratively towards the synthesis of polyketides. Cox recently classified fungal megasynthases into three categories based on the degrees of β-reduction of the final product.14 Fungal aromatic polyketides are mainly synthesized by nonreducing polyketide synthases (NRPKS) in which no reductive domains are employed during the elongation steps of the polyketide chain. In addition to the minimal PKS (KS, MAT and ACP), a typical NRPKS consists of a starter-unit:ACP transacylase (SAT) domain that selects starter units;15 and a PT domain that controls the immediate cyclization steps of the nascent polyketide.16,17 Frequently a thioesterase/Claisen-like cyclase (TE/CLC) is found at the C-terminus of the megasynthase to catalyze additional cyclization steps and product release.18 The cyclization regioselectivities of the PT and TE domains give rise to the F-mode cyclization observed for nearly all the fungal aromatic polyketides.
In addition to the dissociated type II PKSs and type I iterative megasynthase systems, type III PKSs are also involved in the biosynthesis of aromatic polyketides in bacteria and fungi. Since the first identification of a type III PKS in Streptomyces griseus,19 numerous type III PKS genes have been found in bacterial and fungal genomes. As a result of these efforts, type III PKSs that synthesize non-fused compounds with different cyclization regioselectivities (C2–C7, C3–C8 and C1–C6) have been identified.
A series of excellent publications in this journal have reviewed the various aspects of the biosynthesis of aromatic polyketides.1,3,12,20,21 This review focuses on the enzymatic machineries that control the different cyclization modes observed for bacteria and fungi. We will mostly cover the literature from 1999 to 2009, while some pertinent prior studies will be cited for completeness. Recent advancement in the structural biology of polyketide cyclization will also be discussed.
2 Cyclization of bacterial aromatic polyketides
2.1 The first cyclization step
The nascent poly-β-ketone synthesized by the minimal PKS is extremely reactive and prone to spontaneous cyclization. As a result, aromatic PKSs must fix the regioselectivity of the first intramolecular aldol condensation immediately following completion of the polyketide chain. Regioselectivity of the first cyclization event also dictates the structure of the final aglycon in that it determines where the carbon backbone “turns the corner”, as described by Rawlings.20 The first cyclization step and the corresponding cyclases have been well-studied during the past 20 years, among which C7–C12 and C9–C14 cyclizations are shown to be the most widely adopted cyclization patterns. These two cyclization regioselectivities lead to the formation of the most important bacterial aromatic polyketide scaffolds, including tetracyclines, anthracyclines, tetracenomycins, angucyclines and benzoisochromanequinones. Other first ring cyclization regioselectivities are also observed in the presence of nonacetate starter units or longer polyketide backbones.22
2.1.1 C7–C12 first-ring cyclization
Early studies using the heterologous host/vector pairs11 suggest that the minimal PKSs synthesize C7–C12 cyclized polyketides as the major products, such as SEK4 1 synthesized by octaketide act minimal PKS,23 SEK15 2 synthesized by decaketide tetracenomycin (tcm) minimal PKSs,23 and a set of compounds such as TW93c 3 synthesized by the dodecaketide whiE minimal PKS.24 These compounds are all derived from regioselective C7–C12 aldol condensation, followed by spontaneous cyclization of the remaining portions of the polyketide chain. Although minor products with other first-ring cyclization regioselectivities were also observed from these PKSs, such as C10–C15 SEK4b 4,25 C9–C14 SEK15b 5,26 C8–C13 TW93f 6,22 the formations of 1–3 in the absence of additional cyclases suggest that the various KS-CLFs have significant influence on the folding of the polyketide chain and promote the observed C7–C12 cyclizations. In 2004, Keatinge-Clay et al. solved the crystal structure of the act KS-CLF heterodimer and suggested the possible chain length control mechanism and conformation of the growing polyketide chain (Fig. 2a).27 As predicted via previous mutagenesis studies,9 the interface between KS and CLF forms the back wall of the substrate binding pocket and defines the length of the active site tunnel. When the growing poly-β-ketone chain reaches the interface, additional elongation of the chain must be accompanied by buckling of the backbone to fit into the cavity. Therefore, the size of the tunnel not only determines the chain length of the poly-β-ketone intermediate, but also directs where the linear intermediate folds. In the act KS-CLF structure, a water molecule positioned in the middle of the pocket has been suggested to act as a proton donor and to facilitate the C7–C12 aldol condensation. The structure illustrates the possibility of a similar distance between the active site cysteine and the back wall of the substrate binding pocket among all KS-CLFs and rationalizes the preferential C7–C12 cyclization regardless of final chain length.
Fig. 2.
Structures of type II PKS enzymes. (a) Heterodimer of act KS-CLF. (b) Crystal structure of act KR in complex with NADPH. (c) Crystal structure of TcmN. Y35 and R69 play essential roles in orienting the uncyclized polyketide backbone. (d) Crystal structure of SnoaL. (e) Crystal structure of AknH. (f) Crystal structure of TcmI. H26, D27, R40 and H51 are proposed to steer the C2–C19 regioselectivity.
In addition to the possible KS-CLF facilitated C7–C12 cyclization, putative cyclases that can direct first-ring C7–C12 cyclization of unreduced polyketide backbones are found in several gene clusters. These include ZhuI from the R1128 pathway,28 StfQ from the steffimycin pathway,29 and MtmQ from the mithramycin pathway.30 The precise roles of these enzymes have been difficult to confirm as their activities cannot be assayed independently from the C7–C12 cyclization directed by the KS-CLF. The cyclase activities of these enzymes were recently confirmed using a polyketide backbone generated by an engineered fungal minimal PKS derived from the Gibberella fujikuroi PKS4,17 which does not promote C7–C12 cyclization of the nascent polyketide backbone. Addition of these cyclases to the PKS4 minimal PKS resulted in the exclusive synthesis of C7–C12 cyclized nonaketide NonaSEK4 7. Therefore, these enzymes are indeed C7–C12 cyclases, although may not be essential for the biosynthesis of the associated natural products. Instead, these enzymes may serve ancillary roles to ensure that all polyketides are correctly cyclized. The crystal structure of ZhuI was recently reported by Tsai and coworkers, and the structure is similar to that of the C9–C14 first-ring cyclase TcmN (N-terminal).31 Compared to TcmN, ZhuI shows a smaller substrate binding pocket that favors bending of the poly-β-keto backbone between C7 and C12 instead of C9 and C14.32

Another enzyme that can influence the C7–C12 cyclization is the C9-ketoreductase (C9-KR) associated with aromatic polyketides that are reduced at the C9 position, such as anthracyclines and angucyclines. ActIII (act KR) has been the most widely studied C9 KR and reduces polyketide backbones of nearly all lengths. Heterologous host/vector studies have found that polyketides reduced at C9 are cyclized exclusively in the C7–C12 regioselectivity, thereby raising the possibility that the C9 KR may also provide an environment to promote the regioselective aldol condensation.33 For instance, the C9-reduced octaketide, decaketide and dodecaketide all undergo C7–C12 cyclization and spontaneously rearrange to become mutactin 8,11 RM20b/c 9,34 and TW94d 1024 or YT231a 11,35 respectively. Addition of a first-ring aromatase/dehydratase leads to aromatization of the first ring affording compounds such as SEK34 1236 and SEK43 13.37 Dissecting the role of act KR in promoting C7–C12 cyclization was again complicated by the intrinsic activities of the KS-CLFs. In 2008, Tsai and coworkers co-crystallized act KR with the anthraquinone emodin (Fig. 2b) and suggested that act KR may prefer partially cyclized substrates, such as the C7–C12 cyclized polyketide intermediates.38 Recently, Ma et al. demonstrated that the act KR can reduce the polyketide backbone synthesized by G. fujikuroi minimal PKS4 and afford compounds that are not only reduced at C9, but are also regioselectively cyclized between C7–C12.39 Therefore, it is possible that buckling of the polyketide backbone takes place in the active site of C9 KR, which simultaneously positions the correct ketone group for reduction and facilitates the spontaneous C7–C12 cyclization of the first ring.

2.1.2 C9–C14 first-ring cyclization
For unreduced aromatic polyketide backbones that are decaketides or larger, C9–C14 first-ring cyclization is a common cyclization pattern, giving rise to compounds in the tetracenomycin and pentangular families. Addition of a C9–C14 specific cyclase, such as the widely studied TcmN from the tetracenomycin (tcm) gene cluster, to different minimal PKSs can override the C7–C12 cyclization and afford a different set of polyketides. When TcmN is introduced, the act, frenolicin nonaketide synthase (fren), tcm, and whiE PKSs synthesized C9–C14 cyclized products RM77 14,40 PK8 15,41 RM80 16,40 and TW95a 17,24 respectively. The fused, second rings of 14–17 are all derived from subsequent C7–C16 cyclization, which are likely also catalyzed by TcmN. Therefore, TcmN can be considered as a first- and second-ring cyclase for longer polyketide backbones.

The N-terminus, cyclase portion of TcmN (N-TcmN) was successfully crystallized by Ames et al. in 2008.31 They reported that the N-TcmN adopts a helix-grip fold (Fig. 2c) and contains an interior pocket for binding of the polyketide intermediate. The pocket geometry orients the polyketide to buckle between C9 and C14. Docking simulations indicated that the residues within the cavity interact with much of the buckled polyketide, including C9, C13, C14 and C20. In addition, the docked polyketide also adopts a conformation in a narrower portion of the cyclization chamber to position C7 in close proximity of C16, thereby possibly mediate cyclization of the second ring. In contrast, when the poly-β-ketone buckled between C7 and C12 was docked during simulation, a much weaker binding interaction between the polyketide and the cavity is predicted. Site-directed mutagenesis studies showed that two highly conserved residues among cyclases of similar functions, R69 and Y35, are essential for the C9–C14 regioselectivity. Mutation of these residues resulted in the loss of the C9–C14 regioselectivity and led to the synthesis of C7–C12 cyclized products. In comparison to the N-TcmN structure, the crystal structure of WhiE-OrfVI, which directs C9–C14 cyclization of dodecaketides, shows a larger cyclization chamber that is needed to accommodate a longer polyketide backbone.32
2.1.3 Other first-ring cyclization modes
In the presence of minimal PKS alone, most acetate-primed polyketides are subjected to C7–C12 cyclization as discussed in Section 2.1.1. Interestingly, when the starter unit is replaced with amide starter units, such as the malonamate starter unit from the tetracycline pathway, the cyclization regioselectivity is altered. Coexpression of the oxytetracycline (oxy) minimal PKS with the amidotransferase OxyD in a heterologous host yielded a C19 amidated polyketide that adopted C11–C16 first-ring cyclization, followed by C9–C18, and N–C7 cyclization to yield WJ85 18.42 Upon addition of act KR to reduce the C9 ketide, the amidated polyketide cyclized via C13–C18 connectivity, followed by N–C11 cyclization to form the isoquinolone WJ35 19.43 The synthesis of 19 further supports the possibility that C9-KR accepts an uncyclized nascent polyketide intermediate. Formation of 18 and 19 suggests that the amide starter unit has a significant influence on conformation of the polyketide chain in either the KS-CLF or the act KR. The regioselectivities of first-ring cyclases, however, are not affected by the amide starter unit. Addition of C7–C12 or C9–C14 cyclase to either the C9-reduced or unreduced, amidated backbones led to the synthesis of WJ78 20 or WJ150 21, respectively.44 The structures of both compounds reflect the expected regioselectivities of the two cyclases. Interestingly, both products are benzopyrones that are formed through the spontaneous displacement of the amide by a favorably positioned phenol.

2.2 Additional cyclization steps
Following initial cyclization of the first ring through either C7–C12 or C9–C14, additional cyclases are recruited to perform subsequent regioselective cyclizations. The remainder of the polyketide chain can be either folded in a linearly-fused fashion to yield compounds in the tetracycline, anthracycline or tetracenomycin families; or can be folded in an angularly-fused fashion to yield angucycline, pentangular benzo[a]naphthacene, or discoid-folded polyketides. Cyclases, both individually and sometimes synergistically, play crucial roles in controlling the regioselectivities of these successive steps, as well as preventing spontaneous cyclizations.
2.2.1 Linearly cyclized polyketides
Linearly folded polyketides are among the most studied aromatic polyketides, including the well-known antibiotics oxytetracycline 22, the anticancer drug doxorubicin 23,45 and the pigmented actinorhodin 24.6 The aglycons of linearly folded polyketides are typically substituted and oxidized naphthalenes, anthracenes or naphthacenes. Based on the regioselectivities of the first and last rings, this group of compounds can be divided into four groups: (1) tetracyclines and aureolic acids; (2) anthracyclines; (3) tetracenomycins; and (4) benzoisochromanequinones.
2.2.1.1 Tetracyclines and aureolic acids (C7–C12, C5–C14, C3–C16, C1–C18)
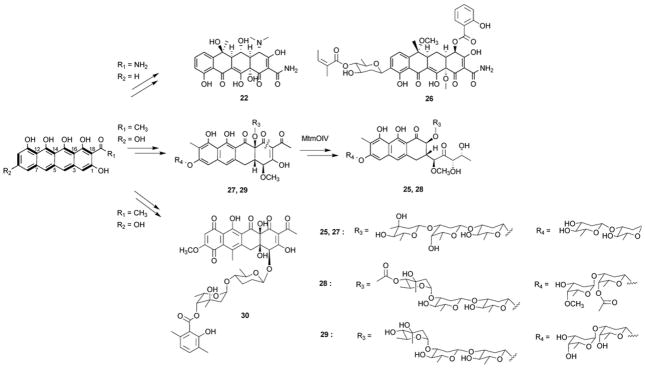
Compounds in the tetracycline and aureolic acid families are derived from decaketide backbones. These two families, although structurally distinct, are both tailored from naphthacene intermediates (Scheme 1). Four successive cyclization steps are required, starting from first-ring C7–C12 closure, followed by formation of C5–C14, C3–C16 and C1–C18 connectivities. Differences in the decorations of the naphthacene core can arise from starter unit choice (amide or acetate), and/or C9 reduction. For example, the tetracyclines are primed with an amide starter unit and are generally ketoreduced at C9; whereas the aureolic acids such as mithramycin 25 are primed with acetate starter units and are not reduced at C9.
Scheme 1.
The tetracyclines are among the most important antibiotics discovered in the last century. It was used widely to treat both Gram-positive and Gram-negative bacterial infections due to their broad-range activities. This family is relatively small, with chlorotetracycline and 22 as the best known examples. The 2-naphthacenecarboxamide scaffold of tetracyclines is derived from the naphthecene intermediates via tailoring steps such as methylation, oxidation and reductive amination. The biosynthetic steps have recently been reconstituted systematically in S. coelicolor strain CH999.42–44,46,47 Cyclization steps leading to the formation of the naphthacene core were investigated, and only two cyclases were shown to be necessary to cyclize all four rings in the presence of the amide starter unit. OxyK was identified as the first-ring cyclase/dehydratase that mediates C7–C12 cyclization of the backbone (note that without OxyK, the polyketide spontaneously cyclizes into 19).48,49 OxyN was identified as the second-ring cyclase that mediates C5–C14 cyclization, while cyclization of the third ring is believed to be spontaneous. No enzyme has been associated with the cyclization of the fourth ring so far. The gene cluster of a third member of the tetracycline family, SF2575 26,50 was recently sequenced from Streptomyces sp. SF2575. Biochemical analysis showed that the aglycon of 26 is also derived from the naphthacene intermediate, which in turn is also cyclized from an amidated backbone. Reconstitution work using enzymes from this gene cluster, however, revealed that cyclization of the last ring requires the action of an acyl-CoA ligase SsfL2. Therefore, the exact mechanism in which the C1–C18 cyclization occurs in this family of compounds is not completely established.
Aureolic acids are a group of linearly-fused, tricyclic aromatic polyketides. The biosynthesis of compounds in this family has been demonstrated to go through a tetracyclic, naphthacene intermediate. Among the aureolic acids, 25 is the best studied example.51,52 Compound 25 was first proposed to be derived from a tetracenomycin-like scaffold based on heterologous expression of the putative last-ring cyclase MtmX from 25 biosynthetic pathway in Streptomyces glaucescens Tu49.53 Subsequently, the key intermediate premithramycin B 27 was isolated from a genetically modified strain of Streptomyces argillaceus.51 The structural similarity between the aglycon of 27 to that of 22 led to the conclusion that 25 is more likely to be derived from a tetracycline-like cyclization pathway. Indeed, the 25 and 22 biosynthetic pathways share a number of highly homologous enzymes, including cyclases and tailoring enzymes.30,43 The oxygenase MtmOIV was identified to transform 27 into the aureolic acid structure via a Baeyer–Villiger oxidative cleavage of the fourth ring.54,55 The MtmOIV homologue also resides in the biosynthetic gene cluster of chromomycin A3 28,56 which is an aureolic acid derived from the tetracyclic intermediate prechromomycin B 29.57
The biosynthetic gene cluster of a related compound polyketomycin 30 was recently identified and sequenced.58 Based on comparative gene cluster analysis and gene knockout, biosynthesis of the aglycon of 30 is expected to parallel closely to that of 22 and 25. 30 contains novel structural features not observed among the tetracyclines, including O-glycosylation and oxidation of the first ring.
2.2.1.2 Anthracyclines (C7–C12, C5–C14, C3–C16, C2–C19)
Anthracycline is an exceptionally important family of bacterial aromatic polyketides, and includes the frontline chemotherapy agent 23 and daunorubicin 31.45 Other anthracyclines of which the biosynthetic gene clusters were recently reported include steffimycin 3229 and aranciamycin 33.59 The tetracyclic aglycon of an anthracycline is derived from a C9-reduced decaketide backbone (Scheme 2). Cyclization of the first three rings of anthracyclines parallels closely to that of tetracyclines, in which cyclase-mediated C7–C12 and C5–C14 cyclizations are followed by spontaneous C3–C16 cyclization and dehydration of the third ring. The second ring of anthracyclines is oxidized by quinone-forming monooxygenases to afford the anthraquinone portion present in all anthracyclines. Hydrolytic release of the anthraquinone followed by methylation yields the key anthraquinone intermediate aklanonic acid methyl ester (AAME, with propionate starter unit) 34 or nogalonic acid methyl ester (NAME, with acetate starter unit) 35.
Scheme 2.
Different from tetracycline compounds, closure of the last ring of anthracyclines proceeds via C2–C19 cyclization. In the biosynthesis of 31, the cyclase that catalyzes this reaction to afford the aldol adduct aklaviketone 36 is DnrD, the AAME cyclase.60 During the synthesis of nogalamycin 37, the cyclase SnoaL converts NAME into nogalaviketone 38; whereas in the synthesis of aclacinomycin A 39, AknH converts NAME into auraviketone 40, which contain the opposite stereochemistry at C19 as 38.61,62 The mechanism and stereoselectivity of NAME cyclases such as SnoaL and AknH have been studied via X-ray crystallography. Distinct from first-ring cyclase such as ZhuI or TcmN, anthracycline cyclases displays an α/β barrel fold (Fig. 2d/e), where the substrate is anchored in the hydrophobic interior of the barrel. The NAME cyclases use a base-catalyzed mechanism to stabilize the enzyme-bound C2 enolate and facilitate the attack at C19 carbonyl group to afford the C2–C19 bond.61,62 Comparison of the sequences and structures of AknH and SnoaL indicate that Tyr/Phe15 and Asn/Leu51 (AknH/SnoaL) play essential roles in determining the stereochemistry of the aldol addition. In AknH, the C19-hydroxyl group forms hydrogen bonds with Asn51 and Tyr15, and fixes the product in the R conformation. On the other hand, the hydrophobic residues Phe15 and Leu51 in SnoaL interact with the C19-methyl group and thereby favor formation of S stereochemistry at C19. Although mutations to these residues in AknH and SnoaL did not invert the C19 stereochemistry of the products, racemic mixtures were isolated.62
Chartreusin 41 is a benzopyrene polyketide synthesized via rearrangement of the anthracycline intermediate 35. The gene cluster responsible for the synthesis of 41 from Streptomyces chartreusis was identified and characterized by Hertweck and coworkers in 2005.63 The biosynthetic pathway of 41 diverges from those of anthracyclines starting from either 38 or 40. Biosynthesis of 41 requires formation of resomycin C 42, which is the dehydrated version of either 38 or 40. Formation of the propionate-primed analog of 42 has been observed as a shunt product of 31 and can take place at increased pH via either dehydration or elimination.60 Genetic studies identified ChaZ, a putative FAD-dependent monooxygenase, as the key enzyme in catalyzing the rearrangement of 42 to chartarin 43. 13C-Acetate incorporation studies revealed that rearrangement may proceed via Baeyer–Villiger oxidative cleavage of C13–C14 in 42, followed by formation of the new C4–C13 bond to afford 43.
2.2.1.3 Tetracenomycins (C9–C14, C7–C16, C5–C18, C2–C19)
Enzymes involved in the biosynthesis of tetracenomycin C 44, such as the minimal tcm PKS and the cyclase TcmN, have been widely studied and utilized in the reconstitution of aromatic PKS enzymes.10 Different from anthracyclines and tetracyclines, biosynthesis of the tetracenomycin scaffold is initiated with a regiospecific C9–C14 first-ring cyclization (Scheme 3). This is followed by C7–C16 and C5–C18 aldol condensations to yield the anthracene carboxylic acid Tcm F2 45.45 Although TcmN is known to catalyze formation of the first two rings, it remains unclear if the C5–C18 third ring cyclization occurs spontaneously or is enzyme-mediated. The combination of tcm minimal PKS and TcmN produces 16 in CH999,40 yet the same enzyme combination produces 45 as the major product in S. lividans.64 A dedicated cyclase TcmI catalyzes the C2–C19 cyclization to yield the naphthacene carboxylic acid Tcm F1 46.65 Interestingly, cyclization regioselectivity of the last ring in 46 is identical to those found in anthracyclines. It is, however, a true aldol condensation, as elimination of water takes place to yield the completely aromatic scaffold. Downstream tailoring steps such as triple hydroxylation furnish the highly oxidized aglycon.66 The antitumor drug elloramycin 47 shares the same aglycon with 44, while the C3 hydroxyl group of 47 is glycosylated with a permethylated L-rhamnose.67,68
Scheme 3.

Structural analysis of TcmI showed it is a dimer consisting of two βαβ ferredoxin-like folds (Fig. 2f).65 Most of the residues forming the catalytic cavity are hydrophobic, thereby providing a favorable environment for binding of the anthracene carboxylic acid 45. Four residues H26, D27, R40 and H51 have been proposed to play important roles in mediating the aldol condensation reaction through mutational analysis, yet none of the single mutants at these positions has complete loss of cyclization activity.65 Therefore, these residues may function to steer the C2–C19 regioselectivity and may not play any catalytic roles. The different three-dimensional structures and catalytic mechanisms indicate fourth-ring cyclases from anthracycline and tetracenomycin pathways may have evolved independently.
2.2.1.4 Benzoisochromanequinones (C7–C12, C5–C14, O3–C15/O15–C3)
Benzoisochromanequinones are typically synthesized from C-9 reduced octaketides and nonaketides (Scheme 4). The best known representatives of this group are the monomeric unit of 24 (an octaketide) and the antiparasitic frenolicin 48 (nonaketide). The aglycon of this family of compounds is linearly fused and contains a lactonized third ring. Cyclization of the first ring is between C7–C12 and a dehydratase/aromatase such as ActVII is required. A second cyclase such as ActIV is required to cyclize the second ring via C5–C14 regioselectivity. If no additional downstream enzymes are present, the third ring of an octaketide will spontaneously cyclize via C2–C15 to yield the well-known anthraquinone 3,8-dihydroxy-1-methylanthraquinone-2-carboxylic acid (DMAC, 49) and its decarboxylated product aleosapanorin.11 Similarly, spontaneous cyclization of the third ring of a nonaketide yields SEK26 50.37
Scheme 4.
During benzoisochromanequinone synthesis, spontaneous cyclization of the third ring is suppressed via release of the naphthalene carboxylic acid from the ACP followed by ketoreduction of one of the uncyclized ketones (C3 or C15). The stereoselectivity of the late-stage KR plays a critical role in the configuration of the final natural product. The med biosynthetic gene cluster encoding the medermycin 51 biosynthetic pathway in Streptomyces sp. K73 was identified in 2003.69 During the biosynthesis of 51, the C3-ketone group is reduced by the C3-KR Med-12, followed by formation of the hemiketal and dehydration to afford (S)-DNPA 52. ActVI catalyzes a similar C3 stereospecific ketoreduction during the biosynthesis of 24.70 ActVI was also found to prefer the hydrolyzed naphthalene β-keto carboxylic acid over the ACP-bound β-keto thioester intermediate, which suggested that the C3-KR does not require ACP for substrate recognition.71 During granaticin 53 biosynthesis, the C3 ketoreduction step takes place with opposite R-stereochemistry. Examination of the gra gene cluster revealed act KR homologs.72 Subsequently, by using different combinations of the KR candidates and the act minimal PKS, it was determined that Gra-ORF5 is the C9-KR; while Gra-ORF6 is the C3-KR that is responsible for the synthesis of (R)-DNPA 54.73 Surprisingly, Gra-ORF6 shows no sequence homology to Med-12 beyond the putative cofactor binding motif.
As an alternative to forming the lactone ring via the C3-ketoreduction step, the recently isolated and characterized alnumycin 55 indicated that the nucleophilic hydroxyl group can also be revealed via reduction of the C15 ketone, which can then attack the C3 carbonyl.74 The gene cluster responsible for the synthesis of 55 was identified in 2008 through heterologous expression. Two enzymes encoded in the pathway, Aln4 and Aln5, were proposed to be involved in the formation of the third ring lactone. Aln4 encodes a putative KR, while Aln5 shows sequence homology to the C-terminus of a type I PKS. Individually knocking out either Aln4 or Aln5 resulted in the loss of 55, and accumulation of K1115A 56 which is spontaneously cyclized from the bicyclic intermediate. Thus the proposed mechanism is that Aln4 acts as the C15 KR and Aln5 functions to either assist Aln4 in the C15-ketoreduction, or promote cyclization of the lactone ring. The stereochemistry of the C15 ketoreduction step in this pathway has not been determined.
2.2.2 Angularly cyclized polyketides
A significant number of bacterial aromatic polyketides, especially those derived from backbones that are dodecaketides and longer, adopts an angularly cyclized scaffold. This difference in cyclization regioselectivity greatly enriches the structural diversity of aromatic polyketides.
2.2.2.1 Angucycline (C7–C12, C5–C14, C4–C17, C2–C19)
Angucyclines are benz[a]anthracenes that have an angularly cyclized third ring, which results in the bending of the fourth ring with respect to the rest of the molecule (Scheme 5). Starting from a C9 reduced decaketide backbone, regioselective cyclizations of C7–C12 and C5–C14 fix the first two rings, in identical fashion as the steps in anthracycline biosynthesis. The unique C4–C17 cyclization angularly directs the orientation of the uncyclized portion of the backbone, and leads to the closure of the last ring via C2–C19 cyclization and spontaneous decarboxylation to afford the common angucycline intermediate UWM6 57.75 Compound 57 can also readily dehydrate and oxidize to afford the well known intermediate rabelomycin 58.75 Therefore, fixing the C4–C17 regioselectivity is a critical step in differentiating the tailoring steps of anthracyclines and angucyclines. The most well-studied angucycline is jadomycin B 59 produced from Streptomyces venezuelae,76 and the cyclase responsible for the angular cyclization steps has been identified as JadI. JadI has been associated with mediating C4–C17 and C2–C19 cyclizations, as well as the dehydration of 57 en route to 58.75
Scheme 5.
Since both anthracyclines and angucyclines are decaketides and the cyclization regioselectivity diverge at the third ring, this presented opportunities to perform combinatorial biosynthesis experiments to probe functional differences between linear and angular cyclases. Mixed results have been obtained using different angucycline cyclases. In a study by Hutchinson and coworkers,77 they demonstrated that replacing the second/third ring cyclase DpsY in the set of genes that afforded aklanonic acid with JadI was insufficient to generate the angucyclic backbone. Similarly, replacing JadI with DpsY in the set of genes that synthesized 58 was inefficient to produce the linear scaffold. Therefore, the presence of heterologous cyclase alone cannot alter the cyclization outcome and additional biosynthetic enzymes are involved in dictating the final configuration of the cyclized polyketide. In contrast, Mäntsälä and coworkers showed angucycline and anthracyclines cyclases can be combinatorially mixed.78 Using the putative angucyclines cyclase PgaF that has sequence homology to JadI, they showed PgaF can function in the presence of anthracyclines PKS from the biosynthetic pathway of 37 and synthesized the expected angucycline aglycons 57 and 58. Therefore, a general model for how angucycline cyclases function in the context of different decaketide PKSs has not been established.
Through tailoring steps such as oxidation, dehydration, methylation, and glycosylation, 57 can be transformed into various bioactive angucyclines and angucyclinones. Conversion of 57 to the urdamycin aglycon 12b-hydroxyl-UWM6 is catalyzed by UrdM, which catalyzes the Baeyer–Villiger oxygenation to form an ε-lactone in the fourth ring. Isolation of the shunt product urdamycin L 60 supports this mechanism.79 Biosynthesis of the aglycon of landomycin A 61, 11-deoxylandomycinone 62, is derived from 58. Rohr and coworkers discovered that synthesis of 58 from tetrangomycin and the conversion of 58 to 62 require a bifunctional oxygenase/reductase enzyme LndM2.80 LndM2 is proposed to first catalyze the attachment of the C15-oxygen to afford 58, then functions as a reductase to convert 58 into 62. The antitumor oviedomycin 63 produced by Streptomyces antibioticus is a heavily oxidized angucycline derived from 58, and the biosynthetic gene cluster was uncovered in 2004.81 The oxygenases required to synthesize 63 have been identified, which also facilitated the combinatorial biosynthesis of a number of angucyclines with enhanced antitumor activities.82 Other compounds derived from 57 are gaudimycin A 64 and gaudimycin B 65, which were identified through heterologous expression of the genes putatively responsible for this aglycon.83
The angucycline scaffold of 57 can also be dramatically rearranged to form other non-linear, fused-ring polyketides, giving rise to greater structural diversity. 59 is synthesized from 57 through oxidative cleavage of the C15–C16 bond in the C ring, followed by the addition of one unit of L-isoleucine.84,85 Gilvocarcin V 66 with a naphthopyran structure is synthesized starting from cleavage of the C15–C16 of a related intermediate, followed by rotation of the C4–C5 bond to facilitate lactonization.84,86 Recently, the gene clusters responsible for the biosynthesis of two gilvocarcin-type anticancer agents ravidomycin 67 and chrysomycin 68 were sequenced.87 Compounds 66, 67 and 68 share an identical polyketide aglycon and differ in the structures of the C-glycosides. Interestingly, a second ACP gene (ravC1/chryC1) is present in the gene cluster of 67 and 68, but not in that of 66. The second ACP has been proposed to be involved in the incorporation of the proprionate primer unit. Benzopyrenomycin 69, the first natural product discovered to possess the carbocyclic benzo[a]pyrene structure, is proposed to be synthesized from the angucycline backbone, as evident from the recovery of several analogues of 57 in the producing culture. Based on the structures of the putative intermediates, it was suggested that the carbon scaffold of 69 maybe formed through fusing one molecule of malonic acid to an angucycline intermediate.88 The natural host of saquayamycin Z 70 is reported to produce the linear polyketide galtamycin B 71.89 However, inactivation of glycosyltransferase saq GT5 or saq GT6 in the gene cluster of 70 eliminated the synthesis of 71, which suggested the biosynthesis of 70 and 71 are related and may both be derived from 57. The possible rearrangement of 57 to produce 71 may therefore be the first example of an angular polyketide serving as the precursor to a linearly-fused aromatic polyketide.
2.2.2.2 Angularly cyclized dodecaketides
Compounds discussed in this group are all cyclized from polyketide backbones that are at least 24 carbons (dodecaketides) or longer (Scheme 6). The cyclization regioselectivities of these compounds are highly similar, which include C9–C14 first-ring cyclization, followed by C7–C16 and C5–C18 cyclizations to yield the linearly fused tricyclic precursor. Cyclization of the fourth ring between C4 and C21 induces bending of the structure, and additional cyclization such as C2–C23 affords the benzo[a]naphthacene quinone skeleton common to many compounds in this family, including pradimicin A 72, benastatin J 73, griseorhodin A 74 and fredericamycin A 75. The biosynthetic gene clusters encoding these PKSs have been elucidated in the last ten years.
Scheme 6.
Pradimicins such as 72 are dodecaketides that show antifungal and antiviral activities.90 Two sequencing efforts resulted in the identification of the 37 kb gene cluster from Actinomadura hibisca P157-2.91,92 The activities of the minimal pdm PKS and the first-ring cyclase PdmD were reconstituted by Khosla and coworkers in S. coelicolor CH999.35 This work verified the dodecaketide chain length of the minimal PKS and the function of PdmD as a C9–C14 and C7–C16 cyclase. Successive heterologous expression experiment by Zhan et al. reconstituted the dihydrobenzo[a]anthraquinone aglycon of pradimicin, G-2A 76.93 Additional enzymes required to afford 76 include two more cyclases PdmK and PdmL, a monooxygenase PdmH and a C19-ketoreductase PdmG. Unexpectedly, PdmK, PdmL and PdmH must work synergistically to oxidize the second ring, and to cyclize the third and fourth rings. Formation of the fifth ring may be spontaneous or equally requiring the action of either PdmK or PdmL.93 Removal of any one of the three enzymes resulted in recovery of the bicyclic product such as 17 synthesized by the minimal PKS and PdmD. A model was proposed in which the three enzymes form a multi-enzyme complex that engulfs the uncyclized portions of the bicyclic intermediate and catalyzes the required tailoring steps simultaneously or in rapid succession. This may serve as a means to lock the entire length of the uncyclized polyketide backbone in place and suppress aberrant cyclizations, which can take place readily for longer polyketide backbones. The C19 KR plays a crucial role in the oxidation state of the fourth ring. The non-aromatic fourth ring in 72 is a result of C19 reduction by PdmG, followed by dehydration and enoylreduction to yield 76. The latter two reactions are catalyzed by unknown enzymes. In the absence of C19 ketoreduction, the pentacyclic product readily aromatizes to afford JX111a 77.93 Reoxidation of C19 and C20 of the fourth ring in 76 by two P450 enzymes install the two hydroxyl groups for downstream tailoring reactions.94
The benastatin family of compounds, such as benastatin A 78 and 73, are synthesized by the ben minimal PKS primed with hexanoate and butyrate, respectively.95 The ben gene cluster was sequenced by Hertweck and coworkers.95 Formation of the benzo[a]anthraquinone portions of 73 and 78 likely proceeds in the same fashion as that of 76. A C19 reductase, BenL, is also found in the gene cluster and has been shown to catalyze the C19 ketoreduction en route to synthesizing 73 and 78.96 The dodecaketide 78 contains an aromatic fourth ring, which suggests that full reduction did not occur.
With the shorter butanoate starter unit, the ben PKS was able to extend the backbone by an additional ketide to yield a tridecaketide backbone of 73. The additional ketide facilities formation of the sixth, distal ring via lactonization.95 Oxidative rearrangement of the third and fourth rings can lead to formation of a spiroketal as observed in the structure of 74. The nonplanar 74 is a member of the rubromycin family that inhibits human telomerase.97 The gene cluster encoding the enzymes responsible for the synthesis of 74 was identified and heterologously reconstituted in 2002.98 Based on this heterologous expression system and inactivation experiments, two FAD-dependent oxygenases GrhO5 and GrhO6 were found to be involved in the cleavage of four carbon–carbon bonds in the third and fourth rings, and formation of the spiroketal scaffold.99
As the longest bacterial aromatic polyketide known to date, the pentadecaketide 75 is produced by S. griseus. The fdm biosynthetic gene cluster was recently reported and the entire pathway was reconstituted in Streptomyces albus.100 The last two ketides of the C30 backbone undergo ketoreduction and dehydration to yield the observed diene, and are proposed to be catalyzed by FdmC. The remaining portions of the backbone undergo identical cyclization steps as 73 with exception of the lactone ring, in which the terminal carboxylic acid is amidated and facilitates formation of the pyridone ring. Amidation of the C1 acid is proposed to be catalyzed by the amidotransferase FdmV. In 2008, Shen and coworkers reported the isolation of fredericamycin E 79,101 the biosynthesis of which was shown to be related to that of 75 via 13C-acetate feeding. A benzilic acid-like rearrangement was proposed to be involved in the conversion of 79 to 75 and thereby establishing the C5 spiro carbon center.
2.3 Other bacterial polyketide cyclization modes
Hedamycin 80 is a member of the pluramycin antitumor family. Biosynthesis of 80 is initiated with the priming of a type II minimal PKS by a 2,4-hexadienate starter unit, which is synthesized by an iterative type I PKS.102 The hed minimal PKS synthesizes a decaketide that is reduced at C9 by a KR, which is then cyclized via C7–C12, C5–C14 and C3–C16 in similar fashion as anthracyclines. The subsequent polyketide intermediate undergoes O15–C19 last ring cyclization to afford the angular aglycon of 80, a structural feature that differentiates the pluramycin antibiotics from both anthracyclines and angucyclines. Das et al. demonstrated that a putative initiation ketosynthase III HedS is not required for priming, and the hed KS-CLF can directly capture the primer unit from the ACP of the type I PKS.103 A bifunctional ACP/aromatase (ARO) HedE was found to participate in both chain elongation and the C7–C12 first-ring cyclization.
Resistomycin 81 possesses a discoid structure unique to other bacterial aromatic polyketides. Three putative cyclases, RemI, RemF and RemL, are present in the rem biosynthetic pathway.104 Using a combination of genetic inactivation and heterologous reconstitution, all three cyclases were shown to be essential and must function in concert to generate the discoid structure. It has been proposed that they form a multienzyme complex with the minimal PKS.105 This observation, along with the synergistic actions of cyclases reported in the biosynthesis of 72, suggests that multienzyme complexes may be a widely adopted strategy for PKSs to direct the regioselective cyclization of reactive backbones.

Erdacin 82 was discovered recently through heterologous expression of type II PKSs gene clusters isolated from environmental DNA.106 The gene cluster was sequenced, and the erd minimal PKS showed high sequence similarity to the type II synthases. The elucidation of a bicyclic octaketide juglomycin F 83 hints that the pentacyclic 82 may arise through joining of two such bicyclic intermediates.

Enterocin 84 derived from a benzoate primed octaketide backbone was isolated from the marine strain Streptomyces maritimus in 1996.107 The enc gene cluster was sequenced and identified by Moore and coworkers.108 The C9-KR was reported to play an indispensible role in the chain elongation of 84, as inactivation of the C9-KR EncD led to the derailment of the enc minimal PKS.109 An FAD-dependent oxygenase EncM was identified to catalyze the Favorskii-like rearrangement that leads to the caged, nonaromatic core of 84.110

2.4 Type I iterative PKSs
As will be discussed in the next section, fungal aromatic polyketides are synthesized from iterative type I PKSs. Iterative type I PKSs are megasynthases in which catalytic domains are linearly juxtaposed in a single polypeptide and the tailoring domains may be used selectively during each chain extension iteration. Intriguingly, bacteria also employs iterative type I PKSs to synthesize simple aromatic functionalities appended to more complex natural product scaffolds, notably members of the enediyne family.111 Examples include orsellinate 85 in calicheamicin 86112 and 2-hydroxyl-5-methyl-naphthoate 87 in neocarzinostatin 88113 (Scheme 7). Gene cluster sequencing efforts have revealed numerous bacterial iterative type I PKSs, such as AviM encoded in the avilamycin A 89 gene cluster,114 CalO5 encoded in the 86 gene cluster,112 AziB encoded in the azinomycin B 90 gene cluster,115 and NcsB encoded in the 88 gene cluster.113 These PKSs include minimal PKS domains such as KS, MAT and ACP; and may have dehydratase (DH) and/or KR domains for selective reduction. These PKSs share considerable sequence homology and are functionally analogous to partially-reducing PKSs (PRPKS) commonly found in fungi, such as the well known 6-methylsalicylic acid synthase (6-MSAS).14
Scheme 7.
Bechthold and coworkers heterologously expressed AviM in both S. lividans TK24 and S. coelicolor CH999, which resulted in the synthesis of 85.114 The synthesis of 85 starts from a tetraketide backbone and is followed by C2-C7 regioselective cyclization. 85 is also synthesized by fungal NRPKSs, which will be discussed in Section 3. Similarly, the type I iterative PKS ChlB1 in the chlorothricin 91 gene cluster synthesized 6-methylsalicylic acid 92 when heterologously expressed in S. lividans TK24.116 92 is derived from a tetraketide backbone which is partially ketoreduced at C5 and cyclized via C2-C7 regioselectivity. The pactamycin 93 gene cluster from Streptomyces pactum has been sequenced by two different groups and contains a putative type I iterative PKS PtmQ (PctS).117,118 PtmQ was heterologously reconstituted in S. lividans T7 and was shown to synthesize 92. Inactivation of PtmQ in the native host resulted in the synthesis of de-6-MSA-pactamycin and de-6-MSA-pactamyate.118 3,6-Dimethyl salicylic acid 94 is found in the tetracyclic quinone glycoside antibiotic 30 and the enediyne antitumor antibiotic maduropeptin 95. Shen and coworkers sequenced the gene cluster of 95 and revealed the presence of a type I iterative PKS MdpB.119 Interestingly, a C-methyltransferase MdpB1 is encoded immediately downstream of MdpB. MdpB was therefore proposed to be responsible for the synthesis of 92, which can be methylated by MdpB1 at C3 to yield 94. Subsequently, the gene cluster of 30 was also shown to encode a type I iterative PKS PokM1 and a C-methyltransferases PokMT1.58 Inactivation of PokMT1 led to substitution of the 3,6-dimethylsalicylate pendant in 30 with 6-methylsalicylate, hence confirming the biosynthetic pathway of 94 as shown in Scheme 7.58
In addition to tetraketides 85 and 92, bacterial type I iterative PKSs such as AziB and NcsB can synthesize and regioselectively cyclize a hexaketide to afford naphthoic acids 87 and 96.113,115 In the case of NcsB, the hexaketide product undergoes C5 and C9-ketoreduction, followed by C2–C7/C6–C11 cyclizations to yield 87. AziB catalyzes three selective ketoreductions at C3, C5 and C9, as well as C2–C7/C6–C11 cyclizations to afford 5-methylnaphthoic acid 96. The C6–C11 cyclization step in the synthesis of these naphthoic acids is unique among known aromatic PKSs.
3 Cyclization of fungal aromatic polyketides
Fungal megasynthases are intrinsically more difficult to study than the dissociated bacterial PKSs due to their large size (in excess of 200 kDa) and the difficulties associated with genetically manipulating the fungal hosts. In recent years, the biochemical studies of fungal aromatic polyketide biosynthesis has been facilitated by: i) rapid accumulation of completed fungal genomes through various genome sequencing projects;120,121 ii) the ability to express intact megasynthases in heterologous hosts such as Escherichia coli,122 Aspergillus oryzae123,138 and Saccharomyces cerevisiae;124 and iii) the ability to excise individual domains as standalone enzymes.16,17 Genome sequencing has revealed that most fungal species encode multiple PKSs, many of which are not associated with known fungal metabolites. Understanding the biochemical basis of PKS enzymatic functions can therefore be important in product prediction and reprogramming of these machineries. Recent studies have shown that the product template (PT) domain125 found in fungal PKSs controls the immediate cyclization regioselectivity of the reactive backbone.16,17 Consistent with the F-mode rule proposed by Thomas, the PT domain mediates the first-ring closure by bridging an even-numbered carbanion carbon and an odd-numbered carbonyl carbon, which leads to two intact acetate units in the first ring (Fig. 1).4 Most of the recent studies on PT domains have focused on that of PksA16 involved in the biosynthesis of norsolorinic acid, and that of PKS417 involved in the biosynthesis of bikaverin. Additional cyclization of the polyketide backbone can be further directed by a C-terminus thioesterase/Claisen-like-cyclase (TE/CLC) which can also offload the polyketide chain from the megasynthases. In the following sections, we will organize the discussion on the cyclization of fungal aromatic polyketides based on the PT regioselectivity and C-terminal TE/CLC function.
3.1 Product template domain mediated cyclization
3.1.1 C2–C7 first-ring cyclization
The tetraketide orsellinic acid 85 is the smallest polyketide produced by a fungal NRPKS (Scheme 8). Although the purification of orsellinic acid synthase (OSAS) dates back to 1968,126 the genetic locus was identified in Aspergillus nidulans only recently.127 The cryptic gene orsA for orsellinic acid biosynthesis was activated through artificially induced fungal–bacterial interactions, which also led to the production of lecanoric acid 97, a typical lichen depside;128 and F-9775A 98 and F-9775B 99, which were initially isolated from Paecilomyces carneus.129 An independent study by Bok et al. on A. nidulans also confirmed the involvement of orsA in the biosynthesis of the orsellinate moieties embedded in 98 and 99.130 The gene orsA encodes a megasynthase that bears the typical NRPKS domain organization. The tetraketide backbone is synthesized by the minimal PKS domains, while the PT domain here is proposed to direct the C2–C7 aldol addition and cyclodehydration to afford the aromatic ring. The orsellinate functionality is also present in the resorcylic acid lactones (RALs) family of fungal polyketides which includes lasiodiplodin 100,131 zearalenone 101,132,133 hypothemycin 102134 and radicicol 103.134,135 Each RAL compound contains a resorcylate moiety fused within a 12- or 14-membered macrolactone ring and requires the synergetic actions of a highly-reducing PKS (HRPKS) and an NRPKS. Sequence alignment revealed high similarity between the RAL NRPKS and OSAS, which hinted that the RAL NRPKS is responsible for providing the resorcylate core of RALs. This was confirmed with in vitro reconstitution studies on PKS13 from Gibberella zeae,136 which is the NRPKS involved in the synthesis of 101. PKS13 was shown to accept a variety of acyl starter units and elongate the chain by three ketides, followed by C2–C7 cyclization to afford resorcylic acids or esters such as 104–106.136 PKS13 was also used to synthesize unnatural RALs such as 107 by using 11-hydroxylundecanoyl-SNAC as the primer unit.136
Scheme 8.
A subset of fungal aromatic polyketides containing α-methyl substitutions are produced by Clade III NRPKS as classified via phylogenetic analysis.120 This family of NRPKS contains a C-methylation domain (C-MeT) and a reductive domain (R) positioned after the ACP domain. Citrinin 108, produced by Aspergillus, Penicillium, and Monascus genera, is the first example in this subset of compounds of which the gene cluster was determined (Scheme 8).137 Following the biosynthetic logic of OSAS, the PT domain most likely catalyzes the C2–C7 regioselective cyclization of the backbone to afford the aromatic ring. The R domain is proposed to catalyze reductive release of the polyketide, which sets up cyclization of the second ring.138 The precise biosynthetic steps leading to formation of 108 have not been completely worked out, including timing of the C-MeT domain and starter unit incorporation. 13C-Acetate incorporation experiments in various fungal strains indicated 108 may be derived from either a pentaketide139 or a tetraketide origin.140 Using degenerate primers designed for KS and C-MeT domain of fungal NRPKSs, the gene encoding 3-methylorcinaldehyde synthase (MOS) was isolated from plant pathogen Acremonium strictum.138 MOS is a Clade III PKS that is involved in the biosynthesis of 3-methylorcinaldehyde 109. The domain organization of MOS matches precisely to that of citrinin synthase in which a homologous PT domain catalyzes C2–C7 aldol condensation. The methionine-derived C4 methyl group is introduced by the C-MeT domain while the C1 aldehyde is likely a result of R-domain-catalyzed reductive release. Another example of a Clade III NRPKS is AfoE found in the asperfuranone 110 biosynthetic pathway, which was mined from A. nidulans through artificial activation of the cryptic pathway.141 AfoE accepts a highly-reduced polyketide intermediate from the HRPKS AfoG and performs four additional rounds of chain extension to afford the octaketide backbone. This is followed by C2–C7 regioselective cyclization and reductive release to yield an intermediate that contains the 3-methylorcinaldehyde fragment. Therefore, the mechanism of AfoE is nearly identical to that of MOS with the exception of starter unit choice. Additional downstream tailoring such as hydroxylation and reduction lead to the formation of the novel furan moiety present in 110.
The bicyclic 1,3,6,8-tetrahydroxynaphthalene 111 is an important precursor for the formation of 1,8-dihydroxynaphthalene (D2HN, 112), the building block for polyketide-derived pigment melanin widely found in fungal species (Scheme 9). In Colletotrichum lagenarium, 111 is directly synthesized by PKS1 from a pentaketide intermediate without any post-PKS modification.12314C-Labeling assays showed that PKS1 solely incorporates malonyl-CoA as starter unit while acetyl-CoA is not recognized. With the intact malonyl starter, the pentaketide backbone undergoes C2–C7 aldol cyclization to afford the first aromatic ring. The second-ring C1–C9 closure is proposed to be catalyzed by the terminal TE/CLC domain via Claisen-like condensation (discussed below). 112 derived from 111 can also assemble together via oxidative coupling to form sporothrin A 113 and B 114 isolated from the mangrove endophytic fungus Sporothrix sp. (#4335).142 In the black fungal pathogen Wangiella dermatitidis, 2-acetyl-1,3,6,8-tetrahydroxynaphthalene (ATHN, 115) is found to be the precursor for 112. The synthesis of hexaketide 115 has been linked to WdPks1 via heterologous reconstitution.143
Scheme 9.
The naphtha-γ-pyrone compound 116 is produced by WA PKS from A. nidulans, which is the precursor of a yellow pigment embedded in the conidial cell walls. [1,2-13C]Acetate feeding studies suggested that the folding pattern of the heptaketide backbone follows the F-mode rule (Scheme 9).144 Furthermore, formation of naphthopyrone scaffold requires second-ring closure by Claisen-like condensation following first-ring cyclization. The TE/CLC domain in WA PKS has been proven to catalyze the Claisen-type cyclization, which will be discussed later. The biosynthetic logic of 116 can also be applied to the biosynthesis of nor-rubrofusarin 117, a dehydration version of 116, which is the precursor of red pigment aurofusarin 118 produced by several strains from Fusarium genus.145–147 Originated from a heptaketide precursor as 116, alternariol 119 isolated from Alternaria genus adopts C2–C7 first-ring cyclization, which is followed by C8–C13 aldol condensation and released via lactonizaton.148
SMA76a 120 is synthesized by PKS4 from Gibberella fujikuroi and is the precursor to red pigment bikaverin 121,122,149,150 which has been shown to possess anticancer properties (Scheme 9).151 Compared to 116, 120 contains an additional aromatic ring linearly fused to the naphtha-γ-pyrone to give the naphthoxanthone structure. Feeding studies suggested that 120 is derived from nine acetate units and the folding pattern of the naphthopyrone portion is identical to that of 116.152 The activities of PKS4 were recently reconstituted in vitro using intact holo-PKS4 purified from Escherichia coli.122 The protein expression level was sufficiently high to allow in vitro synthesis and full characterization of the polyketide product. It was shown that PKS4 synthesized 120 as a predominant product in the presence of malonyl-CoA, hence establishing that the NRPKS possesses all the required activities to synthesize and regioselectively cyclize the nonaketide backbone. Based on a series of studies in vitro, it was confirmed that construction of the nonaketide backbone requires the minimal PKS alone; the PT domain is responsible for catalyzing the first-ring cyclization via aldol condensation between C2 and C7; and the TE/CLC domain facilitates Claisen-like condensation between C10 and C1 to generate the second ring. Subsequently, attack of C13 carbonyl by the nucleophilic C9 phenol results in the tricyclic naphthopyrone, and finally C12–C17 aldol condensation and dehydration afford the last aromatic ring in 120. Timing of third ring closure is critical to form the tetracyclic structure as premature cyclization of the last three ketides will prevent the formation of the naphthoxanthone core. Therefore, it is likely that the TE/CLC domain may facilitate cyclization of both the second and third rings. However, this is yet to be verified.
PKS4 was highly amendable to domain engineering and dissection, which allowed detailed characterization of individual domain functions. When the cyclization domains such as PT and TE were excised from PKS4, the minimal PKS continued to produce ample amounts of the nonaketide backbone. Compound 120 was no longer observed as a product, as the committed C2–C7 cyclization did not take place. Instead, the reactive poly-β-ketone was cyclized into a spectrum of compounds not observed in previously known fungal metabolites. These compounds contain different first-ring cyclization regioselectivities, including the major products 15 cyclized via C9–C14 and C7–C16; and naphthopyrone 122 previously synthesized by a mutant type III PKS153 (Scheme 10). The cyclization steps leading to the formation of 122 is ambiguous since the first cyclization event can take place via either C5–C10 or C10–C15 regioselectivity. Remarkably, none of the F-mode cyclization patterns, including C2–C7, C4–C9 and C6–C11 were observed among the products, suggesting these cyclization events must be catalyzed by PT domains specific to fungal NRPKS. The PKS4 minimal PKS was also able to interact with bacterial cyclases as discussed in Sections 2.1.1 and 3.2.
Scheme 10.

3.1.2 C4–C9 first-ring cyclization
When the nascent polyketide chain is longer than a pentaketide, fungal PT domains can cyclize the first aromatic ring with alternative regioselectivity. A group of fungal aromatic compounds features C4–C9 cyclization of the first ring. Among them is norsolorinic acid 123, which is the anthraquinone precursor of the well-studied mycotoxin aflatoxin B1 124 (Scheme 11). The biosynthesis of 123 is accomplished by an 1.4 × 106 Da enzyme complex (NorS) consisting of an NRPKS (PksA) and a pair of fatty acid synthases-like enzymes.154 Initiated by a hexanoyl starter, PksA performs seven rounds of polyketide extension to afford an octaketide backbone. Townsend and coworkers deconstructed the intact PksA by domain dissection and reassembled in trans to assign the precise roles of different domains.16 Their work identified the PT domain mediates the successive C4–C9 and C2–C11 aldol cyclizations and the TE/CLC domain performs the C14–C1 Claisen-like condensation and product release. Removal of the TE/CLC domain led to the synthesis of a large amount of naphthopyrone 159, confirming the roles of the PT and TE domains in the cyclization reaction. The minimal PKS domains of PksA were inefficient when removed from the PT domain and synthesized only trace amounts of 123 and 159. The loss of product turnover implied that PksA PT domain also plays an important role in the overall efficiency of the chain elongation steps of PksA minimal PKS. From the same study, it is also proposed that the PT domain facilitates release of intermediates attached to the ACP domain.16
Scheme 11.
The structural basis of the PksA PT regioselectivity was recently elucidated by Townsend and coworkers.155 The PksA PT adopts a ‘double hot dog’ (DHD) fold similar to the helix-grip structure of the bacterial cyclase TcmN.31 Evident in the crystal structure is a deep internal pocket that can accommodate the nascent polyketide chain while attached to the phosphopantetheinyl arm (Fig. 3). Extended from the protein surface into the internal pocket is a phosphopantetheine-binding site, followed by a cyclization chamber that can accommodate up to two aromatic rings. The innermost part of the pocket is proposed to be a holding channel for the hexyl moiety, which is the starter unit for PksA. Mutagenesis analysis confirmed the function of the proposed catalytic dyad, Asp1543 and His1345, suggesting the PT domain has a catalytic role in addition to a template role. Furthermore, the PT domain was found to be a dimer as facilitated by a PT-specific insertion sequence. The dimeric structure of PT implies its role as a structural joint for the dimerization of the entire NRPKS structure.
Fig. 3.

Structures of standalone PT domain dissected from PksA. (a) Dimer structure of PT domain. (b) One monomer structure with bound palmitate; and (c) substrate analogue HC8. D1543 and H1345 are the catalytic dyad.
Xanthone is a commonly observed structural moiety in fungal aromatic polyketides, such as in demethylsterigmatocystin 125, an intermediate during the tailoring of 123 to 124 (Scheme 11). The xanthone structure was formed via a series of reactions on the anthraquinone precursor. These steps include aryl epoxidation of the first ring, C7 reduction, Baeyer–Villiger oxidative cleavage of the quinone ring, followed by C2–C3 bond rotation and phenol addition to yield the central 4H-pyran-4-one ring.156
Other C4–C9 cyclized fungal aromatic polyketides include dothistromin 126 produced by Dothistroma septosporum, which is a member of the aflatoxin-producing family. The gene (pksA) encoding an NRPKS responsible for the synthesis of the polyketide precursor was recently cloned.157 The unusual feature of pksA is that the ACP domain in this NRPKS is present in triplicate, with the first two ACP domains sharing more sequence similarity to each other than the C-terminus ACP. Cercosporin 127, belonging to perylenequinone family of compounds, is a phytotoxin produced by many phytopathogenic Cercospora species and is likely formed through the dimerization of two polyketide monomers (Scheme 11). The cloned CTB1 gene encoding an NRPKS with two tandem ACPs is proposed to synthesize the monomeric unit,158 which is a heptaketide that adopts first-ring C4–C9 cyclization and second-ring C2–C11 cyclization features.159 The cyclized dihydronaphthalen-1(4H)-one intermediate attached to ACP may be released via hydrolysis, followed by decarboxylation to afford the naphthalen-1(4H)-one structure 128. Additional modifications such as hydroxylation, methylation, ketoreduction and dimerization generate the final product 127. The biosynthesis of the related elsinochrome A 129 has been assigned to EfPKS1 from Elsinoë fawcettii.160 The close relationship between EfPKS1 and CTB1 based on the phylogenetic analysis suggests that EfPKS1 shares the similar biosynthetic logic to CTB1.
Ascomycone A 130 and B 131 are heptaketide-derived pyranonaphthoquinones (Scheme 11).161 They are structurally similar to fusarubin family of metabolites, such as dehydroherbarin and thysanone.162 Isotope labeling experiment suggested that this family of compounds are also derived via C4–C9 and C2–C11 cyclizations.163 Formation of the pyran portion in 130 and 131 may require reductive release from PKS, possibly catalyzed by an R domain. Often produced together with fusarubin metabolites are 2-azaanthraquinones that are structurally related to pyranonaphthoquinones. Representatives of this nitrogen-containing family of compounds include scorpinone 132164 and bostrycoidin 133.165 It is also observed that production of 133 and fusarubin was similarly affected by the carbon to nitrogen ratio, and pH of the culturing conditions, suggesting these two families of compounds may share a common polyketide precursor but differ in post-PKS modifications.166 One hypothesis for introducing the nitrogen atom into 132 is the participation of a pyridoxal-5′-phosphate-dependent aminotransferase that transfers the amine group into the terminal aldehyde produced via reductive release from ACP.166 Alternatively, 132 may be derived from a hexaketide intermediate via C4–C9 and C2–C11 cyclization, but is released via aminolysis with an alanine followed by last ring closure. Chaetocyclinone A 134 and B 135, isolated from an endosymbiotic fungus, both contain a pyranochromenone core (Scheme 11). It is postulated that both compounds also originated from heptaketide precursors cyclized at C4–C9 and C2–C11.167 Following cyclization, the second ring of the naphthoquinone intermediate is subjected to oxidative ring-opening that leads to formation of the chromone scaffold. The pyran rings present in 134 and 135 may be formed in similar fashion as those present in 130 and 131.
3.1.3 C6–C11 first-ring cyclization
Anthraquinones represent a large family of aromatic polyketides produced by fungi, among which emodin 136 can be viewed as the model compound. 136 and its derivatives have been isolated from several fungal genera, including Dermocybe, Cladosporium, Penicillium and Aspergillus. The NRPKS gene involved in emodin anthrone biosynthesis was identified from A. terreus RED1 by gene disruption.168 Horinouchi and coworkers targeted a homologous gene from A. terreus NIH2624, which encodes an atrochrysone carboxylic acid synthase (ACAS) involved in the biosynthesis of 136.169 The nascent octaketide chain is proposed to bend from the third ketide, adopting C6–C11 cyclization to fix the regioselectivity of the first ring, followed by C4–C13 and C2–C15 aldol condensation to establish the second and third rings, respectively (Scheme 12). This differs from the biosynthesis of 123 in which the anthraquinone is formed via the committed C4–C9 cyclization. Although the exact function of the PT domain involved in the biosynthesis of emodin family of compounds has not been verified, it is highly likely that the PT mediates both the C6–C11 and the C4–C13 ring closures. Closure of the last ring can be spontaneous, which is commonly observed for anthraquinones of bacterial origin. The C6–C11 PT domains therefore represent the third kind of fungal PKS “cyclase”. ACAS lacks a C-terminal TE domain based on sequence analysis, which may lead to the stalling of intermediate assembly on ACP. Alkaline hydrolysis of the ACAS-polyketide intermediate in vitro afforded the carboxylated emodin endocrocin 138.169 Enzymatic offloading of the tricyclic product was found to be catalyzed by a dissociated metal-dependent β-lactamase (ACTE), which is encoded upstream of the ACAS gene on the chromosome.169 Addition of ACTE to ACAS afforded 137, which can dehydrate to yield 138 and can further decarboxylate to yield 136.
Scheme 12.
Genome mining of A. nidulans using different approaches has activated several NRPKSs that synthesize aromatic polyketides with C6–C11 first-ring cyclization. Modification of chromatin in A. nidulans by demethylation of histone led to the activation of a cryptic biosynthetic pathway for 136, which resulted in the unexpected production of monodictyphenone 139 and a number of derivatives of 136.130 The production level of the highly hydroxylated anthraquinone asperthecin 141 was dramatically elevated by removing protein sumoylation in A. nidulans.170 Wang and coworkers located the NRPKS AptA responsible for the biosynthesis of 141 through a highly systematic gene knockout approach. The proposed biosynthetic pathway of 141 also involves 136 as a key intermediate. Structurally similar to 139, sulochrin 140, a co-metabolite produced by A. terreus during lovastatin fermentation,168 is also proposed to be derived from 136 following oxidative ring cleavage. Also produced by A. terreus, (+)-geodin 142 is derived from 136 via a series of post-PKS modifications including oxidative ring opening of 136 to yield 140. The key enzyme catalyzing the intramolecular phenol oxidative coupling that forms the spiro-furanone moiety in 142 has been identified to be dihydrogeodin oxidase (DHGO), a multicopper blue enzyme.171 Chrysophanol 143 of fungal origin was proved to adopt C6–C11 cyclization, of which the formation is similar as that of 136.172 It is observed that 143 is a common biosynthetic precursor to a few xanthone substances, such as secalonic acid 144 and tajixanthone 145 (Scheme 12).173,174 The mechanism for the formation of xanthone core in these compounds is proposed to include epoxidation, reduction and oxidative ring opening, similar to the steps proposed for 125.175
Starting from the C6–C11 first-ring cyclization, tetracyclic aromatic polyketides are also produced by fungi in the Penicillium and Hypomyces genera. Cyclization steps leading to formation of fungal tetracyclic compounds are significantly different than the bacterial steps discussed previously. Hypomycetin 146, an antifungal metabolite from Hypomyces aurantius, is a prenylated polyketide that contains a tetracyclic core (Scheme 12).176 An isotopic labeling experiment established that 146 is derived from an unreduced decaketide with the first-ring cyclization taken place between C6–C11, followed by sequential cyclizations of C4–C13, C2–C15 and a final C1–C18 Claisen-like condensation. Although no genetic locus has been identified for the biosynthesis of 146, a canonical NRPKS in which PT and TE domains play corresponding roles is to be expected.
Structurally similar to 146, viridicatumtoxin 147 and its epoxidized derivative viridicatumtoxin B 148 were isolated from Penicillium (Scheme 12).177,178 The tetracyclic core of 147 is nearly identical to that of anhydrotetracycline, including the signature amide on the distal ring. Despite the striking similarity in structure between the bacterial and fungal compounds, folding of 147 is dramatically different, setting up an intriguing case of convergent evolution towards the natural product scaffold. Isotope feeding experiments suggested that the 147 may contain a nonaketide backbone and exhibit the F-mode folding pattern. In contrast to the oxytetracycline pathway, biosynthesis of 147 is not expected to go through the fully aromatic intermediate pretetramid, but via the intermediate 149. In 149, the first two aromatic rings are formed via C6–C11 and C4–C13 aldol condensations. Formation of the third ring via C2–C15 cyclization without dehydration leaves a tertiary hydroxyl group derived from acetate oxygen. Cyclization of the fourth ring in 147 is also expected to be different than that of 146 based on the labeling studies.176,177 Because of the unknown steps leading to formation of the last ring, as well as the origin of the terminal amide unit, the regioselective cyclization of viridicatumtoxin backbone remains obscure. Therefore, the cyclization regioselectivities of 146 and 147 may be different, which implies that the functions of two PT domains in these NRPKSs may also be distinct.
3.1.4 Other possible cyclizations
Griseofulvin 150, an antifungal antibiotic compound synthesized by Penicillium griseofulvum and related strains, contains a spiro-grisan structure, as does (+)-geodin 142 (Scheme 13). A [2-13C]acetate labeling experiment established the heptaketide origin of 150.179 It is proposed that through a clamp-like folding pattern, a Claisen-like condensation between C1–C6 and an aldol condensation between C8–C13 are required to form the benzophenone intermediate.180 Recently, the biosynthetic gene cluster for 150 has been identified by our group (unpublished), where a NRPKS is confirmed to assemble the polyketide backbone of 150. Bioinformatic analysis of this NRPKS indicates that it bears the typical domain organization but lacks a TE/CLC domain. Since no other domains are likely to catalyze the C1–C6 Claisen-like condensation, the PT domain of this NRPKS was proposed to be involved. The C8–C13 aldol condensation is proposed to occur spontaneously. Sporothrin C 151 is a marine fungal polyketide isolated together with 113 and 114.142 151 is proposed to be assembled from three pentaketide cyclohexatrione units through a series of intermolecular aldol condensations. Biosynthesis of each pentaketide cyclohexatrione unit may involve a NRPKS catalyzing C1–C6 Claisen-type condensation, and may also be facilitated by a PT domain.
Scheme 13.
3.2 Thioesterase domain mediated cyclization
In addition to cyclizations mediated by the PT domain, fungal NRPKSs also use the terminal TE domain to catalyze regioselective cyclization of nascent polyketides. A majority of the fungal TE domains found in NRPKSs catalyze Claisen-like condensations to form C–C bonds, which is accompanied by release of the polyketide product from the megasynthases. Hence these TE domains are also referred to as TE/CLC domains. The Claisen-like condensation activity of TE/CLC is distinct from bacterial TE domains that catalyze lactonization181–183 or hydrolysis,184 and therefore represents an important strategy employed by fungal NRPKS to produce fused ring structures.
The CLC function of fungal NRPKS TE was first elucidated in the study of 116. Whereas the full length WA synthase produced the naphthopyrone 116 when heterologously expressed from Aspergillus oryzae,144 Ebizuka and coworkers observed the biosynthesis of citreoisocoumarin 152 as major product by a version of WA PKS truncated at the C-terminus.185 The differences in the cyclization patterns of the second ring between 116 and 152 suggested the importance of the TE domain in catalyzing the required Claisen-like C1–C10 condensation. Site-directed mutagenesis of the WA PKS TE domain revealed two essential catalytic residues Ser1967 and His2129 and led to the proposed two-step, general base-catalyzed mechanism.18 The similar catalytic role of the PKS1 TE/CLC domain in the synthesis of 111 was confirmed by deletion and site-directed mutagenesis inactivation experiments. These PKS1 mutants produced pentaketide isocoumarin 153 via spontaneous lactonization of the last ring, as well a hexaketide isocoumarin 154. The presence of the latter compound indicated the possible involvement of the functional TE/CLC domain in maintaining chain length of the minimal PKS. The PKS1 TE/CLC domain also displays hydrolytic activity in addition to the CLC activity, as intact PKS1 produced a significant amount of α-acetylorsellinic acid 155, a pentaketide carboxylic acid, as a major byproduct to 111.123 Inactivation of PKS1 TE/CLC similarly led to the loss of 155.

The CLC activity of PKS4 TE/CLC domain in the biosynthesis of 120 was investigated by domain deletion, site-directed mutagenesis and in trans complementation assays in vitro.39 Truncation of PKS4 at the C-terminus of the ACP domain gave rise to the nonaketide product SMA93 156. The same isocoumarin compound was also produced by the PKS4 Ser1830Ala mutant. Therefore, in the absence of the C1–C10 cyclization activity, the C2–C7 cyclized backbone spontaneously undergoes pyrone formation. Interestingly, when the standalone PKS4 TE was incubated with TE-less PKS4, the C1–C10 cyclization was partially restored and both 120 and 159 were recovered in similar yields. Additional PKS4 in vitro assays revealed the substrate specificity of the TE/CLC domain. When supplied with octanoyl-CoA, PKS4 preferentially incorporated the C8 starter unit and synthesized two new isocoumarins SMA76b 157 and SMA76c 158.122 The structures of 157 and 158 illustrated that in the presence of a partial β-keto backbone, whereas the PT can maintain the C2–C7 cyclization regioselectivity, the TE/CLC domain can no longer catalyze the C1–C10 CLC reaction. The incompatibility of the TE/CLC towards the fatty acyl-primed polyketide showed that a full poly-β-ketone backbone is required for binding to the enzyme, which suggests the PKS4 TE/CLC may also dictate the timing of third ring cyclization en route to 120.

Other confirmed TE/CLC domains include that of PksA involved in the biosynthesis of 123.16 When dissected PksA domains were reassembled in trans in the absence of TE/CLC, significant amounts of the naphthopyrone 159 were found in the in vitro assay. Re-introduction of TE/CLC to the full ensemble of PksA catalytic domains restored the production of 123. As expected, the C1–C14 regioselectivity of the PksA TE/CLC differs from the C1–C10 pattern associated with NRPKSs that have C2–C7 first-ring cyclization regioselectivtity.

3.3 Convergence of bacterial and fungal polyketide cyclization
Although divergent cyclization strategies are employed by bacterial and fungal PKSs, the two folding modes can also converge to yield the same final molecule. This is elegantly illustrated in the example of anthraquinone chrysophanol 143, a pigment and chemical defense agent found both from fungi and bacteria.172 Isotope labeling experiments revealed that the synthesis of 143 obeys the respective folding rules in the host organisms (Scheme 13). In a Nocardia strain, the nascent octaketide first undergoes C5–C10 aldol condensation, followed by C3–C12 and C2–C15 cyclizations, which is in accordance with S-mode folding. A novel S mode folding pattern of the nascent octaketide from a Streptomyces strain has been recently characterized: first-ring cyclization occurred between the common C7–C12, followed by C5–C14 and C3–C16 cyclization.186 In the fungal host, the nascent octaketide undergoes cyclization similar to that of 136, in which successive C6–C11, C4–C13 and C2–C15 aldol condensations take place. It is noticeable that S-mode and F-mode patterns follow the same regioselectivity for the last ring. All three pathways yield anthraquinone carboxylic acids, and upon decarboxylation can afford the same product 143. Such convergence has also been observed in compounds such as the previously discussed 111 and 147.
Bacterial cyclases have also been found to interact with the uncyclzed polyketide backbone synthesized by fungal PKS. When G. fujikuroi PKS4 was incubated with act KR, compound 8, which is synthesized by the act minimal PKS and act KR in Streptomyces, was observed.122 The presence of act KR seems to affect the chain length control of PKS4 and promoted the C7–C12 cyclization, while maintaining specific ketoreduction at C9 position. When this reaction system was further supplemented with bacterial cyclase, compounds such as 12, 49 and 50 were synthesized. The appearance of these products that were previously synthesized by bacterial PKSs in Streptomyces demonstrates the successful cross-talking between fungal NRPKS and bacterial cyclases. This study suggests a model in which the completed, ACP-bound polyketide chain synthesized by fungal NRPKS is protected from spontaneous cyclization by PKS4, but still accessible to dissociated bacterial cyclases. The production of these bacterial aromatic polyketides was accompanied by the presence of 120, indicative of the competitive relationship between the built-in cyclization steps catalyzed by PT and the supplemented bacterial cyclases. When the PT domain of PKS4 was removed, cyclization of the nascent polyketide chain can be completely dictated by bacterial cyclases. For example, in the presence of the C9–C14-specific TcmN, the expected 15 was exclusively synthesized; while in the presence of C7–C12-specific cyclase, a new compound 7, which is the nonaketide version of the well-known 1 was formed. These results, together with those reported by Cox and coworkers using the PksA ACP domain,187 show that the fungal NRPKS ACP domains can efficiently interact with bacterial aromatic PKS components. Interestingly, adding a standalone PT domain to the bacterial minimal PKS does not lead to formation of polyketides cyclized via fungal-specific patterns, possibly due to incompatibility between the PT domain and bacterial ACP domains.
4 Aromatic polyketides synthesized by type III PKSs
Type III PKSs are found widely among plants, fungi and bacteria.188 They consist of a homodimer ketosynthase that iteratively condenses malonyl-CoA to give relatively smaller aromatic polyketides than those discussed in the previous sections. Type III PKS differs from type II PKS in two important aspects: 1) the KS directly recruits malonyl-CoA in the absence of an acyltransferase or an ACP; 2) The KS active site catalyzes decarboxylative condensation and chain elongation, and also defines the regioselectivity of intramolecular cyclization. Hence, no additional cyclases or cyclization domains are required to cyclize these aromatic polyketides.
RppA from S. griseus is the first biochemically characterized bacterial type III PKS. RppA catalyzes the formation of 111 which is the building block of polyketide-based melanin pigment.19 Both Claisen and aldol condensations were proposed to be performed by RppA. The functionally equivalent 1,3,6,8-tetrahydroxynaphthlene synthase (THNS) from S. coelicolor was both structurally and biochemically characterized.189 Structural and mutational analyses revealed an unexpected cysteine residue that facilitates elongation of a malonyl-primed polyketide beyond the triketide stage. [1,2-13C]-Isotope incorporation experiment in S. coelicolor revealed the U-shaped folding of linear polyketide skeleton, in which the bridging bond between the two aromatic rings is derived from cyclization instead of an intact acetate unit.189 RppA-like enzymes are also believed to be responsible for the biosynthesis of the naphthoquinone moiety of some polyketides produced by bacteria. The recently isolated azamerone 160 from Streptomyces sp. CNQ-766 is a novel meroterpenoid with a unique phthalazinone ring system.190 Recently, Moore and coworkers have conducted a series of 13C- and 15N-labelling experiments to trace the biosynthesis of 160 in vivo.191 The phthalazinone ring of 160 is proposed to be derived via an oxidative rearrangement of a diazo chlorinated meroterpenoid A80915D 161. The feeding studies revealed that 111 is a precursor of the naphthoquinone core in 161, while the isoprenoid units are derived from the mevalonate pathway. Formation of the diazo functional group of 161 involves a transamination reaction followed by nucleophilic attack of the aminodihydroquinone intermediate on nitrous acid.

Alkylresorcinols are important phenolic lipids detected in the membrane of both bacteria and fungi. Essential for encystment of Azotobacter vinelandii, alkylresorcinols 162 with different alkyl chain lengths are synthesized by synergetic actions of a type I fatty acid synthase (ArsA) and a type III PKS (ArsB).192 ArsA synthesizes long fatty acyl chains that serve as starter units for ArsB. ArsB performs three rounds of chain elongation to yield a fatty acyl primed tetraketide, which is cyclized via C2–C7 aldol condensation to afford the aromatic ring. SrsA from S. griseus was shown to synthesize methylated alkylresorcinol in vitro by accepting acyl-CoAs with various chain lengths as starter unit and methylmalonyl-CoA as extender unit.193 Predicted from genome sequence of Neurospora crassa, 2′-oxoalkylresorcylic acid synthase (ORAS) was the first identified fungal type III PKS.194 ORAS preferentially selects stearoyl-CoA as starter unit and synthesizes a pentaketide intermediate with four rounds of chain extension. C2–C7 aldol condensation closes the resorcylic acid ring, which upon decarboxylation, affords resorcinol 163. The crystal structure of ORAS showed a distinct arrangement of structural elements around the enzyme active site compared to other structurally characterized type III PKSs.195 Structural-based mutational analyses of ORAS altered the cyclization regioselectivity of the PKS and resulted in the synthesis of alkylpyrones.196 Both bacterial ArsB and fungal ORAS have been shown to synthesize bis-5-alkylresorcinols 164 when primed by alkanedioic acid NAC dithioester in vitro.197 During the biosynthesis of 164, two rounds of C2–C7 cyclization were proposed to be performed by the enzymes. To conclude discussion of resorcinol biosynthesis, it is worth mentioning that an alkylresorcinol-producing enzyme (Steely1 or DiPKS1) was discovered in the social amoeba Dictyostelium discoideum, a primitive eukaryote.198,199 Steely1 is a hybrid megasynthases in which a type III PKS is appended at the C-terminus of a type I iterative PKS. Steely1 synthesizes the differentiation regulating factor 4-methyl-5-pentylbenzene-1,3-diol (MPBD, 165).199 The crystal structure of the type III PKS domain of Steely1 showed a conserved αβαβα-fold and a didomain architecture characteristic of other type III PKSs.198 The protein–protein interaction between upstream ACP and the type III PKS part was verified to be crucial in determining the cyclization of final products. Without the presence of upstream ACP, the type III PKS primarily produced alkylpyrones instead of alkylresorcinols.199

In addition to C2–C7 cyclization, type III PKSs can also catalyze aromatic ring formation via C3–C8 aldol condensation. The best-known example is DpgA from the vancomycin-type antibiotics biosynthetic pathways.200,201 DpgA condenses four molecules of malonyl-CoA and mediates C3–C8 cyclization to generate 3,5-dihydroxylphenylacetyl-CoA 166, a key intermediate in the biosynthesis of the nonproteinogenic amino acid building block 3,5-dihydroxyphenylglycine. Two enzymes with weak enoyl-CoA hydratase activity, DpgB and DpgD, were found to significantly increase the turnover rate of DpgA and were implicated to assist the aromatization reaction. Type III PKS Ken2 from the kendomycin biosynthetic pathway is functionally equivalent to DpgA and synthesizes 166 that becomes the starter unit for a type I modular PKS.202

The biosynthesis of phloroglucinol 167, which requires C1–C6 Claisen-type condensation, has been linked to a type III PKS (PhlD) from different strains of Pseudomonas fluorescens.203,204 Usnic acid 168, an antibacterial metabolite produced by many lichens, is composed of two molecules of methylated acetylphloroglucinol 169.205 It is possible that a fungal type III PKS may be involved in the biosynthesis of 169. The C1–C6 Claisen-type condensation is also employed by a hybrid type I fatty acid synthase-type III polyketide synthase Steely2 (DiPKS37) from D. discoideum to synthesize phlorocaprophenone, the precursor of differentiation-inducing factor DIF-1 170.198

Acknowledgments
Due to space constraints, we apologize for any work that we were not able to cite. Related research activities in our lab are supported by the National Science Foundation (CBET-0545860) and the National Institutes of Health (1R01GM085128). We thank Prof. Bradley Moore for helpful discussions.
Biographies
 Hui Zhou obtained her Bachelor’s degree in Biochemistry from Nanjing University, China, in 2003. She then conducted research in analytical biochemistry in the same university for three years and received her Master’s degree in 2006. She is now pursuing her Ph.D. degree in the Department of Chemical and Biomolecular Engineering at University of California, Los Angeles, under the guidance of Prof. Yi Tang. She is currently studying the biochemistry of fungal polyketide megasynthases, with a particular emphasis on fungal macrolactones.
Hui Zhou obtained her Bachelor’s degree in Biochemistry from Nanjing University, China, in 2003. She then conducted research in analytical biochemistry in the same university for three years and received her Master’s degree in 2006. She is now pursuing her Ph.D. degree in the Department of Chemical and Biomolecular Engineering at University of California, Los Angeles, under the guidance of Prof. Yi Tang. She is currently studying the biochemistry of fungal polyketide megasynthases, with a particular emphasis on fungal macrolactones.
 Yanran Li obtained her Bachelor’s degrees in Chemistry from Nankai University, and Chemical Engineering from Tianjin University, China, in 2007. She then spent half a year in the Chemical Engineering program at Rice University before moving to University of California, Los Angeles. She is now in her third year of Ph.D. studies in Professor Yi Tang’s group. She is currently working on the engineered biosynthesis of bacterial and fungal aromatic polyketides.
Yanran Li obtained her Bachelor’s degrees in Chemistry from Nankai University, and Chemical Engineering from Tianjin University, China, in 2007. She then spent half a year in the Chemical Engineering program at Rice University before moving to University of California, Los Angeles. She is now in her third year of Ph.D. studies in Professor Yi Tang’s group. She is currently working on the engineered biosynthesis of bacterial and fungal aromatic polyketides.
 Yi Tang, originally from Shanghai, China, immigrated to the United States in 1990. He obtained his B.S. degrees in Chemical Engineering and Material Science from Penn State University in 1997. He then moved to California Institute of Technology and studied biopolymer biosynthesis under the guidance of Prof. David A. Tirrell. After obtaining his Ph.D. in 2002, he moved to Stanford University to study bacterial aromatic polyketide biosynthesis with Prof. Chaitan Khosla. In 2004, he began his independent academic career in the Department of Chemical and Biomolecular Engineering at the University of California, Los Angeles. His group studies natural product biosynthesis, biocatalysts, and recently, nanobiotechnology.
Yi Tang, originally from Shanghai, China, immigrated to the United States in 1990. He obtained his B.S. degrees in Chemical Engineering and Material Science from Penn State University in 1997. He then moved to California Institute of Technology and studied biopolymer biosynthesis under the guidance of Prof. David A. Tirrell. After obtaining his Ph.D. in 2002, he moved to Stanford University to study bacterial aromatic polyketide biosynthesis with Prof. Chaitan Khosla. In 2004, he began his independent academic career in the Department of Chemical and Biomolecular Engineering at the University of California, Los Angeles. His group studies natural product biosynthesis, biocatalysts, and recently, nanobiotechnology.
Footnotes
This review discusses the enzymatic basis of the different cyclization strategies used by bacteria and fungi in the biosynthesis of aromatic polyketides.
References
- 1.Hertweck C, Luzhetskyy A, Rebets Y, Bechthold A. Nat Prod Rep. 2007;24:162–190. doi: 10.1039/b507395m. [DOI] [PubMed] [Google Scholar]
- 2.Brase S, Encinas A, Keck J, Nising CF. Chem Rev. 2009;109:3903–3990. doi: 10.1021/cr050001f. [DOI] [PubMed] [Google Scholar]
- 3.Rix U, Fischer C, Remsing LL, Rohr J. Nat Prod Rep. 2002;19:542–580. doi: 10.1039/b103920m. [DOI] [PubMed] [Google Scholar]
- 4.Thomas R. ChemBioChem. 2001;2:612–627. doi: 10.1002/1439-7633(20010903)2:9<612::AID-CBIC612>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 5.Das A, Khosla C. Acc Chem Res. 2009;42:631–639. doi: 10.1021/ar8002249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malpartida F, Hopwood DA. Nature. 1984;309:462–464. doi: 10.1038/309462a0. [DOI] [PubMed] [Google Scholar]
- 7.Hopwood DA. Chem Rev. 1997;97:2465–2498. doi: 10.1021/cr960034i. [DOI] [PubMed] [Google Scholar]
- 8.Summers RG, Ali A, Shen B, Wessel WA, Hutchinson CR. Biochemistry. 1995;34:9389–9402. doi: 10.1021/bi00029a015. [DOI] [PubMed] [Google Scholar]
- 9.Tang Y, Tsai SC, Khosla C. J Am Chem Soc. 2003;125:12708–12709. doi: 10.1021/ja0378759. [DOI] [PubMed] [Google Scholar]
- 10.Shen B, Hutchinson CR. Science. 1993;262:1535–1540. doi: 10.1126/science.8248801. [DOI] [PubMed] [Google Scholar]
- 11.McDaniel R, Ebert-Khosla S, Hopwood D, Khosla C. Science. 1993;262:1546–1550. doi: 10.1126/science.8248802. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmeister D, Keller NP. Nat Prod Rep. 2007;24:393–416. doi: 10.1039/b603084j. [DOI] [PubMed] [Google Scholar]
- 13.Khosla C, Tang Y, Chen AY, Schnarr NA, Cane DE. Annu Rev Biochem. 2007;76:195–221. doi: 10.1146/annurev.biochem.76.053105.093515. [DOI] [PubMed] [Google Scholar]
- 14.Cox RJ. Org Biomol Chem. 2007;5:2010–2026. doi: 10.1039/b704420h. [DOI] [PubMed] [Google Scholar]
- 15.Crawford JM, Dancy BC, Hill EA, Udwary DW, Townsend CA. Proc Natl Acad Sci U S A. 2006;103:16728–16733. doi: 10.1073/pnas.0604112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crawford JM, Thomas PM, Scheerer JR, Vagstad AL, Kelleher NL, Townsend CA. Science. 2008;320:243–246. doi: 10.1126/science.1154711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang WJ, Li YR, Tang Y. Proc Natl Acad Sci U S A. 2008;105:20683–20688. doi: 10.1073/pnas.0809084105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujii I, Watanabe A, Sankawa U, Ebizuka Y. Chem Biol. 2001;8:189–197. doi: 10.1016/s1074-5521(00)90068-1. [DOI] [PubMed] [Google Scholar]
- 19.Funa N, Ohnishi Y, Fujii I, Shibuya M, Ebizuka Y, Horinouchi S. Nature. 1999;400:897–899. doi: 10.1038/23748. [DOI] [PubMed] [Google Scholar]
- 20.Rawlings BJ. Nat Prod Rep. 1999;16:425–484. doi: 10.1039/a900566h. [DOI] [PubMed] [Google Scholar]
- 21.Moore BS, Hertweck C. Nat Prod Rep. 2002;19:70–99. doi: 10.1039/b003939j. [DOI] [PubMed] [Google Scholar]
- 22.Shen YM, Yoon P, Yu TW, Floss HG, Hopwood D, Moore BS. Proc Natl Acad Sci U S A. 1999;96:3622–3627. doi: 10.1073/pnas.96.7.3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu H, Ebert-Khosla S, Hopwood DA, Khosla C. J Am Chem Soc. 1994;116:4166–4170. [Google Scholar]
- 24.Yu TW, Shen Y, McDaniel R, Floss HG, Khosla C, Hopwood DA, Moore BS. J Am Chem Soc. 1998;120:7749–7759. [Google Scholar]
- 25.Fu H, Hopwood DA, Khosla C. Chem Biol. 1994;1:205–210. doi: 10.1016/1074-5521(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 26.McDaniel R, Ebertkhosla S, Fu H, Hopwood DA, Khosla C. Proc Natl Acad Sci U S A. 1994;91:11542–11546. doi: 10.1073/pnas.91.24.11542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keatinge-Clay AT, Maltby DA, Medzihradszky KF, Khosla C, Stroud RM. Nat Struct Mol Biol. 2004;11:888–893. doi: 10.1038/nsmb808. [DOI] [PubMed] [Google Scholar]
- 28.Marti T, Hu Z, Pohl NL, Shah AN, Khosla C. J Biol Chem. 2000;275:33443–33448. doi: 10.1074/jbc.M006766200. [DOI] [PubMed] [Google Scholar]
- 29.Gullón S, Olano C, Abdelfattah MS, Braña AF, Rohr J, Méndez C, Salas JA. Appl Environ Microbiol. 2006;72:4172–4183. doi: 10.1128/AEM.00734-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lombó F, Blanco G, Fernández E, Méndez C, Salas J. Gene. 1996;172:87–91. doi: 10.1016/0378-1119(96)00029-7. [DOI] [PubMed] [Google Scholar]
- 31.Ames BD, Korman TP, Zhang WJ, Smith P, Vu T, Tang Y, Tsai SC. Proc Natl Acad Sci U S A. 2008;105:5349–5354. doi: 10.1073/pnas.0709223105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsai SC, Ames BD, David AH. Methods Enzymol. Academic Press; 2009. pp. 17–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korman TP, Hill JA, Vu TN, Tsai SC. Biochemistry. 2004;43:14529–14538. doi: 10.1021/bi048133a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu H, McDaniel R, Hopwood DA, Khosla C. Biochemistry. 1994;33:9321–9326. doi: 10.1021/bi00197a036. [DOI] [PubMed] [Google Scholar]
- 35.Lee TS, Khosla C, Tang Y. J Am Chem Soc. 2005;127:12254–12262. doi: 10.1021/ja051429z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDaniel R, Ebert-Khosla S, Hopwood DA, Khosla C. J Am Chem Soc. 1994;116:10855–10859. [Google Scholar]
- 37.McDaniel R, Ebert-Khosla S, Hopwood DA, Khosla C. Nature. 1995;375:549–554. doi: 10.1038/375549a0. [DOI] [PubMed] [Google Scholar]
- 38.Korman TP, Tan YH, Wong J, Luo R, Tsai SC. Biochemistry. 2008;47:1837–1847. doi: 10.1021/bi7016427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma SM, Zhan J, Xie X, Watanabe K, Tang Y, Zhang W. J Am Chem Soc. 2008;130:38–39. doi: 10.1021/ja078091o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McDaniel R, Hutchinson CR, Khosla C. J Am Chem Soc. 1995;117:6805–6810. [Google Scholar]
- 41.Kramer PJ, Zawada RJX, McDaniel R, Hutchinson CR, Hopwood DA, Khosla C. J Am Chem Soc. 1997;119:635–639. [Google Scholar]
- 42.Zhang W, Watanabe K, Wang CCC, Tang Y. J Nat Prod. 2006;69:1633–1636. doi: 10.1021/np060290i. [DOI] [PubMed] [Google Scholar]
- 43.Zhang W, Ames BD, Tsai SC, Tang Y. Appl Environ Microbiol. 2006;72:2573–2580. doi: 10.1128/AEM.72.4.2573-2580.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang W, Wilke BI, Zhan J, Watanabe K, Boddy CN, Tang Y. J Am Chem Soc. 2007;129:9304–9305. doi: 10.1021/ja0736919. [DOI] [PubMed] [Google Scholar]
- 45.Hutchinson CR. Chem Rev. 1997;97:2525–2536. doi: 10.1021/cr960022x. [DOI] [PubMed] [Google Scholar]
- 46.Zhang W, Watanabe K, Wang CCC, Tang Y. J Biol Chem. 2007;282:25717–25725. doi: 10.1074/jbc.M703437200. [DOI] [PubMed] [Google Scholar]
- 47.Zhang W, Watanabe K, Cai X, Jung ME, Tang Y, Zhan J. J Am Chem Soc. 2008;130:6068–6069. doi: 10.1021/ja800951e. [DOI] [PubMed] [Google Scholar]
- 48.Zhang W, Wilke BI, Zhan J, Watanabe K, Boddy CN, Tang Y. J Am Chem Soc. 2007;129:9304–9305. doi: 10.1021/ja0736919. [DOI] [PubMed] [Google Scholar]
- 49.Petkovic H, Thamchaipenet A, Zhou LH, Hranueli D, Raspor P, Waterman PG, Hunter IS. J Biol Chem. 1999;274:32829–32834. [PubMed] [Google Scholar]
- 50.Pickens LB, Kim W, Wang P, Zhou H, Watanabe K, Gomi S, Tang Y. J Am Chem Soc. 2009;131:17677–17689. doi: 10.1021/ja907852c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prado L, Fernández E, Weibach U, Blanco G, Quirós LM, Braña AF, Méndez C, Rohr J, Salas JA. Chem Biol. 1999;6:19–30. doi: 10.1016/s1074-5521(99)80017-9. [DOI] [PubMed] [Google Scholar]
- 52.Trefzer A, Blanco G, Remsing L, Künzel E, Rix U, Lipata F, Braña AF, Méndez C, Rohr J, Bechthold A, Salas JA. J Am Chem Soc. 2002;124:6056–6062. doi: 10.1021/ja017385l. [DOI] [PubMed] [Google Scholar]
- 53.Künzel E, Wohlert SE, Beninga C, Haag S, Decker H, Hutchinson CR, Blanco G, Mendez C, Salas JA, Rohr J. Chem Eur J. 1997;3:1675–1678. [Google Scholar]
- 54.Gibson M, Nur-e-alam M, Lipata F, Oliveira MA, Rohr J. J Am Chem Soc. 2005;127:17594–17595. doi: 10.1021/ja055750t. [DOI] [PubMed] [Google Scholar]
- 55.Beam MP, Bosserman MA, Noinaj N, Wehenkel M, Rohr J. Biochemistry. 2009;48:4476–4487. doi: 10.1021/bi8023509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Menéndez N, Nur-e-Alam M, Braña AF, Rohr J, Salas JA, Méndez C. Chem Biol. 2004;11:21–32. doi: 10.1016/j.chembiol.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 57.Menéndez N, Nur-e-Alam M, Fischer C, Braña AF, Salas JA, Rohr J, Méndez C. Appl Environ Microbiol. 2006;72:167–177. doi: 10.1128/AEM.72.1.167-177.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Daum M, Peintner I, Linnenbrink A, Frerich A, Weber M, Paululat T, Bechthold A. ChemBioChem. 2009;10:1073–1083. doi: 10.1002/cbic.200800823. [DOI] [PubMed] [Google Scholar]
- 59.Luzhetskyy A, Mayer A, Hoffmann J, Pelzer S, Holzenkämper M, Schmitt B, Wohlert SE, Vente A, Bechthold A. ChemBioChem. 2007;8:599–602. doi: 10.1002/cbic.200600529. [DOI] [PubMed] [Google Scholar]
- 60.Kendrew SG, Katayama K, Deutsch E, Madduri K, Hutchinson CR. Biochemistry. 1999;38:4794–4799. doi: 10.1021/bi9827924. [DOI] [PubMed] [Google Scholar]
- 61.Sultana A, Kallio P, Jansson A, Wang JS, Niemi J, Mantsala P, Schneider G. EMBO J. 2004;23:1911–1921. doi: 10.1038/sj.emboj.7600201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kallio P, Sultana A, Niemi J, Mäntsälä P, Schneider G. J Mol Biol. 2006;357:210–220. doi: 10.1016/j.jmb.2005.12.064. [DOI] [PubMed] [Google Scholar]
- 63.Xu Z, Jakobi K, Welzel K, Hertweck C. Chem Biol. 2005;12:579–588. doi: 10.1016/j.chembiol.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 64.Shen B, Summers RG, Wendt-pienkowski E, Hutchinson CR. J Am Chem Soc. 1995;117:6811–6821. [Google Scholar]
- 65.Thompson TB, Katayama K, Watanabe K, Hutchinson CR, Rayment I. J Biol Chem. 2004;279:37956–37963. doi: 10.1074/jbc.M406144200. [DOI] [PubMed] [Google Scholar]
- 66.Rafanan ER, Hutchinson CR, Shen B. Org Lett. 2000;2:3225–3227. doi: 10.1021/ol0002267. [DOI] [PubMed] [Google Scholar]
- 67.Rafanan ER, Jr, Le L, Zhao L, Decker H, Shen B. J Nat Prod. 2001;64:444–449. doi: 10.1021/np010007+. [DOI] [PubMed] [Google Scholar]
- 68.Ramos A, Lombó F, Braña AF, Rohr J, Méndez C, Salas JA. Microbiology. 2008;154:781–788. doi: 10.1099/mic.0.2007/014035-0. [DOI] [PubMed] [Google Scholar]
- 69.Ichinose K, Ozawa M, Itou K, Kunieda K, Ebizuka Y. Microbiology. 2003;149:1633–1645. doi: 10.1099/mic.0.26310-0. [DOI] [PubMed] [Google Scholar]
- 70.Taguchi T, Itou K, Ebizuka Y, Malpartida F, Hopwood DA, Surti CM, Booker-Milburn KI, Stephenson GR, Ichinose K. J Antibiot. 2000;53:144–152. doi: 10.7164/antibiotics.53.144. [DOI] [PubMed] [Google Scholar]
- 71.Itoh T, Taguchi T, Kimberley MR, Booker-Milburn KI, Stephenson GR, Ebizuka Y, Ichinose K. Biochemistry. 2007;46:8181–8188. doi: 10.1021/bi700190p. [DOI] [PubMed] [Google Scholar]
- 72.Ichinose K, Bedford DJ, Tornus D, Bechthold A, Bibb MJ, Peter Revill W, Floss HG, Hopwood DA. Chem Biol. 1998;5:647–659. doi: 10.1016/s1074-5521(98)90292-7. [DOI] [PubMed] [Google Scholar]
- 73.Taguchi T, Ebizuka Y, Hopwood DA, Ichinose K. J Am Chem Soc. 2001;123:11376–11380. doi: 10.1021/ja015981+. [DOI] [PubMed] [Google Scholar]
- 74.Oja T, Palmu K, Lehmussola H, Leppäranta O, Hännikäinen K, Niemi J, Mäntsälä P, Metsä-Ketelä M. Chem Biol. 2008;15:1046–1057. doi: 10.1016/j.chembiol.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 75.Kulowski K, Wendt-Pienkowski E, Han L, Yang K, Vining LC, Hutchinson CR. J Am Chem Soc. 1999;121:1786–1794. [Google Scholar]
- 76.Han L, Yang K, Ramalingam E, Mosher RH, Vining LC. Microbiology. 1994;140:3379–3389. doi: 10.1099/13500872-140-12-3379. [DOI] [PubMed] [Google Scholar]
- 77.Wohlert SE, Wendt-Pienkowski E, Bao W, Hutchinson CR. J Nat Prod. 2001;64:1077–1080. doi: 10.1021/np010067f. [DOI] [PubMed] [Google Scholar]
- 78.Metsä-Ketelä M, Palmu K, Kunnari T, Ylihonko K, Mäntsälä P. Antimicrob Agents Chemother. 2003;47:1291–1296. doi: 10.1128/AAC.47.4.1291-1296.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rix U, Remsing LL, Hoffmeister D, Bechthold A, Rohr J. ChemBioChem. 2003;4:109–111. doi: 10.1002/cbic.200390002. [DOI] [PubMed] [Google Scholar]
- 80.Zhu L, Ostash B, Rix U, Nur-e-Alam M, Mayers A, Luzhetskyy A, Mendez C, Salas JA, Bechthold A, Fedorenko V, Rohr J. J Org Chem. 2005;70:631–638. doi: 10.1021/jo0483623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lombó F, Braña AF, Salas JA, Méndez C. ChemBioChem. 2004;5:1181–1187. doi: 10.1002/cbic.200400073. [DOI] [PubMed] [Google Scholar]
- 82.Lombó F, Abdelfattah MS, Braña AF, Salas JA, Rohr J, Méndez C. ChemBioChem. 2009;10:296–303. doi: 10.1002/cbic.200800425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Palmu K, Ishida K, Mäntsälä P, Hertweck C, Metsä-Ketelä M. ChemBioChem. 2007;8:1577–1584. doi: 10.1002/cbic.200700140. [DOI] [PubMed] [Google Scholar]
- 84.Kharel MK, Zhu L, Liu T, Rohr J. J Am Chem Soc. 2007;129:3780–3781. doi: 10.1021/ja0680515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen Y, Wang C, Greenwell L, Rix U, Hoffmeister D, Vining LC, Rohr J, Yang KQ. J Biol Chem. 2005;280:22508–22514. doi: 10.1074/jbc.M414229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fischer C, Lipata F, Rohr J. J Am Chem Soc. 2003;125:7818–7819. doi: 10.1021/ja034781q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kharel MK, Nybo SE, Shepherd MD, Rohr J. ChemBioChem. 2010;11:523–532. doi: 10.1002/cbic.200900673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang X, He J, Niu X, Menzel KD, Dahse HM, Grabley S, Fiedler HP, Sattler I, Hertweck C. Angew Chem, Int Ed. 2008;47:3995–3998. doi: 10.1002/anie.200800083. [DOI] [PubMed] [Google Scholar]
- 89.Erb A, Luzhetskyy A, Hardter U, Bechthold A. ChemBioChem. 2009;10:1392–1401. doi: 10.1002/cbic.200900054. [DOI] [PubMed] [Google Scholar]
- 90.Tsunakawa M, Nishio M, Ohkuma H, Tsuno T, Konishi M, Naito T, Oki T, Kawaguchi H. J Org Chem. 1989;54:2532–2536. [Google Scholar]
- 91.Dairi T, Hamano Y, Igarashi Y, Furumai T, Oki T. Biosci, Biotechnol, Biochem. 1997;61:1445–1453. doi: 10.1271/bbb.61.1445. [DOI] [PubMed] [Google Scholar]
- 92.Kim BC, Lee JM, Ahn JS, Kim BS. J Microbiol Biotechnol. 2007;17:830–839. [PubMed] [Google Scholar]
- 93.Zhan J, Watanabe K, Tang Y. ChemBioChem. 2008;9:1710–1715. doi: 10.1002/cbic.200800178. [DOI] [PubMed] [Google Scholar]
- 94.Zhan J, Qiao K, Tang Y. ChemBioChem. 2009;10:1447–1452. doi: 10.1002/cbic.200900082. [DOI] [PubMed] [Google Scholar]
- 95.Xu Z, Schenk A, Hertweck C. J Am Chem Soc. 2007;129:6022–6030. doi: 10.1021/ja069045b. [DOI] [PubMed] [Google Scholar]
- 96.Lackner G, Schenk A, Xu Z, Reinhardt K, Yunt ZS, Piel J, Hertweck C. J Am Chem Soc. 2007;129:9306–9312. doi: 10.1021/ja0718624. [DOI] [PubMed] [Google Scholar]
- 97.Ueno T, Takahashi H, Oda M, Mizunuma M, Yokoyama A, Goto Y, Mizushina Y, Sakaguchi K, Hayashi H. Biochemistry. 2000;39:5995–6002. doi: 10.1021/bi992661i. [DOI] [PubMed] [Google Scholar]
- 98.Li A, Piel J. Chem Biol. 2002;9:1017–1026. doi: 10.1016/s1074-5521(02)00223-5. [DOI] [PubMed] [Google Scholar]
- 99.Yunt Z, Reinhardt K, Li A, Engeser M, Dahse HM, Gutschow M, Bruhn T, Bringmann G, Piel J. J Am Chem Soc. 2009;131:2297–2305. doi: 10.1021/ja807827k. [DOI] [PubMed] [Google Scholar]
- 100.Wendt-Pienkowski E, Huang Y, Zhang J, Li B, Jiang H, Kwon H, Hutchinson CR, Shen B. J Am Chem Soc. 2005;127:16442–16452. doi: 10.1021/ja054376u. [DOI] [PubMed] [Google Scholar]
- 101.Chen Y, Luo Y, Ju J, Wendt-Pienkowski E, Rajski SR, Shen B. J Nat Prod. 2008;71:431–437. doi: 10.1021/np070664n. [DOI] [PubMed] [Google Scholar]
- 102.Bililign T, Hyun CG, Williams JS, Czisny AM, Thorson JS. Chem Biol. 2004;11:959–969. doi: 10.1016/j.chembiol.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 103.Das A, Khosla C. Chem Biol. 2009;16:1197–1207. doi: 10.1016/j.chembiol.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jakobi K, Hertweck C. J Am Chem Soc. 2004;126:2298–2299. doi: 10.1021/ja0390698. [DOI] [PubMed] [Google Scholar]
- 105.Fritzsche K, Ishida K, Hertweck C. J Am Chem Soc. 2008;130:8307–8316. doi: 10.1021/ja800251m. [DOI] [PubMed] [Google Scholar]
- 106.King RW, Bauer JD, Brady SF. Angew Chem, Int Ed. 2009;48:6257–6261. doi: 10.1002/anie.200901209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sitachitta N, Gadepalli M, Davidson BS. Tetrahedron. 1996;52:8073–8080. [Google Scholar]
- 108.Piel J, Hertweck C, Shipley PR, Hunt DM, Newman MS, Moore BS. Chem Biol. 2000;7:943–955. doi: 10.1016/s1074-5521(00)00044-2. [DOI] [PubMed] [Google Scholar]
- 109.Hertweck C, Xiang L, Kalaitzis JA, Cheng Q, Palzer M, Moore BS. Chem Biol. 2004;11:461–468. doi: 10.1016/j.chembiol.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 110.Xiang L, Kalaitzis JA, Moore BS. Proc Natl Acad Sci U S A. 2004;101:15609–15614. doi: 10.1073/pnas.0405508101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shen B. Curr Opin Chem Biol. 2003;7:285–295. doi: 10.1016/s1367-5931(03)00020-6. [DOI] [PubMed] [Google Scholar]
- 112.Ahlert J, Shepard E, Lomovskaya N, Zazopoulos E, Staffa A, Bachmann BO, Huang K, Fonstein L, Czisny A, Whitwam RE, Farnet CM, Thorson JS. Science. 2002;297:1173–1176. doi: 10.1126/science.1072105. [DOI] [PubMed] [Google Scholar]
- 113.Liu W, Nonaka K, Nie L, Zhang J, Christenson SD, Bae J, Van Lanen SG, Zazopoulos E, Farnet CM, Yang CF, Shen B. Chem Biol. 2005;12:293–302. doi: 10.1016/j.chembiol.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 114.Gaisser S, Trefzer A, Stockert S, Kirschning A, Bechthold A. J Bacteriol. 1997;179:6271–6278. doi: 10.1128/jb.179.20.6271-6278.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhao Q, He Q, Ding W, Tang M, Kang Q, Yu Y, Deng W, Zhang Q, Fang J, Tang G, Liu W. Chem Biol. 2008;15:693–705. doi: 10.1016/j.chembiol.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 116.Shao L, Qu XD, Jia XY, Zhao QF, Tian ZH, Wang M, Tang GL, Liu W. Biochem Biophys Res Commun. 2006;345:133–139. doi: 10.1016/j.bbrc.2006.04.069. [DOI] [PubMed] [Google Scholar]
- 117.Kudo F, Kasama Y, Hirayama T, Eguchi T. J Antibiot. 2007;60:492–503. doi: 10.1038/ja.2007.63. [DOI] [PubMed] [Google Scholar]
- 118.Ito T, Roongsawang N, Shirasaka N, Lu W, Flatt PM, Kasanah N, Miranda C, Mahmud T. ChemBioChem. 2009;10:2253–2265. doi: 10.1002/cbic.200900339. [DOI] [PubMed] [Google Scholar]
- 119.Van Lanen SG, Oh TJ, Liu W, Wendt-Pienkowski E, Shen B. J Am Chem Soc. 2007;129:13082–13094. doi: 10.1021/ja073275o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kroken S, Glass NL, Taylor JW, Yoder OC, Turgeon BG. Proc Natl Acad Sci U S A. 2003;100:15670–15675. doi: 10.1073/pnas.2532165100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Galagan JE, Calvo SE, Cuomo C, Ma LJ, Wortman JR, Batzoglou S, Lee SI, Basturkmen M, Spevak CC, Clutterbuck J, Kapitonov V, Jurka J, Scazzocchio C, Farman M, Butler J, Purcell S, Harris S, Braus GH, Draht O, Busch S, D’Enfert C, Bouchier C, Goldman GH, Bell-Pedersen D, Griffiths-Jones S, Doonan JH, Yu J, Vienken K, Pain A, Freitag M, Selker EU, Archer DB, Penalva MA, Oakley BR, Momany M, Tanaka T, Kumagai T, Asai K, Machida M, Nierman WC, Denning DW, Caddick M, Hynes M, Paoletti M, Fischer R, Miller B, Dyer P, Sachs MS, Osmani SA, Birren BW. Nature. 2005;438:1105–1115. doi: 10.1038/nature04341. [DOI] [PubMed] [Google Scholar]
- 122.Ma SM, Zhan J, Watanabe K, Xie X, Zhang W, Wang CC, Tang Y. J Am Chem Soc. 2007;129:10642–10643. doi: 10.1021/ja074865p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fujii I, Mori Y, Watanabe A, Kubo Y, Tsuji G, Ebizuka Y. Biochemistry. 2000;39:8853–8858. doi: 10.1021/bi000644j. [DOI] [PubMed] [Google Scholar]
- 124.Ma SM, Li JW, Choi JW, Zhou H, Lee KK, Moorthie VA, Xie X, Kealey JT, Da Silva NA, Vederas JC, Tang Y. Science. 2009;326:589–592. doi: 10.1126/science.1175602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Udwary DW, Merski M, Townsend CA. J Mol Biol. 2002;323:585–598. doi: 10.1016/s0022-2836(02)00972-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gaucher GM, Shepherd MG. Biochem Biophys Res Commun. 1968;32:664–671. doi: 10.1016/0006-291x(68)90290-8. [DOI] [PubMed] [Google Scholar]
- 127.Schroeckh V, Scherlach K, Nützmann HW, Shelest E, Schmidt-Heck W, Schuemann J, Martin K, Hertweck C, Brakhage AA. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0901870106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stocker-Wörgötter E. Nat Prod Rep. 2008;25:188–200. doi: 10.1039/b606983p. [DOI] [PubMed] [Google Scholar]
- 129.Sato A, Morishita T, Hosoya T, Ishikawa Y. 11001480. Jpn Pat. 1999
- 130.Bok JW, Chiang YM, Szewczyk E, Reyes-Domingez Y, Davidson AD, Sanchez JF, Lo HC, Watanabe K, Strauss J, Oakley BR, Wang CCC, Keller NP. Nat Chem Biol. 2009;5:462–464. doi: 10.1038/nchembio.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kashima T, Takahashi K, Matsuura H, Nabeta K. Biosci, Biotechnol, Biochem. 2009;73:1118–1122. doi: 10.1271/bbb.80878. [DOI] [PubMed] [Google Scholar]
- 132.Kim YT, Lee YR, Jin J, Han KH, Kim H, Kim JC, Lee T, Yun SH, Lee YW. Mol Microbiol. 2005;58:1102–1113. doi: 10.1111/j.1365-2958.2005.04884.x. [DOI] [PubMed] [Google Scholar]
- 133.Gaffoor I, Trail F. Appl Environ Microbiol. 2006;72:1793–1799. doi: 10.1128/AEM.72.3.1793-1799.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Reeves CD, Hu Z, Reid R, Kealey JT. Appl Environ Microbiol. 2008;74:5121–5129. doi: 10.1128/AEM.00478-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang S, Xu Y, Maine EA, Wijeratne EM, Espinosa-Artiles P, Gunatilaka AA, Molnár I. Chem Biol. 2008;15:1328–1338. doi: 10.1016/j.chembiol.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 136.Zhou H, Zhan J, Watanabe K, Xie X, Tang Y. Proc Natl Acad Sci U S A. 2008;105:6249–6254. doi: 10.1073/pnas.0800657105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shimizu T, Kinoshita H, Ishihara S, Sakai K, Nagai S, Nihira T. Appl Environ Microbiol. 2005;71:3453–3457. doi: 10.1128/AEM.71.7.3453-3457.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bailey AM, Cox RJ, Harley K, Lazarus CM, Simpson TJ, Skellam E. Chem Commun. 2007:4053–4055. doi: 10.1039/b708614h. [DOI] [PubMed] [Google Scholar]
- 139.Hajjaj H, Klaébé A, Loret MO, Goma G, Blanc PJ, François J. Appl Environ Microbiol. 1999;65:311–314. doi: 10.1128/aem.65.1.311-314.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Colombo L, Gennari C, Potenza D, Scolastico C, Aragozzini F, Merendi C. J Chem Soc, Perkin Trans 1. 1981:2594–2597. [Google Scholar]
- 141.Chiang YM, Szewczyk E, Davidson AD, Keller N, Oakley BR, Wang CC. J Am Chem Soc. 2009;131:2965–2970. doi: 10.1021/ja8088185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wen L, Cai XL, Xu F, She ZG, Chan WL, Vrijmoed LLP, Jones EBG, Lin YC. J Org Chem. 2009;74:1093–1098. doi: 10.1021/jo802096q. [DOI] [PubMed] [Google Scholar]
- 143.Wheeler MH, Abramczyk D, Puckhaber LS, Naruse M, Ebizuka Y, Fujii I, Szaniszlo PJ. Eukaryotic Cell. 2008;7:1699–1711. doi: 10.1128/EC.00179-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Watanabe A, Fujii I, Sankawa U, Mayorga ME, Timberlake WE, Ebizuka Y. Tetrahedron Lett. 1999;40:91–94. [Google Scholar]
- 145.Kim JE, Han KH, Jin J, Kim H, Kim JC, Yun SH, Lee YW. Appl Environ Microbiol. 2005;71:1701–1708. doi: 10.1128/AEM.71.4.1701-1708.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Malz S, Grell MN, Thrane C, Maier FJ, Rosager P, Felk A, Albertsen KS, Salomon S, Bohn L, Schäfer W, Giese H. Fungal Genet Biol. 2005;42:420–433. doi: 10.1016/j.fgb.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 147.Frandsen RJ, Nielsen NJ, Maolanon N, Sørensen JC, Olsson S, Nielsen J, Giese H. Mol Microbiol. 2006;61:1069–1080. doi: 10.1111/j.1365-2958.2006.05295.x. [DOI] [PubMed] [Google Scholar]
- 148.Dasenbrock J, Simpson TJ. Chem Commun. 1987:1235–1236. [Google Scholar]
- 149.Linnemannstöns P, Schulte J, Prado MD, Proctor RH, Avalos J, Tudzynski B. Fungal Genet Biol. 2002;37:134–148. doi: 10.1016/s1087-1845(02)00501-7. [DOI] [PubMed] [Google Scholar]
- 150.Wiemann P, Willmann A, Straeten M, Kleigrewe K, Beyer M, Humpf HU, Tudzynski B. Mol Microbiol. 2009;72:931–946. doi: 10.1111/j.1365-2958.2009.06695.x. [DOI] [PubMed] [Google Scholar]
- 151.Zhan J, Burns AM, Liu MX, Faeth SH, Gunatilaka AA. J Nat Prod. 2007;70:227–232. doi: 10.1021/np060394t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.McInnes AG, Walter JA, Smith DG, Wright JL, Vining LC. J Antibiot (Tokyo) 1976;29:1050–1057. doi: 10.7164/antibiotics.29.1050. [DOI] [PubMed] [Google Scholar]
- 153.Abe I, Morita H, Oguro S, Noma H, Wanibuchi K, Kawahara N, Goda Y, Noguchi H, Kohno T. J Am Chem Soc. 2007;129:5976–5980. doi: 10.1021/ja070375l. [DOI] [PubMed] [Google Scholar]
- 154.Watanabe CM, Townsend CA. Chem Biol. 2002;9:981–988. doi: 10.1016/s1074-5521(02)00213-2. [DOI] [PubMed] [Google Scholar]
- 155.Crawford JM, Korman TP, Labonte JW, Vagstad AL, Hill EA, Kamari-Bidkorpeh O, Tsai SC, Townsend CA. Nature. 2009;461:1139–1143. doi: 10.1038/nature08475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Henry KM, Townsend CA. J Am Chem Soc. 2005;127:3724–3733. doi: 10.1021/ja0455188. [DOI] [PubMed] [Google Scholar]
- 157.Bradshaw RE, Jin H, Morgan BS, Schwelm A, Teddy OR, Young CA, Zhang S. Mycopathologia. 2006;161:283–294. doi: 10.1007/s11046-006-0240-5. [DOI] [PubMed] [Google Scholar]
- 158.Choquer M, Dekkers KL, Chen HQ, Cao L, Ueng PP, Daub ME, Chung KR. Mol Plant-Microbe Interact. 2005;18:468–476. doi: 10.1094/MPMI-18-0468. [DOI] [PubMed] [Google Scholar]
- 159.Chen H, Lee M, Daub ME, Chung K. Mol Microbiol. 2007;64:755–770. doi: 10.1111/j.1365-2958.2007.05689.x. [DOI] [PubMed] [Google Scholar]
- 160.Liao HL, Chung KR. Mol Plant-Microbe Interact. 2008;21:469–479. doi: 10.1094/MPMI-21-4-0469. [DOI] [PubMed] [Google Scholar]
- 161.Opatz T, Kolshorn H, Thines E, Anke H. J Nat Prod. 2008;71:1973–1976. doi: 10.1021/np800570w. [DOI] [PubMed] [Google Scholar]
- 162.Medentsev AG, Arinbasarova AY, Akimenko VK. Appl Biochem Microbiol. 2005;41:503–507. [Google Scholar]
- 163.Kurobane I, Vining LC, Mcinnes AG, Walter JA. Can J Chem. 1980;58:1380–1385. [Google Scholar]
- 164.Miljkovic A, Mantle PG, Williams DJ, Rassing B. J Nat Prod. 2001;64:1251–1253. doi: 10.1021/np000625a. [DOI] [PubMed] [Google Scholar]
- 165.Arsenault GP. Tetrahedron Lett. 1965;6:4033. [Google Scholar]
- 166.Van Wagoner RM, Mantle PG, Wright JLC. J Nat Prod. 2008;71:426–430. doi: 10.1021/np070614i. [DOI] [PubMed] [Google Scholar]
- 167.Lösgen Sandra, Schlörke Oliver, Meindl Kathrin, Herbst-Irmer Regine, Zeeck Axel. Eur J Org Chem. 2007:2191–2196. [Google Scholar]
- 168.Couch RD, Gaucher GM. J Biotechnol. 2004;108:171–178. doi: 10.1016/j.jbiotec.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 169.Awakawa T, Yokota K, Funa N, Doi F, Mori N, Watanabe H, Horinouchi S. Chem Biol. 2009;16:613–623. doi: 10.1016/j.chembiol.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 170.Szewczyk E, Chiang YM, Oakley CE, Davidson AD, Wang CC, Oakley BR. Appl Environ Microbiol. 2008;74:7607–7612. doi: 10.1128/AEM.01743-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Huang KX, Fujii I, Ebizuka Y, Gomi K, Sankawa U. J Biol Chem. 1995;270:21495–21502. doi: 10.1074/jbc.270.37.21495. [DOI] [PubMed] [Google Scholar]
- 172.Bringmann G, Noll TF, Gulder TA, Grüne M, Dreyer M, Wilde C, Pankewitz F, Hilker M, Payne GD, Jones AL, Goodfellow M, Fiedler HP. Nat Chem Biol. 2006;2:429–433. doi: 10.1038/nchembio805. [DOI] [PubMed] [Google Scholar]
- 173.Franck B, Bringmann G, Flohr G. Angew Chem, Int Ed Engl. 1980;19:460–461. [Google Scholar]
- 174.Ahmed SA, Bardshiri E, Simpson TJ. J Chem Soc, Chem Commun. 1987:883–884. [Google Scholar]
- 175.Henry KM, Townsend CA. J Am Chem Soc. 2005;127:3724–3733. doi: 10.1021/ja0455188. [DOI] [PubMed] [Google Scholar]
- 176.Breinholt J, Jensen GW, Kjær A, Olsen CE, Rosendahl CN. Acta Chem Scand. 1997;51:855–860. [Google Scholar]
- 177.de Jesus AE, Hull WE, Steyn PS, Vanheerden FR, Vleggaar R. J Chem Soc, Chem Commun. 1982:902–904. [Google Scholar]
- 178.Zheng CJ, Yu HE, Kim EH, Kim WG. J Antibiot. 2008;61:633–637. doi: 10.1038/ja.2008.84. [DOI] [PubMed] [Google Scholar]
- 179.Tanabe M, Detre G. J Am Chem Soc. 1966;88:4515–4517. [Google Scholar]
- 180.Harris CM, Roberson JS, Harris TM. J Am Chem Soc. 1976;98:5380–5386. doi: 10.1021/ja00433a053. [DOI] [PubMed] [Google Scholar]
- 181.Giraldes JW, Akey DL, Kittendorf JD, Sherman DH, Smith JL, Fecik RA. Nat Chem Biol. 2006;2:531–536. doi: 10.1038/nchembio822. [DOI] [PubMed] [Google Scholar]
- 182.Akey DL, Kittendorf JD, Giraldes JW, Fecik RA, Sherman DH, Smith JL. Nat Chem Biol. 2006;2:537–542. doi: 10.1038/nchembio824. [DOI] [PubMed] [Google Scholar]
- 183.Tsai SC, Miercke LJ, Krucinski J, Gokhale R, Chen JC, Foster PG, Cane DE, Khosla C, Stroud RM. Proc Natl Acad Sci U S A. 2001;98:14808–14813. doi: 10.1073/pnas.011399198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Strieter ER, Koglin A, Aron ZD, Walsh CT. J Am Chem Soc. 2009;131:2113–2115. doi: 10.1021/ja8077945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Watanabe A, Ono Y, Fujii I, Sankawa U, Mayorga ME, Timberlake WE, Ebizuka Y. Tetrahedron Lett. 1998;39:7733–7736. [Google Scholar]
- 186.Bringmann G, Gulder TA, Hamm A, Goodfellow M, Fiedler HP. Chem Commun. 2009:6810–6812. doi: 10.1039/b910501h. [DOI] [PubMed] [Google Scholar]
- 187.Ma Y, Smith LH, Cox RJ, Beltran-Alvarez P, Arthur CJ, Simpson TJ. ChemBioChem. 2006;7:1951–1958. doi: 10.1002/cbic.200600341. [DOI] [PubMed] [Google Scholar]
- 188.Austin MB, Noel JP. Nat Prod Rep. 2003;20:79–110. doi: 10.1039/b100917f. [DOI] [PubMed] [Google Scholar]
- 189.Austin MB, Izumikawa M, Bowman ME, Udwary DW, Ferrer JL, Moore BS, Noel JP. J Biol Chem. 2004;279:45162–45174. doi: 10.1074/jbc.M406567200. [DOI] [PubMed] [Google Scholar]
- 190.Cho JY, Kwon HC, Williams PG, Jensen PR, Fenical W. Org Lett. 2006;8:2471–2474. doi: 10.1021/ol060630r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Winter Jaclyn M, Jansma Ariane L, Handel Tracy M, Moore Bradley S. Angew Chem, Int Ed. 2009;48:767–770. doi: 10.1002/anie.200805140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Miyanaga A, Funa N, Awakawa T, Horinouchi S. Proc Natl Acad Sci U S A. 2008;105:871–876. doi: 10.1073/pnas.0709819105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Funabashi M, Funa N, Horinouchi S. J Biol Chem. 2008;283:13983–13991. doi: 10.1074/jbc.M710461200. [DOI] [PubMed] [Google Scholar]
- 194.Funa N, Awakawa T, Horinouchi S. J Biol Chem. 2007;282:14476–14481. doi: 10.1074/jbc.M701239200. [DOI] [PubMed] [Google Scholar]
- 195.Rubin-Pitel SB, Zhang H, Vu T, Brunzelle JS, Zhao H, Nair SK. Chem Biol. 2008;15:1079–1090. doi: 10.1016/j.chembiol.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Goyal A, Saxena P, Rahman A, Singh PK, Kasbekar DP, Gokhale RS, Sankaranarayanan R. J Struct Biol. 2008;162:411–421. doi: 10.1016/j.jsb.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 197.Miyanaga A, Horinouchi S. J Antibiot. 2009;62:371–376. doi: 10.1038/ja.2009.44. [DOI] [PubMed] [Google Scholar]
- 198.Austin MB, Saito T, Bowman ME, Haydock S, Kato A, Moore BS, Kay RR, Noel JP. Nat Chem Biol. 2006;2:494–502. doi: 10.1038/nchembio811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ghosh R, Chhabra A, Phatale PA, Samrat SK, Sharma J, Gosain A, Mohanty D, Saran S, Gokhale RS. J Biol Chem. 2008;283:11348–11354. doi: 10.1074/jbc.M709588200. [DOI] [PubMed] [Google Scholar]
- 200.Pfeifer V, Nicholson GJ, Ries J, Recktenwald J, Schefer AB, Shawky RM, Schroder J, Wohlleben W, Pelzer S. J Biol Chem. 2001;276:38370–38377. doi: 10.1074/jbc.M106580200. [DOI] [PubMed] [Google Scholar]
- 201.Chen H, Tseng CC, Hubbard BK, Walsh CT. Proc Natl Acad Sci U S A. 2001;98:14901–14906. doi: 10.1073/pnas.221582098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wenzel SC, Bode HB, Kochems I, Muller R. ChemBioChem. 2008;9:2711–2721. doi: 10.1002/cbic.200800456. [DOI] [PubMed] [Google Scholar]
- 203.Bangera MG, Thomashow LS. J Bacteriol. 1999;181:3155–3163. doi: 10.1128/jb.181.10.3155-3163.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Achkar J, Xian M, Zhao H, Frost JW. J Am Chem Soc. 2005;127:5332–5333. doi: 10.1021/ja042340g. [DOI] [PubMed] [Google Scholar]
- 205.König GM, Wright AD. Phytochem Anal. 1999;10:279–284. [Google Scholar]