Abstract
The emergence of stereotypies was examined in juvenile rhesus monkeys who, at two weeks of age, received selective bilateral ibotenic acid lesions of the amygdala (N=8) or hippocampus (N=8). The lesion groups were compared to age-matched control subjects that received a sham surgical procedure (N=8). All subjects were maternally reared for the first six months and provided access to social groups throughout development. Pronounced stereotypies were not observed in any of the experimental groups during the first year of life. However, between one to two years of age, both amygdala- and hippocampus-lesioned subjects began to exhibit stereotypies. When observed as juveniles, both amygdala- and hippocampus-lesioned subjects consistently produced more stereotypies than the control subjects in a variety of contexts. Interestingly, neonatal lesions of either the amygdala or hippocampus resulted in unique repertoires of repetitive behaviors. Amygdala-lesioned subjects exhibited more self-directed stereotypies and the hippocampus-lesioned subjects displayed more head-twisting. We discuss these results in relation to the neurobiological basis of repetitive stereotypies in neurodevelopmental disorders, such as autism.
Keywords: amygdaloid complex, repetitive behavior, macaque, psychopathology, neurodevelopment, rhesus
INTRODUCTION
Stereotypies are defined as repetitive and topographically invariant acts without a clearly established purpose or function (Ridley & Baker, 1982). Examples of stereotypies in human populations include, hand flapping, body-rocking, head-rolling etc. (Berkson, Gutermuth, & Baranek, 1995; Rojahn, Matlock, & Tasse, 2000; Rojahn, Tasse, & Sturmey, 1997). These repetitive movements are commonly observed in a variety of developmental, psychiatric and neurological disorders, including autism, Rett syndrome, Fragile X syndrome, mental retardation, schizophrenia, obsessive compulsive disorder, Parkinson disease and Tourette syndrome (Berkson, 1983; Berkson et al., 1995; Bodfish, Symons, Parker, & Lewis, 2000; Lachiewicz, Spiridigliozzi, Gullion, Ransford, & Rao, 1994; South, Ozonoff, & McMahon, 2005; Wales, Charman, & Mount, 2004). The use of animal models provides one approach to identifying the neural underpinnings of repetitive behaviors observed in clinical populations.
Models of restricted, repetitive behaviors in animals have relied on three main experimental manipulations to induce stereotypies: 1) Social and/or environmental restrictions, 2) Pharmacological manipulations and 3) Targeted insults to the central nervous system (e.g., genetic mutations, viral exposure, lesions) (Lewis, Tanimura, Lee, & Bodfish, 2007). Across species, restrictions or perturbations of the physical and social environment are consistently associated with the production of stereotypies (Capitanio, 1986; Lutz, Well, & Novak, 2003; Powell, Newman, Pendergast, & Lewis, 1999). Likewise, pharmacological manipulations of the dopamine system have also been used to reliably induce repetitive stereotypies in both rodents and nonhuman primates (Bedingfield, Calder, Thai, & Karler, 1997; Lewis, Baumeister, McCorkle, & Mailman, 1985; Presti, Mikes, & Lewis, 2003; Randrup & Munkvad, 1974). Both socio-environmental restrictions and pharmacological manipulations have implicated dysfunction of circuits linking the neocortex and basal ganglia in the pathophysiology of repetitive behaviors. It is not clear, however, how these manipulations act upon the basal ganglia to induce motor stereotypies (Canales & Graybiel, 2000; Lewis, Gluck, Beauchamp, Keresztury, & Mailman, 1990; Lewis et al., 2007; Martin, Spicer, Lewis, Gluck, & Cork, 1991). Moreover, there is some evidence that targeted insults to non-striatal structures within the medial temporal lobe result in locomotor stereotypies in both rodents and nonhuman primates (Bachevalier, 1994; Lipska & Weinberger, 2000).
Here we evaluate the effects of neonatal amygdala or hippocampus damage on the emergence of stereotypies in mother-reared, group-living rhesus monkeys. We have previously reported that early damage to the amygdala or hippocampus did not alter fundamental features of social development, including the development of mother-infant interactions and the ability to interact with peers (Bauman, Lavenex, Mason, Capitanio, & Amaral, 2004a,, 2004b). Though our initial observations in the first year of development revealed few stereotypies, both amygdala- and hippocampus-lesioned subjects began to produce stereotypies in the second year of life. The control subjects did not develop motor stereotypies, suggesting that the emergence of repetitive behaviors is related to the brain lesion rather than the socio-environmental rearing context. We examine these data in relation to the neurobiology of abnormal repetitive behaviors, and discuss possible implications for animal models of developmental disorders.
METHODS
All experimental procedures were developed in consultation with the veterinary staff at the California National Primate Research Center. All protocols were approved by the UC Davis IACUC.
Subjects and Living Conditions
Current data were collected across a six-month span beginning when the average subject was a little over two years of age. A brief summary of rearing/housing conditions is provided below. Twenty-four infant rhesus monkeys (Macaca mulatta) naturally born of multiparous mothers were randomly assigned to one of three lesion conditions: bilateral amygdala lesions (five females, three males), bilateral hippocampus lesions (five females, three males) or sham-operated controls (four females, four males). All surgeries were performed at 12–16 days after birth. The infants were returned to their mothers following surgery and provided daily access to a socialization group consisting of six mother-infant pairs and one adult male. The animals in these groups were able to interact for a minimum of three hours per day, five days per week. The four socialization groups were each composed of two amygdala-lesioned infants and their mothers, two hippocampus-lesioned infants and their mothers, and two sham-operated infants and their mothers. The age range between the youngest and oldest infant within each group was approximately two months. Three of the socialization groups were comprised of one male and one female per lesion condition, and the fourth cohort consisted of two female amygdala-lesioned infants, two female hippocampus-lesioned infants, one male and one female sham-operated infants. When the youngest subject within a socialization group reached six months of age, the infants were permanently separated from their mothers, but otherwise continued to experience the same housing and group socialization in the absence of their mothers. At this time, a new adult female was added to each socialization cohort to provide continued exemplars of adult female social behavior. At approximately one year of age, subjects became permanently socially housed (24 hours per day) with their original socialization cohort in a 2.13 m W × 3.35 m D × 2.44 m H chain link enclosure.
It is important to note that one male amygdala-lesioned subject died at approximately one year of age due to unrelated health reasons and was subsequently replaced with an alternative, neonatally amygdala-lesioned male. The replacement male was born the same year as the other subjects, but was reared alone with his mother for the first year of life. Following weaning at one year of age, he was housed with an age-matched female infant until being introduced to his current cohort at approximately one year and three months of age.
Surgical Procedures
The surgical procedures are summarized below and are described in detail in previous publications (Bauman et al., 2004a,, 2004b). On the day of surgery, the infants were initially anesthetized with ketamine hydrochloride (15 mg/kg i.m.) and medetomidine (30 μg/kg), then placed in an magnetic resonance imaging (MRI)-compatible stereotaxic apparatus (Crist Instruments Co., Inc., Damascus, MD). The infant’s brain was imaged using a General Electric 1.5 T Gyroscan magnet; 1.0 mm thick sections were taken using a T1-weighted Inversion Recovery Pulse sequence (TR = 21, TE =7.9, NEX 3, FOV = 8cm, Matrix, 256 × 256). From these images, we determined the location of the amygdala or hippocampus and calculated the coordinates for the ibotenic acid injections. Infants were ventilated and vital signs monitored throughout the surgery. A stable level of anesthesia was maintained using a combination of isoflurane (1.0%, varied as needed to maintain an adequate level of anesthesia) and intravenous infusion of fentanyl (7–10 μg/kg/hour). Following a midline incision, the skin was laterally displaced to expose the skull, two craniotomies were made over the amygdala or the hippocampus, depending on the pre-determined lesion condition, and the dura was reflected to expose the surface of the brain. Ibotenic acid (IBO, Biosearch Technologies Inc., 10 mg/ml in 0.1 M phosphate buffered saline) was injected simultaneously bilaterally into the amygdala or hippocampus using 10 μl Hamilton syringes (26 gauge beveled needles) at a rate of 0.2 μl/min. Complete amygdala lesions required a total of 7–12 μl of ibotenic acid per amygdala. Complete hippocampus lesions required a total of 5.5–7 μl of ibotenic acid per hippocampus. Sham-operated controls underwent the same pre-surgical preparations, received a midline incision and the skull was exposed. The control subjects were maintained under anesthesia for the average duration of the lesion surgeries and the fascia and skin were sutured in two separate layers. Following the surgical procedure, all infants were monitored by a veterinarian and returned to their mothers once they were fully alert.
Lesion Analysis
We obtained T2-weighted magnetic resonance (MR) images ten days after surgery to examine the extent of the edema associated with the lesion. The hyperintense T2-weighted signal for each of the sixteen lesioned subjects was evaluated to confirm the general target and extent of the lesions (i.e., amygdala lesion sparing the hippocampus or hippocampus lesion sparing the amygdala). Their brains were imaged using a General Electric 1.5 T Gyroscan magnet; 1.5 mm thick sections were taken using a T2 weighted Inversion Recovery Pulse sequence (TR = 4000, TE = 102, NEX 3, FOV = 8cm, Matrix, 256 × 256). Additional lesion confirmation was provided by T1-weighted MR images obtained at approximately four years of age. The animals’ brains were scanned using a General Electric 1.5T Signa MRI system; 1mm thick sections were taken using a T1 weighted 3D axial spoiled gradient (SPGR) sequence (TR = 22.0ms, TE = 7.9ms, NEX 3, FOV = 16cm, Matrix, 256 × 256).
Behavioral Observations
Subjects were observed in four distinct behavioral contexts: 1) Solo, 2) Paired with familiar conspecifics, 3) Paired with unfamiliar conspecifics and 4) Group housed in their standard living environment (see Table 1). Both social and non-social data were collected during these tests, but only data relevant to stereotypies will be presented here. The social interaction data from these observations will be described in future publications.
Table 1.
Summary of Observational Contexts
| Test Context | Sampling Method | Description |
|---|---|---|
| Solo Observations | 5-min focal samples | 20 samples per animal alone in home enclosure |
| Familiar Dyads | 5-min focal samples; familiar subjects paired for twenty minutes per session | 20 samples per animal while paired in home enclosure |
| Novel Dyads | 5-min focal samples; unfamiliar subjects paired for twenty minutes per session | 72 samples per animal paired with a novel conspecific while in a novel enclosure |
| Social Groups | 5-min focal samples; subjects observed undisturbed in group environment | 34 samples per animal while in home enclosure |
Re-evaluation of Earlier Video Footage
We re-evaluated a subset of our previously reported behavioral observations (Bauman et al., 2004b) using a more sensitive ethogram designed to assess discrete classes of stereotypies (Table 2). The re-scored data are from novel dyads that took place when the subjects were approximately 12 months of age. For each subject, we rescored the first 20 dyadic interactions (400 minutes total observation time per subject).
Table 2.
Definitions of Stereotypic Behavior
| Whole-body Stereotypies | Definition |
|---|---|
| Backflip | At least two consecutive backflips |
| Bounce | Repetitive hopping or bouncing for at least 3 seconds |
| Pace | Repetitive, undirected walking or running with the same path repeated for at least 3 seconds |
| Spin | Repetitive twirling or spinning for at least two rotations |
| Swing | Repetitive swinging with no progressive movement for at least 3 seconds |
| Self-Direct Stereotypies | |
| Rock | Rocking back and forth |
| Salute (Eye-poke) | Hand held next to or over eye; may include actual poking of eye |
| Self-bite | Biting oneself; most often of own limbs |
| Self-clasp | Unusual holding of body part or limb with another body part |
| Other | Abnormal self-directed behavioral patterns not described above (e.g., nipple clasp) |
| Non-categorical Stereotypies | |
| Head-twist | Twisting or rolling of the neck often seen when an animal approaches a corner or barrier |
Solo Context
Subjects were observed alone in their home enclosure to assess stereotypies in the absence of social stimulation. A single test session consisted of observing each animal for two consecutive 5-minute samples. Subjects participated in two test sessions per day over five consecutive days. A total of 20 solo samples were collected on each animal (100 minutes total observation time per subject).
Familiar Dyad Context
Immediately after two subjects from the same social group had concluded solo testing for a particular session, they were paired in their home enclosure and allowed to interact freely for twenty minutes. Each animal participated in two dyads per day for a total of 10 dyadic meetings per animal. Each animal was tested twice with every other animal from its social group according to a predetermined pseudorandom sequence. The identity of the focal animal alternated every 5 minutes during the 20-min period, so that a total of 20 focal observations were collected on each animal across the 10 dyadic meetings (100 minutes total observation time per subject). All dyads were balanced for testing order and time of day.
Novel Dyad Context
The effect of novelty on the occurrence of stereotypies was assessed during pairings of unfamiliar subjects. Novel dyads consisted of two subjects from different social groups that had never met one another. These pairings started two months after the completion of solo and familiar dyad testing. Observations of novel dyads took place in enclosures that were unfamiliar to both subjects but otherwise identical to their home enclosures. Each animal was observed with every animal from a separate social group (two amygdala-lesioned, two hippocampus-lesioned, and two sham-operated control subjects) according to a predetermined pseudorandom sequence. This complete rotation of dyadic meetings was then repeated for five more weeks, resulting in six pairings for each combination of dyad partners or 36 dyads per animal. Each animal participated in two, 20-minute dyads per day, balanced for testing order and time of day. The identity of the focal animal alternated every 5 minutes during a single 20-minute session, resulting in 72 focal observations per animal (360 minutes total observation time per subject).
Social Group Context
Weekly social group observations were conducted within each cohort’s home enclosure in order to evaluate the frequency of stereotypies under normative conditions. Each subject was observed via 5-minute focal observations on 34 separate occasions (170 minutes total observation time per subject).
Scoring Methods
The presence of stereotypies was assessed using a behavioral ethogram of 11 abnormal behaviors known to exist in laboratory rhesus macaques (Berkson, 1968; Capitanio, 1986) (see Table 2). Stereotypies by definition are repetitive behaviors that often occur in rapid succession which makes them challenging to define and accurately quantify (Gardenier, MacDonald, & Green, 2004). Given that stereotypies generally occur as “bouts” of repetitive behavior, we have adopted a scoring procedure that requires two or more repetitions of a target behavior or repetition of a target behavior for more than 3 seconds to be scored as a stereotypy. Likewise, our scoring procedures require a stereotypy to cease for 3 seconds before another stereotypy can be scored. This approach produces reliable indices of stereotypy bouts for the majority of repetitive behaviors. It is important to note, however, that the frequency of head-twisting may reflect inflated values as an artifact of scoring protocols. Head-twists are often observed while an animal is pacing back and forth, occurring each time the animals reaches the end of a cage and turns. In this scenario, the continuous pacing would be scored as one stereotypy (i.e., pacing must stop for 3 seconds before it can be scored again). However, if the time in between turns is longer than three seconds then each head-twist is scored as a discrete behavior. In order to assess any impact that the inflated frequency of head-twisting may have had on the total number of stereotypies, we analyzed the data with and without this category of stereotypy. The overall results (i.e., lesioned subjects produce more stereotypies than controls) were similar with and without the head-twist data, therefore we only present findings that include head-twisting.
Statistical Analysis
Behavioral data were collected with The Observer software (Noldus, Sterling, VA; (Noldus, 1991) by trained observers demonstrating an inter-observer reliability ≥ 90% (agreements/[agreements + disagreements] × 100). All data were transformed using the ln(x+1) transformation to respect normal distribution requirements. Analyses of variance (ANOVAs) followed by Fisher’s protected least significant difference (PLSD) post-hoc tests (with a significance level of p < 0.05) were used for data analyses. Repeated Measures ANOVAs were conducted, with testing context as a within subject factor, in order to assess the effect of testing context (i.e., solo, familiar partner, novel partner, social group) on the expression of stereotypies.
RESULTS
Magnetic Resonance Imaging and Histological Evaluation of Lesions
T2-weighted images of coronal sections are illustrated in previous publications, providing substantial reassurance that the ibotenic acid was injected and was focused in the amygdaloid complex or hippocampal formation (Bauman et al., 2004a,, 2004b). The extent of the targeted lesion was confirmed in one amygdala-lesioned subject that died due to an unrelated illness and whose brain was subjected to histological evaluation of the lesion (see Figure 2 in Bauman et al. 2004b). Analysis of a second series of structural MRIs performed when the subjects were approximately 4 years of age provided additional confirmation of the lesions (see Figure 2 in (Bauman, Toscano, Mason, Lavenex, & Amaral, 2006). Qualitative assessment of the lesion extent revealed that all eight amygdala-lesioned subjects demonstrated substantial bilateral damage to the amygdaloid complex, as indicated by clear shrinkage of the amygdala and/or expansion of the ventricles into space formerly occupied by the amygdala. If there was any sparing of amygdala tissue, it was limited to the most caudal aspects of the amygdala, perhaps including the central nucleus. Analysis of the hippocampus lesions revealed nearly complete bilateral damage for all cases, with minimal sparing of the extreme rostral and caudal portions. These qualitative observations of the lesion extent are further supported by recent PET neuroimaging of these subjects (Machado, Snyder, Cherry, Lavenex, & Amaral, in press).
Presentation of Findings
Data were analyzed and are presented as the average number of stereotypies per five minute observation (Table 3). The frequency of stereotypies was not normally distributed and contained a number of zero values. Therefore, a ln (X+1) transformation was performed in order to normalize the data and respect theoretical assumptions prior to statistical analyses. The graphs represent the non-transformed data in order to better illustrate the animals’ actual behavior.
Table 3.
Stereotypy Mean Table
| Context | Stereotypy | AMY Mean | HIP Mean | CON Mean | Lesion Effect | Post Hoc |
|---|---|---|---|---|---|---|
| ALL CONTEXTS | All Stereotypies | 1.831 ±0.644 | 3.705 ±1.723 | 0.137 ±0.068 | (F(2,21) =4.819, p=0.0189) |
H>C (p=0.0072) A>C (p=0.0348) |
| ALL CONTEXTS | Whole-body | 0.386 ±0.153 | 0.372 ±0.082 | 0.064 ±0.014 | (F(2,21) =3.931, p=0.0355) | H>C (p=0.0289) A>C (p=0.0206) |
| ALL CONTEXTS | Self-directed | 1.419 ±0.589 | 0.287 ±0.121 | 0.011 ±0.007 | (F(2,21) =6.049, p=0.0084) | A>C (p=0.0027) A>H (p= 0.0295) |
| ALL CONTEXTS | Head-twist | 0.022 ±0.012 | 3.023 ±1.670 | 0.062 ±0.062 | (F(2,21) =4.196, p=0.0293) | H>C (p=0.0225) H>A (p=0.0185) |
| SOLO OBSERVATIONS | All Stereotypies | 2.013 ±0.755 | 6.113 ±2.252 | 0.106 ±0.059 | (F(2,21) =8.519, p=0.0020) |
H>C (p=0.0005) A>C (p=0.0321) |
| SOLO OBSERVATIONS | Whole-body | 0.244 ±0.134 | 1.100 ±0.272 | 0.069 ±0.048 | (F(2,21) =12.938, p=0.0002) | H>C (p <0.0001) H>A (p=0.0010) |
| SOLO OBSERVATIONS | Self-directed | 1.750 ±0.770 | 0.575 ±0.451 | 0.000 ±0.000 | (F(2,21) =4.349, p=0.0263) | A>C (p=0.0082) |
| SOLO OBSERVATIONS | Head-twist | 0.019 ±0.019 | 4.438 ±1.995 | 0.037 ±0.037 | (F(2,21) =6.378, p=0.0068) | H>C (p=0.0058) H>A (p=0.0052) |
| FAMILIAR DYADS | All Stereotypies | 2.463 ±0.988 | 3.031 ±1.459 | 0.063 ±0.031 | (F(2,21) =5.285, p=0.0138) |
H>C (p=0.0099) A>C (p=0.0108) |
| FAMILIAR DYADS | Whole-body | 0.481 ±0.235 | 0.469 ±0.210 | 0.013 ±0.008 | (F(2,21) =3.002, p=0.0713) | -------------- |
| FAMILIAR DYADS | Self-directed | 1.875 ±0.967 | 0.487 ±0.155 | 0.038 ±0.025 | (F(2,21) =4.331, p=0.0266) | A>C (p=0.0078) |
| FAMILIAR DYADS | Head-twist | 0.075 ±0.044 | 2.075 ±1.289 | 0.013 ±0.012 | (F(2,21) =2.947, p=0.0745) | -------------- |
| NOVEL DYADS | All Stereotypies | 1.819 ±0.701 | 4.486 ±2.859 | 0.153 ±0.081 | (F(2,21) =2.659, p=0.0934) | -------------- |
| NOVEL DYADS | Whole-body | 0.417 ±0.205 | 0.222 ±0.061 | 0.068 ±0.031 | (F(2,21) =1.860, p=0.1805) | -------------- |
| NOVEL DYADS | Self-directed | 1.391 ±0.613 | 0.186 ±0.094 | 0.000 ±0.000 | (F(2,21) =5.471, p=0.0122) | A>C (p=0.0047) A>H (p=0.0240) |
| NOVEL DYADS | Head-twist | 0.012 ±0.006 | 4.030 ±2.787 | 0.085 ±0.085 | (F(2,21) =2.841, p=0.0809) | -------------- |
| SOCIAL GROUPS | All Stereotypies | 1.379 ±0.491 | 1.033 ±0.328 | 0.165 ±0.098 | (F(2,21) =4.609, p=0.0219) |
A>C (p=0.0091) H>C (p=0.0329) |
| SOCIAL GROUPS | Whole-body | 0.349 ±0.091 | 0.202 ±0.041 | 0.085 ±0.045 | (F(2,21) =4.286, p=0.0275) | A>C (p=0.0080) |
| SOCIAL GROUPS | Self-directed | 1.015 ±0.445 | 0.213 ±0.144 | 0.026 ±0.017 | (F(2,21) =5.408, p=0.0128) | A>C (p=0.0048) A>H (p=0.0270) |
| SOCIAL GROUPS | Head-twist | 0.015 ±0.011 | 0.618 ±0.308 | 0.055 ±0.055 | (F(2,21) =3.335, p=0.0552) | -------------- |
Average number (frequency) of self-directed, whole-body and all stereotypies ± SEM is shown for All Contexts Combined, Solo Observations, Familiar and Novel Dyads and Social Groups per amygdala-lesioned subjects (AMY), hippocampus-lesioned subjects (HIP), and sham-operated controls (CON).
We analyzed the frequency of stereotypies across all contexts and then within each individual testing context (solo, familiar dyads, novel dyads and social groups). We began by analyzing the overall frequency of stereotypies (termed “All Stereotypies” in graphs and tables) for lesion group differences. We then subdivided the stereotypies into three broad categories based on previous descriptions of repetitive behaviors in nonhuman primates (Lutz et al., 2003): 1) Whole-body stereotyped behaviors are active, often repetitive, movements of the animal’s entire body (e.g., back flip, bounce, pace, spin and swing referred to as “Whole Body” in graphs and tables) and 2) Self-directed stereotyped behaviors are directed to the animal’s own body (e.g., rock, salute, self bite, self clasp and other self-directed behaviors referred to as “Self-directed” in graphs and tables) and 3) Non-categorical (head-twisting). In keeping with the definitions of Novak and colleagues (Lutz et al., 2003), we did not include the head-twist stereotypy in either the whole-body or self-directed categories. Finally, we analyzed each individual stereotypy separately for lesion group differences, across all contexts and within each context.
Re-evaluation of Videos from 12 Months of Age
Using the more sensitive ethogram designed for this study to assess discrete classes of stereotypies, we again found no lesion differences among the experimental groups (F(2,21) = 0.851, p = 0.4410) when they were approximately 12 months of age. Only four of the twenty-four subjects produced a consistently identifiable stereotypy at 12 months of age (i.e., averaging at least one stereotypy per five minute observation). These four subjects included one amygdala-lesioned subject, two hippocampus-lesioned subjects and one control subject. The control subject’s repertoire of stereotypies was limited to head-twisting, while the amygdala-lesioned subject demonstrated primarily self-directed stereotypies and the hippocampus-lesioned subjects produced head-twisting combined with swinging and pacing.
All Contexts Combined
Amygdala- and hippocampus-lesioned subjects displayed more total stereotypies (F(2,21) = 4.819, p = 0.0189) than control subjects when all contexts are combined (p = 0.0072, p = 0.0348, respectively) (Figure 1). Both amygdala-lesioned and hippocampus-lesioned subjects displayed more whole-body stereotypies (F(2,21) = 3.931, p = 0.0355) than control subjects (p = 0.0206, p = 0.0289, respectively) (Figure 2a). In contrast, amygdala-lesioned subjects exhibited more self-directed stereotypies (F(2,21) = 6.049, p = 0.0084) than control and hippocampus-lesioned subjects (p = 0.0027 and p = 0.0295, respectively) (Figure 2b). Lesion differences were also found for head-twists. Hippocampus-lesioned animals head-twisted (F(2,21) = 4.196, p = 0.0293) more than control and amygdala-lesioned subjects (p = 0.0225 and p = 0.0185, respectively) (Figure 2c).
Figure 1.
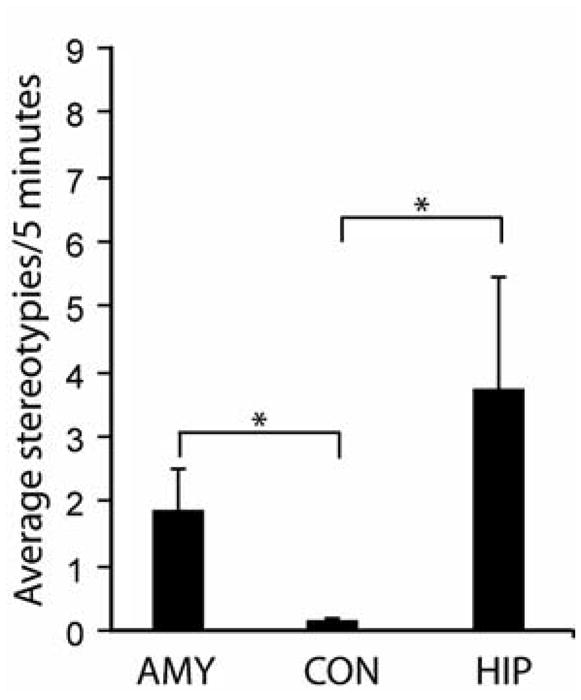
Graph illustrating frequency of stereotypies produced across all behavioral contexts (Mean +/− SEM per 5 min observation period) for all stereotypies combined. Asterisks denote significant post hoc Fisher PLSD test (p< .05).
Figure 2.
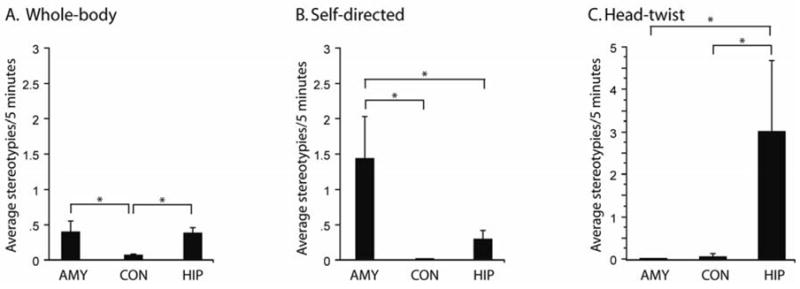
Graph illustrating frequency of stereotypies produced across all behavioral contexts (Mean +/− SEM per 5 min observation period) divided into categories of repetitive behaviors: a) whole body stereotypies, b) self-directed stereotypies, c) head-twist stereotypies. Asterisks denote significant post hoc Fisher PLSD test (p< .05).
Individual Context Differences
Repeated Measures ANOVAs were conducted in order to assess the impact of test context on the overall production of stereotypies. Results revealed a significant effect of test context (F(3,21) = 3.224, p = 0.0284) and a significant interaction of lesion condition and test context (F(6,21) = 2.529, p = 0.0295) on the overall frequency of stereotypies. However, these findings were driven almost entirely by the occurrences of two whole-body stereotypies, pacing and spinning. Both pacing and spinning were affected by the test context (F(3,21) = 8.261, p = 0.0001, F(3,21) = 9.462, p = <0.0001, respectively). Subjects, irrespective of lesion condition, spun more in the group setting compared to the solo, novel dyad and familiar dyadic contexts (p = <0.0001, p = 0.0001, p = 0.0015, respectively). Conversely, subjects paced more in the solo context than in the group setting (p = 0.0037). There was also a significant interaction between lesion condition and context for pacing (F(6,21) = 6.390, p = <0.0001). Hippocampus-lesioned, but not control or amygdala-lesioned subjects, altered the amount of pacing based on the context (F(3,28) = 7.439, p = 0.0008). The hippocampus-lesioned animals paced more in the solo context compared to the group, novel dyad and familiar dyadic contexts (p = 0.0002, p = 0.0008, p = 0.0099, respectively).
DISCUSSION
Delayed Emergence of Stereotypies Following Neonatal Brain Lesions
We have previously reported that monkeys that received lesions of either the amygdala or hippocampus at two weeks of age demonstrated few stereotypies in the first year of life (Bauman et al., 2004a,, 2004b). We have confirmed this finding by re-evaluating videos of the experimental animals at 12 months of age using an ethogram designed to sensitively identify and quantify various stereotyped behaviors. During their second year, it became apparent to staff monitoring these animals that subjects from both lesion groups (but not control subjects) were increasingly producing stereotypies. We therefore formally evaluated the emergence of these behaviors in a variety of experimental conditions, including observations of the juvenile subjects alone, when paired with either familiar or unfamiliar conspecifics and while in their social rearing group. Our observations consistently demonstrated that the amygdala- and hippocampus-lesioned subjects produced more stereotypies than the control subjects (Figure 1) and that the two lesioned groups developed different profiles of stereotypical behaviors (Figure 2). These unique profiles were relatively consistent across different testing paradigms, suggesting that the behavioral context had little influence on the types of stereotypies produced for either lesion group.
Different Profile of Stereotypies for Amygdala and Hippocampus Lesions
Although it may not be surprising that animals with neonatal brain injuries acquire stereotypies after a period of normal development, it is somewhat surprising that damage to the amygdala versus the hippocampus results in a unique profile of repetitive behaviors. Nonhuman primate stereotypies are typically defined as belonging to three broad categories: 1) Whole-body movements (i.e., pacing, bouncing, swinging, etc.), 2) Self-directed movements (i.e., self-clasping, self-biting, etc.) or 3) Non-categorical movements (i.e., head-twisting) (Berkson, 1968; Capitanio, 1986; Lutz et al., 2003). Whole body stereotypies generally involve some form of locomotion, are often associated with small cage size and are potentially reversible; whereas self-directed stereotypies generally do not involve locomotion, are more common in animals that have been socially isolated, and are more resistant to reversal (Mason, 1991). When stereotypies were grouped and analyzed according to these three categories, we found differences between the amygdala- and hippocampus-lesioned subjects. The amygdala-lesioned subjects generally produced a more varied profile of stereotypies compared to hippocampus-lesioned subjects (Table 4). Self-directed stereotypies were more common in the amygdala-lesioned group; two behaviors, salute and self-bite, accounting for more than 65% of the total. In contrast, the hippocampus-lesioned group produced more head twists across testing conditions and more whole-body stereotypies in the solo condition. Although the head twist data appeared to be driven by three of the eight hippocampus-lesioned subjects (Table 4), we have observed that six of the eight hippocampus-lesioned subjects have continued to develop a similar repertoire of stereotypies (consisting primarily of head twisting and pacing) as the subjects reach adulthood (Toscano & Bauman, unpublished observations).
Table 4.
| Lesion Group | ALL | Whole-body | Self-directed | Head-twist |
|---|---|---|---|---|
| AMY SUBJECT #1 | TOTAL = 199 | 90% | 3% | 7% |
| AMY SUBJECT #2 | TOTAL = 57 | 18% | 81% | 2% |
| AMY SUBJECT #3 | TOTAL = 116 | 37% | 53% | 6% |
| AMY SUBJECT #4 | TOTAL = 138 | 1% | 99% | 0% |
| AMY SUBJECT #5* | TOTAL = 762 | 15% | 85% | 0% |
| AMY SUBJECT #6 | TOTAL = 3 | 100% | 0% | 0% |
| AMY SUBJECT #7 | TOTAL = 294 | 9% | 91% | 0% |
| AMY SUBJECT #8 | TOTAL = 570 | 13% | 87% | <1% |
| AMY TOTAL | TOTAL =2139 | 21% | 78% | 1% |
| HIP SUBJECT #1 | TOTAL = 125 | 81% | 18% | <1% |
| HIP SUBJECT #2 | TOTAL = 33 | 67% | 0% | 33% |
| HIP SUBJECT #3 | TOTAL = 634 | 16% | <1% | 83% |
| HIP SUBJECT #4 | TOTAL = 1298 | 5% | 10% | 85% |
| HIP SUBJECT #5 | TOTAL = 163 | 31% | 39% | 30% |
| HIP SUBJECT #6 | TOTAL = 133 | 30% | 70% | 0% |
| HIP SUBJECT #7 | TOTAL = 9 | 89% | 11% | 0% |
| HIP SUBJECT #8 | TOTAL = 1933 | 2% | <1% | 97% |
| HIP TOTAL | TOTAL = 4328 | 10% | 8% | 82% |
| CON SUBJECT #1 | TOTAL = 15 | 100% | 0% | 0% |
| CON SUBJECT #2 | TOTAL = 5 | 100% | 0% | 0% |
| CON SUBJECT #3 | TOTAL = 88 | 17% | 1% | 82% |
| CON SUBJECT #4 | TOTAL = 11 | 100% | 0% | 0% |
| CON SUBJECT #5 | TOTAL = 14 | 100% | 0% | 0% |
| CON SUBJECT #6 | TOTAL = 3 | 100% | 0% | 0% |
| CON SUBJECT #7 | TOTAL = 4 | 0% | 100% | 0% |
| CON SUBJECT #8 | TOTAL = 20 | 60% | 40% | 0% |
| CON SUBJECT | TOTAL = 160 | 47% | 8% | 45% |
Percentage of head-twist, pace, whole-body, self-directed out of total stereotypies exhibited (x/total #) is shown for each amygdala-lesioned subject (AMY), hippocampus-lesioned subject (HIP), and sham-operated control subject (CON) for all Contexts Combined.
Replacement amygdala-lesioned subject reared by mother without access to peers for the first 10 months.
Our observations of different stereotypy repertoires associated with amygdala or hippocampus lesions expands on previous rodent and nonhuman primate models of repetitive behaviors. Rodents with neonatal damage to the amygdala or ventral hippocampus show different behavioral changes in open field paradigms, though these studies have focused on differences in gross locomotor activity rather than specific stereotypies (Daenen, Van der Heyden, Kruse, Wolterink, & Van Ree, 2001; Wolterink et al., 2001). For example, amygdala-lesioned animals demonstrate increased levels of activity characterized by repetitive circling around the perimeter of the test enclosure, while hippocampus-lesioned animals repeatedly explored objects in the center of the open field (Daenen, Wolterink, Gerrits, & Van Ree, 2002).
Previous nonhuman primate lesion models have evaluated the presence of stereotypies following early lesions of medial temporal lobe structures, but have not assessed discrete classes of stereotypies (see Table 5). For example, peer-reared monkeys that received large medial temporal lobe (MTL) ablations early in life (including the amygdala, hippocampus and surrounding cortex) produced locomotor stereotypies and self-directed behaviors when observed at six months of age (Bachevalier, 1994; Bachevalier, Malkova, & Mishkin, 2001). The abnormal behaviors observed following neonatal lesions of the MTL appear to persist into adulthood (Malkova, Mishkin, Suomi, & Bachevalier, 1997). Early onset stereotypies at six months of age were also reported when the lesion was limited to the amygdala and surrounding cortex, but not when the lesion was limited to the hippocampus (Bachevalier, 1994). Although the animals with neonatal hippocampus lesions did not display early onset stereotypies, these subjects did develop locomotor stereotypies in adulthood (Bachevalier, Alvarado, & Malkova, 1999; Beauregard, Malkova, & Bachevalier, 1995).
In contrast to the early onset stereotypies reported by Bachevalier and colleagues, we did not observe differences in the frequency of stereotypies in the first year of life for either the amygdala or hippocampus-lesioned subjects and attribute this difference to the more naturalistic maternal rearing protocol that we have employed (Bauman et al., 2004a, 2004b). We did, however, observe a protracted emergence of stereotypies following neonatal damage to the amygdala or hippocampus that is consistent with the late-onset stereotypies reported for the neonatal hippocampus-lesioned animals in Bachevalier’s later studies. It is important to note that differences in lesion technique (aspiration vs. excitotoxic), rearing conditions and behavioral methodology may preclude direct comparison between results from Bachevalier and colleagues and the present study (see Table 5). For example, our studies utilized ibotenic acid to produce discrete lesions of the amygdala or hippocampus (Bauman et al., 2004a,, 2004b; Bauman et al., 2006). Although observations of these animals is ongoing and histological evaluation of the lesions is not yet possible, previous research indicates that the ibotenic acid lesion technique produces more selective lesions than aspiration lesions by sparing fibers of passage coursing to and from adjacent ventral and medial temporal cortical areas (Meunier, Bachevalier, Murray, Malkova, & Mishkin, 1999) . Our studies have also controlled for social-environmental factors known to contribute to the development of abnormal behaviors in nonhuman primates (i.e., rearing conditions, access to social partners, cage size etc.) (Capitanio, 1986; Lutz et al., 2003). Indeed, the control subjects in our studies displayed more stereotypies than lesioned groups in only one of fifteen testing paradigms conducted in the first year of life (i.e., individual home cage observations between 6–12months of age; (Bauman et al., 2004b). This increase in stereotypies of the control subjects was transient since none of the control subjects have produced consistent stereotypies in any other testing paradigm conducted in the first 7 years of life (Bauman and Toscano, unpublished observations). We thus attribute the development of stereotypies to the lesion condition, rather than socio-environmental restrictions. In contrast, the control subjects in the Bachevalier studies demonstrated stereotypies early in development (Bachevalier, 1994) which became more prominent in adulthood (Bachevalier et al., 1999; Beauregard et al., 1995). Given that the control subjects in the Bachevalier’s studies develop motor stereotypies, it is plausible that the rearing environment may be a contributing factor to the emergence of these behaviors in both lesion and control groups.
Potential Causes of Stereotypies
To assess the underlying cause of delayed-onset stereotypies in the amygdala- and hippocampus-lesioned subjects, it is first necessary to consider factors that are known to induce stereotypies in nonhuman primates. Since it is well known that restricted social and nonsocial environments are associated with behavioral pathology in nonhuman primates, we designed our rearing and housing regimens to facilitate species-typical development within the laboratory environment. Briefly, the subjects in the present study were reared by their mothers for the first six months and experienced daily group socialization in large cages for the first twelve months. All subjects, regardless of lesion condition, developed fundamental aspects of social behavior and displayed species-typical mother-infant and peer interactions (Bauman et al., 2004a,, 2004b). After the first year, the subjects were permanently socially housed with their original rearing group. As discussed above, the control subjects never exhibited pronounced stereotypies, indicating that this rearing strategy provided an environment conducive to species-typical behavioral development.
In spite of these efforts, it is plausible that other factors that we were unable to control may have affected the emergence of stereotypies in the amygdala and hippocampus-lesioned subjects. It has been demonstrated that stress-inducing events (such as frequent immobilization or blood draws) can be associated with stereotypies in laboratory animals (Lutz et al., 2003; Rapp, Vollmer, St Peter, Dozier, & Cotnoir, 2004). Though we have designed our housing protocols and experiments to minimize such known stressors, it is plausible that subjects with neonatal lesions perceive and respond to stress differently than control subjects. In this scenario, the subjects with neonatal brain lesions may develop stereotypies as a coping mechanism for events they perceive as stressful. It is unclear what events may have triggered the onset of stereotypies in both amygdala- and hippocampus-lesioned subjects in the second year of life. The only substantial change in environment between the first year (when stereotypies were rare) and the second year (when stereotypies were pronounced in the subjects with neonatal lesions) was a change in housing from daily 3 hour periods of socialization with members of their rearing group to permanent 24 hour housing with the same group beginning at 12 months of age. While we presume that increased cage size and socialization time is beneficial to all the subjects, we cannot rule out the possibility that this change in socio-environmental complexity may have triggered a stress response in the neonatally lesioned subjects. We have observed that the amygdala-lesioned subjects (but not the hippocampus-lesioned subjects) were consistently the lowest ranking members of their social group (Bauman et al., 2006). We did not, however, observe any behavioral indications that the amygdala- and hippocampus-lesioned subjects were responding adversely to increased socialization. Indeed, our preliminary reports on social interactions of these subjects indicates that both amygdala- and hippocampus-lesioned subjects produced species-typical behavior during this observation period (Bauman, Toscano, Mason, & Amaral, 2007). Comprehensive assessments of these social interactions will be described in future publications.
Our observation that stereotypies emerge in the second year of life, following a period of relatively normal development, suggests that the emergence of stereotypies was not directly caused by damage of the targeted structures, but was possibly due to aberrant maturation of connections and or related structures. Indeed, rodent models have demonstrated that lesions of the amygdala or hippocampus on postnatal day 7 result in a delayed emergence of motor abnormalities that are not seen following similar lesions produced at postnatal day 21 (Daenen et al., 2001; Daenen et al., 2002; Daenen, Wolterink, Van Der Heyden, Kruse, & Van Ree, 2003; Wolterink et al., 2001). Although the precise neural mechanism underlying these late-emerging behavioral changes are not known, evidence from rodent models indicates that neonatal damage to medial temporal lobe structures alters the development of the prefrontal cortex and the subsequent regulation of subcortical dopamine function (Baca et al., 1998; Brake, Sullivan, Flores, Srivastava, & Gratton, 1999; Lillrank, Lipska, Bachus, Wood, & Weinberger, 1996; Lillrank, Lipska, Kolachana, & Weinberger, 1999; Lipska, al-Amin, & Weinberger, 1998; Lipska, Jaskiw, Chrapusta, Karoum, & Weinberger, 1992; Lipska, Jaskiw, & Weinberger, 1994; Lipska & Weinberger, 1998).
Converging results from nonhuman primate models also indicates that dysfunction of the prefrontal cortex and subcortical dopaminergic system may be a consequence of early damage to medial temporal lobe structures. For example, nonhuman primates with neonatal medial temporal lobe lesions display decreased levels of N-acetyl-aspartate (NAA; a marker of neuronal viability) in the prefrontal cortex relative to both normal controls and animals that received similar lesions as adults (Bertolino et al., 1997). These neonatal lesions were also shown to disrupt the manner in which the dorsolateral prefrontal cortex regulates dopamine release by the caudate nucleus (Saunders, Kolachana, Bachevalier, & Weinberger, 1998). Subcortical dopamine systems have been strongly implicated in the production of repetitive stereotypies in animal models (Bedingfield et al., 1997; Canales & Graybiel, 2000; Presti & Lewis, 2005; Presti et al., 2003; Saka, Goodrich, Harlan, Madras, & Graybiel, 2004). Collectively, these studies provide a possible mechanism to account for the delayed emergence of stereotypies following neonatal lesions of either the amygdala or hippocampus via disruption of cortical regulation of dopaminergic systems.
Clinical Implications
Two main findings have emerged from the present study that may provide insight into the neurobiology of repetitive behavior disorders in humans. First, the emergence of stereotypies in nonhuman primates that sustained neonatal damage to the amygdala or hippocampus illustrates how early insults to the developing brain can produce specific psychopathology following an initial period of relatively normal development. These results are in accord with previous rodent models (Lipska & Weinberger, 2000), and clarify data from previous primate models (Bachevalier, 1994) by controlling for other social-environmental factors that are associated with the emergence of stereotypies. These findings may provide insight into the temporal course of repetitive behaviors in clinical disorders such as autism. Recent evidence from young children with autism indicate that while some repetitive behaviors may be clearly manifested as young as age 2, these behaviors may take different forms and/or worsen later in development (Charman et al., 2005; Chawarska, Klin, Paul, & Volkmar, 2007; Chawarska & Volkmar, 2005; Lord, 1995; MacDonald et al., 2007; Richler, Bishop, Kleinke, & Lord, 2007). Second, we have found that damage to different brain structures results in a unique behavioral profile of stereotypies. Although the pathophysiology of repetitive behaviors is unknown, it seems unlikely that repetitive behavior disorders stem from a single common pathogenesis (Lewis & Bodfish, 1998). Our observations that self-directed stereotypies are more frequently observed following neonatal amygdala damage, while head-twisting appears more closely related to hippocampal damage indicates that a unique pathophysiology may underlie different patterns of repetitive behaviors. Unfortunately, it is not clear how our findings relate to repetitive behaviors in humans. Research on stereotypies in human subjects has often relied on indirect measures (i.e., check lists, rating scales, parental reports, etc.) rather than direct observations of stereotypies (Lewis & Bodfish, 1998). Future research in clinical populations that operationally defines and quantifies discrete classes of stereotypies will provide an essential database to determine which features of repetitive behaviors are common across developmental disorders, and which features are unique to specific disorders (Bodfish et al., 2000). This, in turn, will provide information that is needed to develop and evaluate animal models that may reveal the underlying pathology and suggest effective strategies for clinical intervention.
Acknowledgments
This research was supported by a grant from the National Institute of Mental Health (R37MH57502) and by the base grant of the California National Primate Research Center (RR00169). This work was also supported through the Early Experience and Brain Development Network of the MacArthur Foundation. We thank the veterinary and husbandry staff of the CNPRC for excellent care of the animal subjects. We also thank Pierre Lavenex, Jeffrey Bennett and Pamela Tennant for assistance with surgical procedures, and Melissa Marcucci for assistance with behavioral data collection.
Literature Cited
- Baca SM, Lipska BK, Egan MF, Bachus SE, Ferguson JN, Hyde TM. Effects of prefrontal cortical lesions on neuropeptide and dopamine receptor gene expression in the striatum-accumbens complex. Brain Res. 1998;797(1):55–64. doi: 10.1016/s0006-8993(98)00343-6. [DOI] [PubMed] [Google Scholar]
- Bachevalier J. Medial Temporal Lope Structures and Autism - a Review of Clinical and Experimental Findings. Neuropsychologia. 1994;32(6):627–648. doi: 10.1016/0028-3932(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Alvarado MC, Malkova L. Memory and socioemotional behavior in monkeys after hippocampal damage incurred in infancy or in adulthood. Biol Psychiatry. 1999;46(3):329–339. doi: 10.1016/s0006-3223(99)00123-7. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Malkova L, Mishkin M. Effects of selective neonatal temporal lobe lesions on socioemotional behavior in infant rhesus monkeys (Macaca mulatta) Behavioral Neuroscience. 2001;115(3):545–559. doi: 10.1037//0735-7044.115.3.545. [DOI] [PubMed] [Google Scholar]
- Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of mother-infant interactions after neonatal amygdala lesions in rhesus monkeys. J Neurosci. 2004a;24(3):711–721. doi: 10.1523/JNEUROSCI.3263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of social behavior following neonatal amygdala lesions in rhesus monkeys. J Cogn Neurosci. 2004b;16(8):1388–1411. doi: 10.1162/0898929042304741. [DOI] [PubMed] [Google Scholar]
- Bauman MD, Toscano JE, Mason WA, Amaral DG. Continued analysis of social development in rhesus monkeys with neonatal amygdala lesions. Paper presented at the Society for Neuroscience; San Diego, CA. 2007. [Google Scholar]
- Bauman MD, Toscano JE, Mason WA, Lavenex P, Amaral DG. The expression of social dominance following neonatal lesions of the amygdala or hippocampus in rhesus monkeys (Macaca mulatta) Behav Neurosci. 2006;120(4):749–760. doi: 10.1037/0735-7044.120.4.749. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Malkova L, Bachevalier J. Stereotypies and loss of social affiliation after early hippocampectomy in primates. Neuroreport. 1995;6(18):2521–2526. doi: 10.1097/00001756-199512150-00018. [DOI] [PubMed] [Google Scholar]
- Bedingfield JB, Calder LD, Thai DK, Karler R. The role of the striatum in the mouse in behavioral sensitization to amphetamine. Pharmacol Biochem Behav. 1997;56(2):305–310. doi: 10.1016/s0091-3057(96)00331-0. [DOI] [PubMed] [Google Scholar]
- Berkson G. Development of abnormal stereotyped behaviors. Developmental Psychobiology. 1968;1(2):118–132. [Google Scholar]
- Berkson G. Repetitive stereotyped behaviors. Am J Ment Defic. 1983;88(3):239–246. [PubMed] [Google Scholar]
- Berkson G, Gutermuth L, Baranek G. Relative prevalence and relations among stereotyped and similar behaviors. Am J Ment Retard. 1995;100(2):137–145. [PubMed] [Google Scholar]
- Bertolino A, Saunders RC, Mattay VS, Bachevalier J, Frank JA, Weinberger DR. Altered development of prefrontal neurons in rhesus monkeys with neonatal mesial temporo-limbic lesions: A proton magnetic resonance spectroscopic imaging study. Cerebral Cortex. 1997;7(8):740–748. doi: 10.1093/cercor/7.8.740. [DOI] [PubMed] [Google Scholar]
- Bodfish JW, Symons FJ, Parker DE, Lewis MH. Varieties of repetitive behavior in autism: comparisons to mental retardation. J Autism Dev Disord. 2000;30(3):237–243. doi: 10.1023/a:1005596502855. [DOI] [PubMed] [Google Scholar]
- Brake WG, Sullivan RM, Flores G, Srivastava LK, Gratton A. Neonatal ventral hippocampal lesions attenuate the nucleus accumbens dopamine response to stress: an electrochemical study in the adult rat. Brain Res. 1999;831(1–2):25–32. doi: 10.1016/s0006-8993(99)01477-8. [DOI] [PubMed] [Google Scholar]
- Canales JJ, Graybiel AM. A measure of striatal function predicts motor stereotypy. Nat Neurosci. 2000;3(4):377–383. doi: 10.1038/73949. [DOI] [PubMed] [Google Scholar]
- Capitanio J. Behavioral Pathology. In: Mitchell G, Erwin J, editors. Comparative Primate Biology: Behavior, Conservation, and Ecology. 2A. New York: Alan R. Liss; 1986. pp. 411–454. [Google Scholar]
- Charman T, Taylor E, Drew A, Cockerill H, Brown JA, Baird G. Outcome at 7 years of children diagnosed with autism at age 2: predictive validity of assessments conducted at 2 and 3 years of age and pattern of symptom change over time. J Child Psychol Psychiatry. 2005;46(5):500–513. doi: 10.1111/j.1469-7610.2004.00377.x. [DOI] [PubMed] [Google Scholar]
- Chawarska K, Klin A, Paul R, Volkmar F. Autism spectrum disorder in the second year: stability and change in syndrome expression. J Child Psychol Psychiatry. 2007;48(2):128–138. doi: 10.1111/j.1469-7610.2006.01685.x. [DOI] [PubMed] [Google Scholar]
- Chawarska K, Volkmar F. Autism in infancy and early childhood. In: Volkmar F, Paul R, Klin A, Cohen D, editors. Autism and Pervasive Developmental Disorders. Vol. 1. Hoboken, New Jersey: John Wiley and Sons; 2005. pp. 223–246. [Google Scholar]
- Daenen EW, Van der Heyden JA, Kruse CG, Wolterink G, Van Ree JM. Adaptation and habituation to an open field and responses to various stressful events in animals with neonatal lesions in the amygdala or ventral hippocampus. Brain Res. 2001;918(1–2):153–165. doi: 10.1016/s0006-8993(01)02987-0. [DOI] [PubMed] [Google Scholar]
- Daenen EW, Wolterink G, Gerrits MA, Van Ree JM. Amygdala or ventral hippocampal lesions at two early stages of life differentially affect open field behaviour later in life; an animal model of neurodevelopmental psychopathological disorders. Behav Brain Res. 2002;131(1–2):67–78. doi: 10.1016/s0166-4328(01)00350-3. [DOI] [PubMed] [Google Scholar]
- Daenen EW, Wolterink G, Van Der Heyden JA, Kruse CG, Van Ree JM. Neonatal lesions in the amygdala or ventral hippocampus disrupt prepulse inhibition of the acoustic startle response; implications for an animal model of neurodevelopmental disorders like schizophrenia. Eur Neuropsychopharmacol. 2003;13(3):187–197. doi: 10.1016/s0924-977x(03)00007-5. [DOI] [PubMed] [Google Scholar]
- Gardenier NC, MacDonald R, Green G. Comparison of direct observational methods for measuring stereotypic behavior in children with autism spectrum disorders. Res Dev Disabil. 2004;25(2):99–118. doi: 10.1016/j.ridd.2003.05.004. [DOI] [PubMed] [Google Scholar]
- Lachiewicz AM, Spiridigliozzi GA, Gullion CM, Ransford SN, Rao K. Aberrant behaviors of young boys with fragile X syndrome. Am J Ment Retard. 1994;98(5):567–579. [PubMed] [Google Scholar]
- Lewis MH, Baumeister AA, McCorkle DL, Mailman RB. A computer-supported method for analyzing behavioral observations: studies with stereotypy. Psychopharmacology (Berl) 1985;85(2):204–209. doi: 10.1007/BF00428415. [DOI] [PubMed] [Google Scholar]
- Lewis MH, Bodfish JW. Repetitive behavior disorders in autism. Mental Retardation and Developmental Disabilities Research Reviews. 1998;4:80–89. [Google Scholar]
- Lewis MH, Gluck JP, Beauchamp AJ, Keresztury MF, Mailman RB. Long-term effects of early social isolation in Macaca mulatta: changes in dopamine receptor function following apomorphine challenge. Brain Res. 1990;513(1):67–73. doi: 10.1016/0006-8993(90)91089-y. [DOI] [PubMed] [Google Scholar]
- Lewis MH, Tanimura Y, Lee LW, Bodfish JW. Animal models of restricted repetitive behavior in autism. Behav Brain Res. 2007;176(1):66–74. doi: 10.1016/j.bbr.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillrank SM, Lipska BK, Bachus SE, Wood GK, Weinberger DR. Amphetamine-induced c-fos mRNA expression is altered in rats with neonatal ventral hippocampal damage. Synapse. 1996;23(4):292–301. doi: 10.1002/(SICI)1098-2396(199608)23:4<292::AID-SYN7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Lillrank SM, Lipska BK, Kolachana BS, Weinberger DR. Attenuated extracellular dopamine levels after stress and amphetamine in the nucleus accumbens of rats with neonatal ventral hippocampal damage. J Neural Transm. 1999;106(2):183–196. doi: 10.1007/s007020050150. [DOI] [PubMed] [Google Scholar]
- Lipska BK, al-Amin HA, Weinberger DR. Excitotoxic lesions of the rat medial prefrontal cortex. Effects on abnormal behaviors associated with neonatal hippocampal damage. Neuropsychopharmacology. 1998;19(6):451–464. doi: 10.1016/S0893-133X(98)00045-1. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Jaskiw GE, Chrapusta S, Karoum F, Weinberger DR. Ibotenic acid lesion of the ventral hippocampus differentially affects dopamine and its metabolites in the nucleus accumbens and prefrontal cortex in the rat. Brain Res. 1992;585(1–2):1–6. doi: 10.1016/0006-8993(92)91184-g. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Jaskiw GE, Weinberger DR. The effects of combined prefrontal cortical and hippocampal damage on dopamine-related behaviors in rats. Pharmacol Biochem Behav. 1994;48(4):1053–1057. doi: 10.1016/0091-3057(94)90220-8. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Weinberger DR. Prefrontal cortical and hippocampal modulation of dopamine-mediated effects. Adv Pharmacol. 1998;42:806–809. doi: 10.1016/s1054-3589(08)60869-8. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Weinberger DR. To model a psychiatric disorder in animals: schizophrenia as a reality test. Neuropsychopharmacology. 2000;23(3):223–239. doi: 10.1016/S0893-133X(00)00137-8. [DOI] [PubMed] [Google Scholar]
- Lord C. Follow-up of two-year-olds referred for possible autism. J Child Psychol Psychiatry. 1995;36(8):1365–1382. doi: 10.1111/j.1469-7610.1995.tb01669.x. [DOI] [PubMed] [Google Scholar]
- Lutz C, Well A, Novak M. Stereotypic and self-injurious behavior in rhesus macaques: a survey and retrospective analysis of environment and early experience. Am J Primatol. 2003;60(1):1–15. doi: 10.1002/ajp.10075. [DOI] [PubMed] [Google Scholar]
- MacDonald R, Green G, Mansfield R, Geckeler A, Gardenier N, Anderson J, et al. Stereotypy in young children with autism and typically developing children. Res Dev Disabil. 2007;28(3):266–277. doi: 10.1016/j.ridd.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Snyder AZ, Cherry SR, Lavenex P, Amaral DG. Effects of neonatal amygdala or hippocampal lesions on resting brain metabolism in the macaque monkey: A microPET imaging study. NeuroImage. doi: 10.1016/j.neuroimage.2007.09.029. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova L, Mishkin M, Suomi SJ, Bachevalier J. Socioemotional behavior in adult rhesus monkeys after early versus late lesions of the medial temporal lobe. Ann N Y Acad Sci. 1997;807:538–540. doi: 10.1111/j.1749-6632.1997.tb51961.x. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Spicer DM, Lewis MH, Gluck JP, Cork LC. Social deprivation of infant rhesus monkeys alters the chemoarchitecture of the brain: I. Subcortical regions. J Neurosci. 1991;11(11):3344–3358. doi: 10.1523/JNEUROSCI.11-11-03344.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason GJ. Stereotypies: A critical review. Anim Behav. 1991;41:1015–1037. [Google Scholar]
- Meunier M, Bachevalier J, Murray EA, Malkova L, Mishkin M. Effects of aspiration versus neurotoxic lesions of the amygdala on emotional responses in monkeys. Eur J Neurosci. 1999;11(12):4403–4418. doi: 10.1046/j.1460-9568.1999.00854.x. [DOI] [PubMed] [Google Scholar]
- Noldus LP. The Observer: A software system for collection and analysis of observational data. Behavior Research Methods, Instruments & Computers. 1991;23:415–429. doi: 10.3758/bf03195516. [DOI] [PubMed] [Google Scholar]
- Powell SB, Newman HA, Pendergast JF, Lewis MH. A rodent model of spontaneous stereotypy: initial characterization of developmental, environmental, and neurobiological factors. Physiol Behav. 1999;66(2):355–363. doi: 10.1016/s0031-9384(98)00303-5. [DOI] [PubMed] [Google Scholar]
- Presti MF, Lewis MH. Striatal opioid peptide content in an animal model of spontaneous stereotypic behavior. Behav Brain Res. 2005;157(2):363–368. doi: 10.1016/j.bbr.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Presti MF, Mikes HM, Lewis MH. Selective blockade of spontaneous motor stereotypy via intrastriatal pharmacological manipulation. Pharmacol Biochem Behav. 2003;74(4):833–839. doi: 10.1016/s0091-3057(02)01081-x. [DOI] [PubMed] [Google Scholar]
- Randrup A, Munkvad I. Pharmacology and physiology of stereotyped behavior. J Psychiatr Res. 1974;11:1–10. doi: 10.1016/0022-3956(74)90062-4. [DOI] [PubMed] [Google Scholar]
- Rapp JT, Vollmer TR, St Peter C, Dozier CL, Cotnoir NM. Analysis of response allocation in individuals with multiple forms of stereotyped behavior. J Appl Behav Anal. 2004;37(4):481–501. doi: 10.1901/jaba.2004.37-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richler J, Bishop SL, Kleinke JR, Lord C. Restricted and repetitive behaviors in young children with autism spectrum disorders. J Autism Dev Disord. 2007;37(1):73–85. doi: 10.1007/s10803-006-0332-6. [DOI] [PubMed] [Google Scholar]
- Ridley RM, Baker HF. Stereotypy in monkeys and humans. Psychol Med. 1982;12(1):61–72. doi: 10.1017/s0033291700043294. [DOI] [PubMed] [Google Scholar]
- Rojahn J, Matlock ST, Tasse MJ. The stereotyped behavior scale: psychometric properties and norms. Res Dev Disabil. 2000;21(6):437–454. doi: 10.1016/s0891-4222(00)00057-3. [DOI] [PubMed] [Google Scholar]
- Rojahn J, Tasse MJ, Sturmey P. The Stereotyped Behavior Scale for adolescents and adults with mental retardation. Am J Ment Retard. 1997;102(2):137–146. doi: 10.1352/0895-8017(1997)102<0137:TSBSFA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Saka E, Goodrich C, Harlan P, Madras BK, Graybiel AM. Repetitive behaviors in monkeys are linked to specific striatal activation patterns. J Neurosci. 2004;24(34):7557–7565. doi: 10.1523/JNEUROSCI.1072-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders RC, Kolachana BS, Bachevalier J, Weinberger DR. Neonatal lesions of the medial temporal lobe disrupt prefrontal cortical regulation of striatal dopamine. Nature. 1998;393(6681):169–171. doi: 10.1038/30245. [DOI] [PubMed] [Google Scholar]
- South M, Ozonoff S, McMahon WM. Repetitive behavior profiles in Asperger syndrome and high-functioning autism. J Autism Dev Disord. 2005;35(2):145–158. doi: 10.1007/s10803-004-1992-8. [DOI] [PubMed] [Google Scholar]
- Wales L, Charman T, Mount RH. An analogue assessment of repetitive hand behaviours in girls and young women with Rett syndrome. J Intellect Disabil Res. 2004;48(Pt 7):672–678. doi: 10.1111/j.1365-2788.2003.00590.x. [DOI] [PubMed] [Google Scholar]
- Wolterink G, Daenen LE, Dubbeldam S, Gerrits MA, van Rijn R, Kruse CG, et al. Early amygdala damage in the rat as a model for neurodevelopmental psychopathological disorders. Eur Neuropsychopharmacol. 2001;11(1):51–59. doi: 10.1016/s0924-977x(00)00138-3. [DOI] [PubMed] [Google Scholar]


