Abstract
Background
Depression and Genetic variation in serotonin and monoamine transmission have both been associated with Body Mass Index (BMI), but their interaction effects are not well understood. We examined the interaction between depressive symptoms and functional polymorphisms of serotonin transporter (SLC6A4) and monoamine oxidase A (MAOA) on categories of BMI.
Methods
Participants were from the National Longitudinal Study of Adolescent Health. Multiple logistic regression was used to investigate interactions between candidate genes and depression on risk of obesity (BMI≥30) or overweight + obese combined (BMI≥25).
Results
Males with an MAOA active allele with high depressive symptoms were at decreased risk of obesity (OR, 0.22; 95% CI, 0.06 – 0.78) and overweight + obesity (OR, 0.48; 95% CI, 0.26 – 0.89). No similar effect was observed among females.
Conclusions
These findings highlight that the obesity-depression relationship may vary as a function of gender and genetic polymorphism, and suggest the need for further study.
Introduction
Obesity is increasingly a major public health issue and has been associated with a number of chronic health conditions. Genetic factors are believed to play an important role in regulating the development of obesity (Bray, 2006). There has been considerable interest in the serotonin and dopamine neurotransmitter systems, which are hypothesized to regulate behavioral and metabolic responses associated with the development of obesity through feeding and satiety (Barsh & Schwartz, 2002). Recent studies of Argentinean adolescents (Sookoian et al., 2007) and young adult males (Sookoian, Gianotti, Gemma, Burgueno, & Pirola, 2008) found significant associations between a polymorphism of the serotonin transporter SLC6A4 and being overweight (Sookoian et al., 2007). In a U.S. sample of young adults, this gene was also found to be associated with obesity, primarily among men (Fuemmeler et al., 2008). In addition to SLC6A4, the gene that encodes monoamine oxidase A (MAO-A)—an enzyme that metabolizes brain amines including serotonin and dopamine—has been examined as a predictor of obesity. In a large U.K. cohort (n=1,150) of Caucasian females, significant associations were detected between MAOA and Body Mass Index (BMI), with the low-activity u-VNTR genotype (3/3) being more frequent among obese females (Need, Ahmadi, Spector, & Goldstein, 2006). This finding supports a previous family-based study in which preferential transmission of the low activity allele was observed among subjects with BMI >= 35 kg/m2 (Camarena et al., 2004). Also, the association between the low activity allele and obesity was observed among white and Hispanic, but not African-American, men in a U.S. cohort of young adolescents and adults (Fuemmeler et al., 2008).
Despite these promising findings, results from many candidate gene studies are not replicated in other samples. The number of genes found to be consistently associated with obesity-related phenotypes is much smaller than the set of candidate genes investigated in the literature (Rankinen et al., 2006) and effect sizes in many of these studies are small. Thus, it is likely that determining the putative genetic factors of obesity is complex and may involve gene × gene and gene × environment interactions.
The interaction of specific genetic alleles with depressive symptoms could be important to understanding gene × environment interactions, since depressive symptoms have been linked with obesity and disregulation in eating (e.g., both hyperphasia or appetite loss) (Faith, Matz, & Jorge, 2002; Stunkard, Harris, Pedersen, & McClearn, 1990). Depression is one of the more common psychiatric disorders among adolescents and young adults (Birmaher et al., 1996). A recent report from the National Health and Nutrition Examination Survey indicated that nearly 8% of adolescents ages 15-19 experience a major depressive disorder (MDD). This rate was higher (10%) among adults ages 20-24 (Riolo, Nguyen, Greden, & King, 2005). Current models propose that depression and obesity share common pathophysiological elements of the serotoninergic and dopaminergic neurotransmitter systems (Hainer, Kabrnova, Aldhoon, Kunesova, & Wagenknecht, 2006; Kalia, 2005; Lopez-Leon et al., 2007; Lopez Leon et al., 2005). Obesity and depression often co-occur, with some studies suggesting that obesity can influence the development of depression (Anderson, Cohen, Naumova, Jacques, & Must, 2007; Kasen, Cohen, Chen, & Must, 2007; Scott et al., 2007), whereas others find early depressive symptoms predict obesity later in life (Anderson, Cohen, Naumova, & Must, 2006; Goodman & Whitaker, 2002; Hasler et al., 2005; Pine, Goldstein, Wolk, & Weissman, 2001; Richardson et al., 2003). In particular, one prospective cohort study of adolescents found that depressed mood at baseline predicted an increase in BMI among those adolescents that were not obese at baseline (Goodman & Whitaker, 2002).
Notably, gender appears to be important in the relationship between depression and obesity, as several studies have found either a positive association in women but not men (Anderson et al., 2007; Foster, Wadden, Kendall, Stunkard, & Vogt, 1996; Pine et al., 2001; Richardson et al., 2003) or an inverse relationship between depression and obesity among men (Anderson et al., 2006; Carpenter, Hasin, Allison, & Faith, 2000; Scott et al., 2007). A large cohort study of more than 40,000 respondents found that women with higher BMI were at increased risk for both MDD and suicide ideation, but among men, lower BMI was associated with decreased risk for MDD, fewer suicide attempts, and less suicidal ideation (Carpenter et al., 2000). Both obesity and depression are risk factors for similar chronic diseases (i.e. coronary heart disease) (Herva et al., 2006); thus, it is an important public health endeavor to understand this depression-obesity link.
The purpose of this paper is to examine whether depressive symptoms moderate the relationship between candidate genes (SLC6A4 and MAOA) and obesity. In this model, depressive symptoms exert influence on the genotype-obesity association through both unique genetic and environmental pathways. A previous study by our research team has found main effects between specific candidate genes and obesity risk (Fuemmeler et al., 2008). Given our previous finding amid similar studies, other studies suggesting an association between obesity and depression, and the hypothesized overlap in the neurobiological mechanisms underlying these conditions, we hypothesized that candidate genes associated with the regulation of serotonin and dopamine would interact with depressive symptoms to predict obesity and that gender would be important to these relationships.
Methods
Data source
The study population was 20,745 adolescents from the National Longitudinal Study of Adolescent Health (Add Health), a nationally representative study of adolescents. The longitudinal cohort includes 15,197 eligible respondents who completed in-home surveys on three separate occasions (April to December, 1995; April to August, 1996; and August 2001 to August 2002). The mean age of survey participants in the three waves of data collection was 15.65 (SD = 1.75) years, 16.22 (SD = 1.64) years, and 22.96 (SD = 1.77) years, respectively. All survey participants at Wave III were 18 years of age or older. By design, the Add Health survey included a sample stratified by region, urbanicity, school type, ethnic mix, and size to garner a nationally representative sample. Precise details regarding the design and data collection have been described elsewhere (Harris et al., ; Resnick et al., 1997).
Study Sample
At Wave III, a subset of individuals identified to be full siblings or twins at earlier waves (n = 3,787) consented to provide a saliva sample for DNA analysis. The study conformed to local institutional review board (IRB) approved procedures (further details can be obtained at www.cpc.unc.edu/projects/addhealth). For our analyses, we included only unrelated individuals by randomly selecting one sibling from each sibship. Participants who were pregnant were excluded from analysis (n=51). For the analyses comparing normal weight individuals (BMI, 18.5 - 25) to obese individuals (BMI ≥ 30) the total available sample included 1,133 individuals. For the analyses comparing normal weight individuals to overweight and obese individuals (BMI ≥ 25) the total available sample included 1,584 individuals. Genotype was missing for one or more of the genetic markers for some individuals which resulted in variability in the total number of individuals available for each gene-specific analysis.
Genotyping
Buccal samples were collected on the participants and DNA extracted using a modification of procedures previously described (Freeman et al., 1997; Lench, Stanier, & Williamson, 1988; Meulenbelt, Droog, Trommelen, Boomsma, & Slagboom, 1995; Spitz et al., 1998) (further details at www.cpc.unc.edu/projects/addhealth). Six functional polymorphisms were genotyped within six candidate genes that had been previously associated with behavioral and psychological outcomes. However, for the purposes of this study we only focused on two that had previously been associated with obesity in this sample (Fuemmeler et al., 2008): a 44 bp insertion/deletion polymorphism (5HTTLPR) in the promoter of the serotonin transporter (SLC6A4) and a 30 bp VNTR in the promoter of the monoamine oxidase A (MAOA) gene. Details regarding the genotyping procedure are reported elsewhere (Anchordoquy, McGeary, Liu, Krauter, & Smolen, 2003; Timberlake et al., 2006). The genotypes were tested for deviations from Hardy Weinberg Equilibrium (HWE) in the normal weight BMI strata and no deviations were observed (p-values > .05).
Body Mass Index
BMI was calculated based on height and weight (BMI = weight in kilograms/height in meters2) measured by Add Health staff during the in-home interviews at Wave II and Wave III. Height and weight were self-reported at Wave I and thus, analyses of BMI were restricted to Wave II and III.
Depressive Symptoms
The Add Health study included a modified 10-item version of the Center for Epidemiologic Studies – Depression (CES-D). For Add Health, the response scale and tense (i.e., from the first to second person) of some CES-D items were modified, but have been shown to not meaningfully affect the internal structure of the measure (Crockett, Randall, Shen, Russell, & Driscoll, 2005). Respondents are asked to indicate how often they experienced each depressive symptom in the past 7 days. An example of one of the items is “you felt depressed”. Responses range from 0 (never or rarely) to 3 (most of time or all of the time) with a total scores ranging from 0 to 30. This version of the CES-D has demonstrated good internal consistency across waves (Cronbach's α=.87 at Wave III). For our analyses, individuals were classified into one of two groups, those reporting CES-D scores of 10 or greater and those reporting between 0 – 9 symptoms. We used a ≥ 10 cutoff to ensure that participants had at least mild to moderate depressive symptoms. This 10 symptom cutoff was chosen because previous literature has suggested that scores of greater than 10 on a 10-item CES-D represent levels similar to those ≥ 16 on the full-length 20 item version (Andresen, Malmgren, Carter, & Patrick, 1994), which is indicative of mild to moderate depression (Radloff, 1977).
Sociodemographic variables
Covariates included indicators of socioeconomic status (e.g., parental reported education at Wave I), chronological age of participant at Wave III, and self-identified race/ethnicity. American Indians and Asians were excluded from the analyses because they were underrepresented in the available data. Thus, our analyses only included American whites, African-Americans, and Hispanics.
Statistical Analysis
Statistical analyses were conducted using SAS-callable SUDAAN (version 8.0) statistical software (SUDAAN User's Manual, Release 8.0, 2001). SUDAAN allows for control of survey design effects of individuals clustered in sampling unit of school and stratification of geographic region. The specific genotypes were grouped for analysis according to the extant literature with these candidate genes (Munafo, Clark, Johnstone, Murphy, & Walton, 2004; Todd et al., 2005). MAOA alleles were classified into two groups, the low-activity alleles indicated by three copies of the 30-bp repeat sequence and high activity alleles, namely 3.5, 4, or 5 repeats. Participants were classified by genotype as homozygous for low-activity (3/3) or high-activity indicated by carriers of a high-activity allele (homozygous or heterozygous) (Sabol, Hu, & Hamer, 1998). The dialelic model for SLC6A4 was used for classifying participants into s/s, s/l or l/l (Hu et al., 2005). Two separate sets of multiple logistic regressions were conducted. In the first, regression was used to identify the variables which predicted obesity (BMI > 29.9) using normal weight (BMI = 18.5 – 24.9) as a referent in order to identify genetic factors associated with the highest level of risk. In the second, regression was used to predict overweight + obese combined (BMI ≥ 25). This approach allowed for the identification of any risk factors associated with above normal weight. Participants who were underweight (< 18.5; n = 47) were excluded. Each of the two polymorphisms (MAOA or SLC6A4) was evaluated separately. Because we found in a previous analysis that the main effect for the relationship between SLC6A4 and obesity was present among men but not women, we stratified our analysis on gender (Fuemmeler et al., 2008). Because the MAOA gene is located on the X-chromosome we stratified analyses for that genotype on gender. Models included the categorical main effect variable for depressive symptoms (CES-D), the specific allele (MAOA or SLC6A4), age, race, parental education level, and finally the interaction between the allele and categorical variable for depressive symptoms. For significant interaction effects, p-values are presented. To clarify the interpretation of significant interaction effects, stratified analyses were conducted and odds ratios were calculated when samples sizes permitted.
Results
Table I displays the overall socio-demographics by BMI category (normal weight vs. obese vs. overweight + obese). Bivariate chi square analyses revealed significant differences among BMI category for race/ethnicity, parental education and age (p < .05), and a trend for gender (normal vs. overweight + obese, p = .07). About 15.5 percent of the normal weight individuals in this sample had CES-D scores greater than or equal to 10 which is slightly higher than what others have reported using this cut-off among older adults (Andresen et al., 1994); however, this percentage of high levels of depressive symptoms is consistent with rates observed among older adolescents and young adults (Colangelo et al., 2007). In the sample as a whole, a greater proportion of women had CES-D scores greater than or equal to 10 compared to men (10.9% vs. 5.7%; X2 = 27.14, p < .0001). Depressive symptoms were not associated with BMI status at the bivariate level.
Table I.
Gender, Ethnicity, Parental Education, and Age by Normal Weight, Obese, and Overweight + Obesea.
| Normal Weight |
Obese |
Overweight + Obese |
||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Total | 751 | 382 | 833 | |||
| Gender | ||||||
| Male | 350 | 46.61 | 167 | 43.72 | 424 | 50.90 |
| Female | 401 | 53.39 | 215 | 56.28 | 409 | 49.10 |
| Ethnicity | ||||||
| White | 523 | 69.64 | 232 | 60.73b | 524 | 62.90c |
| Black | 139 | 18.51 | 67 | 17.53 | 145 | 17.41 |
| Hispanic | 89 | 11.85 | 83 | 21.72 | 164 | 19.69 |
| Parental Education Level | ||||||
| Less Than High School | 59 | 8.86 | 57 | 14.92b | 102 | 13.73c |
| High School or Equivalent | 146 | 21.92 | 117 | 30.63 | 232 | 31.22 |
| Some College | 202 | 30.33 | 87 | 22.77 | 219 | 29.48 |
| College or Higher | 259 | 38.89 | 76 | 19.90 | 190 | 25.57 |
| CES-D | ||||||
| 0 – 9 | 634 | 84.42 | 314 | 82.20 | 688 | 82.99 |
| ≥ 10 | 117 | 15.58 | 68 | 17.80 | 141 | 17.01 |
|
Mean
|
SD
|
Mean
|
SD
|
Mean
|
SD
|
|
| Age | 21.18 | 0.14 | 22.18d | 0.13 | 22.05e | 0.13 |
Normal Weight = BMI 18.5 - 24.9; Obese = BMI >29.9; Overweight or Obese = BMI >25
Significant X2 comparison between obese vs. normal weight (p < .05)
Significant X2 comparison between overweight + obese vs. normal weight (p < .05)
Significant t-tests between obese vs. normal weight (p < .05)
Significant t-tests between overweight + obese vs. normal weight (p < .05)
Table II describes genotype frequencies overall and by BMI categories included in the analyses. Genotype distributions in the normal weight BMI strata did not deviate from HWE (all p-values > .05). Signficant bi-variate associations were observed between SLC6A4 and obesity and overweight+obesity for both genders. A trend toward significance was observed between MAOA and obesity for both genders.
Table II.
Overall Genotype Frequencies and Frequencies by Normal Weight, Obese, and Overweight + Obesea.
| Total | Normal Weight | Obese | Overweight + Obese | |||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| SLC6A4 (Males) | ||||||||
| s/s | 141 | 18.34 | 48 | 13.79 | 40 | 23.95 | 93 | 22.09 |
| s/l | 360 | 46.81 | 166 | 47.70 | 76 | 45.51 | 194 | 46.08 |
| l/l | 268 | 34.85 | 134 | 38.51 | 51 | 30.54b1 | 134 | 31.83c1 |
| SLC6A4 (Females) | ||||||||
| s/s | 132 | 16.40 | 63 | 15.75 | 39 | 18.31 | 69 | 17.04 |
| s/l | 373 | 46.34 | 202 | 50.50 | 88 | 41.31 | 171 | 42.22 |
| l/l | 300 | 37.27 | 135 | 33.75 | 86 | 40.38b2 | 165 | 40.74c2 |
| MAOA (Males) | ||||||||
| Low active | 314 | 40.94 | 133 | 38.33 | 76 | 45.78 | 181 | 43.10 |
| Active | 453 | 59.06 | 214 | 61.67 | 90 | 54.22b2 | 239 | 56.90 |
| MAOA (Females) | ||||||||
| Low active | 474 | 59.10 | 231 | 58.33 | 138 | 64.49 | 243 | 59.85 |
| Active | 328 | 40.90 | 165 | 41.67 | 76 | 35.51b2 | 163 | 40.15 |
Normal Weight = BMI 18.5 - 24.9; Obese = BMI >29.9; Overweight or Obese = BMI >25
b Significant X2 comparison between obese vs. normal weight (b1 p < .05; b2 p ≤ .1 )
c Significant X2 comparison between overweight + obese vs. normal weight (c1 p < .05; c2 p ≤ .1)
The main effects from the logistic regression analyses with age, race, parental education level, genotype (either SLC6A4 or MAOA) and CES-D can be seen in Table III. Controlling for depressive symptoms and other covariates a significant main effect of MAOA and SLC6A4 on obesity and overweight+obesity was found among men. In general, the relationship between depressive symptoms and greater weight categories tended to show an inverse relationship among men but was positive associated among women; however, these associations were not statistically significant in many of the models.
Table III.
Adjusted Odd Ratioa, Confidence Intervals, and p-values for main effect models
| Normal vs. Obese | Normal vs. Overweight/Obese | |||||
|---|---|---|---|---|---|---|
| OR | CI | p | OR | CI | p | |
| Males | ||||||
| CES-D | ||||||
| < 9 | ||||||
| >= 10 | 0.59 | 0.28 - 1.24 | 0.16 | 0.60 | 0.36 - 1.02 | 0.06 |
| SLC6A4 | ||||||
| L/L | ||||||
| S/L | 0.87 | 0.51 - 1.47 | 0.99 | 0.70 - 1.38 | ||
| S/S | 1.94 | 1.01 - 3.71 | 0.03 | 1.75 | 1.07 - 2.85 | 0.03 |
| Females | ||||||
| CES-D | ||||||
| < 9 | ||||||
| >= 10 | 1.49 | 0.81 - 2.78 | 0.20 | 1.47 | 0.93 - 2.32 | 0.09 |
| SLC6A4 | ||||||
| L/L | ||||||
| S/L | 0.70 | 0.42 - 1.16 | 0.66 | 0.46 - 0.95 | ||
| S/S | 0.96 | 0.56 - 1.64 | 0.12 | 0.87 | 0.54 - 1.38 | 0.06 |
| Males | ||||||
| CES-D | ||||||
| < 9 | ||||||
| >= 10 | 0.59 | 0.30 - 1.17 | 0.13 | 0.58 | 0.34 -0.98 | 0.04 |
| MAOA | ||||||
| High Activity | ||||||
| Low Activity | 1.92 | 1.20 - 3.06 | 0.01 | 1.47 | 1.06 - 2.04 | 0.02 |
| Females | ||||||
| CES-D | ||||||
| < 9 | ||||||
| >= 10 | 1.50 | 0.82 - 2.75 | 0.19 | 0.69 | 0.44 - 1.08 | 0.10 |
| MAOA | ||||||
| High Activity | ||||||
| Low Activity | 0.99 | 0.69 - 1.42 | 0.95 | 0.93 | 0.68 - 1.27 | 0.64 |
models adjusted for race, parental education level and age
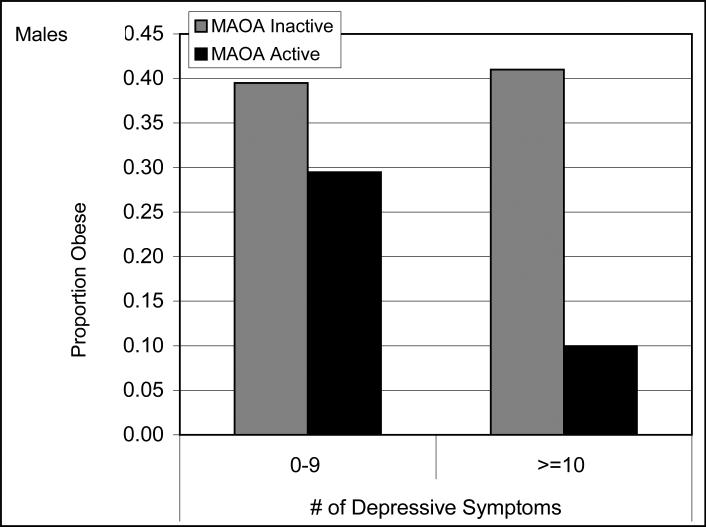
The p-values for the models that included the interaction term and the stratified analyses can be viewed in Tables IV and V. The interaction models yielded a significant interaction between MAOA and depression on both risk of obesity and overweight+obesity among males (p < .05), but not females. In males with the active form of the MAOA gene, higher depressive symptoms were associated with a decreased risk of obesity (OR, 0.22; 95% CI, 0.06 – 0.78) and overweight + obesity (OR, 0.48; 95% CI, 0.26 – 0.89). The predicted marginals for those with ≥ 10 and active MAOA allele was 10.0 % versus 29.5 % among those with a 0-9 CES-D score, p = .002 (Figure 1). Men with the low activity MAOA allele showed a slightly higher risk of obesity relative to those with the active allele in which a decreased risk was present (low active MAOA OR, 1.32; 95% CI, 0.51 – 3.37 vs. Active MAOA OR, .22 (0.06 – 0.78). Nevertheless, among those with the low active allele, depressive symptoms did not appear to play a role (predicted marginals for those ≥ 10 and low active allele = 41.0 % versus those with 0-9 and low active allele = 39.5%, p = .87).
Table IV.
Risk of obesity or overweight + obesity among males and females associated with depressive symptoms and polymorphic markers of the SLC6A4 gene.
| CES-D Levels | SLC6A4 genotype | Interaction p value | ||||||
|---|---|---|---|---|---|---|---|---|
| s/s |
s/l |
l/l |
||||||
| n | ORa (95% CI) | n | ORa (95% CI) | n | ORa (95% CI) | |||
| Normal vs Obese | ||||||||
| Males | ||||||||
| Depressive Symptoms | Low | 69 | 187 | 144 | ||||
| High | 15 | 0.48 (0.13 - 1.82) | 23 | .07 (0.28 - 1.78) | 22 | 0.48 (0.12 - 1.87) | 0.72 | |
| Females | ||||||||
| Depressive Symptoms | Low | 72 | 206 | 152 | ||||
| High | 18 | 1.63 (0.48 - 5.56) | 50 | 1.07 (0.43 - 2.65) | 40 | 2.30 (0.88 - 6.00) | 0.39 | |
| Normal vs Overweight + Obese | ||||||||
| Males | ||||||||
| Depressive Symptoms | Low | 113 | 284 | 211 | ||||
| High | 20 | 0.42 (0.14 - 1.26) | 32 | 0.66 (0.33 - 1.29) | 31 | 0.77 (0.33 - 1.80) | 0.77 | |
| Females | ||||||||
| Depressive Symptoms | Low | 92 | 260 | 210 | ||||
| High | 24 | 1.77 (0.68 - 4.57) | 70 | 1.34 (0.68 - 2.63) | 55 | 1.79 (0.77 - 4.20) | 0.74 | |
OR adjusted for age, race, and parental education level
Table V.
Risk of obesity or overweight + obesity among males and females associated with depressive symptoms and polymorphic markers of the MAOA gene.
| CES-D Levels | MAOA genotype | Interaction p value | ||||
|---|---|---|---|---|---|---|
| Low activity |
High Activity |
|||||
| n | ORa (95% CI) | n | ORa (95% CI) | |||
| Normal vs Obese | ||||||
| Males | ||||||
| Depressive Symptoms | Low | 148 | 250 | |||
| High | 31 | 1.32 (0.51 - 3.37) | 29 | 0.22 (0.06 - 0.78) | 0.04 | |
| Females | ||||||
| Depressive Symptoms | Low | 294 | 197 | |||
| High | 75 | 1.30 (0.61 - 2.75) | 44 | 1.87 (0.76 - 4.57) | 0.57 | |
| Normal vs Overweight + Obese | ||||||
| Males | ||||||
| Depressive Symptoms | Low | 232 | 375 | |||
| High | 40 | 0.77 (0.37 - 1.59) | 42 | 0.48 (0.26 - 0.89) | 0.31 | |
| Females | ||||||
| Depressive Symptoms | Low | 372 | 262 | |||
| High | 101 | 1.21 (0.72 - 2.06) | 62 | 1.85 (0.92 - 3.68) | 0.34 | |
OR adjusted for age, race, and parental education level
Figure 1.
Interaction of MAOA with Depressive symptoms on obesity.
Discussion
The present study assessed relationships among reported depressive symptoms, genotype, and risk of obesity in a U.S. sample of young adults. A genotype × depressive symptoms interaction was observed for the polymorphism in the MAOA gene among males. When this interaction was stratified by allele and depressive symptoms the result revealed a significant protective effect for those reporting elevated depressive symptoms and those who carry the active MAOA allele. The current study extends our previous findings of a significant main effect between MAOA among males and obesity (Fuemmeler et al., 2008) by suggesting that the obesity risk among males is related to an interaction between genetic differences and depressive symptoms.
The active form of the MAOA promoter VNTR, in combination with ≥ 10 CES-D score was associated with a very low risk of obesity (predicted marginals = 10%) which was much lower than those without this form of the gene who also had depressive symptoms. This adjusted proportion of obesity is also much lower than the proportion obese seen among young adults observed in other studies (Hedley et al., 2004). This finding of a decreased obesity risk in the presence of the active form of MAOA and depressive symptoms is of interest, since previous studies have found that men in general who are depressed show either no increased risk of obesity (Istvan, Zavela, & Weidner, 1992; Moore, Stunkard, & Srole, 1962) or show decreased risk relative to women (Carpenter et al., 2000). The present results suggest that the previously observed decreased risk of obesity among depressed men may be due to a decreased risk in a subsample of men who carry the active MAOA allele. The link between obesity-depression and the role of genetic influence deserves further study.
The MAOA polymorphism that we examined is believed to regulate activity of brain MAO-A enzymes which in turn modulates levels of brain amines including dopamine, serotonin and norepinephrine (Buckholtz & Meyer-Lindenberg, 2008). A dysregulation in brain monoamine levels may potentially influence energy balance, as these amines are associated with feeding behaviors and enjoyment of food. Dopamine neurotransmission, for instance, is associated with a range of brain functions including reward, sensorimotor activation, associative learning and emotion, each with putative effects on food reward and feeding behavior (Palmiter, 2007). Further, it has been hypothesized that eating may restore low levels of dopamine signaling (Bassareo & Di Chiara, 1999). If so, a proposed mechanism for the observed finding is that those with the active variant of MAOA—and thus lower tonic levels of dopamine—might be at heightened risk of obesity since eating restores brain dopamine levels. However, in the context of depression and male gender, the relation between dopamine and feeding and ultimately obesity might be attenuated. High brain MAOA levels and dopamine signaling are thought to be central to depression. Decreased dopamine availability is thought to be associated with symptoms of anhedonia (among others) seen in clinical depression (Dunlop & Nemeroff, 2007). Although speculative, the current findings might suggest biologically low tonic levels of dopamine contribute to higher depressive symptoms and lower reward sensitivity whereby little pleasure is derived from eating and feeding resulting in decreased rates of obesity among males in this sample. Individuals without depression and those with higher dopamine availability (low brain MAOA levels) could be more inclined to have obesity rates equal to or higher than the general population. Why this potential mechanism might be at play among males but not females is a question for future studies aimed at elucidating the processes underlying genetic variation and feeding behavior.
It should be noted that there remains debate about the relationship between how enzymatic activity of MAO-A relates to particular MAOA genotypes, as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) studies have found a lack of correspondence between low vs. high activity MAOA genotype and brain MAO-A levels among healthy adult males (Alia-Klein, Kriplani et al., 2008; Fowler et al., 2007). This lack of association between polymorphism in the MAOA gene and brain MAO-A in this cross-sectional study gives rise to the hypothesis that the gene-enzyme relationship could be developmentally mediated (Alia-Klein, Goldstein et al., 2008; Fowler et al., 2007).
Our results indicated some interesting potential gender effects. Different effects of gender were found with regard to the interaction between MAOA genotype and depressive symptoms in predicting obesity. Gender differences are notable in the epidemiology of depression. Women have higher rates especially in the reproductive years and symptom presentation tends to differ, with men more likely to present with non-atypical depressive symptoms (NAD) (e.g., hypophagia, insomnia, psychomotor agitation) and women with atypical symptoms (i.e., hyperphagia, hypersomnia, psychomotor retardation) (Grigoriadis & Robinson, 2007). As noted above, the association between depression and obesity risk also appears to be distinguished by gender. The finding of this study could help explain why previous researchers have mixed results regarding a reduced or null risk of obesity among men compared to women with depressive symptoms. Specifically, the results suggest that a genetic variant of MAOA – an X-linked gene – may be exerting influence in this association. However, replication studies in other samples are needed before definitive conclusions can be made.
While we are enthusiastic about the findings presented here, caution is warranted. Gene × environment interactions are difficult to detect with diminishing sample sizes (Dempfle et al., 2008). While the sample size in this study was fairly robust and many of the cell counts for these models were of sufficient size, we feel replication is warranted before definitive conclusions can be made about the role that depressive symptoms and these genes have on regulating weight and risk of obesity. Further, initial reports from candidate gene studies, in general, may overestimate the effect (Lohmueller, Pearce, Pike, Lander, & Hirschhorn, 2003) and modest yet significant effects are reported here. The hypothesis driven analyses reduce, in part, the Type I error, but continued research is needed. Another limitation of the current study was that other indicators of adiposity or body composition (e.g., waist circumference, skin fold measures) were not present in the Add Health study. In general, BMI is a good proxy, but examining the association between these candidate genes and other indicators of adiposity and body composition would strengthen the findings.
The results underscore the need for additional research examining the role that depression and these neurotransmitter systems have on BMI and other energy-balance behaviors (e.g., diet and physical activity). Furthermore, there may exist other potential complex gene × gene and gene × environment interactions that may further characterize the risk of obesity. Understanding the potential interaction between biological, psychological, and social risk factors is central to generating informed hypotheses about the causes and ultimately prevention of obesity.
Acknowledgments
Portions of this work were supported by grant number NIDA K23DA017261 (FJM), NINDS NS049067 (MG, SHK and AAK), NICHD HD31921 (MG and AAK), and NCI 1K07CA124905 (BFF).
This research uses data from Add Health, a program project designed by J. Richard Udry, Peter S. Bearman, and Kathleen Mullan Harris, and funded by a grant P01-HD31921 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 17 other agencies. Special acknowledgment is due Ronald R. Rindfuss and Barbara Entwisle for assistance in the original design. Persons interested in obtaining data files from Add Health should contact Add Health, Carolina Population Center, 123 W. Franklin Street, Chapel Hill, NC 27516-2524 (addhealth@unc.edu). No direct support was received from grant P01-HD31921 for this analysis.
References
- Alia-Klein N, Goldstein RZ, Kriplani A, Logan J, Tomasi D, Williams B, et al. Brain monoamine oxidase A activity predicts trait aggression. J Neurosci. 2008;28(19):5099–5104. doi: 10.1523/JNEUROSCI.0925-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alia-Klein N, Kriplani A, Pradhan K, Ma JY, Logan J, Williams B, et al. The MAO-A genotype does not modulate resting brain metabolism in adults. Psychiatry Research. 2008 doi: 10.1016/j.pscychresns.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anchordoquy HC, McGeary C, Liu L, Krauter KS, Smolen A. Genotyping of three candidate genes after whole-genome preamplification of DNA collected from buccal cells. Behav Genet. 2003;33(1):73–78. doi: 10.1023/a:1021007701808. [DOI] [PubMed] [Google Scholar]
- Anderson SE, Cohen P, Naumova EN, Jacques PF, Must A. Adolescent obesity and risk for subsequent major depressive disorder and anxiety disorder: prospective evidence. Psychosom Med. 2007;69(8):740–747. doi: 10.1097/PSY.0b013e31815580b4. [DOI] [PubMed] [Google Scholar]
- Anderson SE, Cohen P, Naumova EN, Must A. Association of depression and anxiety disorders with weight change in a prospective community-based study of children followed up into adulthood. Arch Pediatr Adolesc Med. 2006;160(3):285–291. doi: 10.1001/archpedi.160.3.285. [DOI] [PubMed] [Google Scholar]
- Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med. 1994;10(2):77–84. [PubMed] [Google Scholar]
- Barsh GS, Schwartz MW. Genetic approaches to studying energy balance: perception and integration. Nat Rev Genet. 2002;3(8):589–600. doi: 10.1038/nrg862. [DOI] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G. Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neuroscience. 1999;89(3):637–641. doi: 10.1016/s0306-4522(98)00583-1. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Ryan ND, Williamson DE, Brent DA, Kaufman J, Dahl RE, et al. Childhood and adolescent depression: a review of the past 10 years. Part I. J Am Acad Child Adolesc Psychiatry. 1996;35(11):1427–1439. doi: 10.1097/00004583-199611000-00011. [DOI] [PubMed] [Google Scholar]
- Bray GA. Obesity: the disease. J Med Chem. 2006;49(14):4001–4007. doi: 10.1021/jm0680124. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Meyer-Lindenberg A. MAOA and the neurogenetic architecture of human aggression. Trends Neurosci. 2008;31(3):120–129. doi: 10.1016/j.tins.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Camarena B, Santiago H, Aguilar A, Ruvinskis E, Gonzalez-Barranco J, Nicolini H. Family-based association study between the monoamine oxidase A gene and obesity: implications for psychopharmacogenetic studies. Neuropsychobiology. 2004;49(3):126–129. doi: 10.1159/000076720. [DOI] [PubMed] [Google Scholar]
- Carpenter KM, Hasin DS, Allison DB, Faith MS. Relationships between obesity and DSM-IV major depressive disorder, suicide ideation, and suicide attempts: results from a general population study. Am J Public Health. 2000;90(2):251–257. doi: 10.2105/ajph.90.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colangelo LA, Sharp L, Kopp P, Scholtens D, Chiu BC, Liu K, et al. Total testosterone, androgen receptor polymorphism, and depressive symptoms in young black and white men: the CARDIA Male Hormone Study. Psychoneuroendocrinology. 2007;32(8-10):951–958. doi: 10.1016/j.psyneuen.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockett LJ, Randall BA, Shen YL, Russell ST, Driscoll AK. Measurement equivalence of the center for epidemiological studies depression scale for Latino and Anglo adolescents: a national study. J Consult Clin Psychol. 2005;73(1):47–58. doi: 10.1037/0022-006X.73.1.47. [DOI] [PubMed] [Google Scholar]
- Dempfle A, Scherag A, Hein R, Beckmann L, Chang-Claude J, Schafer H. Gene-environment interactions for complex traits: definitions, methodological requirements and challenges. Eur J Hum Genet. 2008;16(10):1164–1172. doi: 10.1038/ejhg.2008.106. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64(3):327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- Faith MS, Matz PE, Jorge MA. Obesity-depression associations in the population. J Psychosom Res. 2002;53(4):935–942. doi: 10.1016/s0022-3999(02)00308-2. [DOI] [PubMed] [Google Scholar]
- Foster GD, Wadden TA, Kendall PC, Stunkard AJ, Vogt RA. Psychological effects of weight loss and regain: a prospective evaluation. J Consult Clin Psychol. 1996;64(4):752–757. doi: 10.1037//0022-006x.64.4.752. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Alia-Klein N, Kriplani A, Logan J, Williams B, Zhu W, et al. Evidence that brain MAO A activity does not correspond to MAO A genotype in healthy male subjects. Biol Psychiatry. 2007;62(4):355–358. doi: 10.1016/j.biopsych.2006.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman B, Powell J, Ball D, Hill L, Craig I, Plomin R. DNA by mail: an inexpensive and noninvasive method for collecting DNA samples from widely dispersed populations. Behav Genet. 1997;27(3):251–257. doi: 10.1023/a:1025614231190. [DOI] [PubMed] [Google Scholar]
- Fuemmeler BF, Agurs-Collins TD, McClernon FJ, Kollins SH, Kail ME, Bergen AW, et al. Genes Implicated in Serotonergic and Dopaminergic Functioning Predict BMI Categories. Obesity (Silver Spring) 2008;16(2):348–355. doi: 10.1038/oby.2007.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman E, Whitaker RC. A prospective study of the role of depression in the development and persistence of adolescent obesity. Pediatrics. 2002;110(3):497–504. doi: 10.1542/peds.110.3.497. [DOI] [PubMed] [Google Scholar]
- Grigoriadis S, Robinson GE. Gender issues in depression. Annals of Clinical Psychiatry. 2007;19(4):247–255. doi: 10.1080/10401230701653294. [DOI] [PubMed] [Google Scholar]
- Hainer V, Kabrnova K, Aldhoon B, Kunesova M, Wagenknecht M. Serotonin and norepinephrine reuptake inhibition and eating behavior. Ann N Y Acad Sci. 2006;1083:252–269. doi: 10.1196/annals.1367.017. [DOI] [PubMed] [Google Scholar]
- Harris KM, Florey F, Tabor J, Bearman PS, Jones J, Udry JR. [February 25, 2008];The National Longitudinal Study of Adolescent Helaht: Research Design. from http://www.cpc.unc.edu/projects/addhealth/design.
- Hasler G, Lissek S, Ajdacic V, Milos G, Gamma A, Eich D, et al. Major depression predicts an increase in long-term body weight variability in young adults. Obes Res. 2005;13(11):1991–1998. doi: 10.1038/oby.2005.244. [DOI] [PubMed] [Google Scholar]
- Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. Jama. 2004;291(23):2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- Herva A, Laitinen J, Miettunen J, Veijola J, Karvonen JT, Laksy K, et al. Obesity and depression: results from the longitudinal Northern Finland 1966 Birth Cohort Study. Int J Obes (Lond) 2006;30(3):520–527. doi: 10.1038/sj.ijo.0803174. [DOI] [PubMed] [Google Scholar]
- Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcohol Clin Exp Res. 2005;29(1):8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- Istvan J, Zavela K, Weidner G. Body weight and psychological distress in NHANES I. Int J Obes Relat Metab Disord. 1992;16(12):999–1003. [PubMed] [Google Scholar]
- Kalia M. Neurobiological basis of depression: an update. Metabolism. 2005;54(5 Suppl 1):24–27. doi: 10.1016/j.metabol.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Kasen S, Cohen P, Chen H, Must A. Obesity and psychopathology in women: a three decade prospective study. Int J Obes (Lond) 2007 doi: 10.1038/sj.ijo.0803736. [DOI] [PubMed] [Google Scholar]
- Lench N, Stanier P, Williamson R. Simple non-invasive method to obtain DNA for gene analysis. Lancet. 1988;1(8599):1356–1358. doi: 10.1016/s0140-6736(88)92178-2. [DOI] [PubMed] [Google Scholar]
- Lohmueller KE, Pearce CL, Pike M, Lander ES, Hirschhorn JN. Meta-analysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nat Genet. 2003;33(2):177–182. doi: 10.1038/ng1071. [DOI] [PubMed] [Google Scholar]
- Lopez-Leon S, Janssens AC, Gonzalez-Zuloeta Ladd AM, Del-Favero J, Claes SJ, Oostra BA, et al. Meta-analyses of genetic studies on major depressive disorder. Mol Psychiatry. 2007 doi: 10.1038/sj.mp.4002088. [DOI] [PubMed] [Google Scholar]
- Lopez Leon S, Croes EA, Sayed-Tabatabaei FA, Claes S, Van Broeckhoven C, van Duijn CM. The dopamine D4 receptor gene 48-base-pair-repeat polymorphism and mood disorders: a meta-analysis. Biol Psychiatry. 2005;57(9):999–1003. doi: 10.1016/j.biopsych.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Meulenbelt I, Droog S, Trommelen GJ, Boomsma DI, Slagboom PE. High-yield noninvasive human genomic DNA isolation method for genetic studies in geographically dispersed families and populations. Am J Hum Genet. 1995;57(5):1252–1254. [PMC free article] [PubMed] [Google Scholar]
- Moore ME, Stunkard A, Srole L. Obesity, social class, and mental illness. Jama. 1962;181:962–966. doi: 10.1001/jama.1962.03050370030007. [DOI] [PubMed] [Google Scholar]
- Munafo M, Clark T, Johnstone E, Murphy M, Walton R. The genetic basis for smoking behavior: a systematic review and meta-analysis. Nicotine Tob Res. 2004;6(4):583–597. doi: 10.1080/14622200410001734030. [DOI] [PubMed] [Google Scholar]
- Need AC, Ahmadi KR, Spector TD, Goldstein DB. Obesity is associated with genetic variants that alter dopamine availability. Ann Hum Genet. 2006;70(Pt 3):293–303. doi: 10.1111/j.1529-8817.2005.00228.x. [DOI] [PubMed] [Google Scholar]
- Palmiter RD. Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. 2007;30(8):375–381. doi: 10.1016/j.tins.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Pine DS, Goldstein RB, Wolk S, Weissman MM. The association between childhood depression and adulthood body mass index. Pediatrics. 2001;107(5):1049–1056. doi: 10.1542/peds.107.5.1049. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psycho Meas. 1977;1:385–401. [Google Scholar]
- Rankinen T, Zuberi A, Chagnon YC, Weisnagel SJ, Argyropoulos G, Walts B, et al. The human obesity gene map: the 2005 update. Obesity (Silver Spring) 2006;14(4):529–644. doi: 10.1038/oby.2006.71. [DOI] [PubMed] [Google Scholar]
- Resnick MD, Bearman PS, Blum RW, Bauman KE, Harris KM, Jones J, et al. Protecting adolescents from harm. Findings from the National Longitudinal Study on Adolescent Health. Jama. 1997;278(10):823–832. doi: 10.1001/jama.278.10.823. [DOI] [PubMed] [Google Scholar]
- Richardson LP, Davis R, Poulton R, McCauley E, Moffitt TE, Caspi A, et al. A longitudinal evaluation of adolescent depression and adult obesity. Arch Pediatr Adolesc Med. 2003;157(8):739–745. doi: 10.1001/archpedi.157.8.739. [DOI] [PubMed] [Google Scholar]
- Riolo SA, Nguyen TA, Greden JF, King CA. Prevalence of depression by race/ethnicity: findings from the National Health and Nutrition Examination Survey III. Am J Public Health. 2005;95(6):998–1000. doi: 10.2105/AJPH.2004.047225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabol SZ, Hu S, Hamer D. A functional polymorphism in the monoamine oxidase A gene promoter. Hum Genet. 1998;103(3):273–279. doi: 10.1007/s004390050816. [DOI] [PubMed] [Google Scholar]
- Scott KM, Bruffaerts R, Simon GE, Alonso J, Angermeyer M, de Girolamo G, et al. Obesity and mental disorders in the general population: results from the world mental health surveys. Int J Obes (Lond) 2007 doi: 10.1038/sj.ijo.0803701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sookoian S, Gemma C, Garcia SI, Gianotti TF, Dieuzeide G, Roussos A, et al. Short allele of serotonin transporter gene promoter is a risk factor for obesity in adolescents. Obesity (Silver Spring) 2007;15(2):271–276. doi: 10.1038/oby.2007.519. [DOI] [PubMed] [Google Scholar]
- Sookoian S, Gianotti TF, Gemma C, Burgueno A, Pirola CJ. Contribution of the functional 5-HTTLPR variant of the SLC6A4 gene to obesity risk in male adults. Obesity (Silver Spring) 2008;16(2):488–491. doi: 10.1038/oby.2007.64. [DOI] [PubMed] [Google Scholar]
- Spitz MR, Shi H, Yang F, Hudmon KS, Jiang H, Chamberlain RM, et al. Case-control study of the D2 dopamine receptor gene and smoking status in lung cancer patients. J Natl Cancer Inst. 1998;90(5):358–363. doi: 10.1093/jnci/90.5.358. [DOI] [PubMed] [Google Scholar]
- Stunkard AJ, Harris JR, Pedersen NL, McClearn GE. The body-mass index of twins who have been reared apart. N Engl J Med. 1990;322(21):1483–1487. doi: 10.1056/NEJM199005243222102. [DOI] [PubMed] [Google Scholar]
- Timberlake DS, Haberstick BC, Lessem JM, Smolen A, Ehringer M, Hewitt JK, et al. An association between the DAT1 polymorphism and smoking behavior in young adults from the National Longitudinal Study of Adolescent Health. Health Psychol. 2006;25(2):190–197. doi: 10.1037/0278-6133.25.2.190. [DOI] [PubMed] [Google Scholar]
- Todd RD, Huang H, Smalley SL, Nelson SF, Willcutt EG, Pennington BF, et al. Collaborative analysis of DRD4 and DAT genotypes in population-defined ADHD subtypes. J Child Psychol Psychiatry. 2005;46(10):1067–1073. doi: 10.1111/j.1469-7610.2005.01517.x. [DOI] [PubMed] [Google Scholar]