Abstract
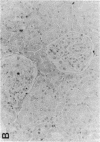
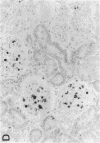
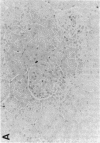
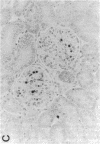
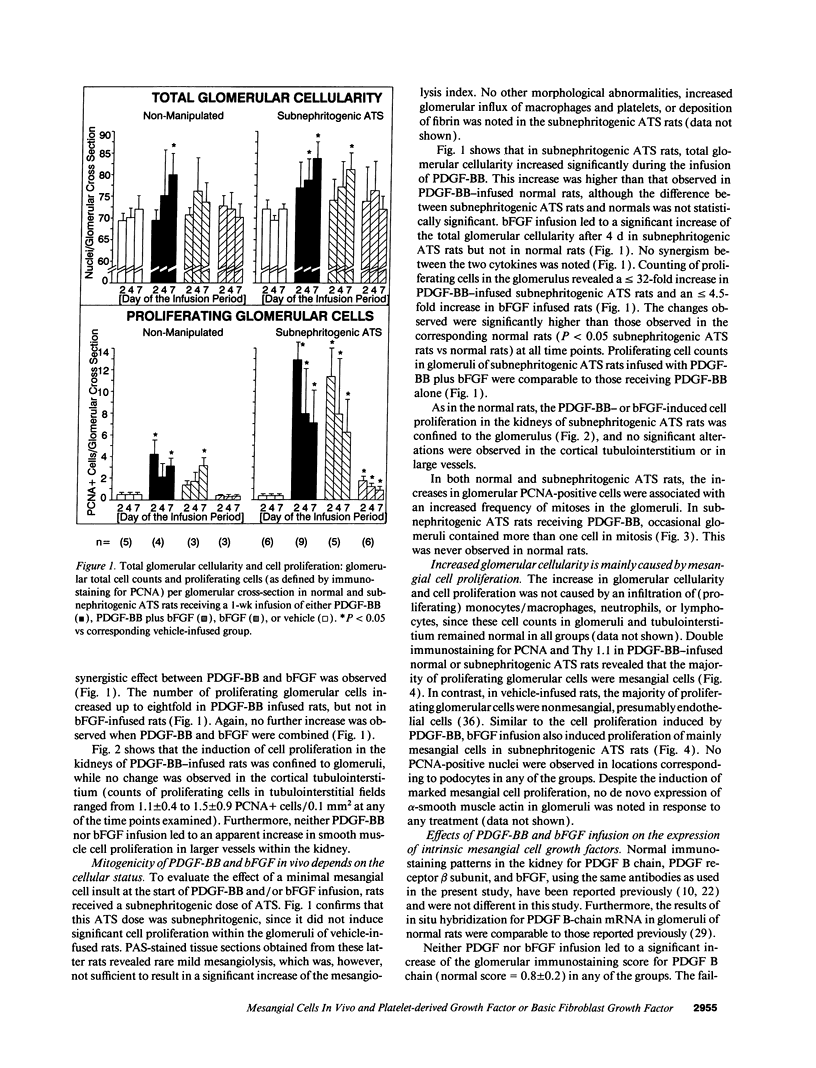
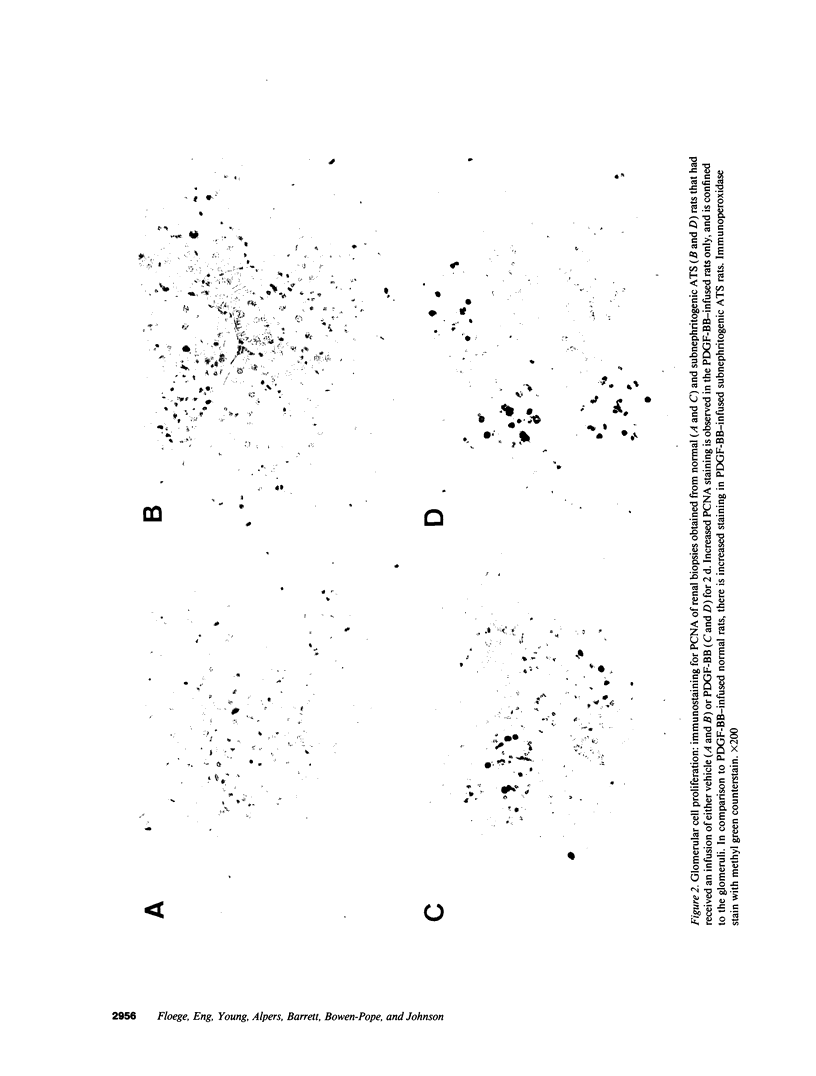
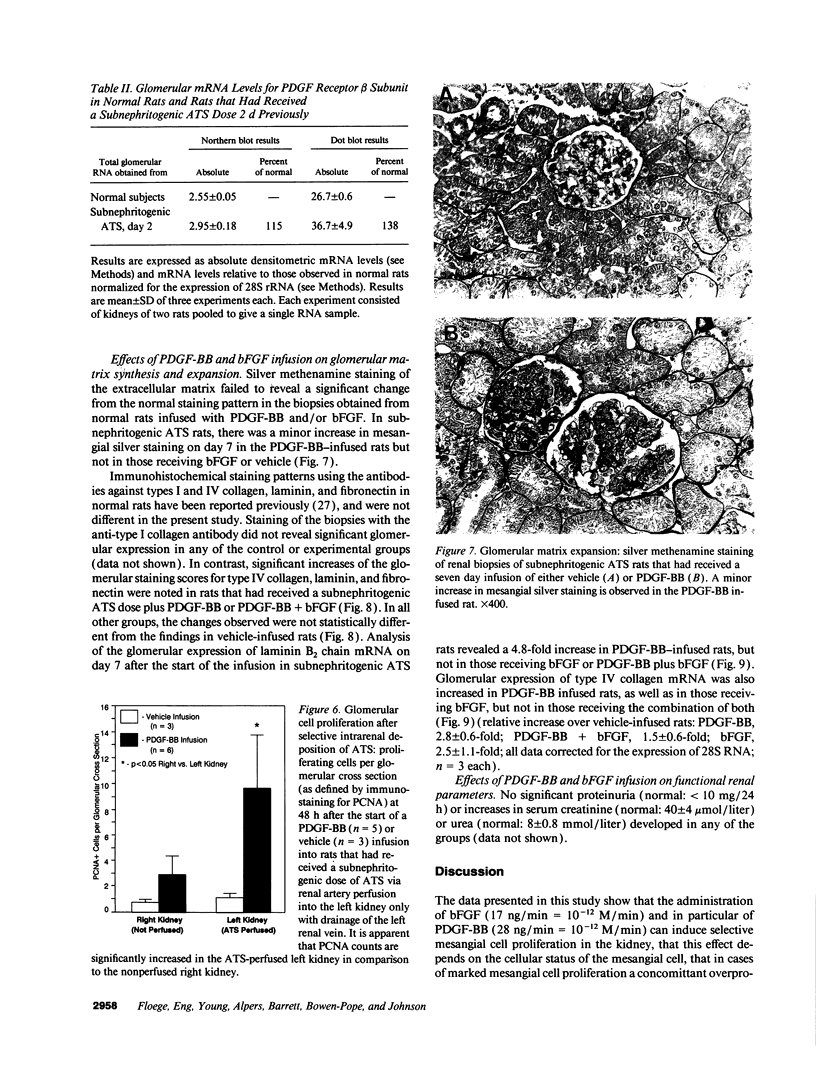
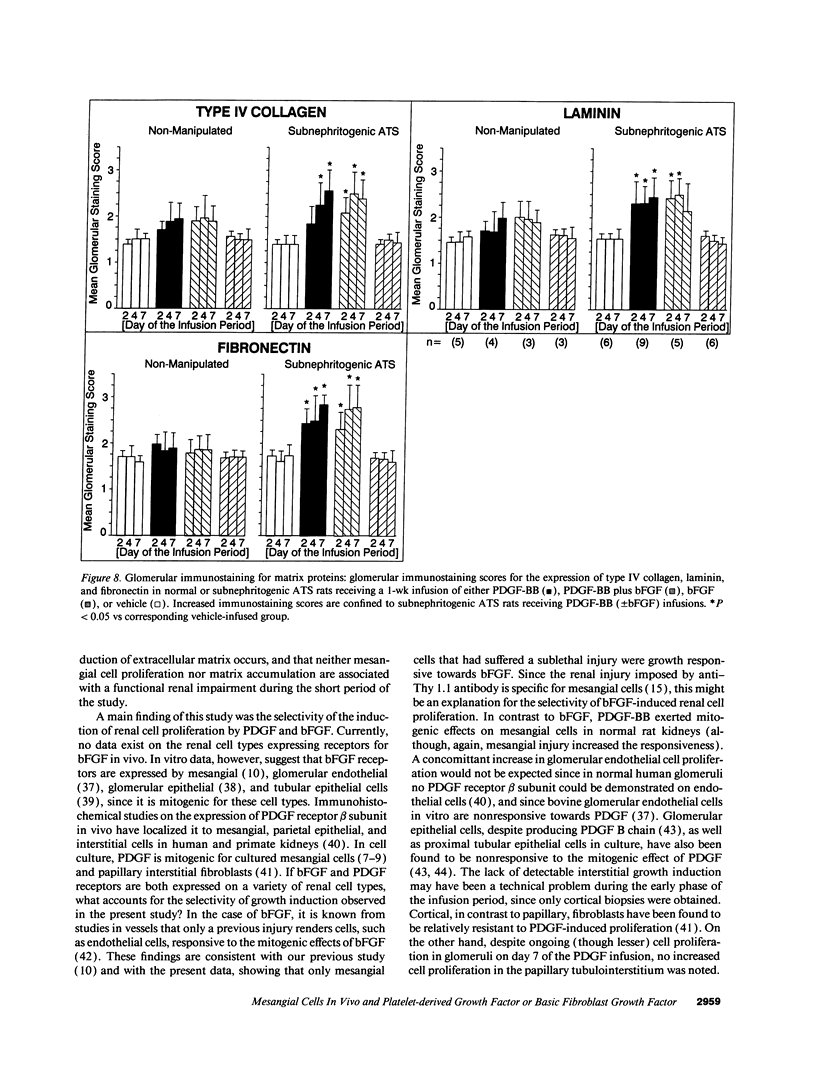
Mesangial cell (MC) proliferation and extracellular matrix expansion are involved in the pathogenesis of glomerulosclerosis and renal failure. In vitro, PDGF and basic fibroblast growth factor (bFGF) regulate MC proliferation and/or matrix production. To elucidate the role of PDGF and bFGF in vivo, equimolar concentrations of recombinant PDGF-BB or bFGF or vehicle were infused intravenously into rats over a 7-d period. Rats were either nonmanipulated ("normals") or had received a subnephritogenic dose of anti-MC antibody ("anti-Thy 1.1 rats") before the infusion period. Glomerular cell proliferation (anti-proliferating cell nuclear antigen immunostaining) on days 2, 4, and 7 was unchanged in vehicle-infused normals or anti-Thy 1.1 rats. PDGF infusion increased glomerular cell proliferation 32-fold in anti-Thy 1.1 rats and an 11-fold in normals on day 2. bFGF increased glomerular cell proliferation fourfold in anti-Thy 1.1 rats but was ineffective in normals. Induction of cell proliferation in all kidneys was limited to the glomerulus. The majority of proliferating cells were identified as MC by double immunolabeling. No significant proteinuria, glomerular leukocyte, or platelet influx developed in any group. Glomerular matrix expansion with increased deposition of type IV collagen, laminin, and fibronectin, as well as upregulated laminin and collagen IV mRNA expression was confined to PDGF-infused anti-Thy 1.1 rats. These results show that PDGF and, to a lesser degree, bFGF are selective MC mitogens in vivo and that previous subclinical injury can enhance this MC response. The data thereby support a role of these cytokines in the pathogenesis of glomerulosclerosis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abboud H. E. Growth factors in glomerulonephritis. Kidney Int. 1993 Jan;43(1):252–267. doi: 10.1038/ki.1993.39. [DOI] [PubMed] [Google Scholar]
- Alpers C. E., Hudkins K. L., Gown A. M., Johnson R. J. Enhanced expression of "muscle-specific" actin in glomerulonephritis. Kidney Int. 1992 May;41(5):1134–1142. doi: 10.1038/ki.1992.173. [DOI] [PubMed] [Google Scholar]
- Alpers C. E., Seifert R. A., Hudkins K. L., Johnson R. J., Bowen-Pope D. F. PDGF-receptor localizes to mesangial, parietal epithelial, and interstitial cells in human and primate kidneys. Kidney Int. 1993 Feb;43(2):286–294. doi: 10.1038/ki.1993.45. [DOI] [PubMed] [Google Scholar]
- Bagchus W. M., Jeunink M. F., Elema J. D. The mesangium in anti-Thy-1 nephritis. Influx of macrophages, mesangial cell hypercellularity, and macromolecular accumulation. Am J Pathol. 1990 Jul;137(1):215–223. [PMC free article] [PubMed] [Google Scholar]
- Bagchus W. M., Jeunink M. F., Rozing J., Elema J. D. A monoclonal antibody against rat platelets. I. Tissue distribution in vitro and in vivo. Clin Exp Immunol. 1989 Feb;75(2):317–323. [PMC free article] [PubMed] [Google Scholar]
- Barclay A. N. Different reticular elements in rat lymphoid tissue identified by localization of Ia, Thy-1 and MRC OX 2 antigens. Immunology. 1981 Dec;44(4):727–736. [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dijkstra C. D., Döpp E. A., Joling P., Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985 Mar;54(3):589–599. [PMC free article] [PubMed] [Google Scholar]
- Doi T., Vlassara H., Kirstein M., Yamada Y., Striker G. E., Striker L. J. Receptor-specific increase in extracellular matrix production in mouse mesangial cells by advanced glycosylation end products is mediated via platelet-derived growth factor. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2873–2877. doi: 10.1073/pnas.89.7.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floege J., Burns M. W., Alpers C. E., Yoshimura A., Pritzl P., Gordon K., Seifert R. A., Bowen-Pope D. F., Couser W. G., Johnson R. J. Glomerular cell proliferation and PDGF expression precede glomerulosclerosis in the remnant kidney model. Kidney Int. 1992 Feb;41(2):297–309. doi: 10.1038/ki.1992.42. [DOI] [PubMed] [Google Scholar]
- Floege J., Eng E., Lindner V., Alpers C. E., Young B. A., Reidy M. A., Johnson R. J. Rat glomerular mesangial cells synthesize basic fibroblast growth factor. Release, upregulated synthesis, and mitogenicity in mesangial proliferative glomerulonephritis. J Clin Invest. 1992 Dec;90(6):2362–2369. doi: 10.1172/JCI116126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floege J., Eng E., Young B. A., Johnson R. J. Factors involved in the regulation of mesangial cell proliferation in vitro and in vivo. Kidney Int Suppl. 1993 Jan;39:S47–S54. [PubMed] [Google Scholar]
- Floege J., Johnson R. J., Alpers C. E., Fatemi-Nainie S., Richardson C. A., Gordon K., Couser W. G. Visceral glomerular epithelial cells can proliferate in vivo and synthesize platelet-derived growth factor B-chain. Am J Pathol. 1993 Feb;142(2):637–650. [PMC free article] [PubMed] [Google Scholar]
- Floege J., Johnson R. J., Gordon K., Iida H., Pritzl P., Yoshimura A., Campbell C., Alpers C. E., Couser W. G. Increased synthesis of extracellular matrix in mesangial proliferative nephritis. Kidney Int. 1991 Sep;40(3):477–488. doi: 10.1038/ki.1991.235. [DOI] [PubMed] [Google Scholar]
- Floege J., Topley N., Hoppe J., Barrett T. B., Resch K. Mitogenic effect of platelet-derived growth factor in human glomerular mesangial cells: modulation and/or suppression by inflammatory cytokines. Clin Exp Immunol. 1991 Nov;86(2):334–341. doi: 10.1111/j.1365-2249.1991.tb05819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouser L., Iruela-Arispe L., Bornstein P., Sage E. H. Transcriptional activity of the alpha 1(I)-collagen promoter is correlated with the formation of capillary-like structures by endothelial cells in vitro. J Biol Chem. 1991 Sep 25;266(27):18345–18351. [PubMed] [Google Scholar]
- Humes H. D., Beals T. F., Cieslinski D. A., Sanchez I. O., Page T. P. Effects of transforming growth factor-beta, transforming growth factor-alpha, and other growth factors on renal proximal tubule cells. Lab Invest. 1991 Apr;64(4):538–545. [PubMed] [Google Scholar]
- Iida H., Seifert R., Alpers C. E., Gronwald R. G., Phillips P. E., Pritzl P., Gordon K., Gown A. M., Ross R., Bowen-Pope D. F. Platelet-derived growth factor (PDGF) and PDGF receptor are induced in mesangial proliferative nephritis in the rat. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6560–6564. doi: 10.1073/pnas.88.15.6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iruela-Arispe M. L., Diglio C. A., Sage E. H. Modulation of extracellular matrix proteins by endothelial cells undergoing angiogenesis in vitro. Arterioscler Thromb. 1991 Jul-Aug;11(4):805–815. doi: 10.1161/01.atv.11.4.805. [DOI] [PubMed] [Google Scholar]
- Issandou M., Darbon J. M. Basic fibroblast growth factor stimulates glomerular mesangial cell proliferation through a protein kinase C-independent pathway. Growth Factors. 1991;5(4):255–264. doi: 10.3109/08977199109000289. [DOI] [PubMed] [Google Scholar]
- Johnson R. J., Floege J., Couser W. G., Alpers C. E. Role of platelet-derived growth factor in glomerular disease. J Am Soc Nephrol. 1993 Aug;4(2):119–128. doi: 10.1681/ASN.V42119. [DOI] [PubMed] [Google Scholar]
- Johnson R. J., Floege J., Yoshimura A., Iida H., Couser W. G., Alpers C. E. The activated mesangial cell: a glomerular "myofibroblast"? J Am Soc Nephrol. 1992 Apr;2(10 Suppl):S190–S197. doi: 10.1681/ASN.V210s190. [DOI] [PubMed] [Google Scholar]
- Johnson R. J., Garcia R. L., Pritzl P., Alpers C. E. Platelets mediate glomerular cell proliferation in immune complex nephritis induced by anti-mesangial cell antibodies in the rat. Am J Pathol. 1990 Feb;136(2):369–374. [PMC free article] [PubMed] [Google Scholar]
- Johnson R. J., Iida H., Alpers C. E., Majesky M. W., Schwartz S. M., Pritzi P., Gordon K., Gown A. M. Expression of smooth muscle cell phenotype by rat mesangial cells in immune complex nephritis. Alpha-smooth muscle actin is a marker of mesangial cell proliferation. J Clin Invest. 1991 Mar;87(3):847–858. doi: 10.1172/JCI115089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. J., Klebanoff S. J., Ochi R. F., Adler S., Baker P., Sparks L., Couser W. G. Participation of the myeloperoxidase-H2O2-halide system in immune complex nephritis. Kidney Int. 1987 Sep;32(3):342–349. doi: 10.1038/ki.1987.215. [DOI] [PubMed] [Google Scholar]
- Johnson R. J., Raines E. W., Floege J., Yoshimura A., Pritzl P., Alpers C., Ross R. Inhibition of mesangial cell proliferation and matrix expansion in glomerulonephritis in the rat by antibody to platelet-derived growth factor. J Exp Med. 1992 May 1;175(5):1413–1416. doi: 10.1084/jem.175.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klahr S., Schreiner G., Ichikawa I. The progression of renal disease. N Engl J Med. 1988 Jun 23;318(25):1657–1666. doi: 10.1056/NEJM198806233182505. [DOI] [PubMed] [Google Scholar]
- Knecht A., Fine L. G., Kleinman K. S., Rodemann H. P., Müller G. A., Woo D. D., Norman J. T. Fibroblasts of rabbit kidney in culture. II. Paracrine stimulation of papillary fibroblasts by PDGF. Am J Physiol. 1991 Aug;261(2 Pt 2):F292–F299. doi: 10.1152/ajprenal.1991.261.2.F292. [DOI] [PubMed] [Google Scholar]
- Kurki P., Vanderlaan M., Dolbeare F., Gray J., Tan E. M. Expression of proliferating cell nuclear antigen (PCNA)/cyclin during the cell cycle. Exp Cell Res. 1986 Sep;166(1):209–219. doi: 10.1016/0014-4827(86)90520-3. [DOI] [PubMed] [Google Scholar]
- Lindner V., Majack R. A., Reidy M. A. Basic fibroblast growth factor stimulates endothelial regrowth and proliferation in denuded arteries. J Clin Invest. 1990 Jun;85(6):2004–2008. doi: 10.1172/JCI114665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madri J. A., Marx M. Matrix composition, organization and soluble factors: modulators of microvascular cell differentiation in vitro. Kidney Int. 1992 Mar;41(3):560–565. doi: 10.1038/ki.1992.82. [DOI] [PubMed] [Google Scholar]
- Montinaro V., Hevey K., Aventaggiato L., Fadden K., Esparza A., Chen A., Finbloom D. S., Rifai A. Extrarenal cytokines modulate the glomerular response to IgA immune complexes. Kidney Int. 1992 Aug;42(2):341–353. doi: 10.1038/ki.1992.295. [DOI] [PubMed] [Google Scholar]
- Pabst R., Sterzel R. B. Cell renewal of glomerular cell types in normal rats. An autoradiographic analysis. Kidney Int. 1983 Nov;24(5):626–631. doi: 10.1038/ki.1983.203. [DOI] [PubMed] [Google Scholar]
- Poelstra K., Hardonk M. J., Koudstaal J., Bakker W. W. Intraglomerular platelet aggregation and experimental glomerulonephritis. Kidney Int. 1990 Jun;37(6):1500–1508. doi: 10.1038/ki.1990.141. [DOI] [PubMed] [Google Scholar]
- Reilly T. M., Taylor D. S., Herblin W. F., Thoolen M. J., Chiu A. T., Watson D. W., Timmermans P. B. Monoclonal antibodies directed against basic fibroblast growth factor which inhibit its biological activity in vitro and in vivo. Biochem Biophys Res Commun. 1989 Oct 31;164(2):736–743. doi: 10.1016/0006-291x(89)91521-0. [DOI] [PubMed] [Google Scholar]
- Sasaki M., Yamada Y. The laminin B2 chain has a multidomain structure homologous to the B1 chain. J Biol Chem. 1987 Dec 15;262(35):17111–17117. [PubMed] [Google Scholar]
- Sekiya S., Gotoh S., Yamashita T., Watanabe T., Saitoh S., Sendo F. Selective depletion of rat neutrophils by in vivo administration of a monoclonal antibody. J Leukoc Biol. 1989 Aug;46(2):96–102. doi: 10.1002/jlb.46.2.96. [DOI] [PubMed] [Google Scholar]
- Shiraishi T., Morimoto S., Itoh K., Sato H., Sugihara K., Onishi T., Ogihara T. Radioimmunoassay of human platelet-derived growth factor using monoclonal antibody toward a synthetic 73-97 fragment of its B-chain. Clin Chim Acta. 1989 Sep 15;184(1):65–74. doi: 10.1016/0009-8981(89)90257-x. [DOI] [PubMed] [Google Scholar]
- Shultz P. J., DiCorleto P. E., Silver B. J., Abboud H. E. Mesangial cells express PDGF mRNAs and proliferate in response to PDGF. Am J Physiol. 1988 Oct;255(4 Pt 2):F674–F684. doi: 10.1152/ajprenal.1988.255.4.F674. [DOI] [PubMed] [Google Scholar]
- Silver B. J., Jaffer F. E., Abboud H. E. Platelet-derived growth factor synthesis in mesangial cells: induction by multiple peptide mitogens. Proc Natl Acad Sci U S A. 1989 Feb;86(3):1056–1060. doi: 10.1073/pnas.86.3.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalli O., Ropraz P., Trzeciak A., Benzonana G., Gillessen D., Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986 Dec;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striker L. J., Peten E. P., Elliot S. J., Doi T., Striker G. E. Mesangial cell turnover: effect of heparin and peptide growth factors. Lab Invest. 1991 Apr;64(4):446–456. [PubMed] [Google Scholar]
- Takeuchi A., Yoshizawa N., Yamamoto M., Sawasaki Y., Oda T., Senoo A., Niwa H., Fuse Y. Basic fibroblast growth factor promotes proliferation of rat glomerular visceral epithelial cells in vitro. Am J Pathol. 1992 Jul;141(1):107–116. [PMC free article] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Yamamoto K., Kawasaki K., Yaoita E., Shimizu F., Kihara I. Immunoelectron microscopic demonstration of Thy-1 antigen on the surfaces of mesangial cells in the rat glomerulus. Nephron. 1986;43(4):293–298. doi: 10.1159/000183857. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Escobedo J. A., Kuang W. J., Yang-Feng T. L., Daniel T. O., Tremble P. M., Chen E. Y., Ando M. E., Harkins R. N., Francke U. Structure of the receptor for platelet-derived growth factor helps define a family of closely related growth factor receptors. Nature. 1986 Sep 18;323(6085):226–232. doi: 10.1038/323226a0. [DOI] [PubMed] [Google Scholar]
- Yoshimura A., Gordon K., Alpers C. E., Floege J., Pritzl P., Ross R., Couser W. G., Bowen-Pope D. F., Johnson R. J. Demonstration of PDGF B-chain mRNA in glomeruli in mesangial proliferative nephritis by in situ hybridization. Kidney Int. 1991 Sep;40(3):470–476. doi: 10.1038/ki.1991.234. [DOI] [PubMed] [Google Scholar]
- Zhang G. H., Ichimura T., Wallin A., Kan M., Stevens J. L. Regulation of rat proximal tubule epithelial cell growth by fibroblast growth factors, insulin-like growth factor-1 and transforming growth factor-beta, and analysis of fibroblast growth factors in rat kidney. J Cell Physiol. 1991 Aug;148(2):295–305. doi: 10.1002/jcp.1041480216. [DOI] [PubMed] [Google Scholar]