Abstract
The objective of this study was to investigate the potential of various formulation strategies to achieve 1-month continuous (improved) release of the novel anti-cancer drug, 2-methoxyestradiol (2-ME), from injectable cylindrical poly(dl-lactide-co-glycolide) (PLGA) implants. PLGA implants were prepared by a solvent extrusion method. PLGA 50:50 (Mw = 51 kDa, end group = lauryl ester) (PLGA–lauryl ester) implants loaded with 3–30 wt% 2-ME exhibited a pronounced lag phase (i.e., corresponding to induction time to polymer mass loss) and triphasic release profile. Incorporation of 5 wt% hydroxypropyl-β-cyclodextrin (HP-β-CD) (~57% release after 28 days) or Pluronic® F127 (~42% release after 28 days) in PLGA–lauryl ester implants reduced the lag-phase and improved the drug release moderately over a period of 28 days. The formation and the incorporation of a 2-ME/polyethylene glycol (PEG) 8000 solid dispersion in PLGA–lauryl ester implants further increased drug release (~21% and 73% release after 1 and 28 days, respectively), attributable to improved drug solubility/dissolution, higher matrix porosity, and accelerated polymer degradation. Blending of PLGA 50:50 (Mw = 24 kDa, end group = COOH) (PLGA–COOH) with the PLGA–lauryl ester also provided moderate enhancement of 2-ME release over a period of 28 days. PLGA–COOH (Mw = 24 kDa) implants with 3–5% w/w pore-forming MgCO3 exhibited the most desirable drug release among all the formulations tested, and, demonstrated 1-month slow and continuous in vitro release of ~80% 2-ME after a minimal initial burst. Hence, these formulation approaches provide several possible avenues to improve release rates of the hydrophobic drug, 2-ME, from PLGA for future application in regional anti-cancer therapy.
Keywords: 2-Methoxyestradiol, Controlled drug release, Cylindrical PLGA injectable implants, Effect of water soluble additives, Solid dispersion, Polymer blending, Effect of MgCO3
1. Introduction
2-Methoxyestradiol (2-ME), a metabolite of 17-β-estradiol, inhibits tumor growth, neovascularization, and growth of cancer cells (angiosarcoma, gastric, cervical, colorectal, hepatocellular, prostate, lung, pancreatic, breast, neuroblastoma, leukemia, multiple myeloma) [1–6]. In addition to inhibiting the proliferation of cancer cells, 2-ME also possesses anti-angiogenic properties [7]. 2-ME induces changes in the levels and activities of many proteins involved in regulation of the cell cycle, including stress kinases, cell division kinases, cyclin B, and regulators of cell cycle arrest and apoptosis [4]. 2-ME has been shown to inhibit growth of ER+ and ER− breast cancer cell lines and HeLa cells [5]. In prostate cancer, 2-ME acts through the modulation of the expression of androgen-regulated genes, mainly by reducing the levels of TSG-6 immunoreactivity [3]. Because of its efficacy in murine tumor models, 2-ME is being evaluated in Phase I and Phase II clinical trials against a variety of human tumors [8,9].
2-ME has been orally administered to cancer patients since 2000 [10]. The steroid was found to be rapidly removed from the plasma, e.g., <~10 ng/ml 1 h after intravenous administration [11]. Due to its poor gastrointestinal absorption and/or rapid metabolic deactivation and rapid removal from plasma [10,11], high oral or intraperitoneal doses of 75 and 150 mg kg−1 day−1, respectively, of 2-ME were necessary to reduce the growth of melanoma or myeloma tumors [12]. Although 2-ME is a relatively safe drug, high doses of 2-ME or its frequent administration may cause hot flashes, fatigue, diarrhea, and reversible liver enzyme elevations. Regional anti-cancer drug delivery offers pharmacokinetic as well as therapeutic advantages via localized delivery [13]. Recently, for choroidal neovascularization, 2-ME loaded silicone rubber (3.0 mm length, 2.0 mm diameter) and polyvinyl alcohol (1.0 × 1.0 × 2.0 mm) implants were developed and evaluated in rabbit and rat models [14,15]. The implants exhibited localized zero-order sustained release of 2-ME over a period of 35 days with no ocular toxicities [14,15]. For diabetic retinopathy, uveitis, and other retinovascular diseases, studies are underway to develop a suitable controlled release system [16]. In local cancers, continuous exposure of tumor cells to 2-ME at a stable level is also necessary to maintain the inhibitory effect [11], signifying the necessity of continuous drug releasing implant formulation for this novel drug.
Biodegradable PLGA injectable implants containing chemotherapeutic agents are clinically useful for both site-specific and systemic drug therapies in cancer [17–19]. Once injected in the body, the implant slowly releases anti-tumor agent, providing desirable drug levels for over one month [19]. By intratumoral administration of an implant, it is possible to maintain drug concentration in the tumor at cytotoxic levels and overcome the drawbacks (toxicity, metabolic deactivation, etc.) associated with oral administration [18]. However, hydrophobic drugs, proteins, and peptides often exhibit a multi-phasic release profile from PLGA-based matrices [20–23]. To achieve a steady release, various formulation strategies have been employed [20,24–33].
2-ME is highly lipophilic with very low water solubility (solubility of 2-ME in water at 37 °C was determined to be 1.8 ± 0.1 µg/mL (mean ± SE, n = 3)). To the best of our knowledge, the effect of formulation parameters on 2-ME release profile from the most widely used biodegradable polymers, PLGA, has not been studied thus far, signifying the necessity of optimization of 2-ME/PLGA formulations. In this study, cylindrical PLGA injectable implants on the millimeter scale (0.8 mm diameter) were selected for development as 2-ME localized sustained release dosage forms in local cancers. The objective of this study was to explore the potential of formulation parameters to achieve a 1-month continuous (improved) release of 2-ME from PLGA–lauryl ester (Mw =51 kDa) and PLGA–COOH (Mw = 24 kDa) implants. To achieve a continuous release of 2-ME from PLGA–lauryl ester implants, parameters such as drug loading, blending of 24 kDa PLGA–COOH with 51 kDa PLGA–lauryl ester, and the incorporation of water soluble additives (HP-β-CD and Pluronic® F127) and 2-ME PEG 8000 SD were investigated. In the current study, HP-β-CD and Pluronic® F127 were selected as release-modulating excipients as they vary in molecular size (1380–1480 Da (HP-β-CD) and 12,600 Da (Pluronic® F127)), hydrophilicity (water soluble (HP-β-CD) and water and polymer solvent (acetone) soluble (Pluronic® F127)) and cavity-forming ability [29]. Excipients with low melting points are suitable carriers for preparing drug/carrier solid dispersion by the melting method [34]. Therefore, PEG 8000 (58–65 °C) was selected as a carrier for preparing 2-ME/PEG 8000 solid dispersion (2-ME PEG 8000 SD). On the other hand, to achieve a continuous release of 2-ME from 24 kDa PLGA–COOH implants, MgCO3, a well-known release enhancer for proteins [35], was also examined.
2. Materials and methods
2.1. Materials
2-ME was purchased from Toronto Research Chemicals, Inc. (North York, Canada). PLGA was purchased from Medisorb, Alkermes, Inc. (Cambridge, USA), with 50:50 d,l-lactide/glycolide ratio, and varied with molecular weight and endcapping as follows: PLGA–lauryl ester (i.v. = 0.57 dl/g, Mw = 51 kDa, end group = lauryl ester), 13 kDa PLGA–COOH (i.v. = 0.16 dl/g, Mw = 13 kDa, end group = COOH), and 24 kDa PLGA–COOH (i.v. = 0.26 dl/g, Mw = 24 kDa, end group = COOH). PLGA 50:50 (i.v. = 0.67 dl/g, end group = carboxylic acid) was purchased from DURECT Corporation (Pelham, USA). Pluronic® F127 (average Mw 12,600) was obtained from BASF (Ludwigshafen, Germany). HP-β-CD was received as a gift sample from Roquette Fre`res (Lestrem, France). PEG 8000 was purchased from Fisher Scientific (New Jersey, USA) and MgCO3 was purchased from Sigma–Aldrich Co. (St. Louis, USA). All other reagents and solvents were of analytical grade or purer and purchased from commercial suppliers.
2.2. HPLC assay of 2-ME
All HPLC assays were performed on a Waters 2695 alliance system (Milford, USA) consisting of a 2996 Photodiode array detector and a personal computer with Empower 2 Software. An Econosphere C18 (4.6 × 250 mm) reverse phase column (Alltech, USA) was used at a flow rate of 1.0 mL/min. The mobile phase was acetonitrile:water (75:25) and detection wavelength was set to 205 nm. The standard curve of 2-ME was established and the concentration of unknown samples was calculated from the standard curve.
2.3. Preparation of 2-ME/PEG 8000 solid dispersion (2-ME PEG 8000 SD)
2-ME PEG 8000 SD was prepared by the melting method at the 2-ME:PEG 8000 mass ratio of 1:1.5. Briefly, weighed amounts of PEG 8000 were taken into a glass vial and heated up to 65 °C. The relative amount of 2-ME was then dispersed in the melted PEG 8000 for 5 min, followed by cooling in an ice bath for 30 min. 2-ME PEG 8000 SD was passed through a stainless steel sieve to obtain the particle size of <90 µm.
2.4. Preparation of 2-ME/PLGA cylindrical implants
2-ME loaded cylindrical implants were prepared by the solvent extrusion method. Briefly, PLGA was dissolved in acetone to make a final polymer concentration of 55% (w/w) with respect to solvent weight. Sieved (<90 µm) 2-ME (3%, 10%, and 30% (w/w)), 2-ME PEG 8000 SD (15% (w/w)), HP-β-CD (5% (w/w)), and Pluronic® F127 (5% (w/w)), were added to polymer solution to form drug/polymer or drug/additives/polymer mixtures. The mixtures were loaded into a 3-ml syringe and slowly extruded into the silicone tubing (I.D = 0.8 mm). The solvent extruded product was dried at room temperature for 48 h and then in a vacuum oven at 40 °C for another 72 h before testing. The various cylindrical implant formulations prepared are listed in Table 1.
Table 1.
Evaluation of 2-ME loaded PLGA cylindrical implant formulations
| Cylindrical implant formulation | Theoretical loading (% w/w) |
Actual loading (%w/w)d |
Loading efficiency (%)d |
Tg (°C) |
|---|---|---|---|---|
| Blank 51 kDa PLGA–lauryl estera | – | – | – | 17.1 |
| 51 kDa PLGA–lauryl estera | ||||
| 3.0 | 3.0 ± 0.1 | 100 ± 3 | 25.3 | |
| 10.0 | 9.9 ± 0.2 | 99 ± 2 | 28.1 | |
| 30.0 | 30.1 ± 0.2 | 102 ±1 | 31.7 | |
| 24 kDa PLGA–COOHb/51 kDa PLGA–lauryl ester blend | ||||
| Blend ratio 1:3 | 10.0 | 10.0 ± 0.3 | 100 ± 3 | 23.8 |
| 1:1 | 10.0 | 10.0 ± 0.2 | 100 ± 2 | 22.7 |
| 3:1 | 10.0 | 10.0 ± 0.3 | 100 ± 3 | 22.0 |
| 51 kDa PLGA–lauryl ester + water soluble additive | ||||
| 4.94 wt% Pluronic F127 | 9.9 | 9.4 ± 0.2 | 95 ± 2 | 31.4 |
| 5 wt% HP-β-CD | 9.9 | 9.3 ± 0.3 | 94 ± 3 | 29.3 |
| 51 kDa PLGA–lauryl ester + 2-ME/PEG 8000 solid dispersion | 6.5 | 6.2 ± 0.2 | 95 ± 2 | 23.2 |
| 24 kDa PLGA–COOH | 10.0 | 9.9 ± 0.2 | 99 ± 2 | 20.3 |
| 13 kDa PLGA–COOHc | 9.9 | 9.7 ± 0.1 | 98 ± 1 | 19.2 |
| 24 kDa PLGA–COOH + 3 wt% Mg CO3 | 10.3 | 9.7 ± 0.3 | 94 ± 3 | 24.1 |
| 24 kDa PLGA–COOH + 5 wt% Mg CO3 | 10.5 | 10.0 ± 0.3 | 95 ± 2 | 25.4 |
PLGA 50:50 (i.v. = 0.57 dl/g, Mw = 51 kDa, end group = lauryl ester).
PLGA 50:50 (i.v. = 0.26 dl/g, Mw = 24 kDa, end group = COOH).
PLGA 50:50 DL2A (i.v. = 0.16 dl/g, Mw = 13 kDa, end group = COOH).
Mean ± SE, n = 3.
2.5. Determination of drug loading and solubility
About 3 mg of 2-ME-loaded PLGA implants were digested in acetonitrile, diluted suitably using acetonitrile and analyzed by HPLC. The 2-ME loading was calculated as the percentage of the amount of 2-ME versus the total weight of the implant mixture (i.e., 2-ME, PLGA, and other excipients if present). To determine the 2-ME solubility in water and 5% w/v PEG 8000 solution, 1 mL of this medium was taken into separate 2-mL scintillation vials. Excess amount of drug was added into the vials, capped tightly and incubated at 37 °C for 72 h (this duration was determined to be sufficient to achieve the equilibrium). After 72 h, drug/water or drug/PEG 8000 suspension was passed through a 0.2-µm filter (Millipore Corporation, Bedford, USA) and drug content (n = 3) was similarly analyzed by HPLC.
2.6. X-ray diffraction (XRD)
To obtain the XRD patterns, we prepared the PLGA, 3, 10, and 30 wt% 2-ME/PLGA, PEG 8000/PLGA, and 2-ME PEG 8000 SD/PLGA films by a solvent casting method. Briefly, drug/polymer/acetone or 2-ME PEG 8000 SD/polymer/acetone mixtures were cast onto glass slides followed by drying at room temperature (2 days) and under vacuum (2 days). The physical state of 2-ME in the polymer was examined by measuring the XRD pattern using a Scintag powder X-ray diffractometer (Scintag Inc., CA, USA). The X-ray source was copper Kα (40 kV, 30 mA), and the scanning speed was 2 deg/min.
2.7. Determination of glass transition temperatures
Glass transition temperatures (Tg) of all implant formulations were measured by a differential scanning calorimeter (Perkin-Elmer® DSC7, Waltham, USA). Implant samples (~7 mg) were sealed in aluminum hermetic pans and thermograms were determined by heating from −50 to 70 °C at a heating rate of 10 °C/min. The startpoint of the second run was used for Tg calculation.
2.8. Evaluation of 2-ME release from PLGA implants
In vitro release studies were conducted in phosphate buffered saline (PBST, pH 7.4) under perfect sink conditions. About 3 mg 2-ME containing PLGA implants were weighed, placed in previously labeled eighteen cellulose dialysis bags (molecular weight cutoff 12–14,000), and tied at the both ends. All the dialysis bags were immersed in a large bottle containing 3 L PBST (pH 7.4) and placed in an incubator maintained at 37 °C. The release medium was stirred with a magnetic stir-bar at 100 RPM. To maintain the sink conditions, the release medium (3 L) was replaced everyday up to 27 days. At predetermined time intervals (1, 3, 7, 14, 21, and 28 days), three dialysis bags were taken out and implants were freeze-dried immediately. Freeze-dried implants were digested in acetonitrile, suitably diluted with acetonitrile and analyzed by HPLC. The cumulative amount of released 2-ME was determined by subtracting the 2-ME content remaining in the implants from the initial drug content.
2.9. Determination of polymer/water partition coefficient of 2-ME
The polymer/water partition coefficient of 2-ME was determined with modifications as per the method described previously [36]. In brief, very thin films (48 ± 7 µm, mean ± SE, n = 10) of PLGA 50:50 with lauryl ester (i.v. = 0.57 dl/g) and carboxylic acid (i.v. = 0.67 dl/g) end-group polymers were prepared by dipping glass tubes into a PLGA acetone solution (400 mg/mL) followed by subsequent quenching in 4 °C water for 1 h, drying in air for 1 h and vacuum-drying for 48 h at room temperature. Each polymer strip (1 × 1 cm2) was placed in one 4-mL scintillation vial, and the entire film was immersed in 1 mL water containing excess 2-ME. Each vial was capped tightly and incubated at 37 °C. At predetermined times (1, 3, 7, and 10 days), the film was removed, washed with water, digested in acetonitrile and 2-ME content in the polymer was measured similarly by HPLC (n = 3). The aqueous medium containing excess drug was passed through a 0.2-µm filter (Millipore Corporation, Bedford, USA) before injection into the HPLC system.
2.10. Scanning electron microscopy
The inner/surface morphologies of 2-ME-loaded cylindrical implants were examined using a Hitachi S3200N scanning electron microscope (Hitachi Ltd., Tokyo, Japan). The implants were fixed previously on a brass stub using double-sided adhesive tape and then were made electrically conductive by coating, in a vacuum, with a thin layer of gold (approximately 3–5 nm) for 60 s at 40 W. The cross-sectional and surface view images of implants were taken at an excitation voltage of 20 kV.
2.11. Measurement of water uptake in the PLGA implants
To determine the water uptake in the cylindrical PLGA implants, implants were incubated in PBST at 37 °C in cellulose dialysis bags as described for the evaluation of 2-ME release from PLGA implants. At 1, 3, 7, and 14 days, implants were blotted with tissue paper, weighed immediately (W1), freeze-dried and then weighed again (W2). The water content of implants was calculated by Eq. (1).
| (1) |
2.12. Gel permeation chromatography
Weight-averaged molecular weight (Mw) of the degraded PLGA was measured by gel permeation chromatography (GPC). The Waters 1525 GPC system (Waters, Milford, MA) consisted of two Styragel columns (HR 1 and HR-5E columns, Waters, Milford, MA) connected in series (7.8 × 300 mm each), a binary HPLC pump, waters 717 plus autosampler, waters 2414 refractive index detector and Breeze™ software to compute molecular weight distribution. Sample solutions in tetrahydrofuran (THF) at a concentration of ~3 mg/mL were filtered through a 0.2-µm hydrophobic fluoropore (PTFE) filter (Millipore Corporation, Bedford, USA) before injection into the GPC system and were eluted with THF at 1 ml/min. The average molecular weight (Mw) of each sample was calculated using monodisperse polystyrene standards, Mw 2330–1,860,000 Da.
3. Results and discussion
3.1. 2-ME loading in PLGA implants
Potential advantages of injectable PLGA millicylindrical implants for localized drug release application relative to common microspheres include: simple preparation, easy adjustment and control of drug loading, and minimal migration of the implant from the implant site following administration [17–19,37]. In the current study, 2-ME loaded PLGA cylindrical implants were prepared by the solvent extrusion method. In all the implants formulations, the drug loading was found to be close to the theoretical value (greater than 94% drug encapsulation efficiency—see Table 1), indicating a very effective loading process.
3.2. Physical state of 2-ME in PLGA implants
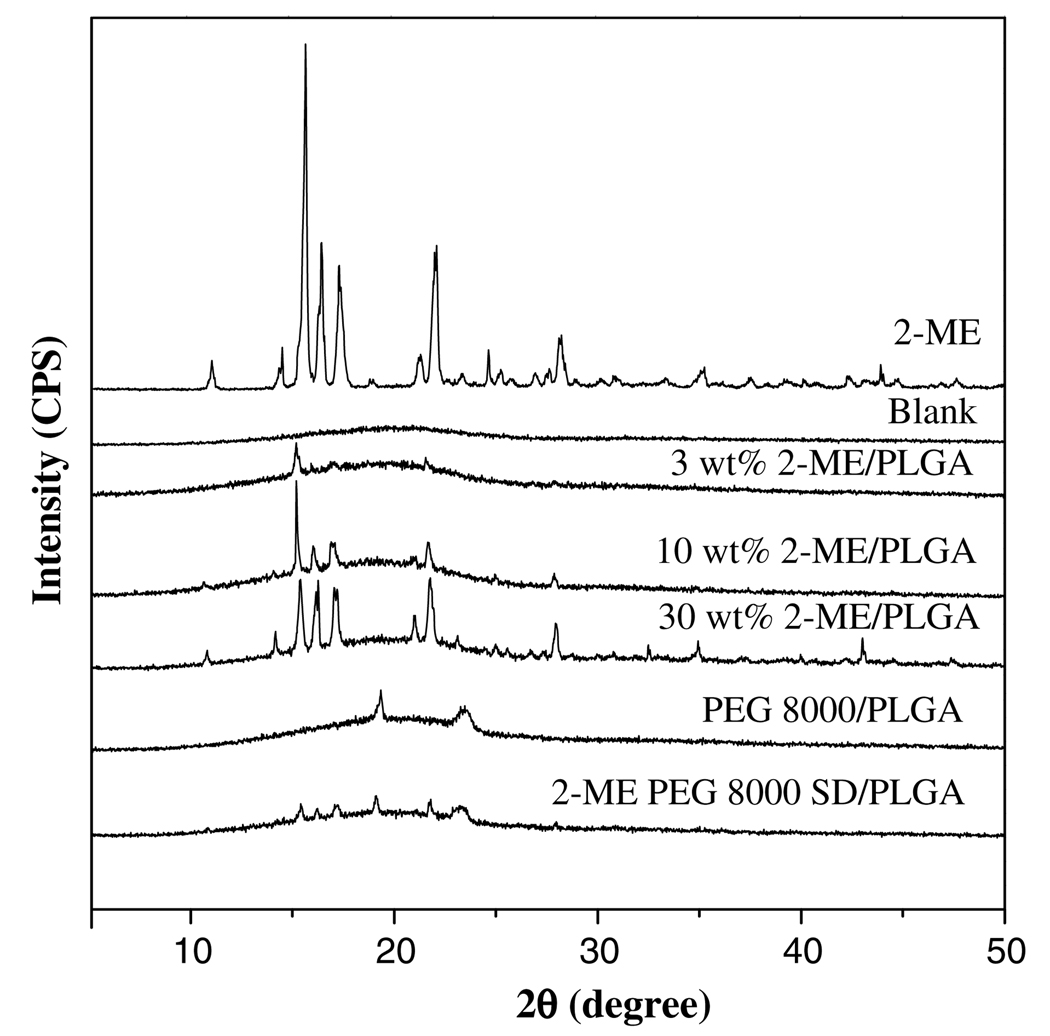
The effect of drug loading on 2-ME release profile from PLGA–lauryl ester (PLGA 50:50, (i.v. = 0.57 dl/g, Mw = 51 kDa, end-group = lauryl ester)) implants was studied at 3, 10, and 30 wt% loading. To further increase the 2-ME release, 2-ME solid dispersions with PEG 8000 (2-ME PEG 8000 SD) were prepared by the melting method and similarly loaded in the same polymer. To understand the physical state of 2-ME in PLGA and 2-ME PEG 8000 SD, XRD patterns of these formulations prepared as films (prepared by a solvent casting method) were obtained, as shown in Fig. 1. The diffractogram of 2-ME exhibited a series of intense peaks (2θ values of 11.2°, 14.6°, 15.8°, 16.6°, 17.5°, 21.4°, 22.1°, and 28.3°), indicating the crystalline character of the drug. At 3, 10, and 30 wt% 2-ME loading in PLGA, crystalline peaks of 2-ME at the same 2θ values were observed indicating the presence of crystalline drug at each loading studied. However, at the lower loading of 3%, the crystalline peak was very low suggestive of either drug partitioning into the polymer phase or the presence of some fraction of amorphous drug.
Fig. 1.
Effect of drug loading (theoretical) (3, 10, and 30 wt% 2-ME) and formation of 2-ME/PEG 8000 solid dispersion on the physical state of 2-ME in PLGA 50:50 (i.v. = 0.57 dl/g, Mw = 51 kDa, end-group = lauryl ester) implants. X-ray diffraction patterns shown for blank, 2-ME, 3–30 wt% 2-ME/PLGA, PEG 8000/PLGA, and 15 wt% 2-ME PEG 8000 SD/PLGA implants. 2-ME loading (theoretical) in 15 wt% 2-ME PEG 8000 SD/PLGA implants was 6.5 wt%.
Examination of the implants by DSC was consistent with these data. As shown in Table 1, the glass transition temperature (Tg) of the blank implant was reduced relative to that of the unprocessed polymer (Tg = 45 °C) owing to residual acetone in the polymer. We observed the same phenomenon when encapsulating N-acetylcysteine previously, particularly in nonporous blank specimens, because of the difficulty of removing polar organic solvents from this polymer prepared on this length scale (~1 mm diameter) [38]. At 3, 10, and 30 wt% 2-ME loadings in PLGA, the Tg of the polymer increased consistently with the presence of a biphasic system (i.e., drug and polymer phases) which in turn improved acetone removal with increased pores formed by drug particles [38]. The effect of altering several additional formulation parameters on the Tg of the polymer is discussed in the in vitro drug release section below. On the other hand, 2-ME PEG 8000 SD/PLGA films exhibited crystalline peaks of 2-ME but with reduced intensity. This finding can be attributed to lower drug loading (6.5 wt%), although the drug was still present in crystalline form in the 2-ME PEG 8000 SD-loaded PLGA implants.
3.3. Modulation of 2-ME release from 51 kDa PLGA–lauryl ester implants
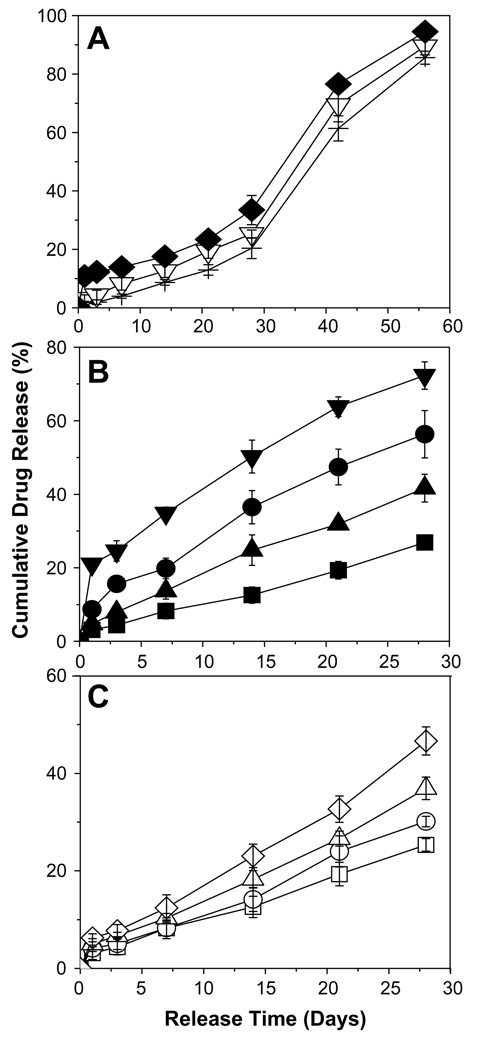
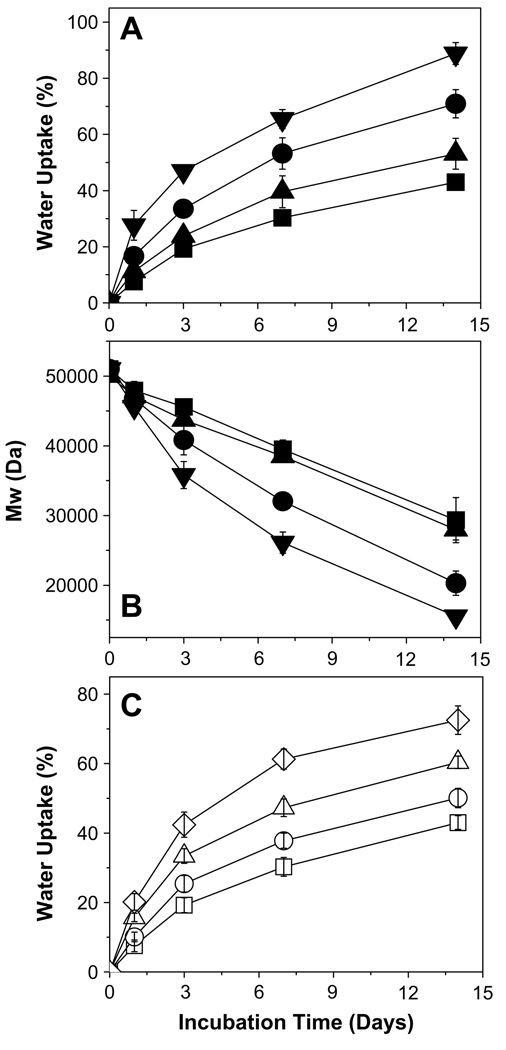
The modulation of 2-ME release from PLGA–lauryl ester (51 kDa) implants was performed by varying the drug-load, incorporating water soluble additives (HP-β-CD and pluronic® F127), forming 2-ME PEG 8000 SD, and blending 24 kDa PLGA-COOH with 51 kDa PLGA–lauryl ester (see Fig. 2). The effect of the above-mentioned variables on water uptake and/or degradation characteristics of PLGA–lauryl ester-based implants is shown in Fig. 3.
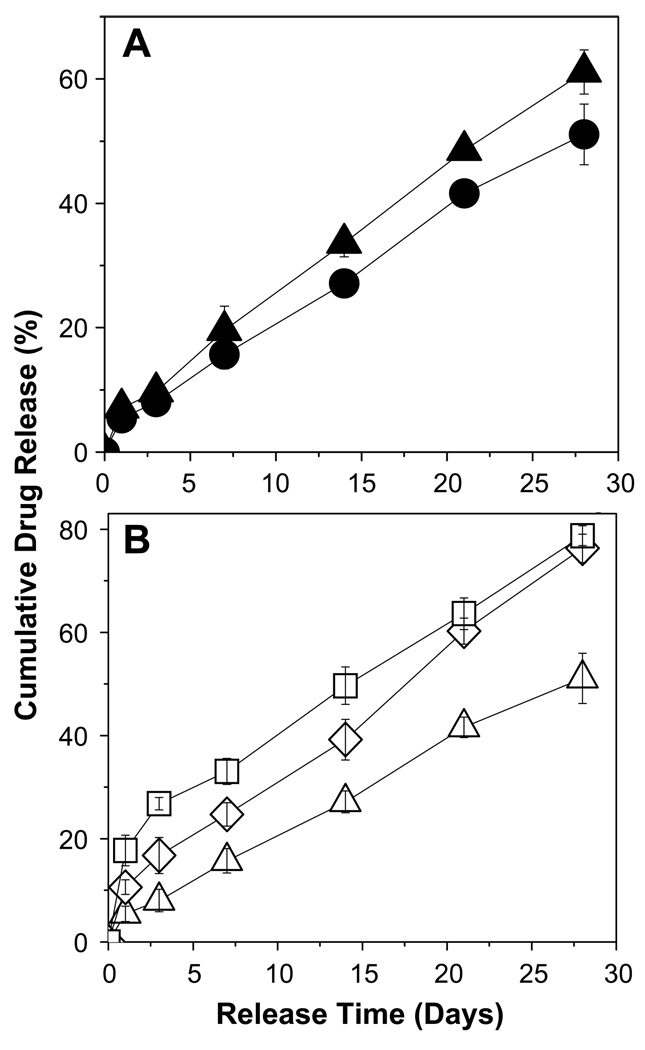
Fig. 2.
Modulation of 2-ME release from cylindrical PLGA–lauryl ester (Mw = 51 kDa) implants. (A) Effect of drug loading (theoretical) (3 (+), 10 (▽), and 30 (♦) wt% 2-ME) on 2-ME release from control implants (no additives or blending). (B) Effect of incorporation of water soluble additives (HP-β-CD or Pluronic® F127) or 2-ME PEG 8000 SD. 2-ME release profiles are shown for 10 wt% 2-ME (■), 10 wt% 2-ME + 5 wt% HP-β-CD (●), 10wt% 2-ME + 5 wt% Pluronic® F127 (▲), and 15wt% 2-ME PEG 8000 SD (▼) loaded implants. 2-ME loading in 15 wt% 2-ME PEG SD/PLGA implants was 6.5 wt%. (C) Effect of blending of 24 kDa PLGA–COOH with PLGA–lauryl ester. 2-ME release profiles are shown for the implants prepared using 0 (□), 0.25 (○), 0.5 (△), and 0.75 (◇) weight fraction of PLGA–COOH. 2-ME loading (theoretical) in non-blend and blend implants was 10 wt%. All studies were carried out in PBST at 37 °C and symbols represent mean ± SE, n = 3.
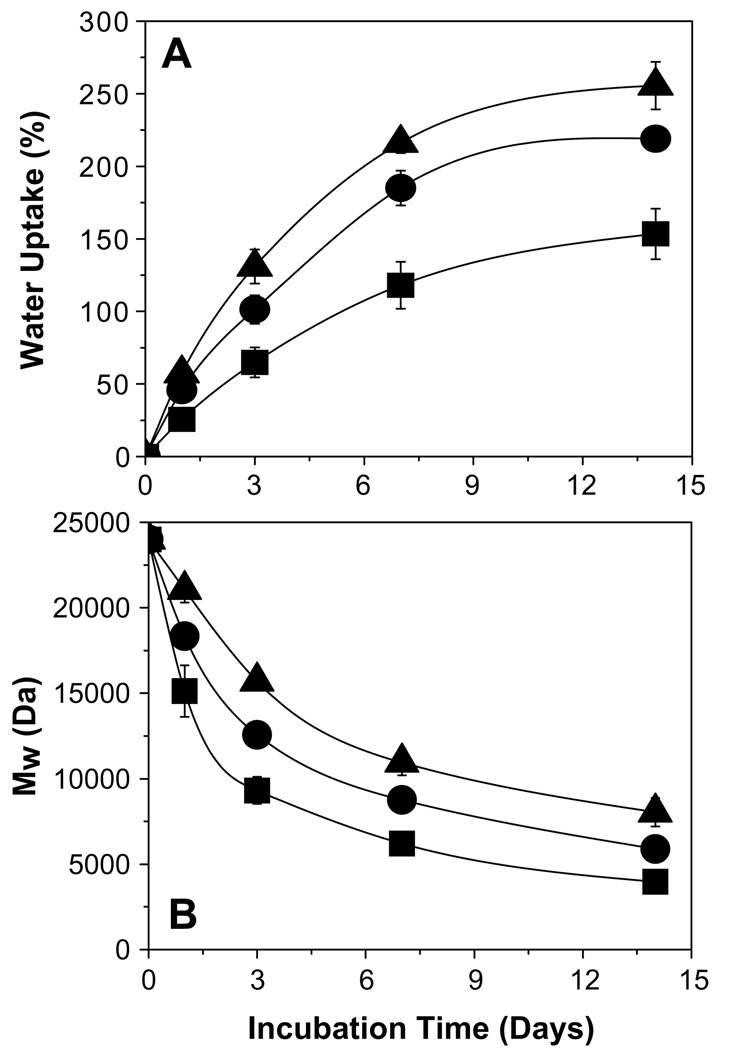
Fig. 3.
Water uptake and degradation characteristics of 2-ME/PLGA–lauryl ester-based cylindrical implants. Effect of incorporation of water soluble additives (HP-β-CD or Pluronic® F127) or 2-ME PEG 8000 SD on water uptake (A) and degradation (B) profiles of PLGA–lauryl ester implants. Profiles are shown for 10 wt% 2-ME (■), 10 wt% 2-ME + 5 wt% Pluronic® F127 (▲), 10wt% 2-ME + 5wt% HP-β-CD (●), and 15wt% 2-ME PEG 8000 SD (▼) loaded implants. 2-ME loading (theoretical) in 15wt% 2-ME PEG SD/PLGA implants was 6.5 wt%. (C) Effect of blending of 24 kDa PLGA–COOH with 51 kDa PLGA–lauryl ester on water uptake profile of implants. Water uptake profiles are shown for the implants prepared using 0 (□), 0.25 (○), 0.5 (Δ), and 0.75 (◇) weight fraction of PLGA–COOH. 2-ME loading (theoretical) in non-blend and blend implants was 10wt%. Implants were incubated in PBST at 37 °C and symbols represent mean ± SE, n = 3.
3.3.1. Effect of drug loading
The drug release from 3, 10, and 30 wt% 2-ME loaded PLGA–lauryl ester implants followed the classic triphasic pattern, as shown in Fig. 2A. An initial release phase (after 1 day, 2%, 4%, and 11% drug release from 3, 10, and 30 wt% 2-ME loaded implants, respectively) followed by a second slow release phase lasting 3 weeks (after 28 days, 21%, 27%, and 34% drug release from 3, 10, and 30 wt% 2-ME loaded implants, respectively) and a third rapid release phase (after 56 days, 86%, 90%, and 95% drug release from 3, 10, and 30 wt% 2-ME loaded implants, respectively). Such a tri-phasic drug release is often undesirable for most drug therapies. An increase in drug loading from 3 to 30 wt% did not increase the release steadily, but instead only slightly increased the initial release phase. Despite the reduction in release corresponding to the second release phase, drug release was still continuous albeit very slow, as is commonly observed for other steroids, e.g., triamcinolone and triamcinolone acetonide [21]. We hypothesized that this finite release was caused by diffusion through the polymer phase. To test this, we sought to determine if 2-ME had an appreciable polymer/water partition coefficient (PC). To accomplish this, we used the PLGA–lauryl ester and PLGA–COOH polymers to explore the potential of 2-ME partitioning in the two extremes of hydrophobicity and hydrophilicity at a common polymer molecular weight (i.v. ~0.57–0.67 dl/g). It was found that 2-ME equilibrated in the PLGA–lauryl ester and PLGA–COOH by 3 and 7 days (data not shown), respectively, to very similar extents (PC ~42–46). As expected, the high partition coefficient and rapid equilibration indicates the capability of the novel steroid to partition and diffuse in PLGAs. Therefore, to diminish the lag-time and obtain an improved continuous release of 2-ME from PLGA–lauryl ester implants, we evaluated various formulation strategies, as described below.
3.3.2. Effect of incorporation of water soluble additives (HP-β-CD and Pluronic® F127)
Effective modulation of drug release from biodegradable matrices can be achieved by incorporating the small amount of water soluble additives (attributable to the formation of drug-releasing pores in the matrix) which vary in lipophilic nature, solubility, molecular weight, location and interaction with the polymer [27,29,31,32,39]. In the present study, we selected commonly used water soluble additives varying in molecular size (1380–1480 Da (HP-β-CD) and 12,600 Da (Pluronic® F127)), hydrophilicity (water soluble (HP-β-CD) and water and polymer solvent (acetone) soluble (Pluronic® F127)) and cavity-forming ability to evaluate their ability to provide improved continuous release of 2-ME from PLGA–lauryl ester implants. About 5 wt% HP-β-CD or Pluronic® F127/PLGA were co-incorporated into 10 wt% 2-ME loaded PLGA–lauryl ester implants.
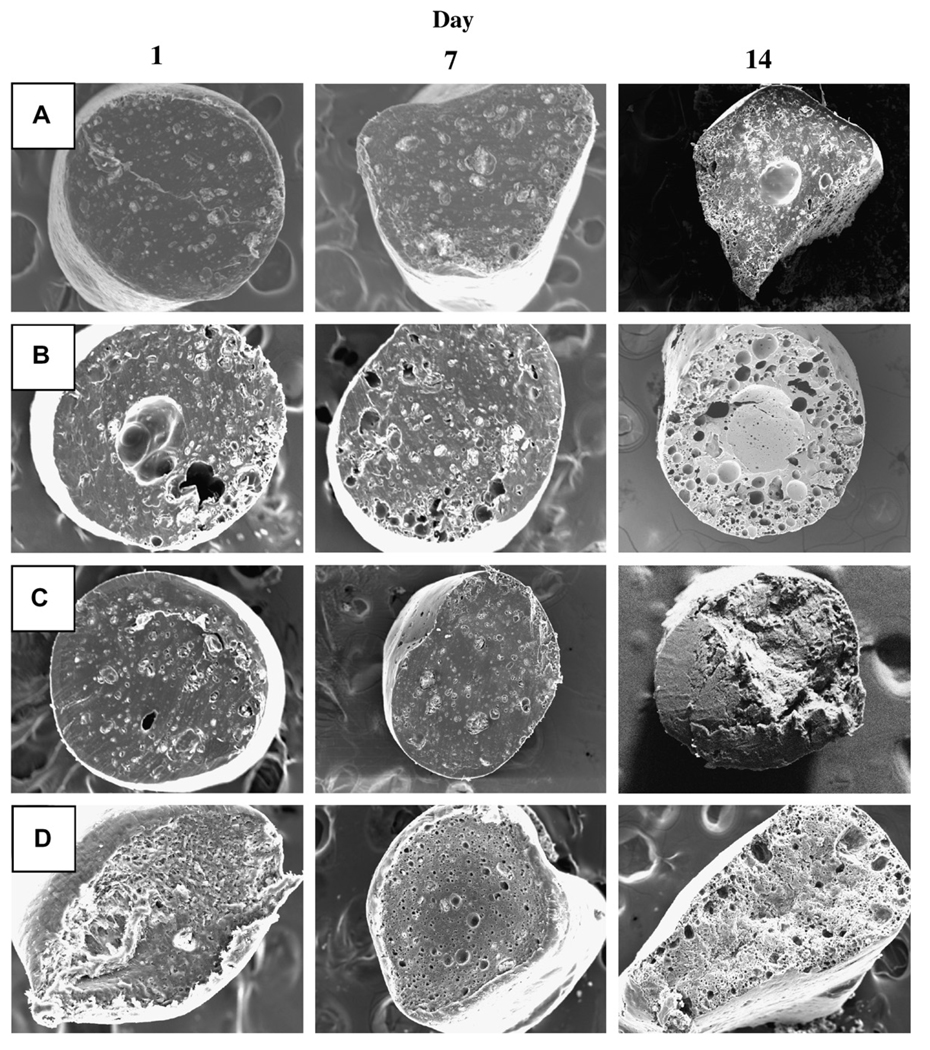
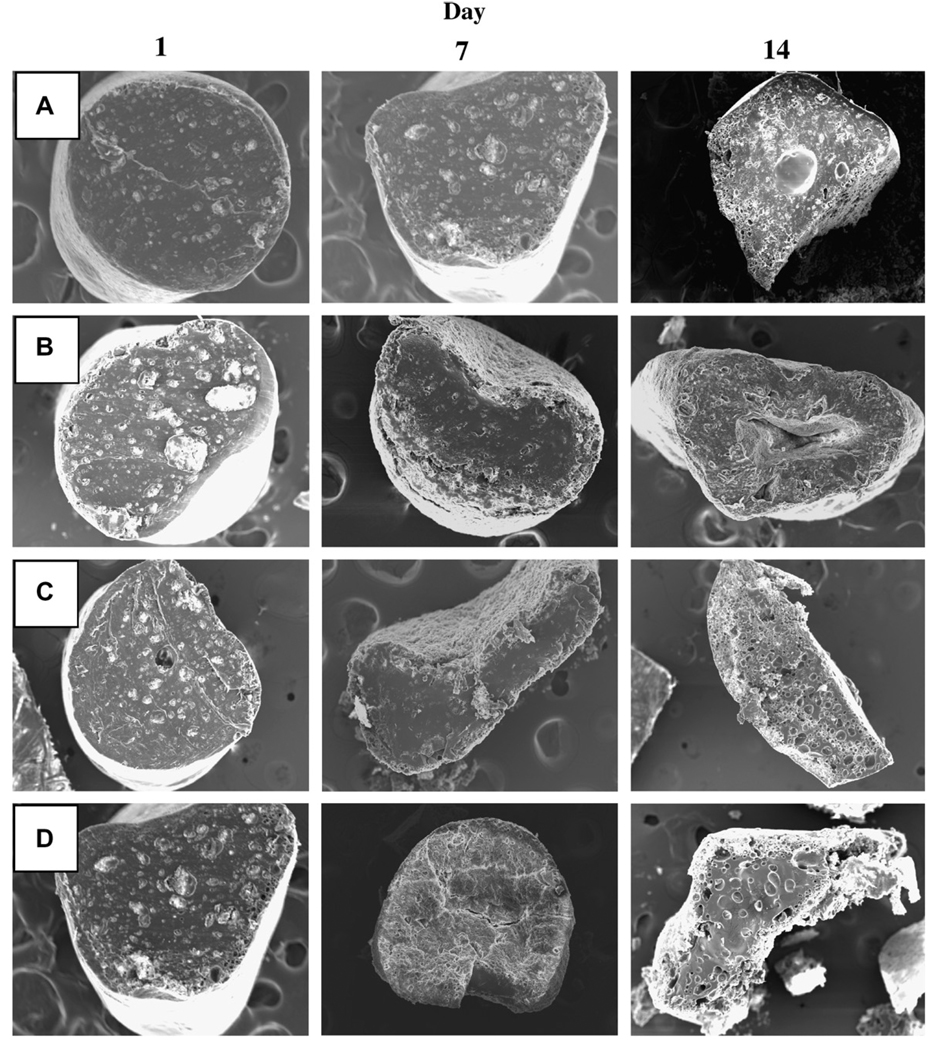
The effect of the incorporation of HP-β-CD or Pluronic® F127 on 2-ME release profile from PLGA–lauryl ester implants is also displayed in Fig. 2B. The release of 2-ME from PLGA–lauryl ester implants without water-soluble additives was very slow (27% drug release after 28 days). When HP-β-CD or Pluronic® F127 was co-incorporated in the PLGA–lauryl ester implants, the 2-ME release rose steadily over 28 days. However, the PLGA–lauryl ester implant loaded with 5 wt% HP-β-CD exhibited higher cumulative drug release (57% drug release after 28 days) than the 5wt% Pluronic® F127/PLGA–lauryl ester implant (42% drug release after 28 days). Consistent with this trend, the rate of water uptake (Fig. 3A) and polymer degradation (Fig. 3B) were each higher for the HP-β-CD/PLGA–lauryl ester implant compared to the Pluronic® F127/PLGA–lauryl ester implant. Similarly, the polymeric matrix of 5 wt% HP-β-CD/PLGA–lauryl ester implant (Fig. 4B) was more porous than the 5 wt% Pluronic® F127/PLGA–lauryl ester implant (Fig. 4C).
Fig. 4.
Effect of incorporation of 5 wt% water soluble additives (HP-β-CD or Pluronic® F127) or 15 wt% 2-ME PEG 8000 SD on inner morphology of 2-ME/PLGA–lauryl ester (Mw = 51 kDa) based cylindrical implants after 1, 7, and 14 days of immersion in PBST at 37 °C. SEM images are displayed for 0 wt% additive (A), 5 wt% HP-β-CD (B), 5 wt% Pluronic® F127 (C), and 15 wt% 2-ME PEG 8000 SD (D) loaded implants. 2-ME loadings (theoretical) were 10 (A, B, and C) and 6.5 (D) wt%.
3.3.3. Effect of the formation of 2-ME/PEG 8000 solid dispersion (2-ME PEG 8000 SD)
Formulation of solid dispersions of poorly soluble drugs with water soluble excipients (polyethylene glycols, polyvinylpyrrolidone, hydroxypropylmethylcellulose, urea, and mannitol, for example) for enhanced oral drug delivery has been extensively demonstrated [34]. The water soluble excipients used to form solid dispersion improve drug’s solubility/dissolution [34]. In the present study, we investigated the potential of this approach to enhance the release of 2-ME from PLGA–lauryl ester cylindrical implants. 2-ME PEG 8000 SD was prepared by the melting method at a 2-ME:PEG mass ratio of 1:1.5 and 15 wt% 2-ME PEG. The SD was loaded in PLGA–lauryl ester cylindrical implants. The melting point of PEG 8000 is low, between 58 and 65 °C [34], and advantageous to form the solid dispersion by the melting method [34].
The effect of the formation of 2-ME PEG SD on 2-ME release from PLGA–lauryl ester implant is depicted in Fig. 2B. The cumulative drug release from 2-ME PEG SD/PLGA–lauryl ester implants was higher (21% and 73% drug release after 1 and 28 days, respectively) than those loaded with water-soluble additives (5 wt% HP-β-CD or Pluronic® F127) or without additive. PEG 8000 increased the water solubility of 2-ME considerably (solubility of 2-ME in water and 5% w/v PEG 8000 solution at 37 °C was determined to be 1.8 ± 0.1 and 9.9 ± 0.6 µg/mL (mean ± SE, n = 3), respectively). Upon the loading of 2-ME PEG 8000 SD in PLGA–lauryl ester implants, the Tg of the polymer was reduced considerably (Table 1) and water uptake (Fig. 3A) in the polymer increased, giving rise to higher degradation of 2-ME PEG 8000 SD/PLGA–lauryl ester implants when compared to HP-β-CD or Pluronic® F127/PLGA–lauryl ester implants (see Fig. 3B). Consistent with these findings, the formation of nearly inter-connected pores in the 2-ME PEG 8000 SD/PLGA–lauryl ester matrix can be clearly observed in SEM images of the implants during the release (Fig. 4D). More continuous release of 2-ME from 2-ME PEG 8000 SD/PLGA–lauryl ester implants can be attributed to the improvement in drug’s solubility/dissolution and hydrophilicity of PLGA–lauryl ester by PEG 8000. These results are in agreement with the study reported by Dorati et al [40]. In their study, co-encapsulation PEG 4000 made the polymer more hydrophilic and improved the release of ovalbumin from PLGA microspheres [40].
3.3.4. Effect of blending of 24 kDa PLGA–COOH with 51 kDa PLGA–lauryl ester
To investigate the potential of blending 24 kDa PLGA–COOH with the 51 kDa PLGA–lauryl ester to provide continuous release of 2-ME, implants with different weight ratios of 24 kDa PLGA–COOH to 51 kDa PLGA–lauryl ester (1:3, 1:1, and 3:1 ratios) were prepared. As the weight fraction of 24 kDa PLGA–COOH increased in the blend, the 2-ME release rose steadily over a period of one month and the lag-time proportionally diminished (Fig. 2C). PLGA–lauryl ester implant loaded with 10 wt% 2-ME exhibited 27% drug-release after 28 days. By contrast, implants prepared with 24 kDa PLGA–COOH: 51 kDa PLGA–lauryl ester blend at 1:3, 1:1, and 3:1 ratios exhibited 31%, 37%, and 47% drug release, respectively. The Tg of the 51 kDa PLGA–lauryl ester implant was determined to be 28.1 °C (Table 1). Upon blending of 24 kDa PLGA–COOH with 51 kDa PLGA–lauryl ester polymer, the Tg of the blend implants decreased considerably, indicating the good mis-cibility and plasticizing effect of 24 kDa PLGA–COOH. For example, the Tg of the PLGA–COOH:PLGA–lauryl ester blend implants at 1:3, 1:1, and 3:1 ratios was determined to be 23.8, 22.7, and 22 °C, respectively (Table 1). Consistent with this result, blending of 24 kDa PLGA–COOH with 51 kDa PLGA–lauryl ester resulted in increased water uptake in the implants (Fig. 3C). Further, as the weight fraction of 24 kDa PLGA–COOH increased in the blend, the cylindrical shape of the implants and matrix integrity was lost (Fig. 5B–D). Therefore, increased 2-ME release from 24 kDa PLGA–COOH: 51 kDa PLGA–lauryl ester blend implants can be attributed to lower glass transition temperatures and increased matrix porosity, providing respective increases in solid-state and pore-diffusion of 2-ME [26].
Fig. 5.
Effect of blending of 24 kDa PLGA–COOH with 51 kDa PLGA–lauryl ester on the inner morphology of the 2-ME loaded cylindrical implants after 1, 7, and 14 days of immersion in PBST at 37 °C. SEM images are displayed for the implants prepared using 0 (A), 0.25 (B), 0.5 (C), and 0.75 (D) weight fraction of 24 kDa PLGA–COOH. 2-ME loading (theoretical) in all the implant formulations was 10 wt%.
3.4. Modulation of 2-ME release from PLGA–COOH implants
Modulation of 2-ME release profile from PLGA–COOH was performed by varying the polymer molecular weight (13 and 24 kDa) (see Fig. 6A) and incorporating 3–5 wt% MgCO3 (see Fig. 6B). The effect of these variables on implants degradation (water uptake and polymer molecular weight decline) and inner/surface morphologies of implants are shown in Figs. 7 and 8, respectively.
Fig. 6.
Modulation of 2-ME release profile from cylindrical PLGA–COOH implants. (A) Effect of polymer molecular weight on 2-ME release profile is shown for implants prepared using 13 kDa PLGA–COOH (▲) and 24 kDa PLGA–COOH (●). (B) Effect of the incorporation of MgCO3 in 24 kDa PLGA–COOH implants on 2-ME release profile is shown for 0 (△), 3 (◇), and 5 (□) wt% MgCO3. 2-ME loading (theoretical) in all the implant formulations was 10 wt%. All studies were carried out in PBST at 37 °C and symbols represent mean ± SE, n = 3.
Fig. 7.
Effect of incorporation of MgCO3 in 2-ME/24 kDa PLGA–COOH cylindrical implants on water uptake (A) and polymer degradation (B) characteristics. MgCO3 was loaded at 0 (■), 3 (●), and 5 (▲) wt%. 2-ME loading (theoretical) in all the implants was 10wt%. Implants were incubated in PBST at 37 °C and symbols represent mean ± SE, n = 3.
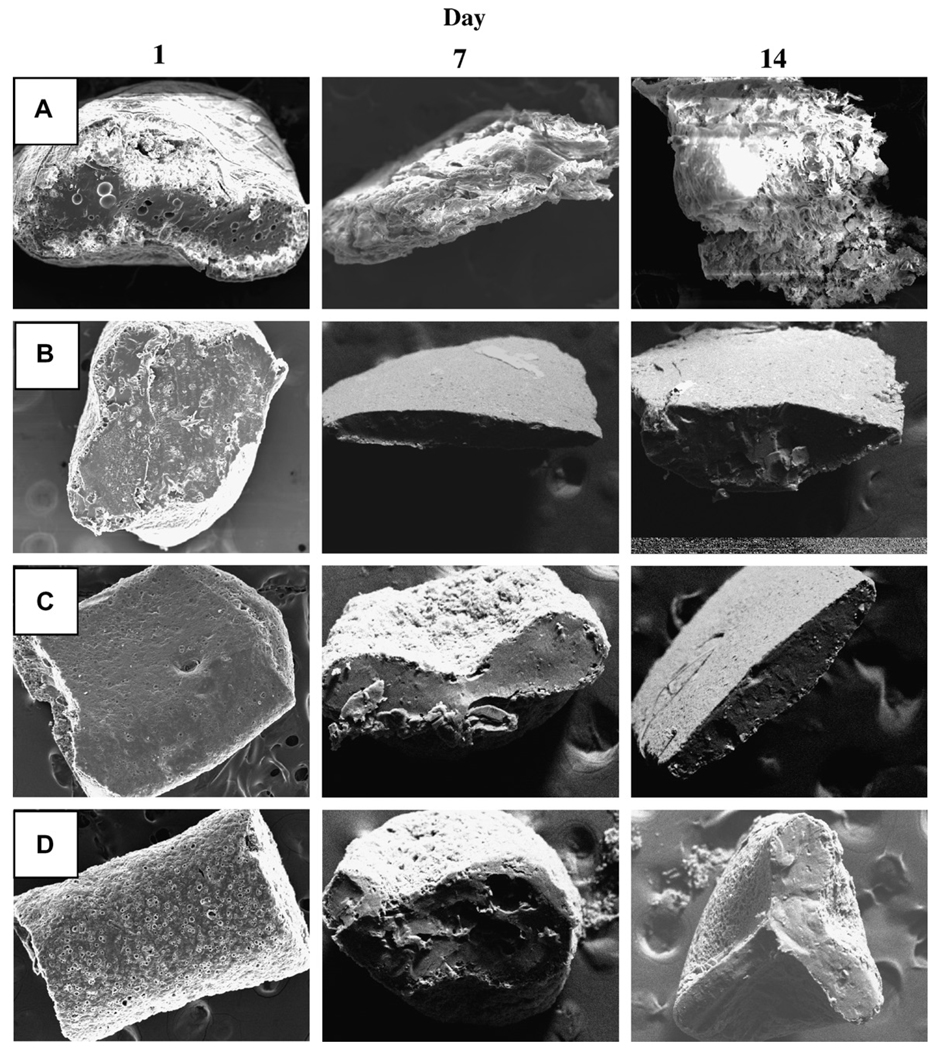
Fig. 8.
Effect of polymer molecular weight and incorporation of MgCO3 on inner/surface morphologies of 2-ME/PLGA–COOH cylindrical implants after 1, 7, and 14 days of immersion in PBST at 37 °C. SEM images are shown for 13 kDa PLGA–COOH (A), 24 kDa PLGA–COOH (B), 3 wt% MgCO3/24 kDa PLGA–COOH (C), and 5 wt% MgCO3/24 kDa PLGA–COOH (D) implants. 2-ME loading (theoretical) in all the implant formulations was 10 wt%.
3.4.1. 2-ME release profile from low molecular weight (13 and 24 kDa) PLGA–COOH implants
We first obtained the release profile of 2-ME from low molecular weight (13 and 24 kDa) PLGA–COOH implants (Fig. 6A). After 1 and 28 days, 10 wt% 2-ME/24 kDa PLGA–COOH implants exhibited ~6% and 51% drug-release, respectively, whereas, 10wt% 2-ME/13 kDa PLGA–COOH implants exhibited ~8% and 63% drug release. The slight difference in 2-ME release profile can be attributed to the difference in degradation profiles of 13 and 24 kDa PLGA–COOH implants (data not shown). Consistent with the drug release behavior, the polymeric matrix of 13 kDa PLGA–COOH implants (Fig. 8A) was more porous than 24 kDa PLGA–COOH implants (Fig. 8B).
3.4.2. Effect of the incorporation of metal salt (MgCO3)
In an attempt to study the effect of known release enhancers of proteins [35] on 2-ME release from PLGA, 3 and 5 wt% MgCO3 was co-encapsulated in 10 wt% 2-ME/24 kDa PLGA–COOH implants. As shown in Fig. 6B, 24 kDa PLGA–COOH implants without MgCO3 exhibited ~5.5% and 52% drug release after 1 and 28 days, respectively. By contrast, 24 kDa PLGA–COOH implants loaded with 3 and 5 wt% MgCO3 exhibited an acceptable initial burst (after 1-day, 11% and 18% drug release, respectively), and nearly complete drug release after 28-days (76% and 79% drug release, respectively). Consistent with the increased release behavior, polymeric matrices with 3 and 5 wt% MgCO3 (Fig. 8C and D) were more porous than the control implant (Fig. 8B).
Generally, the polymer degradation rate increases as the water uptake in the polymer increases [22,41,42]. However, the effects of co-encapsulated salts and bases range from simple changes in the water uptake profile accompanied by no appreciable change in degradation rate (e.g., for water-soluble sodium chloride, magnesium sulfate, and zinc sulfate) to considerable increases in the water uptake profile together with substantial decreases in degradation rate (e.g., for poorly soluble MgCO3 and Mg(OH)2) [43]. To test whether the presence of 2-ME affected the MgCO3-altered water uptake (Fig. 7A) and degradation (Fig. 7B) profiles of PLGA, these polymer properties were similarly evaluated. As shown in Fig. 7, 24 kDa PLGA–COOH implants loaded with 3 and 5 wt% MgCO3 exhibited higher water uptake in the polymer and degraded more slowly than the control implant without base. It is very clear that the encapsulation of 2-ME did not appear to influence the MgCO3-induced distinct PLGA water uptake and degradation profiles.
The distinct polymer degradation (higher water uptake and slower degradation pattern) exhibited by the MgCO3/PLGA matrices have been attributed to the involvement of one or more forces and processes, including: the reaction of the base with the water-soluble acids to form Mg-carboxylate salts (which in turn disrupts autocatalysis by removal of the water-soluble acids from the polymer phase), the osmotic pressure created by the newly formed salts in the polymer pores (which creates new pores in the polymer matrix), and potentially, interactions between Mg ions and polymer functionalities such as carboxylic acid or ester groups (which decreases the reactivity of ester linkage towards hydrolysis) [36,38,43–46]. Co-encapsulation of MgCO3 appears to be a suitable option to achieve continuous release of 2-ME from cylindrical low molecular weight PLGA–COOH implants.
4. Conclusions
The goal of this research was to explore the potential of various formulation strategies to achieve 1-month continuous release of 2-ME from cylindrical PLGA–lauryl ester and PLGA–COOH implants. Incorporation of HP-β-CD or 2-ME PEG 8000 SD in PLGA–lauryl ester implants inhibited the lag-phase time and increased the 2-ME release steadily. By blending varying amount of 24 kDa PLGA– COOH with 51 kDa PLGA–lauryl ester, lag-phase time could be diminished proportionally and moderate enhancement in 2-ME release could be obtained over a period of 28 days. Release of 2-ME was further accelerated by the use of low molecular weight (13 and 24 kDa) PLGA–COOH implants. Implants (24 kDa PLGA–COOH) loaded with 3–5 wt% MgCO3 exhibited more continuous drug release among all formulations tested. The cylindrical PLGA–lauryl ester and PLGA–COOH implants studied in this paper by varying the formulation parameters provide a wide range of strategies to facilitate continuous release, which may be useful for the modulated localized controlled release applications of 2-ME or similar hydrophobic drug molecules. In vivo evaluation of 2-ME release from PLGA implants in a head and neck tumor model is underway and will be reported in a subsequent manuscript.
Acknowledgement
This research was supported by NIH R01 CA 95901.
References
- 1.Barchiesi F, Jackson EK, Fingerle J, Gillespie DG, Odermatt RK, Dubey RK. 2-Methoxyestradiol, an estradiol metabolite, inhibits neointima formation and smooth muscle cell growth via double blockade of the cell cycle. Circ. Res. 2006;99:266–274. doi: 10.1161/01.RES.0000233318.85181.2e. [DOI] [PubMed] [Google Scholar]
- 2.Dobos J, Timar J, Bocsi J, Burian Z, Nagy K, Barna G, Petak I, Ladanyi A. In vitro and in vivo antitumor effect of 2-methoxyest-radiol on human melanoma. Int. J. Cancer. 2004;112:771–776. doi: 10.1002/ijc.20473. [DOI] [PubMed] [Google Scholar]
- 3.Garcia GE, Wisniewski HG, Lucia MS, Arevalo N, Slaga TJ, Kraft SL, Strange R, Kumar AP. 2-Methoxyestradiol inhibits prostate tumor development in transgenic adenocarcinoma of mouse prostate: role of tumor necrosis factor-alpha-stimulated gene 6. Clin. Cancer Res. 2006;12:980–988. doi: 10.1158/1078-0432.CCR-05-2068. [DOI] [PubMed] [Google Scholar]
- 4.Huh JI, Calvo A, Charles R, Green JE. Distinct tumor stage-specific inhibitory effects of 2-methoxyestradiol in a breast cancer mouse model associated with Id-1 expression. Cancer Res. 2006;66:3495–3503. doi: 10.1158/0008-5472.CAN-04-2372. [DOI] [PubMed] [Google Scholar]
- 5.Raobaikady B, Purohit A, Chander SK, Woo LWL, Leese MP, Potter BVL, Reed MJ. Inhibition of MCF-7 breast cancer cell proliferation and in vivo steroid sulphatase activity by 2-methoxyoestradiol-bis-sulphamate. J. Steroid. Biochem. 2003;84:351–358. doi: 10.1016/s0960-0760(03)00049-9. [DOI] [PubMed] [Google Scholar]
- 6.Sato F, Fukuhara H, Basilion JP. Effects of hormone deprivation and 2-methoxyestradiol combination therapy on hormone-dependent prostate cancer in vivo. Neoplasia. 2005;7:838–846. doi: 10.1593/neo.05145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mooberry SL. Mechanism of action of 2-methoxyestradiol: new developments. Drug Resist. Update. 2003;6:355–361. doi: 10.1016/j.drup.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 8.James J, Murry DJ, Treston AM, Storniolo AM, Sledge GW, Sidor C, Miller KD. Phase I safety, pharmacokinetic and pharmacodynamic studies of 2-methoxyestradiol alone or in combination with docetaxel in patients with locally recurrent or metastatic breast cancer. Invest. New Drug. 2007;25:41–48. doi: 10.1007/s10637-006-9008-5. [DOI] [PubMed] [Google Scholar]
- 9.Sweeney C, Liu G, Yiannoutsos C, Kolesar J, Horvath D, Staab MJ, Fife K, Armstrong V, Treston A, Sidor C, Wilding G. A phase II multicenter, randomized, double-blind, safety trial assessing the pharmacokinetics, pharmacodynamics, and efficacy of oral 2-methoxyestradiol capsules in hormone-refractory prostate cancer. Clin. Cancer Res. 2005;11:6625–6633. doi: 10.1158/1078-0432.CCR-05-0440. [DOI] [PubMed] [Google Scholar]
- 10.Sidor C, D’Amato R, Miller KD. The potential and suitability of 2-methoxyestradiol in cancer therapy. Clin. Cancer Res. 2005;11:6094–6095. doi: 10.1158/1078-0432.CCR-05-0724. [DOI] [PubMed] [Google Scholar]
- 11.Ireson CR, Chander SK, Purohit A, Perera S, Newman SP, Parish D, Leese MP, Smith AC, Potter BVL, Reed RJ. Pharmacokinetics and efficacy of 2-methoxyoestradiol and 2-methoxyoestradiolbis-sulphamate in vivo in rodents. Br. J. Cancer. 2004;90:932–937. doi: 10.1038/sj.bjc.6601591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dingli D, Timm M, Russell SJ, Witzig TE, Rajkumar SV. Promising preclinical activity of 2-methoxyestradiol in multiple myeloma. Clin. Cancer Res. 2002;8:3948–3954. [PubMed] [Google Scholar]
- 13.Dhanikula AB, Panchagnula R. Localized paclitaxel delivery. Int. J. Pharm. 1999;183:85–100. doi: 10.1016/s0378-5173(99)00087-3. [DOI] [PubMed] [Google Scholar]
- 14.Robinson MR, Baffi J, Yuan P, Sung C, Byrnes G, Cox TA, Csaky KG. Safety and pharmacokinetics of intravitreal 2-methoxyestradiol implants in normal rabbit and pharmacodynamics in a rat model of choroidal neovascularization. Exp. Eye Res. 2002;74:309–317. doi: 10.1006/exer.2001.1132. [DOI] [PubMed] [Google Scholar]
- 15.Ross ML, Lutz RJ, Yuan P, King BA, Whitcup SM, Robinson MR. Sustained-release intraocular implant for 2-methoxyestradiol: an angiostatic agent for the treatment of choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 2000;41:S770. [Google Scholar]
- 16.Ciulla TA, Walker JD, Fong DS, Criswell MH. Corticosteroids in posterior segment disease: an update on new delivery systems and new indications. Curr. Opin. Ophthalmol. 2004;15:211–220. doi: 10.1097/01.icu.0000120711.35941.76. [DOI] [PubMed] [Google Scholar]
- 17.Mallery SR, Pei P, Kang JC, Ness GM, Ortiz R, Touhalisky JE, Schwendeman SP. Controlled-release of doxorubicin from poly(lactide-co-glycolide) microspheres significantly enhances cytotoxicity against cultured AIDS-related kaposi’s sarcoma cells. Anticancer Res. 2000;20:2817–2825. [PubMed] [Google Scholar]
- 18.Weinberg BD, Ai H, Blanco E, Anderson JM, Gao JM. Antitumor efficacy and local distribution of doxorubicin via intratumoral delivery from polymer millirods. J. Biomed. Mater. Res. A. 2007;81A:161–170. doi: 10.1002/jbm.a.30914. [DOI] [PubMed] [Google Scholar]
- 19.Weinberg BD, Blanco E, Lempka SF, Anderson JM, Exner AA, Gao JM. Combined radiofrequency ablation and doxorubicineluting polymer implants for liver cancer treatment. J. Biomed. Mater. Res. A. 2007;81A:205–213. doi: 10.1002/jbm.a.30926. [DOI] [PubMed] [Google Scholar]
- 20.Luan XS, Bodmeier R. Modification of the tri-phasic drug release pattern of leuprolide acetate-loaded poly(lactide-co-glycolide) microparticles. Eur. J. Pharm. Biopharm. 2006;63:205–214. doi: 10.1016/j.ejpb.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Zhou TH, Lewis H, Foster RE, Schwendeman SP. Development of a multiple-drug delivery implant for intraocular management of proliferative vitreoretinopathy. J. Control. Release. 1998;55:281–295. doi: 10.1016/s0168-3659(98)00061-3. [DOI] [PubMed] [Google Scholar]
- 22.Witt C, Kissel T. Morphological characterization of microspheres, films and implants prepared from poly(lactide-co-glycolide) and ABA triblock copolymers: is the erosion controlled by degradation, swelling or diffusion? Eur. J. Pharm. Biopharm. 2001;51:171–181. doi: 10.1016/s0939-6411(01)00130-8. [DOI] [PubMed] [Google Scholar]
- 23.Elkharraz K, Faisant N, Guse C, Siepmann F, Arica-Yegin B, Oger JM, Gust R, Goepferich A, Benoit JP, Siepmann J. Paclitaxel-loaded microparticles and implants for the treatment of brain cancer: preparation and physicochemical characterization. Int. J. Pharm. 2006;314:127–136. doi: 10.1016/j.ijpharm.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 24.Gopferich A. Bioerodible implants with programmable drug release. J. Control. Release. 1997;44:271–281. [Google Scholar]
- 25.Klose D, Siepmann F, Elkharraz K, Krenzlin S, Siepmann J. How porosity and size affect the drug release mechanisms from PLGA-based microparticles. Int. J. Pharm. 2006;314:198–206. doi: 10.1016/j.ijpharm.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 26.Lyu SP, Sparer R, Hobot C, Dang K. Adjusting drug diffusivity using miscible polymer blends. J. Control. Release. 2005;102:679–687. doi: 10.1016/j.jconrel.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Redenti E, Szente L, Szejtli J. Drug/cyclodextrin/hydroxy acid multicomponent systems. Properties and pharmaceutical applications. J. Pharm. Sci. 2000;89:1–8. doi: 10.1002/(SICI)1520-6017(200001)89:1<1::AID-JPS1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 28.RothenWeinhold A, Besseghir K, Gurny R. Analysis of the influence of polymer characteristics and core loading on the in vivo release of a somatostatin analogue. Eur. J. Pharm. Sci. 1997;5:303–313. [Google Scholar]
- 29.Song CX, Labhasetwar V, Levy RJ. Controlled release of U-86983 from double-layer biodegradable matrices: effect of additives on release mechanism and kinetics. J. Control. Release. 1997;45:177–192. [Google Scholar]
- 30.Vogelhuber W, Rotunno P, Magni E, Gazzaniga A, Spruss T, Bernhardt G, Buschauer A, Gopferich A. Programmable biodegradable implants. J. Control. Release. 2001;73:75–88. doi: 10.1016/s0168-3659(01)00282-6. [DOI] [PubMed] [Google Scholar]
- 31.Yue IC, Poff J, Cortes ME, Sinisterra RD, Faris CB, Hildgen P, Langer R, Shastri VP. A novel polymeric chlorhexidine delivery device for the treatment of periodontal disease. Biomaterials. 2004;25:3743–3750. doi: 10.1016/j.biomaterials.2003.09.113. [DOI] [PubMed] [Google Scholar]
- 32.Wang FJ, Blanco E, Ai H, Boothman DA, Gao JM. Modulating β-lapachone release from polymer millirods through cyclodextrin complexation. J. Pharm. Sci. 2006;95:2309–2319. doi: 10.1002/jps.20721. [DOI] [PubMed] [Google Scholar]
- 33.Wang FJ, Saidel GM, Gao JM. A mechanistic model of controlled drug release from polymer millirods: effects of excipients and complex binding. J. Control. Release. 2007;119:111–120. doi: 10.1016/j.jconrel.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 34.Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur. J. Pharm. Biopharm. 2000;50:47–60. doi: 10.1016/s0939-6411(00)00076-x. [DOI] [PubMed] [Google Scholar]
- 35.Bernstein H, Zhang Y, Khan MA, Tracy MA. Modulated release from biocompatible polymers. 6,749,866. US Patent. 2004
- 36.Ding AG, Shenderova A, Schwendeman SP. Prediction of microclimate pH in poly(lactic-co-glycolic acid) films. J. Am. Chem. Soc. 2006;128:5384–5390. doi: 10.1021/ja055287k. [DOI] [PubMed] [Google Scholar]
- 37.Zhu GZ, Mallery SR, Schwendeman SP. Stabilization of proteins encapsulated in injectable poly (lactide-co-glycolide) Nat. Biotechnol. 2000;18:52–57. doi: 10.1038/71916. [DOI] [PubMed] [Google Scholar]
- 38.Desai KGH, Mallery SR, Schwendeman SP. Formulation and characterization of injectable poly(dl-lactide-co-glycolide) implants loaded with N-acetylcysteine, a MMP inhibitor. Pharm. Res. 2008;25:586–597. doi: 10.1007/s11095-007-9430-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szente L, Mikuni K, Hashimoto H, Szejtli J. Stabilization and solubilization of lipophilic natural colorants with cyclodextrins. J. Inclus. Phenom. Mol. 1998;32:81–89. [Google Scholar]
- 40.Dorati R, Genta I, Montanari L, Cilurzo F, Buttafava A, Faucitano A, Conti B. The effect of gamma-irradiation on PLGA/PEG microspheres containing ovalbumin. J. Control. Release. 2005;107:78–90. doi: 10.1016/j.jconrel.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 41.Gopferich A. Mechanisms of polymer degradation and erosion. Biomaterials. 1996;17:103–114. doi: 10.1016/0142-9612(96)85755-3. [DOI] [PubMed] [Google Scholar]
- 42.Von Burkersroda F, Schedl L, Gopferich A. Why degradable polymers undergo surface erosion or bulk erosion. Biomaterials. 2002;23:4221–4231. doi: 10.1016/s0142-9612(02)00170-9. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Zale S, Sawyer L, Bernstein H. Effects of metal salts on poly(dl-lactide-co-glycolide) polymer hydrolysis. J. Biomed. Mater. Res. 1997;34:531–538. doi: 10.1002/(sici)1097-4636(19970315)34:4<531::aid-jbm13>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 44.Mauduit J, Bukh N, Vert M. Gentamicin poly (lactic-acid) blends aimed at sustained-release local antibiotic-therapy administered per-operatively. 3. The case of gentamicin sulfate in films prepared from high and low-molecular-weight poly(dl-lactic acids) J. Control. Release. 1993;25:43–49. [Google Scholar]
- 45.Kang JC, Schwendeman SP. Determination of diffusion coefficient of a small hydrophobic probe in poly(lactide-co-glycolide) microparticles by laser scanning confocal microscopy. Macromolecules. 2003;36:1324–1330. [Google Scholar]
- 46.Zhu GZ, Schwendeman SP. Stabilization of proteins encapsulated in cylindrical poly(lactide-co-glycolide) implants: mechanism of stabilization by basic additives. Pharm. Res. 2000;17:351–357. doi: 10.1023/a:1007513425337. [DOI] [PubMed] [Google Scholar]