Abstract
Dendritic cells (DC) play a central role in the induction of immunity and also in tolerance in their role as professional antigen-presenting cells (APC). In the absence of DC, a fatal autoimmunity develops in animal models. While the role of DC has been investigated extensively in the pathogenesis of Rheumatoid arthritis (RA), it remains unclear if DC initiate autoimmunity in this disease. Nevertheless, evidence points towards a significant role for DC in its maintenance and progression. Current biological therapies of RA are designed to ameliorate disease by targeting downstream products of APC such as TNFα, IL-1 and IL-6. Emerging therapies for RA are exploiting the tolerogenic capacity of DC. “Tolerogenic” DC can be generated from myeloid precursors ex vivo, loaded with antigen and manipulated to suppress autoimmune responses in vivo, through the induction of activation induced cell death (AICD), anergy, and/or regulatory T cells. Cells that are primed by DC such as B cells, T helper (Th)-1(Th1) and Th17 cells, and which have been implicated in certain models of autoimmunity, are also being considered as additional targets for immune based therapy. Studies to validate these approaches to ameliorate autoimmunity will be necessary before they are applied in the clinic.
Background
Rheumatoid arthritis (RA) is an autoimmune disease marked by infiltration of synovia and synovial compartments with dendritic cells (DC), monocytes, T cells, B cells, neutrophils and NK cells1. In this perspective we discuss the role of DC in RA pathogenesis in the context of other immune cells and soluble mediators, and evaluate emerging approaches to treat RA that focus on DC.
DC play a central role in the induction of immunity (Box 1; Figure 1). In peripheral tissues DC exist as immature cells, and undergo differentiation after exposure to pro-inflammatory cytokines, immune complexes containing autoantibodies, or pathogens and endogenous inflammatory factors (e.g. heat shock proteins, high mobility group box (HMGB)-1 protein,) recognized by Toll like receptors (TLR), a family of pattern recognition receptors expressed by DC. Following migration to lymph nodes, DC process and present acquired antigens onto MHC molecules to naive T cells and secrete cytokines resulting in skewing of naïve T cells toward T helper (Th) -1(Th1), Th2, or Th17 cells2. They also promote differentiation and maturation of antibody-producing B cells (Figure 1A).
Box 1.
DCs are professional antigen presenting cells (APC), abundant at body surfaces and within tissues where they sense microbes and sample the environment for antigens.
Upon antigen capture DC migrate to lymphoid tissues, where they present processed antigens to naïve T cells, and induce immunity or tolerance.
DC must undergo a process of “maturation”, exemplified by the up regulation of MHC and costimulatory molecules (CD80/86), activation markers and cytokine production in order to activate T cells.
Depending upon thee stimuli, maturation of DC confers them with the ability to differentiate naïve T cells into Th1, Th2 or Th17 cells. Maturing DC also express cytokines that enable the activation of B cells and NK cells.
For antigen uptake, DCs express a variety of receptors: C type lectin receptors (CLR) which recognize carbohydrate moieties of glycoproteins on microbes and Toll like receptors (TLR), pattern recognition receptors that recognize an array of molecules expressed by pathogens, Fcγ receptors (FcγR) which recognize Ig-containing immune complexes (IC) and as a result constitute a link between humoral and cell-mediated immunity.
There are two major subsets of DCs, plasmacytoid DC (pDC, CD123+, CD45RA+) and myeloid DC (mDC, CD11c+, CD45RO+) characterized by distinct origins, receptors and functions. mDC can be subdivided into further subsets based on their location and function (e.g. Langerhans cells).
DCs play a critical role in the maintenance of tolerance in the thymus and in the periphery. Constitutive ablation of DC breaks self-tolerance of CD4+ T cells and results in autoimmunity.
DCs acquire self-antigens in the form of apoptotic cells undergoing physiologic turnover. DCs have multiple receptors for the uptake of apoptotic cells, the ligation of which renders them tolerogenic.
Tolerogenic DC retain the ability to migrate to lymph nodes and cross present antigens to T cells, however, there is a decrease in co-stimulatory molecules (CD80/CD86), resistance to maturation stimuli, low production of IL-12, and high production of immunosuppressive mediators such as TGFβ, IL-10 and Indoleamine 2,3-dioxygenase (IDO).
Tolerogenic DC can induce cell death, anergy or regulatory T cells.
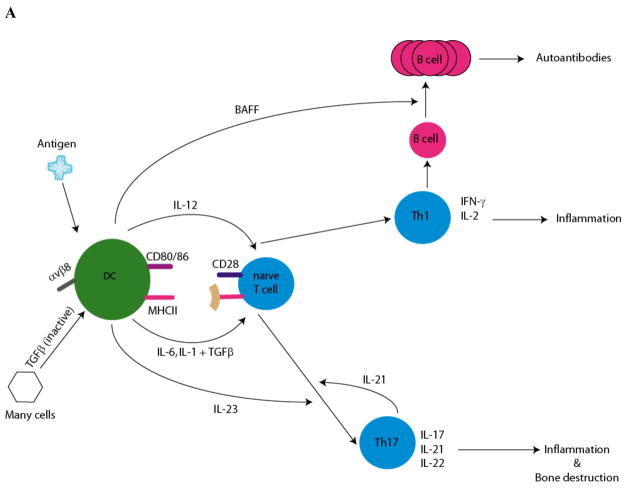
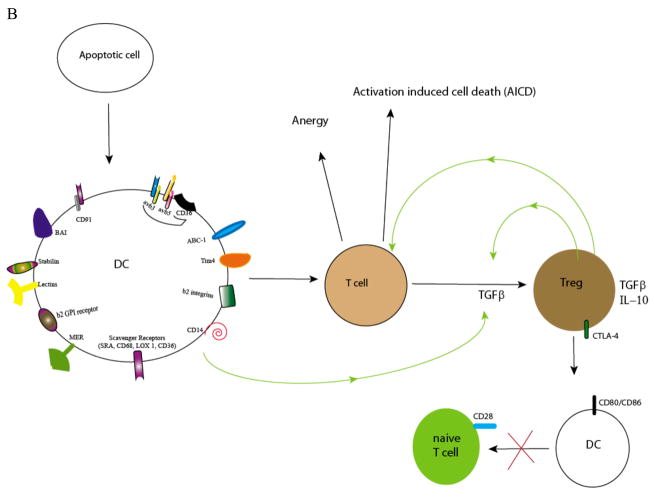
Figure 1. DC: mediators of Immunity and tolerance.
A. Role of DC in differentiation of TH1/TH17 cells: potential effectors in RA. Cytokine production by DC and the subsequent differentiation of naïve T cells is determined by locality and exposure to environmental stimuli (pathogens, necrotic debris, and immune complexes). DCs activate naïve T cells through three signals: presentation of antigenic peptide on MHC molecules, co-stimulatory molecules (CD80/86) and immunomodulatory cytokines. The production of IL-12 by DC strongly favors differentiation of naïve T cells towards the Th1 pathway. Th1 cells produce IFNγ, which is required for cell mediated immunity. Th1 cells also activate B cells to differentiate into plasma cells. DCs produce B cell activating factor (e.g. BAFF) mediating proliferation of the antibody producing B cells. DC products, activated TGFβ and the pro-inflammatory cytokines (IL1, IL-6) induce the differentiation of Th17 cells from naïve T cells. DC can augment local TGFβ levels by converting exogenously produced latent TGFβ (TGFβ associated with latency associated protein (LAP)). The binding of LAP to αvβ8 on DC results in its processing and activation of TGFβ 60. IL-23, an IL-12 family member produced by DC, and IL-21 produced endogenously by Th17 cells, are necessary for proliferation and further maturation. Th17 cells produce IL-17, a pro-inflammatory cytokine that drives inflammation and bone resorption. IL-17 induces expression of RANK on precursor osteoclasts. RANKL expression is upregulated on osteoblasts and mesenchymal cells by IL-17, while TNFα and IL-1 induce RANKL expression on synovial fibroblasts. Interaction of RANKL on these cells with RANK on osteoclast progenitors induces osteoclastogenesis, generation of multinucleated osteoclasts which initiate bone resorption. In addition, cytokines secreted by DC, Th1 and Th17 cells activate macrophages and induce nonhematopoeitic cells to produce inflammatory cytokines (IL-1, IL-6 and TNFα), chemokines and matrix metalloproteinases (MMP) which altogether lead to tissue destruction and inflammation.
B. For apoptotic cell uptake, DCs express a variety of receptors: CD91, the integrins αvβ3 and αvβ5, scavenger receptors (e.g. CD36), the β2 integrins, MER, β2 GPI receptor, CD14, ABC-1, Lectins, Stabilin, BAI, and Tim-4. Upon uptake of apoptotic cells or apoptotic microparticles, DCs are rendered tolerogenic. Tolerogenic DC downregulate co-stimulatory molecules (CD80/86) and on interaction with T cells, induce anergy or activation induced cell death. DCs can also produce TGFβ, a cytokine necessary for differentiation of naïve T cells to Treg 61. Treg have a critical role in the maintenance of self-tolerance by several mechanisms including the production of anti-inflammatory cytokines TGFβ and IL-10. These and other factors block the proliferation and induce the death of CD4+ T cells. Cytotoxic T lymphocyte antigen 4 (CTLA-4), a molecule constitutively expressed on Treg, has a key task in maintaining self tolerance19. CTLA-4 interacts with the co-stimulatory molecules CD80 and CD86 on DCs, delivering inhibitory signals which result in their down-regulation. This interaction also inhibits maturation of DC thereby diminishing the potency of DC-dependent activation of effector T cells.
DC also maintain intrathymic and peripheral tolerance, and in animal models their depletion is associated with the onset of fatal autoimmune-type disease3. Under steady state conditions, immature DC recognize and phagocytose dying apoptotic cells (AC) during physiologic cell turnover4, 5, rendering DC tolerogenic: they produce immunosuppressive cytokines and promote “cross-tolerance”. Responding T cells specific for self-antigens contained within AC are rendered “cross-tolerant” becoming anergic, acquiring a regulatory immunosuppressive phenotype (Treg) or undergoing activation induced cell death (AICD; Figure 1B)6. These tolerogenic DC (TDC) induce hyporesponsiveness even in memory CD4+ and CD8+ T cells7. Generally this process does not mature DC, but aberrations in this pathway, either failed clearance of dead cells and/or exposure of DC to maturation signals (e.g. endogenous inflammatory factors, pathogens) abrogates their tolerogenic capacity6, 7.
Preclinical data
Rheumatoid synovium is characterized by accumulation of immature and mature DC subsets perivascularly, in close association with T cells and B cell follicles1, 8, 9. Synovial fluid (SF) contains significant numbers of myeloid DC (mDC)10 and plasmacytoid DC (pDC; Box 1) compared to blood, signifying a role for these APC in disease perpetuation11. In vitro studies suggest that DC migrate into the joint in response to locally produced cytokines and chemokines, or differentiate locally from myeloid progenitors in response to growth factors contained within synovial fluid (SF)12, 13.
DC may contribute to ongoing inflammation through presentation of autoantigens, as suggested by animal models of autoimmune arthritis14, or production of pro-inflammatory factors (Figure 1A). Joint DC and monocyte-derived DC can present human cartilage glycoprotein 39 (HCgp39), and epitopes from SF to antigen-specific T cells, respectively15, 16. Indeed, synovial DC show evidence of activation in vivo: upregulation of MHC, co-stimulatory molecules, RelB 9, expression of receptor activator of nuclear factor-κB (RANK) and its ligand (RANKL)17, and heightened production of pro-inflammatory cytokines when stimulated ex vivo (IL-1, IL-6, TNFα) with immune complexes or TLR agonists1. DC migrating into SF may undergo activation in response to locally produced cytokines, or to endogenous factors released from dying cells during inflammation18. DC may also indirectly contribute to RA pathogenesis. CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) is a negative regulator of T cell activation also expressed on Treg19. CTLA-4 expression on Treg suppresses DC activation by downregulating the costimulatory molecules CD80 and CD8619. CTLA-4 polymorphisms have been associated with RA, reflecting either a lack of effector T cell blockade or reduced Treg activity20.
RA synovium is characterized by formation of ectopic lymphoid organs resembling germinal centers (GC). They contain plasma cells expressing activation-induced cytidine deaminase (AID) and producing anti-cyclic citrinullated peptide (ACPA) antibodies21. ACPA and rheumatoid factor (RF) are presumed to bind to Fc receptors on macrophages and DC, inducing their activation and production of pro-inflammatory cytokines22. pDC also accumulate in rheumatoid synovium and through production of type I IFN may enhance autoantibody production23. Indeed a subset of RA patients express a type I IFN signature associated with ACPA production24.
RA was initially considered to be Th1 driven. Identification of Th17 cells and the cytokines IL-17 and IL-23 within affected tissues and/or fluids, has implicated both Th1 and Th17 cells in its pathogenesis25, 26, particularly as the p40 subunit (common to both IL-12 and IL-23), and the IL-23 specific subunit p19 are essential for joint inflammation in the collagen-induced arthritis mouse model27. DC are required for differentiation of Th1 and Th17 subsets from naïve precursors (Figure 1A). Th1 cells produce IFNγ, activating macrophages and enhancing production of pro-inflammatory cytokines (IL-1, IL-6 and TNFα). IL-17 stimulates fibroblasts, endothelial and epithelial cells to produce IL-6 and IL-8, recruits neutrophils and monocytes 28, 29, and in animal models, induces osteoclastic bone resorption30, 31. Synovial tissue DC express IL-12p70 and IL-23p19, cytokines essential for full differentiation of Th1 and Th17 cells respectively, providing a mechanism by which their production is locally facilitated or perpetuated23(Figure 1A). Although IL-17 has been located in RA synovial fluid and membrane, with expression predicting disease progression radiologically30, 32, other reports suggest that it and Th17 cells are not abundant in rheumatoid synovial fluid33. Furthermore, variants of IL12B (encoding the IL-12p40 subunit) and IL23R, previously associated with inflammatory diseases, do not appear to play a major role in RA risk34. Recently, Brentano et al., found abundant expression of the p19 but not the p40 subunit of IL-23 in RA synovial lining, indicating that bioactive IL-23 is not produced by synoviocytes35. Furthermore, levels of bioactive IL-23 were not statistically signfiicant between RA and osteoarthritis synovial fluids35. As transgenic expression of p19 in mice leads to systemic inflammation36, it may contribute to RA through p40 independent mechanisms35. In summary, further verification for a role of Th17 cells and related cytokines in RA pathogenesis is required.
With the development of animal models that selectively deplete DC in vivo, the specific role of DC in pathogenesis of autoimmune arthritis can be addressed3, 37. Nevertheless, DC can be exploited to induce tolerance in vivo. In animal models, immunomodulation of immature DC with immunosuppressive cytokines, genetic engineering, proteins and drugs propagates tolerance by skewing immune responses away from pro-inflammatory Th1 responses toward Th2 responses, or through induction of IL-10 producing Treg, anergy or AICD (reviewed in38). Human immature DC can be rendered tolerogenic in vitro by pre-exposure to autologous AC (which express self-antigens) skewing T cell priming and preventing the activation of memory T cells6. Ligation of individual apoptotic cell receptors on human DC (CR3, CR4; Figure 1B) inhibits IL-12 production, elicits TGFβ and specifically causes T cell anergy and AICD (Figure 1B;7). DC can also be targeted in situ using antibodies towards C-type lectin receptors39. When complexed to antigens, antibodies to DEC-205 induce Treg in animal models40. Similarly, C-type lectin receptors like Dcir (dendritic cell immunoreceptor) negatively regulate DC41. Comparison of these different approaches to induce TDC is warranted to identify those most efficacious in inducing tolerance.
Future clinical development
Patients who do not respond to disease modifying anti-rheumatic drugs (DMARDs; e.g. methotrexate) require addition of biological agents targeting TNFα, IL-1 (produced by joint constituents, including DC), B cells or co-stimulatory molecules on DC. Anti-TNF targeted therapies ameliorate clinical symptoms and also reduce frequencies of peripheral activated mDC and pDC in vivo, and in vitro diminish their maturation and their ability to produce pro-inflammatory cytokines and chemokines13, 42. These observations reinforce the strategy of targeting mediators of inflammation and bone resorption, particularly at the level of the DC (reviewed in Table 1). Below we review emerging therapies focusing on DC and related targets to treat RA.
Tolerogenic DC
The administration of TDC, or even Treg, may be options for recalcitrant disease. In the first human study to test TDC, we showed that injection of influenza matrix protein peptide-pulsed immature DC transiently induced antigen-specific, IL-10 producing, CD8+ Treg that blocked IFNγ production and cytolytic function by effector CD8+ T cells43. Thomas et al. (University of Queensland, Brisbane, Australia) are injecting autologous, monocyte-derived DC pulsed with a mixture of four citrullinated peptide antigens derived from vimentin, fibrinogen alpha chain, fibrinogen beta chain and collagen type (II) into patients with RA who express RA-associated DR-B1 alleles and are ACPA+44. The DC are pretreated with BAY 11-7082, an inhibitor of RelB nuclear translocation, which renders them tolerogenic and then subsequently pulsed with citrullinated peptides45. If determined to be safe, it may be possible to advance TDC therapy with the addition of costimulatory blockade e.g. abatacept which maintains DC in their immature, tolerogenic form and has proven synergistic effects in animal models of transplantation46.
Inhibitors of costimulation
Treg employ several mechanisms to maintain self-tolerance: production of TGFβ and IL-10, inhibition of cell metabolism and blockade of DC function via CTLA-4 and sequestration from effector T cells (reviewed in19, 47). Abatacept, a CTLA4-Ig fusion protein that modulates T cell costimulation, has proven efficacious in DMARD- and anti-TNF therapy resistant RA patients. The fusion protein may also enhance the induction in mDC and pDC of indoleamine 2′,3′ dioxygenase (IDO) an enzyme which metabolizes tryptophan to kyenurenine and which induces the differentiation of Treg from naïve T cells48–50.
Inflammatory cytokines
IL-6
We and others identified IL-6, a prototypic inflammatory cytokine in RA synovial fluid 51. Tocilizumab, an antibody that binds the IL-6 receptor, has been proven efficacious for the treatment of RA in combination with DMARDs and is currently in phase III trials52 (Table 1).
IL-23
IL-23, is required for the maturation of Th17 cells (Figure 1A). Neutralizing antibodies to p40, ustekinumab and ABT-874, have shown promise in patients with psoriasis and inflammatory bowel disease but await testing in RA53. Targeting the p19 component of IL-23 may be a more selective way to block Th17 differentiation while keeping Th1 cells unaffected, as they are also required for immunity against pathogens.
IL-17
Clinical trials are underway targeting IL-17 as well as IL-22, a product of Th17 cells. Unlike Th1 cells, Th17 cells are resistant to Treg-mediated suppression in animal models of autoimmunity54. However, they directly stimulate production of cytokines (IL6 and TNFα) at the site of inflammation, which abrogate Treg-mediated suppression. Current anti-IL-1 and IL-6 therapeutics may ameliorate inflammation by also inhibiting Th17 differentiation.
Inhibitors of osteoclastogenesis
RANKL is expressed by DC, T cells (e.g. Th17 cells) and fibroblast-like cells and interaction with RANK on osteoclast progenitors induces osteoclastogenesis. Denosumab, an antibody against RANKL has shown retardation of radiologic progression in RA55.
Small molecule inhibitors
The intracellular kinases JAK-3 and Syk kinases are involved in signaling through the common γ chain of cytokine receptors and/or receptors, which have immuno-receptor tyrosine-based activation motifs (e.g. Fcγ receptor). As rheumatoid synovial tissue DC express JAK3, (in addition to STAT1, STAT4 and STAT656), these inhibitors could conceivably block DC activation53. JAK3 and Syk inhibitors in RA have shown encouraging results53. Mutations in STAT4, a transcription factor that transduces signals from IL-12, IL-23 and type 1 interferon in T cells and monocytes, and STAT3, a critical transcription factor for Th17 cell differentiation have been linked to RA. These transcription factors in addition to those governing Th17 differentiation (RORγt, aryl hydrocarbon receptors) are potential attractive targets for RA. A caveat of inhibiting transcription factors, however, is the potential of side effects since the pathways are common across multiple cells.
Outlook
DC are potential tools to treat autoimmunity because of their natural ability to induce tolerance in vivo. The identification of relevant antigens in RA and optimal approaches to generate TDC will be required to maximize their immunomodulatory activity. Studies linking genetic alterations with RA make it conceivable that therapy can be further tailored to gain maximum benefit. Approaches to target inhibitory receptors on DC (e.g. CR3, DEC-205) with antigen fused to antibodies are another option. Advantage could be taken of other DC subsets (pDC), to induce tolerance. When activated via TLRs or following engagement of CD80/CD86 with CTLA-4, pDC express IDO and induce Treg49, 57. Induction of IDO in animal models of arthritis controls accumulation of pathogenic T cells at the site of inflammation58, 59, and IDO-expressing DC are able to reverse arthritis 59. The combination of established therapies along with active immunomodulation through the use of DC-based therapies may further improve our ability to ameliorate autoimmunity in RA.
Acknowledgments
Some of the studies cited in this review were supported by NIH grant RO1 AIO71078 and an Alliance for Lupus research grant to NB.
Footnotes
Search strategy: Our search efforts included PubMed and Cochran Library using the term “rheumatoid arthritis” and associated specific terms including dendritic cells and autoimmunity, dendritic cells and regulatory T cells, and tolerogenic dendritic cells. We reviewed primary papers, and review articles from June 1999 to June 2009. We reviewed all types of articles including original papers, reviews, case reports, etc. Efforts were made to refer to primary papers whenever possible and to comprehensive reviews. In addition, we searched clinicaltrials.gov to identify ongoing clinical trials using dendritic cells to treat autoimmune diseases and current therapies in clinical trials for rheumatoid arthritis.
Competing interests: None
References
- 1.Wenink MH, Han W, Toes RE, Radstake TR. Dendritic cells and their potential implication in pathology and treatment of rheumatoid arthritis. Handb Exp Pharmacol. 2009:81–98. doi: 10.1007/978-3-540-71029-5_4. [DOI] [PubMed] [Google Scholar]
- 2.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–55. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Ohnmacht C, et al. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J Exp Med. 2009;206:549–59. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Somersan S, Bhardwaj N. Tethering and tickling: a new role for the phosphatidylserine receptor. J Cell Biol. 2001;155:501–4. doi: 10.1083/jcb.200110066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobayashi N, et al. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity. 2007;27:927–40. doi: 10.1016/j.immuni.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skoberne M, Beignon AS, Larsson M, Bhardwaj N. Apoptotic cells at the crossroads of tolerance and immunity. Curr Top Microbiol Immunol. 2005;289:259–92. doi: 10.1007/3-540-27320-4_12. [DOI] [PubMed] [Google Scholar]
- 7.Skoberne M, et al. The apoptotic-cell receptor CR3, but not alphavbeta5, is a regulator of human dendritic-cell immunostimulatory function. Blood. 2006;108:947–55. doi: 10.1182/blood-2005-12-4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas R, et al. Dendritic cells and the pathogenesis of rheumatoid arthritis. J Leukoc Biol. 1999;66:286–92. doi: 10.1002/jlb.66.2.286. [DOI] [PubMed] [Google Scholar]
- 9.Pettit AR, MacDonald KP, O’Sullivan B, Thomas R. Differentiated dendritic cells expressing nuclear RelB are predominantly located in rheumatoid synovial tissue perivascular mononuclear cell aggregates. Arthritis Rheum. 2000;43:791–800. doi: 10.1002/1529-0131(200004)43:4<791::AID-ANR9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 10.Zvaifler NJ, Steinman RM, Kaplan G, Lau LL, Rivelis M. Identification of immunostimulatory dendritic cells in the synovial effusions of patients with rheumatoid arthritis. J Clin Invest. 1985;76:789–800. doi: 10.1172/JCI112036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jongbloed SL, et al. Enumeration and phenotypical analysis of distinct dendritic cell subsets in psoriatic arthritis and rheumatoid arthritis. Arthritis Res Ther. 2006;8:R15. doi: 10.1186/ar1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santiago-Schwarz F, Anand P, Liu S, Carsons SE. Dendritic cells (DCs) in rheumatoid arthritis (RA): progenitor cells and soluble factors contained in RA synovial fluid yield a subset of myeloid DCs that preferentially activate Th1 inflammatory-type responses. J Immunol. 2001;167:1758–68. doi: 10.4049/jimmunol.167.3.1758. [DOI] [PubMed] [Google Scholar]
- 13.van Lieshout AW, et al. Inhibition of TNF alpha during maturation of dendritic cells results in the development of semi-mature cells: a potential mechanism for the beneficial effects of TNF alpha blockade in rheumatoid arthritis. Ann Rheum Dis. 2005;64:408–14. doi: 10.1136/ard.2004.023259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leung BP, et al. A novel dendritic cell-induced model of erosive inflammatory arthritis: distinct roles for dendritic cells in T cell activation and induction of local inflammation. J Immunol. 2002;169:7071–7. doi: 10.4049/jimmunol.169.12.7071. [DOI] [PubMed] [Google Scholar]
- 15.Tsark EC, et al. Differential MHC class II-mediated presentation of rheumatoid arthritis autoantigens by human dendritic cells and macrophages. J Immunol. 2002;169:6625–33. doi: 10.4049/jimmunol.169.11.6625. [DOI] [PubMed] [Google Scholar]
- 16.Steenbakkers PG, et al. Localization of MHC class II/human cartilage glycoprotein-39 complexes in synovia of rheumatoid arthritis patients using complex-specific monoclonal antibodies. J Immunol. 2003;170:5719–27. doi: 10.4049/jimmunol.170.11.5719. [DOI] [PubMed] [Google Scholar]
- 17.Page G, Miossec P. RANK and RANKL expression as markers of dendritic cell-T cell interactions in paired samples of rheumatoid synovium and lymph nodes. Arthritis Rheum. 2005;52:2307–12. doi: 10.1002/art.21211. [DOI] [PubMed] [Google Scholar]
- 18.Martin CA, et al. Aberrant extracellular and dendritic cell (DC) surface expression of heat shock protein (hsp)70 in the rheumatoid joint: possible mechanisms of hsp/DC-mediated cross-priming. J Immunol. 2003;171:5736–42. doi: 10.4049/jimmunol.171.11.5736. [DOI] [PubMed] [Google Scholar]
- 19.Wing K, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–5. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 20.Gregersen PK, Behrens TW. Genetics of autoimmune diseases--disorders of immune homeostasis. Nat Rev Genet. 2006;7:917–28. doi: 10.1038/nrg1944. [DOI] [PubMed] [Google Scholar]
- 21.Humby F, et al. Ectopic lymphoid structures support ongoing production of class-switched autoantibodies in rheumatoid synovium. PLoS Med. 2009;6:e1. doi: 10.1371/journal.pmed.0060001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klareskog L, Catrina AI, Paget S. Rheumatoid arthritis. Lancet. 2009;373:659–72. doi: 10.1016/S0140-6736(09)60008-8. [DOI] [PubMed] [Google Scholar]
- 23.Lebre MC, et al. Rheumatoid arthritis synovium contains two subsets of CD83-DC-LAMP- dendritic cells with distinct cytokine profiles. Am J Pathol. 2008;172:940–50. doi: 10.2353/ajpath.2008.070703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Pouw Kraan TC, et al. Rheumatoid arthritis subtypes identified by genomic profiling of peripheral blood cells: assignment of a type I interferon signature in a subpopulation of patients. Ann Rheum Dis. 2007;66:1008–14. doi: 10.1136/ard.2006.063412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chabaud M, Fossiez F, Taupin JL, Miossec P. Enhancing effect of IL-17 on IL-1-induced IL-6 and leukemia inhibitory factor production by rheumatoid arthritis synoviocytes and its regulation by Th2 cytokines. J Immunol. 1998;161:409–14. [PubMed] [Google Scholar]
- 26.Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. Type 17 T helper cells-origins, features and possible roles in rheumatic disease. Nat Rev Rheumatol. 2009 doi: 10.1038/nrrheum.2009.80. [DOI] [PubMed] [Google Scholar]
- 27.Murphy CA, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–7. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fossiez F, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shahrara S, Pickens SR, Dorfleutner A, Pope RM. IL-17 induces monocyte migration in rheumatoid arthritis. J Immunol. 2009;182:3884–91. doi: 10.4049/jimmunol.0802246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kotake S, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–52. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koenders MI, et al. Blocking of interleukin-17 during reactivation of experimental arthritis prevents joint inflammation and bone erosion by decreasing RANKL and interleukin-1. Am J Pathol. 2005;167:141–9. doi: 10.1016/S0002-9440(10)62961-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirkham BW, et al. Synovial membrane cytokine expression is predictive of joint damage progression in rheumatoid arthritis: a two-year prospective study (the DAMAGE study cohort) Arthritis Rheum. 2006;54:1122–31. doi: 10.1002/art.21749. [DOI] [PubMed] [Google Scholar]
- 33.Yamada H, et al. Th1 but not Th17 cells predominate in the joints of patients with rheumatoid arthritis. Ann Rheum Dis. 2008;67:1299–304. doi: 10.1136/ard.2007.080341. [DOI] [PubMed] [Google Scholar]
- 34.Chang M, et al. The inflammatory disease-associated variants in IL12B and IL23R are not associated with rheumatoid arthritis. Arthritis Rheum. 2008;58:1877–81. doi: 10.1002/art.23492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brentano F, et al. Abundant expression of the interleukin (IL)23 subunit p19, but low levels of bioactive IL23 in the rheumatoid synovium: differential expression and Toll-like receptor-(TLR) dependent regulation of the IL23 subunits, p19 and p40, in rheumatoid arthritis. Ann Rheum Dis. 2009;68:143–50. doi: 10.1136/ard.2007.082081. [DOI] [PubMed] [Google Scholar]
- 36.Wiekowski MT, et al. Ubiquitous transgenic expression of the IL-23 subunit p19 induces multiorgan inflammation, runting, infertility, and premature death. J Immunol. 2001;166:7563–70. doi: 10.4049/jimmunol.166.12.7563. [DOI] [PubMed] [Google Scholar]
- 37.Jung S, et al. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–20. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610–21. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 39.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 40.Mahnke K, Qian Y, Knop J, Enk AH. Induction of CD4+/CD25+ regulatory T cells by targeting of antigens to immature dendritic cells. Blood. 2003;101:4862–9. doi: 10.1182/blood-2002-10-3229. [DOI] [PubMed] [Google Scholar]
- 41.Fujikado N, et al. Dcir deficiency causes development of autoimmune diseases in mice due to excess expansion of dendritic cells. Nat Med. 2008;14:176–80. doi: 10.1038/nm1697. [DOI] [PubMed] [Google Scholar]
- 42.Balanescu A, et al. Early and late effect of infliximab on circulating dendritic cells phenotype in rheumatoid arthritis patients. Int J Clin Pharmacol Res. 2005;25:9–18. [PubMed] [Google Scholar]
- 43.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–8. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hill JA, et al. Cutting edge: the conversion of arginine to citrulline allows for a high-affinity peptide interaction with the rheumatoid arthritis-associated HLA-DRB1*0401 MHC class II molecule. J Immunol. 2003;171:538–41. doi: 10.4049/jimmunol.171.2.538. [DOI] [PubMed] [Google Scholar]
- 45.Martin E, O’Sullivan B, Low P, Thomas R. Antigen-specific suppression of a primed immune response by dendritic cells mediated by regulatory T cells secreting interleukin-10. Immunity. 2003;18:155–67. doi: 10.1016/s1074-7613(02)00503-4. [DOI] [PubMed] [Google Scholar]
- 46.Lan YY, et al. “Alternatively activated” dendritic cells preferentially secrete IL-10, expand Foxp3+CD4+ T cells, and induce long-term organ allograft survival in combination with CTLA4-Ig. J Immunol. 2006;177:5868–77. doi: 10.4049/jimmunol.177.9.5868. [DOI] [PubMed] [Google Scholar]
- 47.Bluestone JA, Thomson AW, Shevach EM, Weiner HL. What does the future hold for cell-based tolerogenic therapy? Nat Rev Immunol. 2007;7:650–4. doi: 10.1038/nri2137. [DOI] [PubMed] [Google Scholar]
- 48.Fallarino F, Gizzi S, Mosci P, Grohmann U, Puccetti P. Tryptophan catabolism in IDO+ plasmacytoid dendritic cells. Curr Drug Metab. 2007;8:209–16. doi: 10.2174/138920007780362581. [DOI] [PubMed] [Google Scholar]
- 49.Manches O, et al. HIV-activated human plasmacytoid DCs induce Tregs through an indoleamine 2,3-dioxygenase-dependent mechanism. J Clin Invest. 2008;118:3431–9. doi: 10.1172/JCI34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chung DJ, et al. Indoleamine 2,3-dioxygenase-expressing mature human monocyte-derived dendritic cells expand potent autologous regulatory T cells. Blood. 2009 doi: 10.1182/blood-2008-11-191197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhardwaj N, et al. IL-6/IFN-beta 2 in synovial effusions of patients with rheumatoid arthritis and other arthritides. Identification of several isoforms and studies of cellular sources. J Immunol. 1989;143:2153–9. [PubMed] [Google Scholar]
- 52.Genovese MC, et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum. 2008;58:2968–80. doi: 10.1002/art.23940. [DOI] [PubMed] [Google Scholar]
- 53.Stanczyk J, Ospelt C, Gay S. Is there a future for small molecule drugs in the treatment of rheumatic diseases? Curr Opin Rheumatol. 2008;20:257–62. doi: 10.1097/BOR.0b013e3282fa13ee. [DOI] [PubMed] [Google Scholar]
- 54.Oukka M. Th17 cells in immunity and autoimmunity. Ann Rheum Dis. 2008;67(Suppl 3):iii26–9. doi: 10.1136/ard.2008.098004. [DOI] [PubMed] [Google Scholar]
- 55.Cohen SB, et al. Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum. 2008;58:1299–309. doi: 10.1002/art.23417. [DOI] [PubMed] [Google Scholar]
- 56.Walker JG, et al. Expression of Jak3, STAT1, STAT4, and STAT6 in inflammatory arthritis: unique Jak3 and STAT4 expression in dendritic cells in seropositive rheumatoid arthritis. Ann Rheum Dis. 2006;65:149–56. doi: 10.1136/ard.2005.037929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mellor AL, Munn DH. Creating immune privilege: active local suppression that benefits friends, but protects foes. Nat Rev Immunol. 2008;8:74–80. doi: 10.1038/nri2233. [DOI] [PubMed] [Google Scholar]
- 58.Criado G, Simelyte E, Inglis JJ, Essex D, Williams RO. Indoleamine 2,3 dioxygenase-mediated tryptophan catabolism regulates accumulation of Th1/Th17 cells in the joint in collagen-induced arthritis. Arthritis Rheum. 2009;60:1342–51. doi: 10.1002/art.24446. [DOI] [PubMed] [Google Scholar]
- 59.Bianco NR, Kim SH, Ruffner MA, Robbins PD. Therapeutic effect of exosomes from indoleamine 2,3-dioxygenase-positive dendritic cells in collagen-induced arthritis and delayed-type hypersensitivity disease models. Arthritis Rheum. 2009;60:380–9. doi: 10.1002/art.24229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Travis MA, et al. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–5. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stuart LM, et al. Inhibitory effects of apoptotic cell ingestion upon endotoxin-driven myeloid dendritic cell maturation. J Immunol. 2002;168:1627–35. doi: 10.4049/jimmunol.168.4.1627. [DOI] [PubMed] [Google Scholar]