Abstract
Biosynthesis of lysine and meso-diaminopimelic acid in bacteria provides essential components for protein synthesis and construction of the bacterial peptidoglycan cell wall. The dapE operon enzymes synthesize both meso-diaminopimelic acid and lysine and, therefore, represent a potential targets for novel antibacterials. The dapE-encoded N-succinyl-L,L-diaminopimelic acid desuccinylase functions in a late step of the pathway and converts N-succinyl-L,L-diaminopimelic acid (L,L-SDAP) to L,L-diaminopimelic acid and succinate. Deletion of the dapE gene is lethal to Helicobacter pylori and Mycobacterium smegmatis indicating that DapE’s are essential for cell growth and proliferation. Since there are no similar pathways in humans, inhibitors that target DapE may have selective toxicity against only bacteria. A major limitation in developing antimicrobial agents that target DapE has been the lack of structural information. Herein we report the high-resolution X-ray crystal structures of the DapE from Haemophilus influenzae with one and two zinc ions bound in the active site, respectively. These two forms show different activity. Based on these newly determined structures we propose a revised catalytic mechanism of peptide bond cleavage by DapE enzymes. These structures provide important insight into catalytic mechanism of DapE enzymes as well as a structural foundation that is critical for the rational design of DapE inhibitors.
The meso-diaminopimelate (mDAP)/lysine biosynthetic pathway offers several potential antibacterial enzyme targets that have yet to be explored.1,2,3 One of the products of this pathway, lysine, is required in protein synthesis and is also used in the peptidoglycan layer of Gram-positive bacterial cell walls. A second product of this pathway, mDAP is an essential component of the peptidoglycan cell wall for Gram-negative bacteria, providing a link between polysaccharide strands. It has been shown that deletion of the gene encoding one of the enzymes in this pathway, the dapE-encoded N-succinyl-L,L-diaminopimelic acid desuccinylase (DapE; EC 3.5.1.18), is lethal in Helicobacter pylori and Mycobacterium smegmatis.4,5 Notably, the H. pylori dapE deletion mutant was unable to grow in lysine supplemented media, implying that lysine can’t be effectively imported. These results strongly suggest that the mDAP/lysine biosynthetic pathway is the only source for lysine and can’t be compensated by other pathways or import. Lysine is an essential amino acid which is not synthesized in humans hence it must be ingested as lysine or lysine-containing proteins. In contrast, most bacteria, plants and algae can synthesize lysine and mDAP from aspartic acid.1,2,6 Since there are no similar biosynthetic pathways in mammals, including humans, inhibitors that target one or more of the enzymes in the mDAP/lysine pathway are hypothesized to exhibit selective toxicity against bacteria.1
Genes encoding DapE’s have been identified in several pathogenic Gram-positive and Gram-negative bacteria such as Acinetobacter baumannii (MDRAB), Mycobacterium tuberculosis, Escherichia coli (O157:H7), Bordetella pertussis, Vibrio cholerae, Rickettsia prowazekii, Pseudomonas aeruginosa, Yersinia pestis, H. pylori, Haemophilus influenzae, Staphylococcus aureus (strain MRSA252), Enterococcus faecium, Salmonella enterica, and Streptococcus pneumoniae.4,5,7,8,9,10 The fact that the DapE gene has been discovered in several multi-drug resistant bacteria suggests that inhibitors of DapE enzymes may provide a new class of broad-spectrum antibiotics. Alignment of the DapE proteins listed above show a minimum of 49% sequence identity.11 Significantly, all DapE proteins characterized to date are medium sized, dimeric enzymes (41.6 kDa/subunit) that require zinc ions for their activity.6,12 The amino acid residues that function as metal ligands in the structurally characterized M28 family members, the carboxypeptidase from Pseudomonas sp strain-RS-16 (CPG2) and the leucine aminopeptidase from Aeromonas proteolytica (AAP),6,13 are fully conserved in all DapE sequences. Both CPG2 and AAP possess a (μ-aquo)(μ-carboxylato)dizinc(II) core with one terminal carboxylate and one histidine residue at each metal site 14,15 and a similar active site has been proposed for DapE.6,11,12,16,17 Interestingly, it has been reported that the “as purified” DapE enzyme contains only one tightly bound Zn(II) ion and exhibits ~60% of its total activity, similar to AAP.6,12 Thus, both metal ions seem to be required for full enzymatic activity, but their individual catalytic roles appear to differ markedly.
A major limitation in understanding the catalytic mechanism of DapE and in developing a novel inhibitors that specifically can target DapE is the lack of knowledge about their structure and an active site architecture.6,18 The only X-ray crystal structure reported for any DapE enzyme is an apo-form of the DapE from Neisseria meningitidis.19 The absence of metal ions in the structure makes it difficult to determine the spatial arrangement of the catalytically relevant residues that constitute the active site. While the current lack of structural data preclude definitive assignment of catalytically relevant residues, we have recently reported a three-dimensional homology model of the DapE from H. influenzae, using the crystal structure of the DapE from N. meningitidis as a template which exhibits ~54% identity to the DapE from H. influenzae.11 In an effort to clearly define the structure of DapE enzymes along with the catalytically relevant residues that constitute the active site, we have solved the 2.0 and 2.3 Å resolution structures of the mono and dinuclear zinc DapE enzymes from H. influenzae, respectively. Now that active forms of DapE have been crystallographically characterized, the critical components of the active site including substrate binding can be elucidated.
Protein purification and X-ray structure of the DapE from H. influenzae
The recombinant DapE from H. influenzae was expressed and purified, as previously described, with minor modifications12 from a stock culture kindly provided by Professor John Blanchard.6 In order to obtain ultra-pure protein suitable for crystallization, ~50 mg of DapE was further purified by loading onto a Mono-Q column (5/50GL, GE Healthcare, Amersham Biosciences Corp., Piscataway, NJ, USA), that was pre-equilibrated with 10 mM, Chelex-100 treated, Tricine buffer at pH 7.8. A flow rate of 0.5 ml/min was used with a linear gradient of NaCl (0-0.25 M). Ultra-pure DapE eluted as the first peak at ~0.15 M NaCl. The pure fractions were concentrated using a Centricon Plus-20 (Millipore Corp., Billerica, MA, USA). Purified DapE from H. influenzae exhibited a single band on SDS-PAGE indicating a Mr = 41,500. Protein concentrations were determined from the absorbance at 280 nm using a molar absorptivity (ε280 = 36,040 M−1 cm−1), calculated using the method developed by Gill and Hippel.20 The protein concentration determined using this molar absorptivity was in good agreement to that obtained using a Bradford assay. The concentration of DapE samples used for crystallization was ~15 mg/ml. Individual aliquots of purified DapE were stored in liquid nitrogen until needed.
DapE’s catalyze the hydrolysis of N-succinyl-L,L-diaminopimelic acid (L,L-SDAP), forming L,L-diaminopimelic acid and succinate.6,12 Initial rates were fit directly to the Michaelis-Menten equation to obtain the catalytic constants Km and kcat. The kcat and Km values for DapE were determined in triplicate by monitoring amide bond cleavage of L,L-SDAP10,12,21 at 225 nm in Chelex-100 treated 50 mM potassium phosphate buffer, pH 7.5, and found to be 140 ± 10 s−1 and 730 ± 15 mM, respectively.12
Crystals of the dizinc form of DapE (ZnZn_DapE) were grown at 16 ° C by vapor diffusion in hanging drops containing 1 mL of precipitant solution (1 M ammonium sulfate, 0.2 M NaCl, 0.1 M Na Acetate pH 4.4) and 1 μL of 13 mg×mL−1 of DapE with three equivalents of zinc. The crystals grew within two weeks and reached a size of 0.1 mm × 0.1 mm × 0.05 mm. These crystals belonged to the primitive orthorhombic space group P212121 with unit cell parameters a = 44.7 Å, b = 95.7 Å, c = 185.4 Å. The asymmetric unit contains two molecules with a Vm value of 2.4 Å (solvent content 48%). In order to determine the structure of a monometaled form of DapE, a second batch of protein equilibrated with only one equivalent of zinc was used and crystals were grown under the same conditions as described above. Crystals of the monozinc form of DapE (Zn_DapE) also grew within 10 days and reached a similar size with an identical morphology as crystals of ZnZn_DapE (space group P212121 with unit cell dimensions a=45.2 Å, b=95.7 Å, c=181.2 Å).
Prior to data collection, an X-ray fluorescence spectrum was recorded for both Zn_DapE and ZnZn_DapE , which identified the presence of Zn ions in the protein crystals. Data collection was carried out on the 19-ID beamline of the Structural Biology Center at the Advanced Photon Source according to the procedure described previously.22 Data to 2.0 and 2.3 Å were collected for Zn_DapE and ZnZn_DapE , respectively, at a wavelength of 0.9794 Å from the single crystals and were processed using HKL2000 (Table 1).23
Table 1.
Data and Refinement Statistics
| Data collection statistics | Zn_DapE | ZnZn_DapE |
|---|---|---|
| space group | P212121 | P212121 |
| unit cell (Å) | a = 45.2, b =95.7 c = 181.2 | a = 44.7, b =95.7 , c = 185.4 |
| wavelength (Å) | 0.9794 | 0.9794 |
| resolution (Å) | 30-2.03 | 35-2.30 |
| number of observed reflections | 232530 | 165276 |
| number of unique reflections | 51964 | 34773 |
| Rmergeb (%) | 7.7 (69.2) | 8.6(56.7) |
| Completeness (%) | 99.9 (99.9) | 95.9(99.7) |
| I/σ | 20.2 (2.5) | 17.8(3.4) |
| Phasing | ||
| phasing method | MR | MR |
| resolution range (Å) | 30-3.0 | 35-3.0 |
| MR statistics | CC 0.5 Rfac 0.46 | CC 0.43 Rfac 0.52 |
| Refinement statistics | ||
| resolution range (Å) | 40.0-2.03 | 40.0-2.30 |
| Rcryst (%) | 20.2 | 19.4 |
| Rfree (%) | 25.4 | 25.0 |
| number of non-hydrogen atoms | 5718 | 5718 |
| Zn ions/SO4 | 2/2 | 4/5 |
| solvent | 401 | 212 |
| Rmsd from target values | ||
| bond lengths (Å) | 0.017 | 0.015 |
| bond angles (deg) | 1.57 | 1.43 |
| Average B factors (Å2) | ||
| protein main chain | 31.2 | 39.3 |
| protein side chains and solvent | 35.1 | 41.5 |
| Zn1/Zn2 | 31.7/- | 34.3/35.7 |
| average metal occupancy | 1.0/- | 1.0/0.45 |
| Ramachandran Plot b(%) | ||
| favored/allowed/outliers | 97.3/100/0 | 97.5/100/0 |
| PDB ID | ||
| 3ISZ | 3IC1 | |
The values in parentheses are for the highest-resolution shell.
Rmerge = ΣhΣi|Ih,i – <Ih>|/ΣhΣiIh,i.
As defined by MOLPROBITY.
The structure of Zn_DapE was determined by molecular replacement using the crystal structure of the DapE from N. meningitidis as a template (PDB id 1vgy) which exhibits ~54% identity with the DapE from H. influenzae, as described previously.11 Molecular replacement searches were completed with MOLREP (R-factor of 0.46%, correlation coefficient 0.50) using the CCP4 suite.24,25 Several rounds of rebuilding and readjusting using COOT and ARP/wARP were required to improve the initial model.26,27 The final model was refined against all reflections using the program REFMAC 5.528 in the resolution range 30 to 2.0 Å except for 5% of the reflections, which were randomly selected and used to monitor Rfree. The final refinement statistics are presented in Table 1.
The structure of ZnZn_DapE was determined by molecular replacement with MOLREP (R-factor of 0.43%, correlation coefficient 0.52) using the catalytic domain of Zn_DapE in the first round and then the dimerization domain of Zn_DapE. Several cycles of model building and water picking with COOT followed by refinement with REFMAC 5.5 were undertaken to build a final structural model (Table 1). Analysis and validation of the structures was performed with the aid of the MOLPROBITY, COOT validation tools, SSM and DALI servers.29,30,31 Figures were prepared using the program Pymol.32
The crystal structures of Zn_DapE and ZnZn_DapE from H. influenzae were determined at 2.0 Å and 2.3 Å resolution, respectively. The final model for both enzymes includes two monomers in the asymmetric unit forming a dimer. The model of Zn_DapE contains 735 residues out of a possible 752 residues, 414 water molecules, two zinc ions, and two sulfate ions. The model of ZnZn_DapE contains 749 residues out of 752 residues, four zinc ions, 212 water molecules, 2 glycerol molecules and 5 sulfate ions. The electron density maps obtained for both structures were of high quality and allowed reliable modeling of both monomers, except in the loop regions (residues 192-198 in chain A and 193-196 and 241-245 in chain B), which are disordered and were not included in the final model. The final models exhibited good crystallographic and geometric statistics and were refined against 2.0 Å data for Zn_DapE with a final Rwork of 20.2 and Rfree 25.4 and against 2.3 Å data for ZnZn_DapE to an Rwork/Rfree of 19.4/25.0 (Table 1).
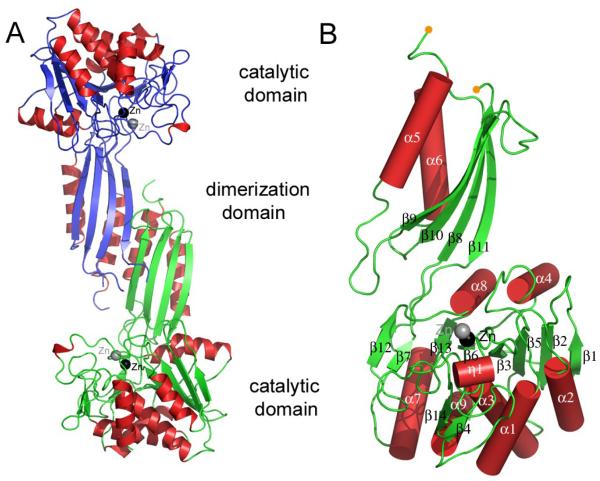
The DapE enzyme from H. influenzae is organized as a dimer and closely resembles the structure of the DapE from N. meningitides. The search for structural homologues of DapE using the DALI and SSM programs30,31 identified several closely related homologs with the apo- form of DapE form N. meningitides (PDB 1vgy, Z-score 47.830, rmsd 2.031 ),19 acetylornithine deacetylase from Bacteroides thetaiotaomicron VPI-5482 (PDB 3ct9, Z-score 32.830, rmsd 2.731),33 and the CPG2 from Pseudomonas sp. strain RS-16 (PDB 1cg2, Z-score 31.630, rmsd 3.031)34 being the top three hits. Each subunit of the dimer consists of two functional domains: a large catalytic domain, which supplies the ligands for the zinc ions in the active site and a smaller dimerization domain that contributes to dimer formation (Figure 1). The domains are connected by small hinge region (residues 176-179, 298-293) allowing movement of the dimerization domain with respect to the catalytic domain. The shape of the DapE dimer resembles a rotary style phone receiver with the larger catalytic domain being placed on the periphery of the dimmer and separated by ~47 Å. The subunit topology is illustrated in Figure 1A.
Figure 1.
Structure of DapE from H. influenzae. A) Ribbon diagram showing the overall structure of the DapE dimer, with monomers shown in different colors (red and blue, and red and green). Individual domains are labeled and the secondary structure elements are colored (blue and green-beta strand, red-alpha helices). B) Diagram of the DapE monomer. The alpha helices are represented as cylinders, zinc ions are label in black (Zn1-the catalytic zinc) and grey (Zn2) and orange dots indicate a disordered loop which has not been modeled.
The dimerization domain of DapE consists of a 114-residue insertion between strand β7 and helix α7 of the catalytic domain (Figure 1) and comprises residues 180–292. The insertion folds into a two-layer α+β sandwich fold (β8, α5, β9, β10, and α6). One layer is formed by the four β-strands arranged into an antiparallel β-sheet with an ~45° twist across its length, while two helices sit on one site of the molecule forming a second layer. The secondary elements are organized into two βαβ motifs revealing a ferredoxin-like fold.
Active site of the DapE from H. influenzae
The catalytic domain is composed of residues 1–179 and 293–376. The core of the catalytic domain consists of an eight-stranded twisted β-sheet that is sandwiched between seven α helices. The mixed β-sheet is formed by two small antiparallel β-strands (β1 and β2) followed by four centrally located parallel strands (β5, β3, β6, and β13) as well as smaller parallel strands (β7 and β12). The last two strands are rotated 180° and are facing in an opposite direction from the centrally located strands. The connecting helices are located below (α1, α2, α3, α9, and α7) and above (α4 and α8) the plane of the β-sheet. In addition to the central sheet (β1, β2, β5, β3, β6, β13, β7, β12) there is also a second small β-sheet located on the surface of the molecule. This β-sheet consists of two shorter antiparallel β-strands (β4 and β14) (Figure 1A). The zinc ion or ions are located near the C-terminal end of the catalytic domains at the surface of the protein (Figures 1A and 1B).
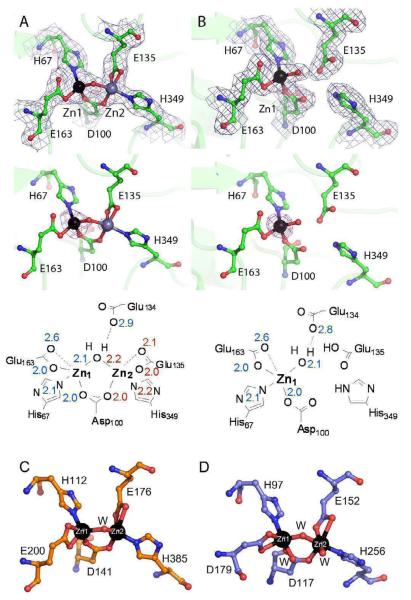
The active-site cleft is located in the center of the catalytic domain above the centrally located parallel strands of the β-sheet (β3, β6, β13) and is covered be the loops. The location and the architecture of the active site in ZnZn_DapE is strikingly similar to the dinuclear active sites of CPG and AAP (Figure 2).34,35 2 The two zinc ions in ZnZn_DapE are 3.36 Å apart compared with 3.45 Å for AAP and 3.25 Å for CPG2 .15,36 Like AAP and CPG2, each of the zinc ions in ZnZn_DapE adopts a distorted tetrahedral or TBP geometry with the Nε nitrogen of His67 for Zn1 and H349 for Zn2 along with a bridging water/hydroxide oxygen atom making up the axial positions of a potential TBP geometry. Identical to CPG2 and AAP, each zinc ion is coordinated by one imidazole group (H67 for Zn1 and H349 for Zn2) and the carboxylate oxygens of E163 (OE1 and the dangling oxygen OE2) for Zn1 and E135 for Zn2 (OE1 and the dangling oxygen OE2). Both zinc ions are bridged by D100 and a water/hydroxide. Interestingly, in the ZnZn_DapE structure the Zn2 binding site exhibits only ~50% occupancy. This structure confirms that the zinc ions in DapE form a (μ-aquo)(μ-carboxylato)dizinc(II) core similar to AAP and CPG2 and are consistent with EXAFS data.16
Figure 2.
Close-up view showing the active site of ZnZn_DapE (A) and Zn_DapE (B) with 2FO – FC electron density map (blue) contoured at 1.0σ, zinc anomalous difference Fourier maps contoured at 4σ (magenta) and drawings of the active sites with distances displayed in angstroms. Zinc ions are labeled in black (Zn1-the catalytic zinc) and grey (Zn2). C) Active site of carboxypeptidase G2 (PDB id 1cg2) and D) close-up view of the active site of a bacterial leucyl aminopeptidase (PDB id 2DEA).
Similar to AAP, it was shown that the as-purified DapE enzyme contains only one tightly bound zinc ion and exhibits ~60% of its total activity in the presence of one zinc ion.12,37 Because DapEs can be activated with one zinc ion it has been hypothesized that one divalent metal binding site is likely filled preferentially. This begs the question regarding which metal ion binding site is filled first in the active site and which site is critical for catalysis? Therefore, we attempted to obtain structural data for a mono-zinc form of DapE (Zn_(DapE)) by limiting the amount of divalent metal ions present during the crystallization process (Figure 2). The Zn(II) ion in Zn_DapE resides in a distorted tetrahedral or TBP geometry with the Nε nitrogen of His67 (2.1 Å) and the bridging water/hydroxide oxygen atom (2.1 Å) making up the axial positions of a potential TBP geometry. The remaining ligands are coordinated by the carboxylate oxygens of D100 (OD1, 2.0 Å) and E163 (OE, 2.0 Å and the dangling oxygen OE2; 2.6 Å). Therefore, this structure confirms that the H67 site (Zn1) of the DapE active site is the high-affinity site and is occupied first and hence, corresponds to the catalytic zinc. This observation is also consistent with ZnZn_DapE structural data where we observed the same site being fully occupied and the second site is only partly occupied.
The structure of the mono-metalated form of DapE is only the second mono-metalated structure for any M28 family metalloprotease38 and is reminiscent of the mono-metalated structures of M24 family metalloproteases, namely the aminopeptidase P from E. coli and the methionine aminopeptidase from E. coli (EcMetAP).39,40 Given that the vast majority of proteases that can bind two metal ions are active in the presence of one metal ion, the X-ray structure of the Zn_DapE enzyme reported herein provides a structural model for the mono-metalated forms of these enzymes.
The structures of Zn_DapE and ZnZn_DapE along with the previously reported divalent metal binding studies,12 provide insight into the observed metal binding properties of DapE as well as all M28 metallopeptidases.12 Based on our X-ray crystallographic data, metal binding to DapE occurs in a sequential fashion with the first zinc binding site (Zn1) bound to the H67 site of the active site and the second zinc ion binding to H349 (Zn2). EPR and electronic absorption data on the Co(II)-substituted forms of WT and mutant DapE enzymes also indicate very clearly that the first metal ion to bind to DapE resides in the H67 site of the active site whereas the second metal binding site corresponds to the H349 site of the active site,11,12 consistent with our X-ray crystallographic data. These data are significant because substrate and also inhibitor zinc-binding groups (ZBG’s) have been hypothesized to bind to Zn1.11 Also of importance is that these data highlight the potential formation of heterodimetallic sites in DapE similar to AAP.41 The H67 residue in DapE corresponds to H97 in AAP while H349 corresponds to H256 in AAP. Based on the crystal structure of the butane boronic acid inhibited form of AAP,42 H97 (H67 in DapE) was proposed to function as a ligand in the second metal binding site. This apparent reversal of the zinc ion binding sites in DapE vs. AAP may likely indicate that the catalytic roles of the active site zinc ions in DapE’s are switched from that proposed for AAP. The latter suggestion is not necessarily surprising since AAP cleaves from the N-terminus while DapE cleaves the equivalent of a C-terminal carboxylate group.
Finally, several potentially important hydrogen-bonding interactions also exist in the active site of both Zn_DapE and ZnZn_DapE. Perhaps most notably, there is at least one interaction between the metal bound water/hydroxide molecule and the carboxylate oxygen atoms of Glu134. Glu134 was recently shown to be essential for catalysis and functions as a general acid/base.17 Glu134 forms a strong hydrogen bond (~2.8 Å) with the terminal metal bound water/hydroxide in Zn_DapE and also with the bridging water/hydroxide (~2.9 Å) in ZnZn_DapE . Other potentially important hydrogen bonding interactions occur between the Nδ proton of His67 with a side chain oxygen of Asp69 forming an Asp-His-Zn triad that has been postulated to decrease the Lewis acidity of zinc ions43 and may further assist in facilitating the coordination of a double bonded oxygen to Zn2. Similar arrangement and a fully conserved Asp-His-Zn triad is observed for both AAP and CPG2 and are postulated to regulate the Lewis acidity of the zinc ion.36
Inhibitor design implications for DapE enzymes
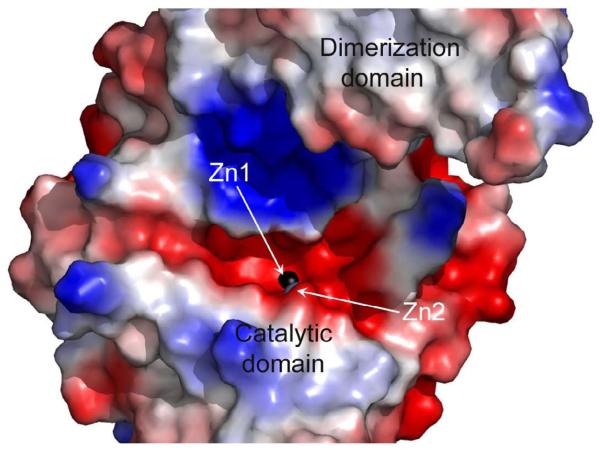
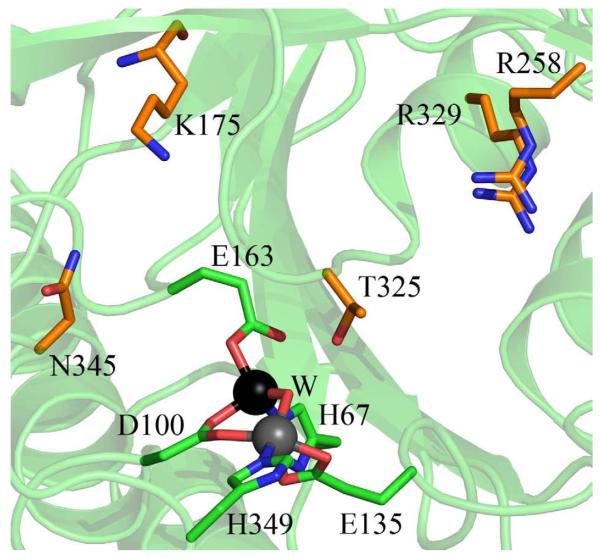
Inspection of the Zn_DapE and ZnZn_DapE structures, combined with surface analysis reveals a smile-shaped cavity that extends along the catalytic domain and surrounds the active site (Figure 3). This well-defined and negatively charged cavity is shaped from the top by strand β12 and α8 and in the middle by the loop connecting these two elements. The bottom of the cavity is formed by loops (loop connecting β-strands β6 and β7, and loop connecting β5 and α4) and α4. Taking into account the linear character of the substrate, it is likely that the substrate binds in an extended conformation along the smile-shaped groove with the peptide bond positioned over the active site metal ions while the rest of the substrate could be further stabilized by the interaction of carboxyl groups of the substrate with the surrounding residues (Figure 4). Assuming that the carbonyl moiety of the peptide bond binds to one or both metal ions in the active site, two residues, E134 and T325, likely form hydrogen bonds with the carbonyl group and/or the backbone amine group of L,L- SDAP. E134 had been shown to function as the general acid/base during catalysis suggesting that T325, which is centrally positioned on the loop overhanging the active site, may play an important role in substrate recognition and transition-state stabilization. It is easy to envision this loop undergoing movements upon substrate binding.
Figure 3.
A) Surface rendering of the ZnZn_DapE molecule (molecule A is shown) showing the charge distribution and depicting the smiled-shaped active site cavity. The surface charge distribution was determined using PyMOL B) Close-up view of the active site cavity of ZnZn_DapE, molecule A, showing charged residues that may interact with the substrate. The zinc ions are represented as grey and black (catalytic zinc) spheres. Side chains of residues that may be involved in recognizing and binding the substrate are shown as orange sticks. Figures were prepared using Pymol.
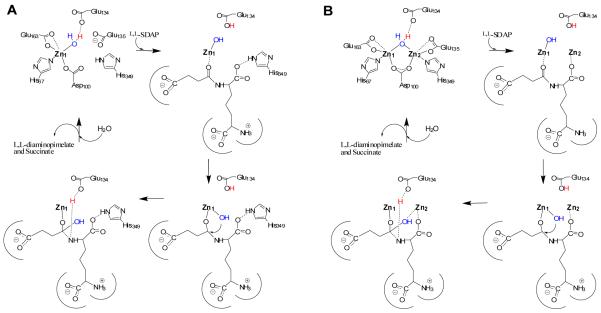
Figure 4.
Proposed catalytic reaction mechanisms of (A) monozinc DapE and (B) dizinc DapE.
DapE enzymes have been shown to exhibit strict specificity for the L,L-isoform of SDAP indicating that the DapE active site is highly specific in both functional group recognition as well as stereochemistry.12 This specificity is built into the substrate binding pocket, which will minimize the non-specific interactions of DapE with non-productive inhibitors.44,45 Therefore it is quite likely that substrate binding is further controlled by interactions of the substrate carboxylic groups with positively charged amino acid side chains. The potential positively charged residues that could interact with the carboxylic groups of the substrate are K175, R178, and N345 at one end and R258, R329 and K139 at the opposite end. The K175 side chain is within ~6 Å of the center of the active site, so it is easy to envision the ε-amino group of the lysine side chain within the appropriate distance to form a salt bridge interaction with a substrate carboxylate group and, thus, be involved in recognizing and binding substrate. On the opposite side, carboxylate groups of L,L- SDAP could also be further stabilized by an interaction with R258 and/or R329. In one of the monomers of the ZnZn_DapE structure, both of these residues form a charged dipole interaction with a sulfate ion, a possible mimic of the carboxylic group of the substrate. R329 is centrally positioned in a positively charged pocket that it forms together with R258 and is further complimented by the N-terminus of α8. These data, in combination with previously reported inhibitor binding studies,12,16,44,45 indicate that DapE represents an excellent target for a highly specific drug that should have high efficacy and low toxicity.
Mechanistic Implications
The X-ray structures of Zn_DapE and ZnZn_DapE presented herein provide a structural foundation for the corroboration of the proposed reaction mechanism of DapE.6,12,17 Analysis of structures and previously reported kinetic and spectroscopic studies on DapE enzymes, allows us to propose a refined mechanism of catalysis for DapE’s (Figure 4). Based on the recently proposed mechanism for AAP,46,47 the first step in catalysis for DapE’s is likely recognition of the L,L-SDAP side chain by the smile-shaped cavity adjacent to the Zn1 site. Next, the peptide carbonyl oxygen of L,L-SDAP coordinates to Zn1, expanding its coordination number from four to five, activating it for nucleophilic attack. Deprotonation of the metal-bound water molecule by E134 to form a nucleophilic hydroxide moiety is consistent with the postulated pKa of the zinc-bound water molecule.6 Once the zinc-bound hydroxide is formed, it can attack the activated carbonyl carbon of the substrate, forming an η-1-μ-transition-state complex.16 Solvent kinetic isotope effect studies yielded an inverse isotope effect that was explained by the attack of a zinc-bound hydroxide on the amide carbonyl.6 E134 may provide a proton to the penultimate amino nitrogen, similar to that observed for AAP, returning it to its ionized state thus facilitating product release. Once the products are released, a water molecule bridging the two metal ions is replaced. In the absence of a second metal ion, the catalytic mechanism does not likely change markedly as H349 is in position to assist in orienting the substrate properly in the active site through the formation of a hydrogen bond with a carboxylate side chain of the substrate, thereby stabilizing the transition-state intermediate reminiscent to proposals for the mono-metalated forms of AAP and EcMetAP.40,48,49 In the presence of a dinuclear site, the second metal ion likely coordinates either the peptide carbonyl oxygen in a bridging fashion or a carboxylate side chain of the substrate.
Protein Data Bank accession numbers
The atomic coordinates and structure factor files for the structures of Zn_DapE and ZnZn_DapE from H. influenzae have been deposited in the RCSB Protein Bank with accession code 3ISZ and 3IC1, respectively.
Acknowledgements
Authors are grateful to all members of the Structural Biology Center at Argonne National Laboratory for their help in conducting these experiments. This work was supported by the National Science Foundation (CHE- 0652981, RCH), the National Institutes of Health (GM074942, AJ) and by the U.S. Department of Energy, Office of Biological and Environmental Research, under contract DE-AC02-06CH11357 (AJ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Scapin G, Blanchard JS. Enzymology of bacterial lysine biosyntesis. Adv. Enzymol. 1998;72:279–325. doi: 10.1002/9780470123188.ch8. [DOI] [PubMed] [Google Scholar]
- 2.Born TL, Blanchard JS. Structure/function studies on enzymes in the diaminopimelate pathway of bacterial cell wall synthesis. Cur. Opin. Chem. Biol. 1999;3:607–613. doi: 10.1016/s1367-5931(99)00016-2. [DOI] [PubMed] [Google Scholar]
- 3.Girodeau J-M, Agouridas C, Masson M, R. P, LeGoffic F. The lysine pathway as a target for a new genera of synthetic antibacterial antibiotics? J. Med. Chem. 1986;29:1023–1030. doi: 10.1021/jm00156a021. [DOI] [PubMed] [Google Scholar]
- 4.Karita M, Etterbeek ML, Forsyth MH, Tummuru MR, Blaser MJ. Characterization of Helicobacter pylori dapE and construction of a conditionally lethal dapE mutant. Infect. Immun. 1997;65:4158–4164. doi: 10.1128/iai.65.10.4158-4164.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pavelka MS, Jacobs WR. Biosynthesis of diaminopimelate, the precursor of lysine and a component of peptidoglycan, is an essential function of Mycobacterium smegmatis. J. Bacteriol. 1996;178:6496–6507. doi: 10.1128/jb.178.22.6496-6507.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Born TL, Zheng R, Blanchard JS. Hydrolysis of N-succinyl-L,-Ldiaminopimelic acid by the Haemophilus influenzae dapE-encoded desuccinylase: metal activation, solvent isotope effects, and kinetic mechanism. Biochemistry. 1998;37:10478–10487. doi: 10.1021/bi9806807. [DOI] [PubMed] [Google Scholar]
- 7.Bouvier J, Richaud C, Higgins W, Bögler O, Stragier P. Cloning, characterization, and expression of the dapE gene of Escherichia coli. J. Bacteriol. 1992;174:5265–5271. doi: 10.1128/jb.174.16.5265-5271.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuchs TO, Schneider B, Krumbach K, Eggeling L, Gross R. Characterization of the Bordetella pertussis diaminopimelate (DAP) biosynthesis locus identifies dapC, a novel gene coding for an N-succinyl-L,L-DAP aminotransferase. J. Bacteriol. 2000;182:3626–3631. doi: 10.1128/jb.182.13.3626-3631.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw-Reid CA, McCormick MM, Sinskey AJ, Stephanopoulos G. Flux through the tetrahydrodipicolinate succinylase pathway is dispensible for L-lysine production in Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 1999;51:325–333. doi: 10.1007/s002530051398. [DOI] [PubMed] [Google Scholar]
- 10.Lin Y, Myhrman R, Schrag ML, Gelb MH. Bacterial N-succinyl-L-diaminopimelic acid desuccinylase. Purification, partial characterization, and substrate specificity. J. Biol. Chem. 1988;263:1622–1627. [PubMed] [Google Scholar]
- 11.Gillner DM, Bienvenue DL, Nocek BP, Joachimiak A, Zachary V, Bennett B, Holz RC. The dapE-encoded N-succinyl-L,L-Diaminopimelic Acid Desuccinylase from Haemophilus influenzae Contains two Active Site Histidine Residues. J. Bio. Inorg. Chem. 2009;14:1–10. doi: 10.1007/s00775-008-0418-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bienvenue DL, Gilner DM, Davis RS, Bennett B, Holz RC. Substrate Specificity, Metal Binding Properties, and Spectroscopic Characterization of the dapE-encoded-N-succinyl-L,L-Diaminopimelic Acid Desuccinylase from Haemophilus influenzae. Biochemistry. 2003;42:10756–10763. doi: 10.1021/bi034845+. [DOI] [PubMed] [Google Scholar]
- 13.Makarova KS, Grishin NV. The Zn-peptidase superfamily: functional convergence after evolutionary divergence. J. Mol. Biol. 1999;292:11–17. doi: 10.1006/jmbi.1999.3059. [DOI] [PubMed] [Google Scholar]
- 14.Chevrier B, Schalk C, D’Orchymont H, Rondeau J-M, Moras D, Tarnus C. Crystal structure of Aeromonas proteolytica aminopeptidase: a prototypical member of the co-catalytic zinc enzyme family. Structure. 1994;2:283–291. doi: 10.1016/s0969-2126(00)00030-7. [DOI] [PubMed] [Google Scholar]
- 15.Greenblatt HM, Almog O, Maras B, Spungin-Bialik A, Barra D, Blumberg S, Shoham G. Streptomyces griseus Aminopeptidase: X-ray crystallographic structure at 1.5A resolution. J. Mol. Biol. 1997;265:620–636. doi: 10.1006/jmbi.1996.0729. [DOI] [PubMed] [Google Scholar]
- 16.Cosper NJ, Bienvenue DL, Shokes J, Gilner DM, Tsukamoto T, Scott* R, Holz* RC. The dapE-encoded N-succinyl-L,L-Diaminopimelic Acid Desuccinylase from Haemophilus influenzae is a Dinuclear Metallohydrolase. J. Am. Chem. Soc. 2004;125:14654–14655. doi: 10.1021/ja036650v. [DOI] [PubMed] [Google Scholar]
- 17.Davis R, Bienvenue D, Swierczek SI, Gilner DM, Rajagopal L, Bennett B, Holz RC. Kinetic and spectroscopic characterization of the E134A- and E134D- altered dapE-encoded N-succinyl-L,L-diaminopimelic acid desuccinylase from Haemophilus influenzae. J Biol Inorg Chem. 2006;11:206–16. doi: 10.1007/s00775-005-0071-8. [DOI] [PubMed] [Google Scholar]
- 18.Velasco AM, Leguina JI, Lazcano A. Molecular evolution of the lysine biosynthetic pathways. J. Mol. Evol. 2002;55:445–459. doi: 10.1007/s00239-002-2340-2. [DOI] [PubMed] [Google Scholar]
- 19.Badger J, Sauder JM, Adams JM, Antonysamy S, Bain K, Bergseid MG, Buchanan SG, Buchanan MD, Batiyenko Y, Christopher JA, Emtage S, Eroshkina A, Feil I, Furlong EB, Gajiwala KS, Gao X, He D, Hendle J, Huber A, Hoda K, Kearins P, Kissinger C, Laubert B, Lewis HA, Lin J, Loomis K, Lorimer D, Louie G, Maletic M, Marsh CD, Miller I, Molinari J, Muller-Dieckmann HJ, Newman JM, Noland BW, Pagarigan B, Park F, Peat TS, Post KW, Radojicic S, Ramos A, Romero R, Rutter ME, Sanderson WE, Schwinn KD, Tresser J, Winhoven J, Wright TA, Wu L, Xu J, Harris TJ. Structural analysis of a set of proteins resulting from a bacterial genomics project. Proteins. 2005;60:787–796. doi: 10.1002/prot.20541. [DOI] [PubMed] [Google Scholar]
- 20.Gill SC, von Hippel PH. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 1989;182:319–326. doi: 10.1016/0003-2697(89)90602-7. [DOI] [PubMed] [Google Scholar]
- 21.Bergmann M, Stein WH. Naphthalene-β-sulfonic acid as a reagent for amino acids. J. Biol. Chem. 1939;129:609–618. [Google Scholar]
- 22.Nocek B, Mulligan R, Bargassa M, Collart F, Joachimiak A. Crystal structure of aminopeptidase N from human pathogen Neisseria meningitidis. Proteins. 2008;70:273–9. doi: 10.1002/prot.21276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Macromolecular crystallography, Pt A. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 24.Vagin A, Teplyakov A. An approach to multi-copy search in molecular replacement. Acta Crystallogr D Biol Crystallogr. 2000;56:1622–4. doi: 10.1107/s0907444900013780. [DOI] [PubMed] [Google Scholar]
- 25.The CCP4 suite: programs for protein crystallography. Acta Crystallogr D Biol Crystallogr. 1994;50:760–3. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 26.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–32. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 27.Morris RJ, Perrakis A, Lamzin VS. ARP/wARP and automatic interpretation of protein electron density maps. Methods Enzymol. 2003;374:229–44. doi: 10.1016/S0076-6879(03)74011-7. [DOI] [PubMed] [Google Scholar]
- 28.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53:240–55. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 29.Davis IW, Leaver-Fay A, Chen VB, Block JN, Kapral GJ, Wang X, Murray LW, Arendall WB, 3rd, Snoeyink J, Richardson JS, Richardson DC. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 2007;35:W375–83. doi: 10.1093/nar/gkm216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holm L, Sander C. Touring protein fold space with Dali/FSSP. Nucleic Acids Res. 1998;26:316–9. doi: 10.1093/nar/26.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krissinel E, Henrick K. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr D Biol Crystallogr. 2004;60:2256–68. doi: 10.1107/S0907444904026460. [DOI] [PubMed] [Google Scholar]
- 32.DeLano WL. The PyMOL Molecular Graphics System. DeLano Scientific LLC; San Carlos, CA, USA: http://www.pymol.org. [Google Scholar]
- 33.JCSG Crystal structure of a Putative Zinc Peptidase ( NP_812461.1) from Bacteroides thetaiotaomicron VPI-5482 at 2.31 A resolution. PDB_code 3CT9. 2008.
- 34.Rowsell S, Pauptit RA, Tucker AD, Melton RG, Blow DM, Brick P. Crystal structure of carboxypeptidase G2, a bacterial enzyme with applications in cancer therapy. Structure. 1997;5:337–347. doi: 10.1016/s0969-2126(97)00191-3. [DOI] [PubMed] [Google Scholar]
- 35.Chevrier B, D’Orchymont H, Schalk C, Tarnus C, Moras D. The structure of the Aeromonas proteolytica aminopeptidase complexed with a hydroxamate inhibitor. Involvement in catalysis of Glu151 and two zinc ions of the cocatalytic unit. Eur. J. Biochem. 1996;237:393–398. doi: 10.1111/j.1432-1033.1996.0393k.x. [DOI] [PubMed] [Google Scholar]
- 36.Desmarais W, Bienvenue DL, Bzymek KP, Petsko GA, Ringe D, Holz RC. The 0.95 Å Resolution and Low pH Crystal Structures of the Aminopeptidase from Aeromonas proteolytica. J Biol Inorg Chem. 2006;11:398–408. doi: 10.1007/s00775-006-0093-x. [DOI] [PubMed] [Google Scholar]
- 37.Prescott JM, Wilkes SH, Wagner FW, Wilson KJ. Aeromonas Aminopeptidase improved isolation and some physical properties. J. Biol. Chem. 1971;246:1756–1764. [PubMed] [Google Scholar]
- 38.Shi D, Yu X, Roth L, Tuchman M, Allewell NM. Structure of a novel N-acetyl-L-citrulline deacetylase from Xanthomonas campestris. Biophys Chem. 2007;126:86–93. doi: 10.1016/j.bpc.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 39.Graham SC, Bond CS, Freeman HC, Guss JM. Structural and functional implications of metal ion selection in aminopeptidase P, a metalloprotease with a dinuclear metal center. Biochemistry. 2005;44:13820–13836. doi: 10.1021/bi0512849. [DOI] [PubMed] [Google Scholar]
- 40.Ye QZ, Xie SX, Ma ZQ, Huang M, Hanzlik RP. Structural Basis of Catalysis by Monometalated Methionine Aminopeptidase. Proc Natl Acad Sci U S A. 2006;103:9470–9475. doi: 10.1073/pnas.0602433103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bennett B, Holz RC. Spectroscopically distinct cobalt(II) sites in heterodimetallic forms of the aminopeptidase from Aeromonas proteolytica: Characterization of substrate binding. Biochemistry. 1997;36:9837–9846. doi: 10.1021/bi970735p. [DOI] [PubMed] [Google Scholar]
- 42.DePaola C, Bennett B, Holz RC, Ringe D, Petsko G. 1-Butaneboronic Acid Binding to Aeromonas proteolytica Aminopeptidase: A Case of Arrested Development. Biochemistry. 1999;38:9048–9053. doi: 10.1021/bi9900572. [DOI] [PubMed] [Google Scholar]
- 43.Christianson D, Cox D. Catalysis by Metal-Activated Hydroxide in Zinc anc Manganese Metalloenzymes. Annu. Rev. Biochem. 1999;68:33–57. doi: 10.1146/annurev.biochem.68.1.33. [DOI] [PubMed] [Google Scholar]
- 44.Gillner DM, Armush N, Holz RC, Becker D. Inhibitors of Bacterial N-Succinyl-L,L-diaminopimelic Acid Desuccinylase (DapE) and Demonstration of in vitro Antimicrobial Activity. Bioorg. Med. Chem. Lett. 2009;19:6350–6352. doi: 10.1016/j.bmcl.2009.09.077. [DOI] [PubMed] [Google Scholar]
- 45.Vanĕk V, Pícha J, Budĕšínský M, Šanda M, Jiráček J, Gilner DM, Holz RC, Hlaváček J. Synthesis of N-succinyl-L,L-diaminopimelic acid mimetics via asymmetric protection. Protein Pept. Lett. 2010 doi: 10.2174/092986610790780387. in press. [DOI] [PubMed] [Google Scholar]
- 46.Ustynyuk L, Bennett B, Edwards T, Holz RC. Inhibition of the Aminopeptidase from Aeromonas proteolytica by Aliphatic Alcholols. Characterization of the Hydrophobic Substrate Recognition Site. Biochemistry. 1999;38:11433–11439. doi: 10.1021/bi991090r. [DOI] [PubMed] [Google Scholar]
- 47.Stamper C, Bienvenue D, Moulin A, Bennett B, Ringe D, Petsko G, Holz RC. Spectroscopic and X-ray Crystallographic Characterization of the Bestatin Bound Form of the Aminopeptidase from Aeromonas proteolytica. Biochemistry. 2004;43:9620–9628. doi: 10.1021/bi049126p. [DOI] [PubMed] [Google Scholar]
- 48.Copik AJ, Swierczek SI, Lowther WT, D’souza V, Matthews BW, Holz RC. Kinetic and Spectroscopic Characterization of the H178A Mutant of the Methionyl Aminopeptidase from Escherichia coli. Biochemistry. 2003;42:6283–6292. doi: 10.1021/bi027327s. [DOI] [PubMed] [Google Scholar]
- 49.Holz RC. The Aminopeptidase from Aeromonas proteolytica: Structure and Mechanism of Co-Catalytic Metal Centers Involved in Peptide Hydrolysis. Coord. Chem. Rev. 2002;232:5–26. [Google Scholar]