Abstract
Metamorphosis is one of the most common, yet dramatic of life history strategies. In insects, complete metamorphosis with morphologically distinct larval stages arose from hemimetabolous ancestors that were more direct developing. Over the past century, several ideas have emerged that suggest the holometabolous pupa is developmentally homologous to the embryonic stages of the hemimetabolous ancestor. Other theories consider the pupal stage to be a modification of a hemimetabolous nymph. To address this question, we have isolated an ortholog of the pupal determinant, broad (br), from the hemimetabolous milkweed bug and examined its role during embryonic development. We show that Oncopeltus fasciatus br (Of'br) is expressed in two phases. The first occurs during germ band invagination and segmentation when Of'br is expressed ubiquitously in the embryonic tissues. The second phase of Of'br expression appears during the pronymphal phase of embryogenesis and persists through nymphal differentiation to decline just before hatching. Knock-down of Of'br transcripts results in defects that range from posterior truncations in the least-affected phenotypes to completely fragmented embryonic tissues in the most severe cases. Analysis of the patterning genes engrailed and hunchback reveal loss of segments and a failure in neural differentiation after Of'br depletion. Finally, we show that br is constitutively expressed during embyrogenesis of the ametabolous firebrat, Thermobia domestica. This suggests that br expression is prominent during embryonic development of ametabolous and hemimetabolous insects but was lost with the emergence of the completely metamorphosing insects.
Keywords: Evolution of metamorphosis, Holometabola, BTB domain, Oncopeltus fasciatus, Direct development
Introduction
The insect world is divided into groups according to life history strategy. Ametabolous insects are direct developers and simply reiterate the first nymphal form, as they grow and molt, then gain genitalia to produce the adult stage. Hemimetabolous insects, which include crickets and true bugs, also reiterate the first nymphal form, but these insects produce wings as well as genitalia at the last nymphal molt. Holometabolous insects, with compete metamorphosis, produce a morphologically distinct larval stage, and the adult form is not generated until the last stages of postembryonic development.
Since the fossil record established that holometabolous insects emerged from hemimetabolous ancestors, a debate on the origin of metamorphosis has persisted among entomologists (for reviews, see Hemming 2003; Erezyilmaz 2006). With the discovery of the metamorphic hormones, more specific hypotheses arose to argue that the pupal stage emerged after an endocrine-induced shift in ontogeny (Novak 1966; Truman and Riddiford 1999, 2002). The metamorphic hormones include the steroid hormone, 20-hydroxyecdysone (20E), which is released in pulses to trigger molts between stages, and the sesquiterpenoid, juvenile hormone (JH). Juvenile hormone was named for its role in maintaining the juvenile state of all insects; loss of JH results in precocious metamorphosis, while exogenous JH promotes the juvenile state instead of the adult fate at the final molt (Riddiford 1996). The ‘pronymph hypothesis’ (Truman and Riddiford 1999, 2002) suggests that the trigger for the evolution of the holometabolous larva was an advancement of JH production into earlier stages of embryonic development. The “morphostatic action” of JH would abbreviate differential growth but promote precocious differentiation (Truman and Riddiford 2007).
Whether the pupal stage arose by a shift in ontogeny, or by addition of new traits, can be resolved by comparing the role of genes that regulate pupal development between the hemi- and holometabolous insects. In holometabolous insects, the br (broad) gene is considered a metamorphic gene and, except for some central neurons (Zhou et al. 2009), is not expressed in tissues until the last larval stage. The br gene produces four transcripts by alternate splicing so that the protein products have one of four (in Drosophila) or five (in Tribolium) different C2H2 zinc fingers (Z1, Z2, Z3, Z4, Z5) spliced to a common BTB (Broad, Tramtrack, Bric-a-brac) core domain (DiBello et al. 1991; Konopova and Jindra 2008; Suzuki et al. 2008; Parthasarathy et al. 2008). The expression pattern of br in flies (Karim et al. 1993), the moths Manduca sexta and Bombyx mori (Zhou et al. 1998; Zhou and Riddiford 2001, 2002), the beetle Tribolium castaneum (Konopova and Jindra 2008; Suzuki et al. 2008; Parthasarathy et al. 2008), and Chrysopa perla (Konopova and Jindra 2008) have shown that br expression is prominent during the larval-pupal transition but lost as the pupa initiates adult differentiation. In Manduca, the restriction of br expression to pupal development occurs through the combined action of JH and 20E (Zhou et al. 1998; Zhou and Riddiford 2001, 2002). During the larval stages, br expression is repressed by the presence of JH. Its expression is first activated by a small peak of 20E, the “commitment peak”, that occurs in the absence of JH during the final larval stage. Once br expression is initiated, the inhibitory relationship between JH and br appears to be reset. At the molt to the pupal stage, JH maintains br expression, and br is not shut off until the molt to the adult stage when 20E appears again in the absence of JH. Exogenous application of JH at the onset of the adult molt, when it is normally absent, allows re-induction of br expression by 20E. The significance of this pattern was seen when heat-shock-driven expression of Br isoforms in Drosophila at the adult molt induced a second pupal cuticle, while heat-shock-driven expression of Br during a larval molt resulted in the precocious expression of pupal genes (Zhou and Riddiford 2002). These data show that JH acts as a binary switch at molts to restrict br expression to pupal development when it regulates pupal-specific gene activity. This role for br in pupal specification has been confirmed with RNAi-based loss of function studies in other holometabolous insects. Knock-down of br in the silkmoth B. mori (Uhlirova et al. 2003), the beetle T. castaneum (Konopova and Jindra 2008; Suzuki et al. 2008; Parthasarathy et al. 2008), and the basal holometabolan C. perla (Konopova and Jindra 2008) also results in defects at the onset of metamorphosis.
To investigate the role of this pupal determinant during embryogenesis of a hemimetabolous insect, we isolated a portion of the br gene from the milkweed bug Oncopeltus fasciatus. In a previous paper, we found that Of'br is expressed at nymphal molts and that it is required for progressive changes that occur during the immature stages (Erezyilmaz et al. 2006). Here, we show that Of'br is expressed during two phases of embryonic development. A transient phase of expression occurs during the process of germ band invagination, while a second episode of Of'br expression appears during pronymphal development, and this persists until hatching. Surprisingly, we find that Of'br is required for a number of early developmental events that follow germ band invagination. We also show that br is expressed throughout embryonic development of the firebrat Thermobia domestica, an ametabolous insect. Taken together, these data show that expression of br is a basal feature of insect embryonic development and has been lost from embryonic development in the Holometabola. We suggest that this loss was instrumental in the evolution of the holometabolous larval form.
Materials and methods
Animal husbandry and staging
Oncopeltus were reared at 26°C under long-day conditions (17L:7D). For staged embryos, fresh cotton was left with adults for no longer than 4 h, and the midpoint of this period was taken as hour zero.
Thermobia were reared on Pablum (Gerber Products, Fremont, MI, USA) supplemented with cat chow (MaxCat, Nutro Products, City of Industries, CA, USA) at 37°C in an atmosphere of 70% humidity maintained by saturated KCl in the bottom of the incubator. Eggs were collected daily and incubated until use under the same conditions.
Cloning
The highly conserved broad BTB domain was amplified from Thermobia genomic DNA using degenerate primers (Zollman et al. 1994). The 3′ end of the divergent br core was amplified with a primer designed from the previously sequenced BTB domain (5′-GTTTTCGCTGGAATAATTACCAAAG) and a degenerate primer designed from the Z1 region of an alignment of br from Bombyx, Drosophila, Acheta domesticus, and Aedes aegypti (5′-CGRTGRTAGATRCTYYTRTGGTTGC).
RT–PCR and RNA isolation, staging
To determine broad expression during embryonic development, total RNA was extracted from embryos collected at 24-h intervals using Trizol (Invitrogen, Carlsbad, CA, USA), and 1 μg was used to generate cDNA synthesized using random hexamer primers with M-MuLV Reverse Transcriptase (Fermentas, Glen Burnnie, MD, USA). The primers and conditions used to detect Of'br expression during embryonic development were tailored to the linear range and these conditions were described previously (Erezyilmaz et al. 2006).
To detect the expression of Td'br, we designed primers from within the br core—forward: 5′-GAGTGTCACGGGCTCATCAGGGTTTTC and reverse: 5′-CTTGCTACGAATATCTCCGCTCTGG. PCR conditions for broad core: 58°C annealing temperature, 30 cycles. We used 18S ribosomal RNA as a loading control, and RT–PCR was performed with an annealing temperature of 61°C and with 13 cycles. To detect 18S, we used the following primers—forward: 5′-TGACTCAACACGGGAAACCTCACCA, reverse: 5′-ACAAAGGGCAGGGACGTAATCAACGC. For both Of'br and Td'br, we did not detect the presence of genomic contamination in RNA tested in sham reactions without reverse transcriptase for each RNA sample. For both insects, the expression that is shown is representative of at least two biological replicates.
In situ methods
The in situ hybridizations were performed on germ band stage embryos after chorion cracking as described in Liu and Kaufman (2004a), except that the proteinase K and detergent stages were omitted, and embryos of all stages were boiled prior to fixation and hybridized within the yolk. Germ band stage embryos were mounted in Fluoromount (Diagnostic Biosystems, Pleasanton, CA, USA). The Of'en digoxigenin-labeled probe was a generous gift of Paul Liu (Indiana University, Bloomington).
dsRNA injections
Separate RNA strands were made from plasmids containing Of'br gene fragments using MEGAscript kit (Ambion, Austin, TX, USA). The sense and antisense strands were annealed as described in Hughes and Kaufman (2000). Parental dsRNAi injections were performed as in Liu and Kaufman (2004a, b), except that females were injected while mating. Then, 10–50 μg of dsRNA was injected in a volume of 5–10 μl into each female. When Of'brRNAi embryos were used for in situ hybridization, we used clutches that were preceded and followed by affected clutches to assure that the clutch we used would produce the Of'br phenotype.
Results
Of'br expression during embryonic development of Oncopeltus
Within insects, segmentation may occur progressively from a small primordium (short germ development) or through subdivision of an existing primordium that surrounds the entire egg (long germ). Embryonic development of Oncopeltus is of an intermediate mode that begins at the surface of the egg but later occurs progressively within the yolk. Embryogenesis begins as daughter nuclei of the fusion nucleus divide, and then migrate to the periphery of the egg where they cellularize. Starting at approximately 1 day after egg deposition, the prospective germ band appears as “two longitudinal plates of cuboidal cells that converge at the posterior pole” of the blastoderm (Butt 1949). At this stage, the two lateral plates consist of presumptive head and thoracic segments only (Liu and Kaufman 2004a). Shortly after the appearance of these plates, a dimple in the blastoderm appears at the posterior pole just above a cluster of cells that Butt (1949) considered germ cells. Over the next several hours, this pit deepens as the outer layer of the blastoderm pushes into the center of the egg; first the prospective thorax enters, followed by the gnathal segments, and finally the head lobes. This process, where embryonic development switches from superficial development on the surface of the blastoderm, to progressive development within the egg is called germ band invagination. Once germ band invagination is completed, growth and segmentation of the abdomen begins. Segments appear progressively in an anterior to posterior wave of segmentation, as the more anterior segments begin to differentiate before the posterior segments have emerged from a posterior “growth zone”.
Oncopeltus embryos produce three cuticles during embryogenesis (Dorn and Hoffman 1981; Konopova and Zrzavy 2005). The first embryonic cuticle appears on day 3 of embryonic development, after the completion of segmentation, but before the onset of katatrepsis, a movement that inverts the orientation of the embryo as it brings the segmented germ band to the surface of the yolk. The second embryonic or “pronymphal” cuticle appears on the fourth day of embryonic development, at the completion of dorsal closure, when the dorso-lateral halves of the embryo grow around the yolk. The first nymphal cuticle is the final cuticle that is produced during embryogenesis, and is first detected on the fifth day of embryonic development (Dorn and Hoffman 1981).
We used primers designed to amplify the broad core domain to follow the expression of Of'br transcripts during the 6 days of embryonic development with semiquantitative RT–PCR. We found two discrete phases of Of'br expression. The first phase appears 24 h after egg deposition and can be occasionally detected on day 2 (Fig. 1 and data not shown). This transient expression coincides with germ band invagination. The second episode of Of'br expression begins on day 3, following the formation of the E1 cuticle, but prior to the time of pronymphal cuticle formation. This expression persists throughout nymphal development but declines by the time of hatching (Fig. 1).
Fig. 1.
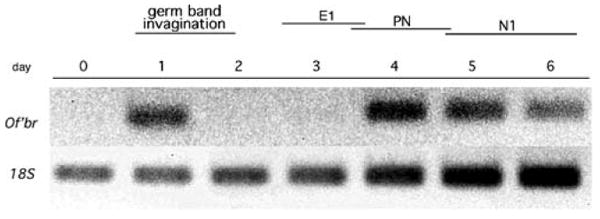
The expression of Of'br core during the 6 days (0–6) of Oncopeltus embryogenesis. Relevant developmental events are indicated above: E1 duration of the E1 cuticle stage, PN duration of the pronymphal cuticle stage, N1 duration of the first embryonic cuticle stage. Top Of'br expression. Bottom 18S ribosomal RNA expression is used as a loading control for the same cDNA reactions used to measure Of'br transcript levels. Apparently, the amount of 18S rRNA transcribed per microgram total RNA increases during the 6 days of development
To determine the spatial distribution of Of'br transcripts during germ band invagination and segmentation, we examined Of'br core expression at the blastoderm stage. Of'br is first discernable at about 28–30 h as the invagination pore forms (Fig. 2a, arrowhead). The expression becomes more pronounced by 32 h when we first detect the gnathal and thoracic stripes of Of'en at the blastoderm stage (data not shown, Liu and Kaufman 2004a, b). At this stage, Of'br is diffusely expressed across the embryonic primordium, which consists of two lateral plates that will form the head and thorax. By 38 h, the presumptive thorax has entered the blastoderm, and Of'br messages are abundant throughout the embryonic tissues (Fig. 2c, g). Of'br expression disappears at the completion of germ band invagination between 44 and 48 h after egg deposition (Fig. 2d, h). A sense-strand probe hybridized to 38-h-old embryos at an identical concentration did not show appreciable staining (Fig. 2e).
Fig. 2.
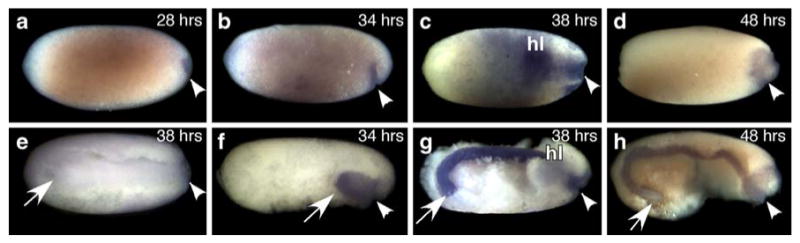
Of'br in situ expression during germ band invagination in whole blastoderms (a–d) and in blastoderms with the lateral half of the yolk removed (e–h). For each panel, the site of invagination is indicated with an arrowhead, the posterior-most tip of the embryo is labeled with an arrow, head lobes (hl) are labeled above a lateral half of the head primordium. In each panel except for (e), the dorsal half of the embryo is facing upwards. The prospective anterior end is to the left in each panel, although the anterior end of the developing germ band may be at the right or left, depending upon the point in germ band invagination and katatrepsis. Faint Of'br is seen at the forming invagination pore at 28–30 h (a) and in the invaginating embryonic primordia at 34 h (b, f). Of'br levels are elevated at 38 h (c, g), at the latest stages of germ band invagination. Of'br transcripts are diminished by 48 h (d, h) after the completion of germ band invagination. e A ventral view of an embryo at 38 h hybridized with an equal amount of Of'br sense strand that did not reveal any stain
The second phase of Of'br expression occurs as the pronymphal cuticle is produced. Since the presence of this cuticle prevents hybridization in epidermal cells, we were unable to determine the spatial distribution of the second phase of Of'br transcripts.
The effects of Of'br depletion on embryonic development
We used parental RNAi to knock down Of'br transcript levels in early development. Of'br dsRNA was made with 5–10 μl of either (1) the 151 base pair (bp) region of the Of'br BTB domain or (2) 218 bp of the Of'br 5′UTR plus the first 107 bp of the BTB domain. We did not detect a significant difference in the activity of either gene fragment (data not shown). To control for non-specific knock-down, we used a syntenic piece of the broad BTB domain from the cricket, Acheta (151 bp of the Acheta BTB domain or 124 bp of the 5′UTR plus about 250 bp of the Acheta BTB domain). Embryos from Ach'br dsRNA injected females developed normally (Fig. 3a).
Fig. 3.
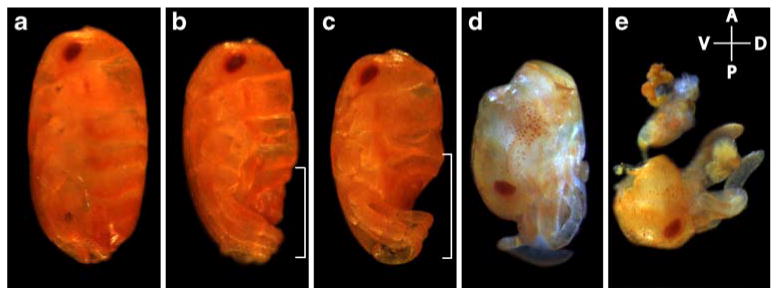
Embryos from mothers injected with Of'br dsRNA exhibited posterior truncation and failed to undergo katatrepsis. In each case, the embryo is shown as it is found within the egg. The anterior of the egg is to the top, and the dorsal side is to the right. Embryos in (d) and (e) failed to complete katatrepsis and are found with their anterior ends at the posterior pole. a A control embryo. b In moderately affected phenotypes, the most posterior segments of the abdomen were missing, although defects were apparent after the third thoracic segment. This area is indicated with a bracket. c Increasingly affected embryos failed to make more than half of the posterior abdominal segments, and compressed segments appeared after the second thoracic segment. The affected region is indicated with a bracket. The more severely affected embryos consisted of head and trunk only (d), or only head segments (e). These more severely affected embryos were often found with the head at the posterior pole of the egg
The effects of Of'br parental RNAi typically appeared in clutches that were laid 2–3 days after injection. At the time of hatching, the mildest of these embryos were missing the posterior-most segments of the abdomen (Fig. 3b). As the phenotype increased in severity over the next several days, embryos with increasing posterior truncation appeared (Fig. 3c, d) so that, as the phenotype peaked, only the most anterior structures were found. In some of the more extreme cases of posterior truncation, the embryos failed to undergo katatrepsis so that the anterior segments remained at the posterior end of the egg (Fig. 3d, e). In the most extreme phenotypes, only eyes, antennae, or mouthparts were found with disorganized tissue and yolk (data not shown).
To determine if these truncations were due to defects in germ band growth or to a failure of germ band differentiation, we examined engrailed (Of'en) expression, which marks the posterior half of each segment (Liu and Kaufman 2004a). Embryos from mothers injected with Of'br dsRNA were fixed 3 days after egg deposition, when segmentation is normally completed but before the onset of pronymphal cuticle deposition, and hybridized with a labeled Of'en RNA probe. The most common phenotype that we observed had disorganized stripes in abbreviated growth zones (Fig. 4). We also observed embryos that appeared to consist of head, or head and thoracic segments only (data not shown).
Fig. 4.
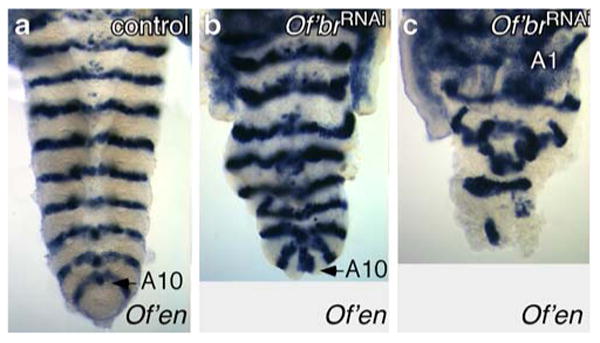
In situ analysis of engrailed mRNA in the abdomens of Oncopeltus embryos from a mother injected with Of'br dsRNA. In (a), the embryo successfully formed all 10 abdominal segments (A10). The abdomen in (b) has also produced 10 engrailed stripes, although posterior growth apparently failed after the last engrailed stripe and the tissue closed in upon itself (arrow). c A highly disorganized posterior growth zone at the completion of segmentation. The first abdominal segment is indicated (A1)
In addition to Of'en, we monitored the expression of the patterning genes, even-skipped (Of'eve), E75A (Of'E75A), Kruppel (Of'Kr), and hunchback (Of'hb) at the blastoderm and germ band stages (Liu and Kaufman 2005; Erezyilmaz et al. 2009; Liu and Kaufman 2004a, b). We were unable to detect differences in the spatial distribution Of'eve, Of'E75A, and Of'Kr (data not shown), although we found changes in the location of Of'hb transcripts, which are transcribed in both the developing central nervous system and in the growth zone. At the onset of abdominal segmentation, Of'hb is found in two tracks of neural epithelium and in emerging neuroblasts that extend from the head to the posterior extent of the thorax where the two tracks become distinct (Patel et al. 2001; Liu and Kaufman 2004a; Fig. 5a). As segments are produced anterior to the growth zone, neural Of'hb expression extends posteriorly so that the point where neural Of'hb expression becomes apparent lies three to four segments anterior to the growth zone expression. In germ bands taken from strongly affected Of'brRNAi clutches, the two tracks of Of'hb expression form a “Y” of neuronal expression, and the gap between neural and posterior growth zone expression is expanded; 13 of 21 Of'br-depleted embryos showed a gap of nine segments or greater (Fig. 5b). In some cases, Of'hb-expressing cells were found only in the anterior-most segments of the head, although the growth zone had nearly produced all 10 abdominal segments (data not shown). These germ bands were usually much thinner than control germ bands, and the morphology of each segment was poorly defined.
Fig. 5.
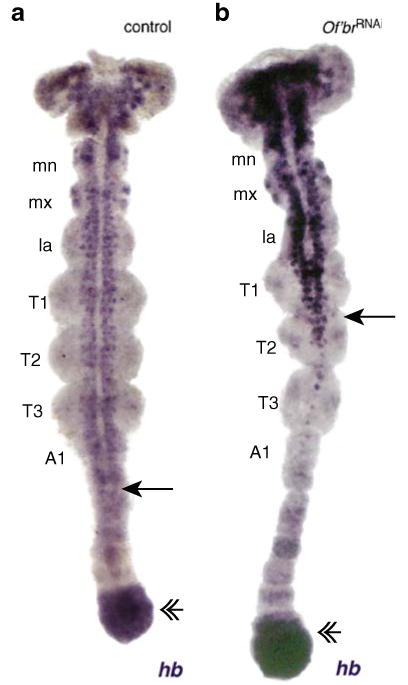
Of'hb stains the posterior growth zone (double arrowheads) and the neuroectoderm and newly formed neuroblasts (mn mandibular segment, mx maxillary segment, la labial segment, T1–T3 first to third thoracic segments, A1 first abdominal segment). a Of'hb in a normal germ band stage embryo. The posterior extent of neural Of'hb expression is indicated by an arrow. b In the Of'br-depleted germ band, the posterior extent of neural Of'hb expression lags behind growth zone expression by several additional segments. In addition, Of'hb expression shows that the two tracks of neural tissue are joined in T2 (arrow)
We did not detect defects in cuticle identity that might be due to the loss of the second peak of Of'br expression, which is coincident with pronymphal cuticle formation.
Embryonic expression of br in T. domestica, an ametabolous insect
broad expression is prominent during embryonic development of the hemimetabolous cricket (Erezyilmaz 2004) and milkweed bug, but this expression has been lost in the Holometabola, where Br protein is limited to a handful of neurons (Zhou et al. 2009). To determine whether embryonic expression of br is ancestral, we isolated a portion of br from the firebrat, T. domestica, an ametabolous insect. This sequence contained all but the first two amino acids of the N terminus BTB domain, the entire zinc finger, and the intervening linker region (GenBank accession number GQ983556). Each of the separate regions had the greatest identity to br from A. domesticus: 99% identity in the BTB domain, 33% identity in the linker region, and 64% identity in the zinc finger region of the Z1 isoform.
The embryonic stages of Thermobia culminate in production of the pronymphal cuticle, and unlike the hemimetabolous insects, the first nymphal cuticle is produced after hatching (Konopova and Zrzavy 2005). At the outset of the E1 phase, the developing germ band sinks into the yolk where it elongates and adds segments from a posterior growth zone. The final abdominal segments differentiate at the onset of katatrepsis, the morphogenetic movement that brings the segmented germ band to the surface of the yolk and reorients the embryo within the egg at around 5–6 days of embryonic development. Just before the eighth day of embryonic development, the lateral halves of the embryo envelop the yolk during dorsal closure, which occurs just before deposition of the pronymphal cuticle. This developmental period is dominated by terminal differentiation and organogenesis, which continue until the time of hatching (Konopova and Zrzavy 2005). We find that the Td'br core is expressed on the first day of development, that it is constitutively expressed throughout the early stages of segmentation and morphogenesis, and that it persists during the later stages of differentiation (Fig. 6).
Fig. 6.
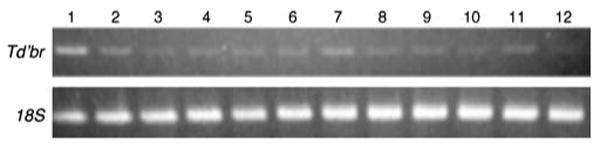
Td'br expression during development of the firebrat, Thermobia. In our culture conditions, Thermobia embryos hatch after 12 days. Top Td'br core expression. Bottom 18S ribosomal RNA used as a loading control
Discussion
Here we report a novel role for br during embryonic development of a hemimetabolous insect (Fig. 7). We find that Of'br is expressed during two discrete phases prior to hatching. The first transient phase coincides with germ band invagination and segmentation, and in situ hybridization reveals that Of'br is ubiquitously expressed during this phase. Of'br is next expressed during pronymphal development. Knock-down of Of'br results in posterior truncations, and Of'en expression in germ band stage embryos shows that this truncation occurs through loss of segments. Analysis of Of'hb expression in neuroectoderm shows that differentiation of this tissue is arrested in strong Of'br knock-down germ bands. Finally, we isolated a br ortholog from the ametabolous insect, T. domestica, and find that Td'br is expressed throughout embryonic development (Fig. 7). The timing of Of'br expression supports the idea that the holometabolous pupa is homologous to the embryonic nymphal stage of hemimetabolous insects, and that the pupa evolved as br expression was lost from embryonic development (Fig. 7).
Fig. 7.
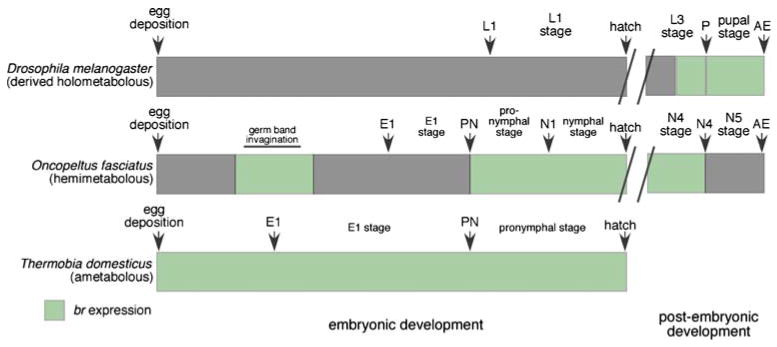
Summary of br expression, shown in green, during embryonic development (at left), and postembryonic development (at right) from representatives of the three types of insect developmental strategy. At bottom, br is constitutively expressed throughout embryonic development of Thermobia domestica, an ametabolous insect. The function of embryonic Td'br is not known. The postembryonic expression of br has not been determined for this insect. Center We have shown that br is expressed in two discrete phases during development of the hemimetabolous milkweed bug Oncopeltus fasciatus. The first coincides with germ band invagination, and loss of Of'br expression during this stage disrupts germ band invagination and germ band maturation. The second bout of expression appears during the pronymphal stage and persists until hatching. br is then expressed at each nymphal molt, where it is required for transitions in morphology, but it is not present at the molt to the adult stage during Oncopeltus development. Br is not detected in the epidermis of Drosophila embryos (top; Zhou et al. 2009). Although most holometabolous insects hatch after production of three cuticles, the pronymphal (or prolarval) cuticle has been lost in Drosophila, and an E1 cuticle has not been described for this insect. At this time, data for just one member of each group are available. During postembryonic development of holometabolous insects, br is expressed at the larval–pupal transition and is required for metamorphosis. E1 first embryonic cuticle; PN pronymphal cuticle; N1, N4, N5 first, fourth, and fifth nymphal cuticles, respectively; L1, L3 first and third larval cuticles, respectively; AE adult eclosion. The embryonic stages, but not the postembryonic stages, are drawn to scale
broad and the maturation of embryonic tissues
When we examined germ band stage embryos from more strongly affected clutches, we found that the germ band embryos were thinner laterally (Fig. 5b) and had poor morphological differentiation. In those severely affected embryos stained with the Of'hb probe, we found that the two tracks of neuroectoderm were fused along the midline in anterior segments in a “Y”. In the grasshopper, Hb marks the neuroectoderm and transiently marks all the delaminating neuroblasts (Patel et al. 2001). Of'hb strongly resembles this pattern in wild-type Oncopeltus embryos (Liu and Kaufman 2004a). The fusion of two tracks of neuroectoderm may reflect a failure in the formation of this tissue after loss of Br. In this scenario, strong failure in cell division in the neuroectodermal region would cause a narrowing of this field, until the two primordia fused, resulting in the “Y” of Of'hb expression.
A failure in cell division might also cause the poor morphological differentiation that we observe in germ bands and ultimately result in the most severe end-point phenotypes. With poor morphological differentiation, the tissues would be unable to withstand the movements of katatrepsis. The onset of katatrepsis is marked by fusion between the amnion, which encases the germ band, and the serosa, which encases the yolk. These membranes fuse at the anterior and posterior ends of the embryo, and the embryo is subsequently flipped into the opposite orientation within the egg (Dorn 1976). We speculate that, without proper formation of the sites of fusion and morphological differentiation, the embryo would be mangled by the subsequent morphogenetic movement of katatrepsis.
We have only observed segment loss or posterior truncations in morphologically differentiated germ bands and nymphs (Figs. 3b–e and 4b, c). On the other hand, early germ bands appear to produce all segments, although these are deficient in growth and in morphology (Fig. 5b and data not shown). We, therefore, suggest that Of'br-depleted germ bands produce segments normally, but portions are subsequently lost. In this scenario, regions of the germ band that have sub-threshold levels of br do not mature, then degenerate or persist as weak embryonic tissue. In support of this, we frequently observe portions of undifferentiated embryonic tissue in severely affected late-stage embryos. For instance, a bridge of such tissue connects the head with a posterior mass of differentiated and undifferentiated tissue in Fig. 3e.
Ecdysteroid involvement during early embryogenesis and embryonic Of'br expression
During postembryonic development of Oncopeltus, br expression is activated at molts as the ecdysteroid titer rises (Erezyilmaz et al. 2006). Ecdysteroids are also present during embryogenesis and may play a role in regulating the phases of embryonic development. Maternally derived conjugated ecdysteroids are present in the freshly oviposited eggs of most insects. In orthopteroid insects, these have been shown to be hydrolyzed and used to regulate production of the serosal layer (Lagueux et al. 1979) and the first embryonic cuticle, which both appear before the embryo's prothoracic glands are formed (Hoffmann and Lagueux 1985; Lanot et al. 1989; Sbrenna-Micciarelli and Sbrenna 1972). In Oncopeltus, ecdysone conjugates are likewise maternally loaded, and the ecdysteroid titer on the first day of embryogenesis is twice the level of the ecdysteroid content on the second day (Dorn and Romer 1976; Dorn 1983). The pronymphal cuticle appears on the fourth day of embryonic development, and the appearance of this cuticle correlates with a modest rise in ecdysteroids. The first nymphal cuticle is present on the fifth day development, and its deposition is preceded by a large surge in embryonic ecdysteroids (Dorn and Romer 1976; Dorn and Hoffman 1981). Although JH titers are not available for Oncopeltus, detailed work on the embryonic locust shows that JH is only present at the molt to the first nymphal stage (Temin et al. 1986). Expression of Of'br follows the modest peak of ecdysteroids observed on the first day of development. In addition, the expression of a second ecdysone response gene, Of'E75A, precedes Of'br expression by several hours, when it also plays a role in patterning the segmenting germ band (Erezyilmaz et al. 2009). This pair of genes is activated by ecdysteroids and co-expressed at the onset of metamorphosis in holometabolous insects. These two genes also appear during oogenesis of Drosophila, where their expression also depends upon the production of 20E (Buszczak et al. 1999). Although Of'E75A and Of'br are not found at the E1 molt, both are re-induced at the time of the pronymphal molt. Of'E75 reappears at 72 h (D.F.E., H. Kelstrup, and L.M.R, unpublished data), and Of'br is re-expressed on or before day 4 of embryonic development (Fig. 1). Therefore, the two genes, which have been characterized as “ecdysone response” genes in other systems, may be regulated by ecdysteroids during embryonic development of Oncopeltus.
During the first two days of embryogenesis, Dorn found that the predominant ecdysteroids are conjugates of ecdysone and 20E with makisterone A, the predominant ecdysteroid of the nymph (Feldlaufer and Svoboda 1986), only appearing at the time of katatrepsis and thereafter. Interestingly, injection of makisterone A, but not of 20E, into females accelerated the onset of katatrepsis in subsequently laid eggs by a few hours (Dorn and Buhlmann 1982). The only studies to suggest a possible role for ecdysteroids in early germ band development have been in M. sexta embryos. In this system, the appearance of 26-hydroxyecdysone from a maternally provided conjugate is temporally correlated with gastrulation and segmentation (Lanot et al. 1989). When isolated germ bands were incubated in vitro, their growth was retarded. The addition of various ecdysteroids (e.g., makisterone A, 20-hydroxyecdysone, and ecdysone) restored the longitudinal growth, suggesting that ecdysteroids are required for proper germ band formation. These data, and the role we have uncovered for Of'br, may implicate a connection between the ecdysone signaling system and germ band growth.
The significance of pronymphal expression of broad to the pronymph hypothesis
The pronymph hypothesis suggests that the holometabolous insects arose after JH production appeared at an earlier stage of embryonic development as the ancestral holometabolan arose from a hemimetabolous ancestor. This encroachment would cause (1) a precocious differentiation of the pronymphal cuticle and (2) an inhibition of differential growth. The combination of these two effects would produce the morphologically divergent larval stage that was endowed with terminal features for life outside the eggshell. If the pronymph is homologous to the holometabolous larva, then br, which first appears during the last larval instar of holometabolous insects and is necessary for formation of the pupa (Fig. 7), should be expressed during the pronymphal stage of hemimetabolous embryos. We found that Of'br is re-expressed on the fourth day of embryonic development and is present at the molt to the first nymphal stage on day 5. Therefore, these expression data support the pronymph hypothesis (Truman and Riddiford 1999, 2002)
The pronymph hypothesis is based upon the JH titers in embryonic Holometabola (Bergot et al. 1981) and the effects of JH treatment at the pronymphal stage of more basal hemimetabolous embryos. A recent electron microscopy study of embryonic cuticles of basal holometabolous insects, however, showed that these insects also hatch after making three cuticles (Konopova and Zrzavy 2005), suggesting that the first larval cuticle is serially homologous to the first nymphal cuticle, not the pronymphal cuticle, as suggested by the pronymph hypothesis. This does not, however, detract from the possibility that JH has transformed the pronymphal cuticle to generate the novel larval form. We suggest that this occurred as JH suppressed the onset of br expression during embryonic development. Detailed studies of endocrine regulation of br in Manduca show that br is activated by an elevation in ecdysteroid that occurs in the absence of JH. Once activated, JH maintains, rather than inhibits, br expression at the ensuing pupal molt. The appearance of 20E in the absence of JH then turns off br expression during the adult molt. Our data show that Of'br is reactivated at the molt to the pronymphal stage, when ecdysteroids appear in the absence of JH. If the same rules of JH/ecdysteroid regulation of br occur in this hemimetabolous insect, the earlier appearance of JH at the pronymphal stage of the ancestral holometabolan would repress this br expression at the pronymphal molt. Due to technical limitations, we were unable to assess the role of Of'br expression at this stage. However, Of'br is present (Fig. 7) and is required for changes in identity and proportion during the postembryonic nymphal stages (Erezyilmaz et al. 2006). By extrapolation, loss of Of'br at the pronymphal molt would freeze the proportions of the pronymph, resulting in a larva with more embryonic proportions. Therefore, the regulation of Of'br at the pronymphal–nymphal transition of Oncopeltus supports developmental homology with br expression at the larval-pupal transition seen in holometabolous insects. However, these ideas await methods that are able to address Of'br function during mid–late embryogenesis of Oncopeltus.
The constitutive expression of Td'br during embryonic development of the firebrat indicates that br may also play a prominent role during embryogenesis of ametabolous insects, although this expression did not provide any clues to the role of Td'br in Thermobia development. Future studies will be required to determine whether this expression appears in the embryonic epidermis, or whether it is restricted to a handful of neurons, as in the Drosophila embryo (Zhou et al. 2009). If Td'br expression were also found in the embryonic epidermis as it is in Oncopeltus, this would support the idea that embryonic br expression was lost specifically in the Holometabola.
Acknowledgments
We thank Dr. Paul Liu and Thom Kaufman (Indiana University, Bloomington) for a gift of the digoxigenin-labeled Of'en probe as well as cDNAs for Of'Kr, Of'hb, and Of'eve. We also thank Bilal Ahmad and Shaunna Harris for the initial isolation of the Thermobia broad gene, and Dr. Takashi Koyama for consultation on molecular techniques. This study was supported by NSF grant IBN-9904959 to JWT and NIH grant GM60122 to LMR.
References
- Bergot BJ, Baker FC, Cerf DC, Jamieson G, Schooley DA. Qualitative and quantitative aspects of juvenile hormone titers in developing embryos of several insect species: discovery of a new JH-like substance extracted from eggs of Manduca sexta. In: Pratt GE, Brooks GT, editors. Juvenile Hormone Biochemistry. Elsevier; Amsterdam: 1981. pp. 35–45. [Google Scholar]
- Buszczak M, Freeman MR, Carlson JR, Bender M, Cooley L, Segraves WA. Ecdysone response genes govern egg chamber development during mid-oogenesis in Drosophila. Development. 1999;126:4581–4589. doi: 10.1242/dev.126.20.4581. [DOI] [PubMed] [Google Scholar]
- Butt FH. Embryology of the milkweed bug, Oncopeltus fasciatus (Hemiptera) Cornell Experiment Station Memoir. 1949;238 [Google Scholar]
- DiBello PLR, Withers DA, Bayer CA, Fristrom JW, Guild GM. The Drosophila broad-complex encodes a family of related proteins containing zinc fingers. Genetics. 1991;129:385–397. doi: 10.1093/genetics/129.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn A. Ultrastructure of embryonic envelopes and integument of Oncopeltus fasciatus Dallas (Insecta, Heteroptera). I. Chorion, amnion, serosa, integument. Zoomorphologie. 1976;85:111–113. [Google Scholar]
- Dorn A. Hormones during embryogenesis in the milkweed bug, Oncopeltus fasciatus. Entomol Gen. 1983;8:193–214. [Google Scholar]
- Dorn A, Buhlmann KJ. Exogenous makisterone A accelerates early embryonic development in the milkweed bug Oncopeltus fasciatus. Experientia. 1982;38:367–368. [Google Scholar]
- Dorn A, Hoffman P. The ‘embryonic molts’ of the milkweed bug as seen by the S.E.M. Tiss Cell. 1981;13:461–473. doi: 10.1016/0040-8166(81)90019-7. [DOI] [PubMed] [Google Scholar]
- Dorn A, Romer F. Structure and function of prothoracic glands and oenocytes in embryos and last larval instars of Oncopeltus fasciatus Dallas (Insecta, Heteroptera) Cell Tissue Res. 1976;171:331–350. doi: 10.1007/BF00224658. [DOI] [PubMed] [Google Scholar]
- Erezyilmaz DF. PhD thesis. University of Washington; Seattle, WA: 2004. The genetic and endocrine bases for the origins of insect metamorphosis. [Google Scholar]
- Erezyilmaz DF. Imperfect eggs and oviform nymphs: a history of ideas about the origins of insect metamorphosis. Integ Comp Biol. 2006;46:795–807. doi: 10.1093/icb/icl033. [DOI] [PubMed] [Google Scholar]
- Erezyilmaz DF, Riddiford LM, Truman JW. The pupal specifier broad directs progressive morphogenesis in a direct-developing insect. Proc Nat Acad Sci U S A. 2006;103:6925–6930. doi: 10.1073/pnas.0509983103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erezyilmaz DF, Kelstrup HC, Riddiford LM. The nuclear receptor E75A has a novel pair-rule-like function in patterning the milkweed bug, Oncopeltus fasciatus. Dev Biol. 2009;334:300–310. doi: 10.1016/j.ydbio.2009.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldlaufer MF, Svoboda JA. Makisterone A: a 28-carbon insect ecdysteroid. Insect Biochem. 1986;16:45–48. [Google Scholar]
- Hemming BS. Insect Development and Evolution. Cornell University Press; Ithaca: 2003. [Google Scholar]
- Hoffmann JA, Lagueux M. Endocrine aspects of embryonic development in insects. In: Kerkut GA, Gilbert LI, editors. Comprehensive Insect Physiology, Biochemistry and Pharmacology. Vol. 1. Pergamon; Oxford: 1985. pp. 435–460. [Google Scholar]
- Hughes CL, Kaufman TC. RNAi analysis of Deformed, proboscipedia, and Sex combs reduced in the milkweed bug Oncopeltus fasciatus: novel roles for Hox genes in the hemipteran head. Development. 2000;127:3683–3694. doi: 10.1242/dev.127.17.3683. [DOI] [PubMed] [Google Scholar]
- Karim FD, Guild GM, Thummel CS. The Drosophila Broad-Complex plays a role in controlling ecdysone-regulated gene expression at the onset of metamorphosis. Development. 1993;118:977–988. doi: 10.1242/dev.118.3.977. [DOI] [PubMed] [Google Scholar]
- Konopova B, Jindra M. Broad-complex acts downstream of Met in juvenile hormone signaling to coordinate primitive holometabolan metamorphosis. Development. 2008;135:559–568. doi: 10.1242/dev.016097. [DOI] [PubMed] [Google Scholar]
- Konopova B, Zrzavy J. Ultrastructure, development, and homology of insect embryonic cuticles. J Morphol. 2005;264:339–362. doi: 10.1002/jmor.10338. [DOI] [PubMed] [Google Scholar]
- Lagueux M, Hetru C, Goltzene F, Kappler C, Hoffmann JA. Ecdysone titre and metabolism in relation to cuticulogenesis in embryos of Locusta migratoria. J Insect Physiol. 1979;25:709–723. [Google Scholar]
- Lanot R, Dorn A, Gunster B, Thiebold J, Lagueux M, Hoffmann JA. Functions of ecdysteroids in oocyte maturation and embryonic development of insects. In: Koolman J, editor. Ecdysone. From Chemistry to Mode of Action. Georg Thieme; Stuttgart: 1989. pp. 262–269. [Google Scholar]
- Liu PZ, Kaufman TC. hunchback is required for suppression of abdominal identity, and for proper germband growth and segmentation in the intermediate germband insect, Oncopeltus fasciatus. Development. 2004a;131:1515–1527. doi: 10.1242/dev.01046. [DOI] [PubMed] [Google Scholar]
- Liu PZ, Kaufman TC. Krüppel is a gap gene in the intermediate germband insect Oncopeltus fasciatus and is required for development of both blastoderm and germband-derived segments. Development. 2004b;131:4567–4579. doi: 10.1242/dev.01311. [DOI] [PubMed] [Google Scholar]
- Liu PZ, Kaufman TC. even-skipped is not a pair-rule gene but has segmental and gap-like functions in Oncopeltus fasciatus, an intermediate germband insect. Development. 2005;132:2081–2092. doi: 10.1242/dev.01807. [DOI] [PubMed] [Google Scholar]
- Novak VJA. Insect Hormones. Methuen; London: 1966. [Google Scholar]
- Parthasarathy R, Tan A, Bai H, Palli SR. Transcription factor broad suppresses precocious development of adult structures during larval–pupal metamorphosis in the red flour beetle, Tribolium castaneum. Mech Dev. 2008;125:299–313. doi: 10.1016/j.mod.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel NH, Hayward DC, Lall S, Pirkl NR, DiPietro D, Ball EE. Grasshopper hunchback expression reveals conserved and novel aspects of axis formation and segmentation. Development. 2001;128:3459–3472. doi: 10.1242/dev.128.18.3459. [DOI] [PubMed] [Google Scholar]
- Riddiford LM. Juvenile hormone: the status of its “status quo” action. Arch Insect Biochem Molec Biol. 1996;32:271–286. doi: 10.1002/(SICI)1520-6327(1996)32:3/4<271::AID-ARCH2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Truman JW, Riddiford LM. The role of Broad in the development of Tribolium castaneum: implications for the evolution of the holometabolous insect pupa. Development. 2008;135:569–577. doi: 10.1242/dev.015263. [DOI] [PubMed] [Google Scholar]
- Sbrenna-Micciarelli A, Sbrenna G. The embryonic apolyses of Schistocerca gregaria (Orthoptera) J Insect Physiol. 1972;18:1027–1037. [Google Scholar]
- Temin G, Zander M, Roussel JP. Physio-chemical (GC–MS) measurements of juvenile hormone III titres during embryogenesis of Locusta migratoria. Internat J Invert Reprod Dev. 1986;9:105–112. [Google Scholar]
- Truman JW, Riddiford LM. The origins of insect metamorphosis. Nature. 1999;401:447–452. doi: 10.1038/46737. [DOI] [PubMed] [Google Scholar]
- Truman JW, Riddiford LM. Endocrine insights into the evolution of metamorphosis in insects. Ann Rev Entomol. 2002;47:467–500. doi: 10.1146/annurev.ento.47.091201.145230. [DOI] [PubMed] [Google Scholar]
- Truman JW, Riddiford LM. The morphostatic actions of juvenile hormone. Insect Biochem Mol Biol. 2007;37:761–770. doi: 10.1016/j.ibmb.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Uhlirova M, Foy BD, Beaty BJ, Olson KE, Riddiford LM, Jindra M. Use of Sindbis virus-mediated RNA interference to demonstrate a conserved role of Broad-Complex in insect metamorphosis. Proc Natl Acad Sci U S A. 2003;100:15607–15612. doi: 10.1073/pnas.2136837100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Riddiford LM. Hormonal regulation and patterning of the broad-complex in the epidermis and wing discs of the tobacco hornworm, Manduca sexta. Dev Biol. 2001;231:125–137. doi: 10.1006/dbio.2000.0143. [DOI] [PubMed] [Google Scholar]
- Zhou X, Riddiford LM. Broad specifies pupal development and mediates the ‘status quo’ action of juvenile hormone on the pupal–adult transformation in Drosophila and Manduca. Development. 2002;129:2259–2269. doi: 10.1242/dev.129.9.2259. [DOI] [PubMed] [Google Scholar]
- Zhou B, Hiruma K, Shinoda T, Riddiford LM. Juvenile hormone prevents ecdysteroid-induced expression of Broad Complex RNAs in the epidermis of the tobacco hornworm, Manduca sexta. Dev Biol. 1998;203:233–244. doi: 10.1006/dbio.1998.9059. [DOI] [PubMed] [Google Scholar]
- Zhou B, Williams DW, Altman J, Riddiford LM, Truman JW. Temporal patterns of Broad isoform expression during the development of neuronal lineages in Drosophila. Neural Dev. 2009;4:39. doi: 10.1186/1749-8104-4-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollman S, Godt D, Prive GG, Couderc JL, Laski FA. The BTB domain, found primarily in zinc finger proteins, defines an evolutionarily conserved family that includes several developmentally regulated genes in Drosophila. Proc Natl Acad Sci U S A. 1994;91:10717–10721. doi: 10.1073/pnas.91.22.10717. [DOI] [PMC free article] [PubMed] [Google Scholar]


