Abstract
Right ventricular (RV) function is closely linked to outcomes in pulmonary hypertension (PH). The authors sought to evaluate RV myocardial strain in 3 groups of patients: normal, PH with compensated RV function (PH-C), and PH with decompensated RV function (PH-D). Fifty-six patients (aged 56±12 years; 40 women; mean pulmonary artery pressure [MPAP] range, 13–82 mm Hg) underwent right heart catheterization and 2-dimensional echocardiography with tissue Doppler imaging of the RV. Right atrial pressures were 6±3, 5±2, and 14±4 mm Hg; MPAP values were 19±3, 44±15, and 56±13 mm Hg; pulmonary vascular resistances were 1.4±0.4, 7.9±5.1, and 11.5±6.6 Wood units; and cardiac indices were 3.4±0.9, 2.8±0.8, and 2.2±0.7 L/min/m2 (P<.05 for all for normal, PH-C, and PH-D patients), respectively. RV free wall strain decreased significantly among all 3 groups (− 26%±6%, − 19%±7%, and − 14%±5%; P<.0001). RV free wall strain decreases in PH without hemodynamically decompensated RV function suggesting it may be a preceding step in the development of RV failure. This may be of particular use in following patients sequentially.
Assessment of right ventricular (RV) function is essential for assessing prognosis and monitoring therapeutic interventions in pulmonary hypertension, (PH) as RV failure is a major determinant of poor outcomes.1 The RV is structurally different from the left ventricle (LV) and operates under different hemodynamic conditions. Thus, the RV is likely to respond differently to chronically elevated afterload. Current standard methods of evaluating RV function are limited due to its complex geometry, which hinders our understanding of RV adaptation to, and eventual failure from, pressure overload.2 Tissue Doppler imaging has been used to quantify myocardial velocities and strain to assess both global and regional LV and RV function.3,4 Longitudinal myocardial velocity and strain of the RV free wall has been reported to describe RV systolic function.5–7 We sought to use strain imaging to gain insight into the mechanisms of RV adaptation to the chronic pressure overload state of PH. RV peak longitudinal systolic strain was evaluated in 3 groups of patients: normal and PH with hemodynamically compensated or decompensated RV function (PH-C and PH-D, respectively), which we defined with a right atrial (RA) pressure threshold of ≥ 10 mm Hg.8 We hypothesized that a decrease in RV peak longitudinal systolic strain would occur before overt signs of RV failure.
Methods
Study Setting
Patients referred to the University of Pittsburgh Pulmonary Hypertension Program and undergoing right heart catheterization (RHC) for evaluation of PH were eligible for enrollment. This protocol was approved by the University of Pittsburgh institutional review board, and all patients provided informed consent. Inclusion criteria were: (1) age 18 years or older and (2) clinically indicated RHC and transthoracic echocardiography. Exclusion criteria were: (1) international normalized ratio >1.6; (2) platelet count <50,000; (3) inability to lay flat for procedures; (4) the presence of a prosthetic valve or valvular heart disease not including tricuspid regurgitation; (5) a pacer or defibrillator wire in the RV; (6) cardiac transplant; (7) complex congenital heart disease; and (8) evidence of an intracardiac shunt such as atrial septal defect.
While the hemodynamic definition of PH is standardized (mean pulmonary artery (PA) pressure ≥ 25 mm Hg), abnormal RV filling pressures are not and so clinicians somewhat arbitrarily use a threshold of ≥ 10 mm Hg based on limited prognostic data after treatment.8 Yet, clearly, RV dysfunction associated with PH portends poor prognosis. We therefore divided patients into 3 groups based on clinically used hemodynamic parameters: normal (normal PA pressure, defined as mean PA pressure ≤ 25 mm Hg), PH-C (PH with hemodynamically compensated RV function, defined as mean PA pressure >25 mm Hg and RA pressure <10 mm Hg), and PH-D (PH with hemodynamically decompensated RV function, defined as mean PA pressure ≥ 25 mm Hg and RA pressure ≥ 10 mm Hg). There were no patients with mean PA pressure ≤ 25 mm Hg and RA pressure ≥ 10 mm Hg.
Transthoracic Echocardiography
Echocardiography was performed with a GE-Vingmed Vivid 7 system (GE Vingmed Ultrasound, Horten, Norway). Standard 2-dimensional views and pulsed and continuous wave Doppler measurements were obtained as per American Echocardiography Association recommendations.9,10 The examination was recorded digitally. Results were confirmed by an independent echocardiographer (NR). RV end-diastolic areas (RVEDAs) and end-systolic areas (RVESAs) were measured from the apical 4-chamber view to calculate RV fractional area change (RVFAC) {RVFAC = [(RVEDA − RVESA)/RVEDA] × 100}.11 Peak PA systolic pressures were estimated by calculating the systolic pressure gradient between the RV and RA by the maximum velocity of the tricuspid regurgitant jet using the modified Bernoulli equation and then adding to this gradient an estimated RA pressure based on the size of the inferior vena cava and its variation with respiration.12
Color-coded tissue Doppler cine loops were captured and analyzed for peak RV myocardial strain, as previously reported.13 Briefly, 3 beats were obtained in the apical 4-chamber view at depths of 14±2 cm with pulse repetition frequency set at 1 kHz, Nyquist velocity range ±16 cm/sec, and frame rates 99±9 Hz. The imaging angle was adjusted to obtain a parallel alignment of the ultrasound beam with the segment of interest. Offline tissue Doppler analysis was performed using the commercially available software EchoPAC PC version 6.00 (GE Vingmed Ultrasound). Initial length for longitudinal strain assessment was set at 12 mm, and regions of interest (18±2 mm by 7±1 mm) were placed in the basal and midventricular segments of the RV free wall and interventricular septum. The region of interest was tracked to remain within the myocardial segment of interest throughout the cardiac cycle. The systolic ejection interval from pulmonic valve opening to pulmonic valve closing was indicated from the RV outflow tract spectral Doppler signal, and this ejection period appeared on the time-velocity/strain analysis screen. Peak myocardial strain during the systolic ejection period was obtained in each segment.14 Values obtained in the basal and mid-ventricular segments were averaged together to obtain a measure of strain in both the interventricular septum and RV free wall.
Right Heart Catheterization
Access to the right internal jugular vein was obtained using the standard modified Seldinger technique. A 7-French Swan-Ganz balloon-tip catheter was inserted into the right internal jugular vein and advanced through the right-heart chambers into the PA under fluoroscopy in the cardiac catheterization laboratory. The PA wedge position was confirmed by the appearance of a typical wedge pressure tracing in addition to anatomic position as seen via fluoroscopy. Pressure was measured in the RA, RV, main PA, and PA wedge position. Cardiac output was measured by the Fick method. All hemodynamic and tissue Doppler data were recorded by operators blinded to the results of the other test.
Statistics
Between-group differences were calculated using ANOVA with a Fisher’s least significant difference or Games-Howell (for variables with equal and unequal variances, respectively) post hoc analysis. Receiver operating characteristic analysis was performed to determine test characteristics for distinguishing patients with from those without PH and to distinguish RV hemodynamic state among patients with PH. All statistical calculations were made using SPSS for Mac, version 11 (SPSS, Inc, Chicago, IL).
Results
Patient Characteristics
A total of 56 patients were evaluated. Mean age was 56±12 years, 40 (71%) were female. Echocardiography and RHC were performed at 2.1±4.6 days (median, 1.0 days; 86% within 48 hours). Ten patients had normal PA pressures (mean PA pressure ≤ 25 mm Hg). Clinical characteristics of the 3 groups are presented in Table I. Groups were similar in age, sex distribution, and serum sodium and blood urea nitrogen concentrations. The 3 groups had a stepwise decrease in functional status as measured by World Health Organization (WHO) classification and 6-minute walking distance. Resting heart rate was significantly higher in the PH-D group than in either of the other groups.
Table I.
Clinical Characteristics of the 3 Groups
| Characteristic | Normal | PH-C | PH-D | P Value |
|---|---|---|---|---|
| No. | 10 | 20 | 26 | |
| Age, y | 51±10 | 54±12 | 51±12 | NS |
| Female | 7 (70%) | 15 (75%) | 18 (69%) | NS |
| WHO functional class | 1.6±0.7 | 2.7±1.0a | 3.4±0.6a,b | <.0001 |
| 6-min walking distance, m | 37±395 | 265±115a | 165±97a,b | .0002 |
| Resting heart rate, bpm | 71±8 | 77±15 | 88±14a,b | .002 |
| Etiology | ||||
| WHO group I | ||||
| Idiopathic | 5 (25%) | 7 (27%) | ||
| Familial | 0 | 1 (4%) | ||
| Connective tissue disease | 7 (35%) | 7 (27%) | ||
| Portopulmonary hypertension | 0 | 1 (4%) | ||
| WHO group III | ||||
| Chronic lung disease | 4 (20%) | 8 (31%) | ||
| WHO group IV | ||||
| CTED | 4 (20%) | 2 (8%) | ||
| Blood chemistry | ||||
| Serum sodium, mEq/L | 141±2 | 139±3 | 139±3 | NS |
| BUN, mg/dL | 14±3 | 20±12 | 22±14 | NS |
| Serum creatinine, mg/dL | 0.8±0.1 | 1.1±0.3a | 1.1±0.4a | .03 |
Abbreviations: bpm, beats per minute; BUN, serum urea nitrogen; CTED, chronic thromboembolic disease; NS, not significant; PH-C, pulmonary hypertension and hemodynamically compensated right ventricular function; PH-D, pulmonary hypertension and hemodynamically decompensated right ventricular function; WHO, World Health Organization. Data are mean ± standard deviation.
P<.05 PH-C vs normal or PH-D vs normal.
P<.05 PH-D vs PH-C.
Overall, 24 patients were receiving treatment for PH; 17 were on monotherapy, 6 were on a combination of 2 medications, and 1 was on a combination of 3 medications. Of these, 5 were receiving sildenafil, 7 were receiving prostacyclin therapy, 9 were receiving endothelin receptor antagonist therapy, and 13 were receiving calcium channel blockers. Several patients had PH diagnosed at the time of this study and subsequently started therapy. There were no significant differences in treatment among groups (Table II).
Table II.
Pulmonary Hypertension Treatment of the 3 Groups
| Medications | Normal | PH-C | PH-D | P Value |
|---|---|---|---|---|
| No. | 10 | 20 | 26 | |
| No. of patients treated | 3a | 8 | 13 | NS |
| Monotherapy | 3a | 4 | 10 | NS |
| Combination of 2 medications | 0 | 4 | 2 | NS |
| Combination of 3 medications | 0 | 0 | 1 | NS |
| Sildenafil | 0 | 1 | 4 | NS |
| Prostacyclins | 0 | 3 | 4 | NS |
| Endothelin receptor antagonist | 0 | 4 | 5 | NS |
| Calcium channel blocker | 3 | 4 | 6 | NS |
Abbreviations: NS, not significant; PH-C, pulmonary hypertension and hemodynamically compensated right ventricular function; PH-D, pulmonary hypertension and hemodynamically decompensated right ventricular function.
Not being used for the indication of pulmonary hypertension.
Hemodynamic differences among groups were as expected (Table III), consistent with the group definitions. There was a stepwise increase in mean PA pressure, transpulmonary gradient, and pulmonary vascular resistance from the normal group to the PH-C group to the PH-D group. Hemodynamic decompensation was evident in the PH-D group by a significantly higher RA pressure and lower cardiac index. RV stroke work index was higher in both PH groups than in the normal group, but it was lower in the PH-D group than in the PH-C group, which indicates hemodynamic decompensation in the PD-D group compared with the PH-C group. Left-sided filling pressures were similar across groups and were within the normal range, indicating that PH was not due to LV dysfunction.
Table III.
Invasive Hemodynamics of the 3 Groups
| Characteristic | Normal | PH-C | PH-D | P Value |
|---|---|---|---|---|
| No. | 10 | 20 | 26 | |
| Right atrial pressure, mm Hg | 6±3 | 5±2 | 14±4a,b | <.0001 |
| Mean PA pressure, mm Hg | 19±3 | 44±15a | 56±13a,b | <.0001 |
| PCWP, mm Hg | 10±2 | 10±3 | 13±7 | NS |
| TPG, mm Hg | 9±3 | 34±16a | 43±15a,b | <.0001 |
| PVR, Wood units | 1.4±0.4 | 7.9±5.1a | 11.5±6.6a,b | <.0001 |
| Cardiac index, L/min/m2 | 3.4±0.9 | 2.8±0.8 | 2.2±0.7a,b | .0005 |
| RVSWI, g-m/m2 | 8.3±2.0 | 18.6±6.7a | 14.0±5.0a,b | <.0001 |
Abbreviations: NS, not significant; PA, pulmonary artery; PCWP, pulmonary capillary wedge pressure; PH-C, pulmonary hypertension and hemodynamically compensated right ventricular function; PH-D, pulmonary hypertension and hemodynamically decompensated right ventricular function; PVR, pulmonary vascular resistance; RVSWI, right ventricular stroke work index; TPG, transpulmonary gradient. Data are mean ± standard deviation.
P<.05 PH-C vs normal or PH-D vs normal.
P<.05 PH-D vs PH-C.
Echocardiographic findings are presented in Table IV. All patients had normal LV ejection fraction (EF) and end-diastolic dimensions. Estimated tricuspid valve regurgitation severity was significantly greater in the PH-D group. RV areas progressively increased across the groups, while RVFAC was lower in patients with PH.
Table IV.
Echocardiographic Characteristics of the 3 Groups
| Characteristic | Normal | PH-C | PH-D | P Value |
|---|---|---|---|---|
| No. | 10 | 20 | 26 | |
| Estimated LVEF, % | 56±4 | 57±4 | 55±8 | NS |
| LV end-diastolic diameter, cm | 4.4±0.5 | 3.9±0.7 | 3.7±0.9 | NS |
| Estimated TR severity | 1.1±1.0 | 1.6±0.9 | 2.3±0.9a,b | .002 |
| RV end-diastolic area, cm2 | 17±3 | 25±9a | 32±10a,b | .0001 |
| RV end-systolic area, cm2 | 8±2 | 18±9a | 24±8a,b | <.0001 |
| RVFAC, % | 51±10 | 32±12a | 24±9a,b | <.0001 |
Abbreviations: LV, left ventricular; LVEF, left ventricular ejection fraction; NS, not significant; PH-C, pulmonary hypertension and hemodynamically compensated right ventricular function; PH-D, pulmonary hypertension and hemodynamically decompensated right ventricular function; RV, right ventricular; RVFAC, right ventricular fractional area change; TR, tricuspid regurgitation. Data are mean ± standard deviation.
P<.05 PH-C vs normal or PH-D vs normal.
P<.05 PH-D vs PH-C.
Peak RV Free Wall Longitudinal Strain by Tissue Doppler Imaging
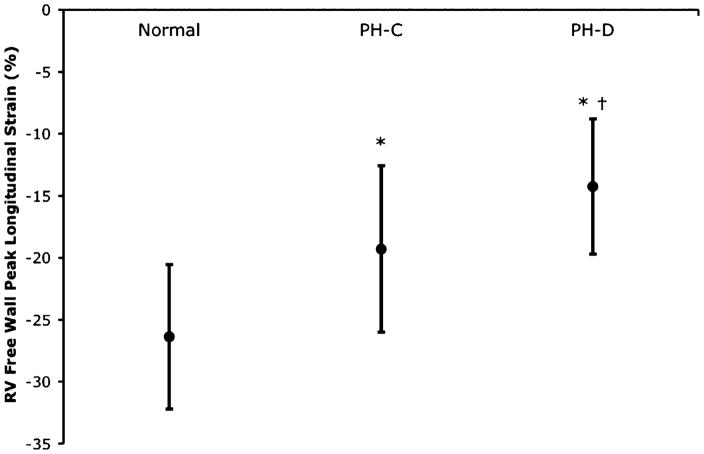
Peak RV free wall longitudinal strain by tissue Doppler imaging is presented in the Figure. There was a significant decrease in the measured strain between the normal group (mean 0 ± standard error, − 26%±2%) and the PH-C group (− 19%±1%) and a further significant decrease from the PH-C group to the PH-D group (− 14%±1%). Similar results were seen in subgroup analysis stratified by WHO group classification of PH. Peak RV free wall longitudinal strain by tissue Doppler imaging correlated highly with RV systolic pressure, PA systolic pressure, PA mean pressure, transpulmonary gradient, pulmonary vascular resistance, and cardiac index (R=0.64, 0.65, 0.68, 0.71, 0.73, − 0.68, respectively; all P<.001).
Figure.
Right ventricular (RV) free wall peak longitudinal strain as measured by tissue Doppler imaging. There was a highly significant stepwise decrease in peak longitudinal strain from patients with normal pulmonary pressures to patients with pulmonary hypertension and hemodynamically compensated RV function (PH-C) to patients with pulmonary hypertension and hemodynamically decompensated RV function (PH-D) (P<.0001). Data are mean with standard deviation bars. *P<.05 PH-C vs normal or PH-D vs normal; †P<.05 PH-D vs PH-C.
By receiver operating characteristic analysis, peak RV free wall longitudinal strain by tissue Doppler imaging ≤ −27.2% (more negative or greater strain) detected normal PA pressure with a sensitivity of 70% and specificity of 93.5% (area under the curve, 0.878; P<.001). Positive predictive value was 70% and negative predictive value was 93.5%. In patients with PH, peak RV free wall longitudinal strain by tissue Doppler imaging ≥ −14.9% detected hemodynamically decompensated RV function (PH-D vs PH-C) with a sensitivity of 61.5% and specificity of 85% (area under the curve, 0.727; P=.009). Positive predictive value was 84% and negative predictive value was 63%. The test characteristics of RVEDA, RVESA, and RVFAC with respect to the PH-C and PH-D groups had lower specificity (67%, 67%, and 72%, respectively for RVEDA ≥ 27.7 cm2, RVESA ≥ 19.99 cm2, and RVFAC ≤ 26.75%) and lower positive predictive values (73%, 76%, and 77%, respectively) for detecting hemodynamically decompensated RV function (PH-D) at comparable sensitivity (67%, 79%, and 71%, respectively) and area under the curve (0.701, 0.713, and 0.722, respectively).
Interobserver reproducibility of peak RV free wall longitudinal strain by tissue Doppler imaging was 1±3% as assessed in a subset of 10 patients by 2 observers (NR and AL) blinded to clinical information. Intraobserver reproducibility was 1%±3% as assessed by one observer (NR) repeating measurements of the same 10 patients at least 30 days later.
Discussion
Our results demonstrate a significant stepwise decrease in the peak RV free wall longitudinal strain as measured by tissue Doppler imaging among the normal, PH-C, and PH-D groups. These groups, defined hemodynamically by mean PA and RA pressures, demonstrated progressive RV dilation and decline in functional status with worsening PH. PH-D was characterized by the highest PA pressures, high RV filling pressures, and low cardiac index. Our finding that peak RV free wall longitudinal strain differentiates these groups of patients suggests that it could be a useful noninvasive test for identifying hemodynamically significant PH and monitoring RV function in such patients. While there is overlap of peak RV free wall longitudinal strain by tissue Doppler imaging among groups, this test distinguishes patients with PH from those without in 93.5% of cases and, further, can distinguish hemodynamically decompensated RV function from compensated RV function in PH in 84% of cases. However, we hypothesize that the most significant clinical benefit of these findings will be in the sequential monitoring of RV strain with disease progression or medical intervention due to the simplicity and reproducibility of RV tissue Doppler imaging. Further, attenuated peak developed RV myocardial strain may be a mechanism of RV failure in the setting of chronic pressure overload, linking observed remodeling with decline in functional status despite preserved cardiac output until late in the disease.
We and others have previously shown good correlation between peak RV free wall longitudinal strain as measured by tissue Doppler imaging and invasively measured hemodynamics including transpulmonary gradient, pulmonary vascular resistance, cardiac index, and RV stroke volume in patients with PH.6,15 The current findings show that peak RV free wall longitudinal strain can further distinguish PH patients with hemodynamically compensated RV function from those with decompensated RV function. This distinction is critical to the evaluation and management of PH in the current era of multiple therapies. Further, the mechanisms of progressive RV dysfunction in response to chronic pressure overload are poorly understood. Our findings suggest that RV myocardial function is compromised on both regional and global levels prior to the development of hemodynamic RV failure, which is associated with further deterioration in peak RV myocardial strain. While PH treatment is known to improve hemodynamics and outcomes, the effect on RV myocardial strain is unknown. Improvement in peak RV myocardial strain with PH therapy might be a good indicator of response to treatment, and failure to respond might be an indication to add an additional therapeutic agent. Responsiveness of regional RV function to therapy in relation to markers of global RV function also remains to be elucidated. These are areas of ongoing investigation.
RVFAC was decreased significantly in both PH groups compared to the normal group and was associated with diminished peak RV free wall longitudinal strain. This demonstrates that global RV function deteriorates along with regional RV function. Thus, regional RV dysfunction has immediate global consequences. Decrement in global RV function may be explained by reduced regional peak developed RV myocardial strain and may relate more directly to underlying mechanisms of RV myocardial failure in the setting of chronic pressure. Tissue Doppler imaging of RV myocardial strain could potentially localize early changes in RV myocardial function and be useful in future studies of the pathogenesis of RV failure in chronic pressure overload.
LVEF was similar among groups and, in particular, normal despite decompensated RV function. This phenomenon is due to decreased filling of the LV due to RV dilation and septal shift (ventricular interdependence) concomitant with decreased ejection volume, therefore maintaining normal LVEF even in the setting of overall decreased cardiac output. The current results further substantiate this finding, which we and others previously reported in patients with PH.16–18
Limitations
The sample size and the cohort design of single-point-in-time measures limit the interpretation of these data. Sequential imaging of patients from early in disease through the development of decompensated RV function would be ideal for documenting regional RV functional response to chronic pressure overload. While we focused on analysis of myocardial strain, tissue Doppler imaging also provides velocity and strain rate data; however, our preliminary studies found strain data to have the most optimal signal-to-noise ratio. Our results may not be applicable to PH caused by left-sided heart disease or intracardiac shunts, as patients with these conditions were excluded from the present study. The definition of hemodynamic RV compensation was based on what clinicians use and derived from limited prognostic data after treatment8: an RA pressure of 10 mm Hg; while this definition is not standardized, it did cosegregate with cardiac index and functional status. The use of calcium channel blockers, though not statistically different between groups, could affect contractility and measured myocardial strain. Although the mean strain values were significantly different in compensated and decompensated PH, there remains a fair amount of overlap in the distribution of values, which limits its diagnostic utility as a “screening tool” for early RV decompensation. However, the simplicity and reproducibility of RV tissue Doppler imaging allows for sequential studies of RV strain with disease progression or medical intervention. We hypothesize that the change in RV strain over time will be of much greater clinical utility than a single “snapshot” in time. The utility of serial assessment of RV function by tissue Doppler imaging and the impact of medical therapy requires further investigation.
Conclusions
Longitudinal RV free wall strain decreases with PH despite hemodynamically compensated RV function and further deteriorates as the RV fails. RV free wall strain appears to be useful in identifying abnormal RV deformation in PH and RV failure and should be further assessed as a potential mechanism of RV failure in the setting of chronic pressure overload. These findings should be confirmed in larger numbers and ideally in a longitudinal study.
Footnotes
Disclosures: Dr Simon reports receiving consulting fees or serving on paid advisory boards for Gilead and receiving lecture fees from Actelion and Gilead. Dr Mathier reports receiving research funding from Actelion; receiving consulting fees or serving on paid advisory boards for Gilead, United Therapeutics, and Actelion; and receiving lecture fees from United Therapeutics, Actelion, Encysive, and GlaxoSmithKline. This work was supported in part by the National Institutes of Health (NIH) Roadmap Multidisciplinary Clinical Research Career Development Award (KL2 RR024154), NIH grants (HL067181, HL07820), and McGinnis Chair endowment funds. This publication was made possible by grant number KL2 RR024154 from the National Center for Research Resources (NCRR), a component of NIH, and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on reengineering the clinical research enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
References
- 1.McLaughlin VV, Presberg KW, Doyle RL, et al. Prognosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126:78S–92S. doi: 10.1378/chest.126.1_suppl.78S. [DOI] [PubMed] [Google Scholar]
- 2.Cheitlin MD, Armstrong WF, Aurigemma GP, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography–summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography) J Am Coll Cardiol. 2003;42(5):954–970. doi: 10.1016/s0735-1097(03)01065-9. [DOI] [PubMed] [Google Scholar]
- 3.Stoylen A, Heimdal A, Bjornstad K, et al. Strain rate imaging by ultrasound in the diagnosis of regional dysfunction of the left ventricle. Echocardiography. 1999;16:321–329. doi: 10.1111/j.1540-8175.1999.tb00821.x. [DOI] [PubMed] [Google Scholar]
- 4.Weidemann F, Eyskens B, Jamal F, et al. Quantification of regional left and right ventricular radial and longitudinal function in healthy children using ultrasound-based strain rate and strain imaging. J Am Soc Echocardiography. 2002;15:20–28. doi: 10.1067/mje.2002.116532. [DOI] [PubMed] [Google Scholar]
- 5.Jamal F, Bergerot C, Argaud L, et al. Longitudinal strain quantitates regional right ventricular contractile function. Am J Physiol Heart Circ Physiol. 2003;285:H2842–H2847. doi: 10.1152/ajpheart.00218.2003. [DOI] [PubMed] [Google Scholar]
- 6.Urheim S, Cauduro S, Frantz R, et al. Relation of tissue displacement and strain to invasively determined right ventricular stroke volume. Am J Cardiol. 2005;96:1173–1178. doi: 10.1016/j.amjcard.2005.06.049. [DOI] [PubMed] [Google Scholar]
- 7.Lopez-Candales A, Dohi K, Bazaz R, et al. Relation of right ventricular free wall mechanical delay to right ventricular dysfunction as determined by tissue doppler imaging. Am J Cardiol. 2005;96:602–606. doi: 10.1016/j.amjcard.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 8.Sitbon O, Humbert M, Nunes H, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol. 2002;40:780–788. doi: 10.1016/s0735-1097(02)02012-0. [DOI] [PubMed] [Google Scholar]
- 9.Henry WL, DeMaria A, Gramiak R, et al. Report of the American Society of Echocardiography committee on nomenclature and standards in two-dimensional echocardiography. Circulation. 1980;62(2):212–217. doi: 10.1161/01.cir.62.2.212. [DOI] [PubMed] [Google Scholar]
- 10.Quiñones MA, Otto CM, Stoddard M, et al. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15(2):167–184. doi: 10.1067/mje.2002.120202. [DOI] [PubMed] [Google Scholar]
- 11.Schenk P, Globits S, Koller J, et al. Accuracy of echocardiographic right ventricular parameters in patients with different end-stage lung diseases prior to lung transplantation. J Heart Lung Transplant. 2000;19:145–154. doi: 10.1016/s1053-2498(99)00121-7. [DOI] [PubMed] [Google Scholar]
- 12.Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984;70:657–662. doi: 10.1161/01.cir.70.4.657. [DOI] [PubMed] [Google Scholar]
- 13.Rajagopalan N, Dohi K, Simon MA, et al. Right ventricular dyssynchrony in heart failure: a tissue Doppler imaging study. J Card Fail. 2006;12(4):263–266. doi: 10.1016/j.cardfail.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Gorcsan J, III, Gulati VK, Mandarino WA, et al. Color-coded measures of myocardial velocity throughout the cardiac cycle by tissue Doppler imaging to quantify regional left ventricular function. Am Heart J. 1996;131:1203–1213. doi: 10.1016/s0002-8703(96)90097-6. [DOI] [PubMed] [Google Scholar]
- 15.Rajagopalan N, Simon MA, Mathier MA, et al. Identifying right ventricular dysfunction with tissue Doppler imaging in pulmonary hypertension. Int J Cardiol. 2008;128:359–363. doi: 10.1016/j.ijcard.2007.06.094. [DOI] [PubMed] [Google Scholar]
- 16.Rajagopalan N, Simon MA, Suffoletto MS, et al. Noninvasive estimation of pulmonary vascular resistance in pulmonary hypertension. Echocardiography. 2009;26(5):489–494. doi: 10.1111/j.1540-8175.2008.00837.x. [DOI] [PubMed] [Google Scholar]
- 17.Huez S, Vachiery JL, Unger P, et al. Tissue Doppler imaging evaluation of cardiac adaptation to severe pulmonary hypertension. Am J Cardiol. 2007;100:1473–1478. doi: 10.1016/j.amjcard.2007.06.047. [DOI] [PubMed] [Google Scholar]
- 18.Louie EK, Lin SS, Reynertson SI, et al. Pressure and volume loading of the right ventricle Have opposite effects on left ventricular ejection fraction. Circulation. 1995;92:819–824. doi: 10.1161/01.cir.92.4.819. [DOI] [PubMed] [Google Scholar]