Abstract
Objective
Examining the degree to which disease severity and domains of self-efficacy (pain, function, other symptoms) explain pain and functioning in rheumatoid arthritis patients.
Methods
Patients (N=263) completed the Arthritis Impact Measurements Scales-2 to assess pain and functioning (physical, affective, and social), the Arthritis Self-Efficacy Scale to assess three self-efficacy domains (pain, physical function, other); disease severity was assessed with C-reactive protein, physician's rating, abnormal joint count. Structural equation modeling was used to examine hypotheses: 1) does disease severity have a direct relationship with pain and each area of functioning, 2) does disease severity have a direct relationship with each arthritis self-efficacy domain, and 3) do the self-efficacy domains mediate the relationship between disease severity and RA pain and each area of functioning.
Results
Disease severity was related to pain, physical functioning, and each self-efficacy domain (β's=.28-.56; p's<.001). Each self-efficacy domain was related to its respective domain of functioning (e.g., self-efficacy for pain was related to pain) (β's=.36-.54; p's<.001). Self-efficacy mediated the relationship between disease severity and pain and functioning (β's=.12-.19; p's<.001). Self-efficacy for pain control and to perform functional tasks accounted for 32-42% of disease severity's total effect on their respective outcomes (e.g., self-efficacy for pain control accounted for 32% of disease severity's total effect on pain). Variance accounted for by the total model was 52% for pain, 53% for physical functioning, and 44% for affective and social functioning.
Conclusions
Disease severity and self-efficacy both impact RA functioning and intervening in these areas may lead to better outcomes.
Over the past 15 years arthritis self-efficacy has emerged as one of the most important psychosocial variables in understanding pain and disability among people with rheumatoid arthritis (RA) (1-4). Self-efficacy refers to an individual's belief that they have the ability to successfully perform a certain behavior to achieve a desired outcome (5, 6). Self-efficacy is conceptualized as domain specific; that is, self-efficacy assesses one's confidence that they can perform a particular task (e.g. managing arthritis pain) rather than a global sense of control or mastery (7). Consistent with this conceptualization, Lorig et al. (8) developed an Arthritis Self-Efficacy Scale (ASES) to measure three key domains of arthritis self-efficacy: self-efficacy for pain control, self-efficacy to perform functional tasks, and self-efficacy to control other symptoms (i.e., fatigue, affect, remain active, enjoy activity). This standardized instrument has been shown to be reliable and is widely used to measure self-efficacy in psychosocial studies of RA patients (2, 3, 8-14). Research has shown that RA patients who report higher levels of self-efficacy experience less pain, physical disability, fatigue, and negative mood (8, 10, 15-17). Increases in self-efficacy that occur over the course of self-management training or pain coping skills training significantly predict improvements in RA pain, health status, and depression (11).
Most rheumatologists focus on evaluating and reducing disease severity in order to reduce pain and disability. They are generally less focused on psychosocial factors, such as self-efficacy, that also potentially influence pain and disability. Likewise, many researchers who study self-efficacy have not sufficiently considered the effects of disease severity on self-efficacy. Given the recognized importance of both of these variables – disease severity and self-efficacy – it is critical to understand their relative relationship with RA outcomes. Further, a consideration of disease severity is especially important in RA because it potentially can influence both arthritis self-efficacy and RA outcomes.
The current study used a structural equation model (SEM) to examine the relationships between disease severity, domains of arthritis self-efficacy, and measures of pain and functioning. SEM allows for simultaneous examination of the direct effects of disease severity and self-efficacy on measures of pain and function as well the degree to which self-efficacy explains observed relationships between disease severity and measures of pain and function (i.e., indirect effects). Our first hypothesis was that disease severity would have a direct relationship with pain and functioning; that is, patients with higher levels of disease severity would experience greater levels of pain and lower functioning. Our second hypothesis was that disease severity would have a direct relationship with self-efficacy; that is, RA patients with higher levels of disease severity would report lower self-efficacy across all self efficacy domains. Finally, we hypothesized that each type of domain specific self-efficacy would mediate the relationship between disease severity and its corresponding pain or functioning measure. Specifically, we predicted that self-efficacy for pain control would mediate the relationship of disease severity to ratings of pain and self-efficacy to perform functional tasks would mediate the relationship of disease severity to physical functioning. We also predicted that self-efficacy to control other symptoms would mediate the relationship between disease severity and both affective and social functioning because self-efficacy to control other symptoms (fatigue, activity regulation, feeling blue, manage pain during activities, manage pain to do things one enjoys, deal with frustration of pain) measures constructs that may be related to both affective (tension levels, mood) and social functioning (social activity, perceived social support).
Methods
Procedures
This study used baseline data from participants taking part in a randomized, clinical trial aimed at decreasing pain and distress in RA patients. Recruitment sources were the metropolitan Detroit area (Wayne State University) and the rheumatology clinics of the Duke University Medical Center. Participants provided informed consent between March 2005 and December 2007; procedures were approved by each Institutional Review Board. In total, 1118 participants were screened for this study. Of these 1118, 321 were in ineligible, 534 were eligible but declined, and 263 were eligible and consented. This research was in compliance with the Helsinki Declaration. Data were collected at the baseline evaluation prior to treatment randomization treatment (pain coping skills training (18) and/or emotional disclosure (19)).
Participants had a physical examination by study rheumatologists. Patients were included if they: a) were diagnosed with RA according to 1987 American College of Rheumatology (ACR) criteria and b) reported pain or stiffness in the past week and excluded if they: a) had a significant rheumatic disorder other than RA or another organic disease that affected functioning; b) were known or judged by the physician to have cognitive impairment or were illiterate; c) were currently participating in a behavioral pain management program; d) were unable to ambulate (walking aides were acceptable); or e) were unable to write.
The physical exam provided measures of disease severity. Physicians determined each patient's abnormal joint count and rated each patient's disease activity level based on their physical exam. Finally, blood samples were used to assess the C-reactive protein level in participants. Patients provided demographic (age, gender, race, education) and medical information (duration of RA, current RA medications) and completed self-report measures.
Self-Report Measures
Self-reported patient outcome assessment tools (i.e., Arthritis Impact Measurement Scales-2 [AIMS2], Arthritis Self-Efficacy Scale [ASES]) have been recommended by the ACR as reliable and valid tools to assess patient outcomes in rheumatology research (20).
Pain and physical, psychological, and social disability
The AIMS2 is designed to measures health status in arthritis patients (21). It provides five summary scales: physical functioning, affective functioning, pain, social functioning, and role functioning. The 5 items that measure pain were used. Physical functioning was assessed with three of the six subscales (13 items) that make up the physical functioning summary scale (mobility level, self-care, household task). Three scales (walking and bending, hand and finger movement, function and arm function) were excluded due to significant item overlap with self-efficacy assessment items. The two subscales that measure affective functioning were used (10 items; tension, mood) and the two subscales that measure social functioning were used (9 items; social activity, support). Reliability and validity of the scales have been demonstrated (22,23). The scales demonstrated good internal consistency in this sample (Cronbach's alpha from .79 to .91).
Arthritis Self-Efficacy
The ASES was used to assess patients' perceived confidence in their abilities to perform behaviors that would control arthritis pain and minimize disability (8). This measure has three subscales: self-efficacy for pain control (5 items), self-efficacy to perform functional tasks (9 items), and self-efficacy to control other symptoms (6 items). Items on the self-efficacy to control other symptoms scale address one's ability to control fatigue, affect, and to remain active and enjoy activity. Patients indicated their responses on a 10 = very uncertain to 100 = very certain Likert-type scale. Subscale scores are obtained by averaging the responses to each item within a subscale and range from 10 to 100. Self-efficacy for pain control scale sample items are: “How certain are you that you can make a large reduction in your arthritis pain by using methods other than taking extra medication?” and “How certain are you that you can decrease your pain quite a bit?” Self-efficacy to perform physical tasks scale sample items are: “How certain are you that you can walk 100 feet on flat ground in 20 seconds?” and “As compared to other people with arthritis like yours, how certain are you that you can manage arthritis during your daily activities?” Self-efficacy to control other symptoms scale sample items are: “How certain are you that you can manage your arthritis symptoms so that you can do the things you enjoy doing?” and “How certain are you that you can do something to help yourself feel better if you are feeling blue?” The ASES has demonstrated good reliability and validity (8, 24). The subscales demonstrated good internal consistency in this sample (Cronbach's alpha from.88 to .92).
Disease Severity Measures
Disease severity measures in this study are consistent with the ACR's recommended disease activity measures for RA clinical trials (25).
Physician's global rating of disease activity
A visual analog scale (VAS) was used by the rheumatologist to rate each participant's disease activity. The VAS is a horizontal line measuring 100 mm with anchors of “no disease activity” to “high disease activity”. Scores range from 0-100. Following a physical exam, the rheumatologist marked the line at the point representing their disease activity global rating. The VAS score is determined by measuring the distance, in mm, from the beginning of the line to the marked point. Similar methods have been used in past studies (26). Physicians participated in standardized training methods and were blind to other study assessment measures.
Abnormal Joint Counts
The rheumatologist determined patient's abnormal joint count. Swelling, tenderness, and deformity were assessed in each of the following 32 joints bilaterally: proximal interphalangeal joints, metacarpal phalangeal joints, wrist, elbow, shoulder, hip, knee and ankle. Scores range from 0-32. Past research has used similar methods (27-29).
C-Reactive Protein
Serum fractions were assayed in order to get a measure of C-reactive protein (CRP). This is an index of RA inflammation and activity; a higher score indicates more disease activity. CRP values in this sample were 0.12 mg/L to 112 mg/L. The examining physician did not know the CRP values during their assessment.
Data Analyses
Pearson correlations were used for continuous study variables and point-biserial correlations were used for one continuous variable and one dichotomous variable. We used SEM to evaluate the relationships between disease severity, domain specific self-efficacy, and pain and domains of functioning. We represented disease severity, physical functioning, affective functioning, and social functioning as latent constructs in our model. Latent variables are unobserved variables that are implied by the covariances among two or more indicators (i.e., factors) (30). Three observed variables were used to create the latent disease severity variable: CRP, physician's disease assessment, and abnormal joint count. Three subscales from the AIMS2 were used to create the physical functioning latent variable and the two subscales from the AIMS2 that measure affective (tension, mood) and social (social activity, social support) functioning created the latent variables of affective functioning and social functioning. Observed variables factor loadings onto a latent variable can be interpreted in a manner similar to conventional common factor analysis. Figure 1 shows the structure of the model. Each arrow represents a hypothesis about the relationship among the variables. Mplus software 5.01 (31) was used to calculate maximum likelihood estimates for model fit. We managed missing data with the full-information maximum likelihood method. Full information likelihood method for missing data is a direct method in which model parameters and standard errors are estimated directly from available data. It is superior to other techniques for handling missing data (32). Disturbance terms were allowed to correlate between the self-efficacy variables. Age, education, race, and opioid use were included as control variables because these variables were related (p<.05) to disease severity, the self-efficacy variables, or the dependent variables in bivariate analyses.
Figure 1.
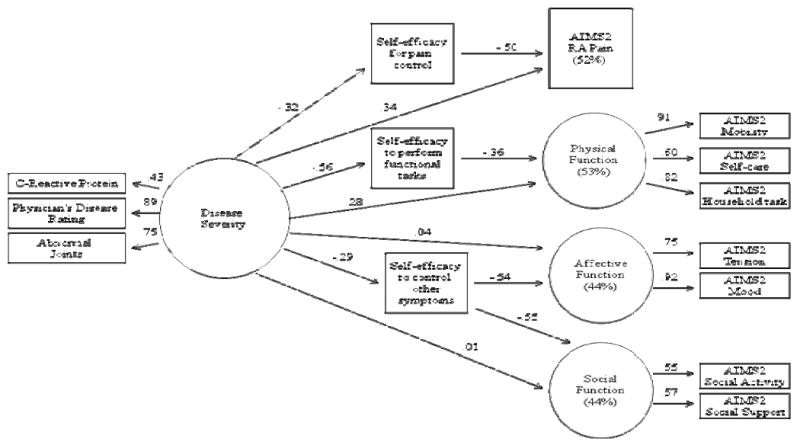
Note. Structural Equation Model; Fit Indices:; CFI = 0.96;; RMSEA = .06 (90% CI = 0.047-0.074); SRMSR = .04. Age, education, race, and opioid use are controlled for in the model. Direct paths were specified between control variables and disease severity, self-efficacy variables, pain, physical function, affective function, and social function. These paths are not displayed in the figure for ease of readability. All paths displayed in the figure are significant except disease Severity to Affective Function and Disease Severity to Social Function.
The Sobel test (33) was used to test the significance of indirect effects. We also examined the significance of indirect effects using a bootstrap approach (with 5000 resamples) to obtain 95% confidence intervals for the direct and indirect effects (34,35). Power was computed for each model parameter using the Monte Carlo study approach (36) and was carried out using Mplus 5.1 (three seeds with 10,000 replications were used for each analysis to ensure that stability was reached). Power ranged from 0.81 to 0.99 for each parameter (i.e., path coefficients) specified in the model with a two-tailed alpha = .05.
The primary task in the model testing procedure is to determine the “goodness of fit” between the hypothesized model and the sample data. The hypothesized model is imposed on the data to examine the observed data fit (e.g., goodness of fit). Several fit indices were used to examine the omnibus fit of the model: root means square error of approximation (RMSEA), the standardized root mean square residual (SRMSR), the comparative fit index, and the Tucker-Lewis Index (TLI). The RMSEA provides an estimate of the average size of the residual adjusting for the degree of freedom; values less than or equal to 0.05 indicate good model fit to the data. The SRMSR is a standardized version of the average size of the residuals, where values less than 0.05 are considered a good fit and those between 0.05 and 0.08 are considered a fair fit (37). The CFI is a measure of how well the model is improved in comparison to the null model; values greater than or equal to 0.95 are considered a good fit of the model.
Results
Participants
The study sample included 263 RA patients who had a mean age of 55.03 (SD = 12.0; Med = 56; IQR = 16; Range = 22 to 82). Table 1 summarizes the demographic and disease characteristics of the sample and provides the means, standard deviations, and possible scale ranges of the study variables. Intercorrelations between demographic/disease variables and model variables are presented in Table 2, and intercorrelations between the model variables themselves are presented in Table 3.
Table 1.
Descriptive statistics for study variables (N =263).
| Mean (SD) | %(n) | |
|---|---|---|
| Age (years) | 55.03 (12.0) | |
| Female | 213 (81%) | |
| Race | ||
| African American | 73 (28%) | |
| White | 180 (68%) | |
| Other | 10 (4%) | |
| Highest Education | ||
| Less than high school | 8 (3%) | |
| High school or GED | 138 (54%) | |
| College degree | 65 (25%) | |
| Graduate degree | 46 (18%) | |
| Duration of RA (years) | 15.63 (12.0) | |
| Current NSAID use | 171 (65%) | |
| Current non-opioid analgesic use | 87 (33%) | |
| Current opioid analgesic use | 74 (28%) | |
| Current steroid used | 120 (46%) | |
| Current DMARD use | 181 (69%) | |
| Current Biological Response Modifier Use | 136 (52%) | |
| Possible Scale Range | ||
| Physician's Disease Assessment | 28.72 (22.48) | 0-100 |
| C-Reactive Protein | 9.00 (13.91) | .12-112a |
| Abnormal Joint Count | 12.20 (8.80) | 0-32 |
| Self-efficacy for pain control | 28.35 (10.76) | 10-100 |
| Self-efficacy to perform functional tasks | 59.26 (21.76) | 10-100 |
| Self-efficacy to control other symptoms | 37.46 (13.04) | 10-100 |
| Pain | 2.91 (1.00) | 1-5 |
| Mobility | 1.64 (.74) | 1-5 |
| Self-care | 1.27 (.64) | 1-5 |
| Household tasks | 1.70 (.91) | 1-5 |
| Tension | 2.60 (.87) | 1-5 |
| Mood | 1.74 (.60) | 1-5 |
| Social Activity | 3.10 (.74) | 1-5 |
| Social Support | 2.00 (.99) | 1-5 |
Note. This indicates the range in this sample.
Table 2.
Correlations between study variables.
| Age | Gender | Race | Education | NSAID use | Non-opioid analgesic | Opioid analgesic | Steroid | DMARD | Biological Response Modifier | |
|---|---|---|---|---|---|---|---|---|---|---|
| CRP | .07 | -.08 | .04 | -.14* | -.01 | .07 | .20** | .09 | -.10 | -.05 |
| Disease Rating | .02 | .01 | -.15* | -.17** | -.05 | .02 | .23** | .13* | .02 | -.02 |
| Joints | .09 | .01 | -.06 | -.19** | -.03 | .12 | .20** | .13* | .01 | .09 |
| SE Pain | .17** | .09 | -.19** | .07 | .01 | -.04 | -.23** | -.08 | -.03 | -.01 |
| SE Function | -.11 | -.08 | -.19** | .15* | .11 | -.14* | -.30** | -.10 | .06 | -.04 |
| SE Other | .21** | .03 | -.14* | .07 | -.02 | -.01 | -.20** | -.07 | .01 | -.02 |
| Pain | -.18** | .01 | .26** | -.18 | -.03 | .09 | .34** | .06 | -.11 | -.10 |
| Mobility | -.10 | .05 | .31** | -.16* | -.10 | .05 | .32** | .05 | -.15* | -.09 |
| Self-care | -.04 | -.02 | .27** | -.07 | -.07 | .01 | .19** | -.06 | -.18** | -.17** |
| Household tasks | -.08 | .03 | .29** | -.07 | .02 | .02 | .27** | .04 | -.09 | -.04 |
| Tension | -.38** | -.03 | .08 | -.04 | .02 | .01 | .07 | .02 | -.03 | -.10 |
| Mood | -.18** | -.03 | .17** | -.19** | .01 | .03 | .17** | .09 | -.12 | -.09 |
| Social Activity | -.13* | -.09 | .12 | .02 | -.05 | -.01 | .10 | .04 | -.02 | .01 |
| Social Support | -.18** | .01 | .05 | -.08 | .07 | .03 | -.01 | .07 | -.07 | .02 |
Note. Correlation is significant at the 0.01 level.
Correlation is significant at the .05 level. CRP = C-reactive protein. SE = Self-efficacy. DMARD = disease-modifying antirheumatic drug.
Table 3.
Correlations between study variables.
| CRP | Disease Rating | Joints | SE Pain | SE Function | SE Other | Pain | Mobility | Self-care | Household Tasks | Tension | Mood | Social Activity | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRP | - | ||||||||||||
| Disease Rating | .40** | - | |||||||||||
| Joints | .32** | .67** | - | ||||||||||
| SE Pain | -.22** | -.33** | -.22** | - | |||||||||
| SE Function | -.26** | -.53** | -.46** | .60** | - | ||||||||
| SE Other | -.19** | -.30** | -.17** | .83** | .60** | - | |||||||
| Pain | .22** | .48** | .35** | -.60** | -.57** | -.55** | - | ||||||
| Mobility | .20** | .51** | .30** | -.45** | -.56** | -.46** | .56** | - | |||||
| Self-care | .10 | .31** | .21** | -.25** | -.39** | -.24** | .43** | .54** | - | ||||
| Household tasks | .10 | .39** | .27** | -.40** | -.53** | -.46** | .48** | .75** | .49** | - | |||
| Tension | -.05 | .10 | .04 | -.38** | -.20** | -.49** | .43** | .29** | .19** | .31** | - | ||
| Mood | .13* | .22** | .17** | -.43** | -.43** | -.60** | .54** | .49** | .30** | .47** | .70** | - | |
| Social Activity | .07 | .10 | .03 | -.28** | -.28** | -.37** | .28** | .33** | .13* | .33** | .33** | .43** | - |
| Social Support | -.01 | .07 | .03 | -.19** | -.19** | -.35** | .19** | .24** | .11 | .29** | .31** | .38** | .33** |
Note. Correlation is significant at the 0.01 level.
Correlation is significant at the .05 level. CRP = C-reactive protein. SE = Self-efficacy.
Structural Equation Modeling
The factor loadings for the latent variable of disease severity were as follows: CRP = .43, physician's disease assessment = .89, abnormal joint count = .75 (see Figure 1). Loadings can be interpreted in a manner similar to conventional common factor analysis. Physician's disease assessment and abnormal joint count load relatively high on disease severity; CRP does not load as highly, but is still within an acceptable range. The factor loadings for the latent variable of physical function were: mobility = .91, self-care = .60, and household task = .82. The factor loadings for the latent variable of affective function variable were: tension = .75 and mood = .92. The factor loadings for the latent variable of social function were: social activity = .55 and social support = .57. These loadings are all within an acceptable range (38).
The overall fit of our hypothesized model was good: CFI = 0.96; TLI = 0.93; RMSEA = .06 (90% CI = 0.047-0.074); SRMSR = .03. This indicates that the data fit the hypothesized model well. The path coefficients (see Figure 1) indicate that disease severity was significantly related to pain (β=.34; p<.001) and physical functioning (β=.28; p<.001). Thus, patients with higher levels of disease severity reported higher levels of pain and lower levels of physical functioning. Disease severity also was significantly related to each of the three domains of self-efficacy (β's=.29-.56; p's<.001). Thus, patients with higher levels of disease severity reported lower self-efficacy for pain control, self-efficacy to perform functional tasks, and self-efficacy to control other symptoms. Disease severity did not have a direct relationship with affective functioning or social functioning. The path model shows that each specific self-efficacy domain was significantly associated with its respective domain of RA functioning (β's=.36-.54; p's<.001).
SEM allows for a convenient means of calculating indirect effects such as the relation between a predictor and outcome by the way of an intervening, or mediating, variable. A statistically significant indirect effect can be interpreted as support of the hypothesis that an intervening variable mediates the relation between a predictor and an outcome. The present model shows two indirect effects that were statistically significant: 1) Disease Severity→ Self-efficacy for pain control → Pain (β=.12; p<.001; 95% CI = .05-.19; Sobel Z = 4.50) and 2) Disease Severity→ Self-efficacy to perform functional tasks → Physical functioning (β=.19; p<.001; 95% CI = .11-.29; Sobel Z = 4.95). Disease severity was not significantly associated with affective function or social function; indirect effects are not reported for these outcomes.
The indirect relationship between disease severity and RA pain via self-efficacy for pain control accounted for 32% of disease severity's total effect (direct + indirect relationship) on pain. This suggests that the relationship between disease severity and pain is partially mediated by self-efficacy for pain control. The indirect relationship between disease severity and RA physical function via self-efficacy to perform functional tasks accounted for 42% of disease severity's total effect on RA physical functioning. This suggests that the relationship between disease severity and functioning is partially mediated by self-efficacy to perform functional tasks.
The SEM model accounted for 52% of the variance in RA pain, 53% of the variance in RA physical functioning, 44% of the variance in RA affective functioning, and 44% of the variance in RA social functioning. Figure 1 summarizes the path coefficients and total variance in the outcomes accounted for by the proposed model.
Discussion
The findings of this study add to growing evidence demonstrating that self-efficacy is important in understanding the impact of RA. We examined three relationships. First, we examined whether disease severity impacts RA pain and functioning. As hypothesized, our study results suggest that patients with higher levels of disease severity experience high levels of pain and lower levels of physical functioning. This finding fits with current models of RA, suggesting that disease severity is a very important factor that influences pain and physical functioning. It also underscores the importance of current clinical practice which is focused on careful assessments of disease severity and the use of treatments designed to reduce disease severity.
Second, we examined whether or not disease severity had direct relationships with self-efficacy. Our study results suggest that RA patients with more severe disease had lower levels of self-efficacy for pain control, self-efficacy to perform functional tasks, and self-efficacy to control other symptoms. Disease severity had the strongest impact on self-efficacy to perform functional tasks, followed by self-efficacy for pain control and then self-efficacy to control other symptoms. To our knowledge, the current study is unique in that it systematically examines the relationship between rheumatoid arthritis disease severity and self-efficacy. These findings have important clinical implications. First, rheumatologists and other clinicians working with RA patients should be aware that those patients with more severe disease may be prone to report much lower levels of self-efficacy. Furthermore, health educators and psychosocial intervention specialists need to be alert to the fact that patients reporting low self-efficacy may be doing so because of their high disease severity. Rheumatologists should consider that referrals to services providing strategies to increase self-efficacy (e.g., pain coping skills training) in patients with high disease severity may provide decreases in pain and improved functioning beyond what is achieved with even optimal medical management. It is important that all healthcare providers (rheumatologists, psychosocial interventionist) be aware of the potentially positive synergistic effects of adequately managing disease severity and self-efficacy to decrease pain and improve functioning. Finally, it should also be acknowledged that enhancing self-efficacy to perform certain functional tasks (e.g., walking) may not be realistic for some RA patients with high levels of disease severity and/or specific physical limitations (e.g., the ability to walk). In these cases, RA patients may find the most benefit from improvements in self-efficacy for pain control and self-efficacy to control other symptoms.
A third goal of this study was to examine whether each type of domain specific self-efficacy would mediate the relationship between disease severity and its corresponding pain or functioning measure. We found that self-efficacy for pain control mediated the relationship of disease severity to ratings of pain (indirect effect = .12) and self-efficacy to perform functional tasks mediated the relationship of disease severity to physical functioning (indirect effect = .19). The amount of disease severity's impact that was explained by self-efficacy varied across these two outcomes. Self-efficacy to perform functional tasks accounted for almost half of disease severity's total effect on physical functioning (42%). It is often assumed that disease severity is directly linked to poor physical functioning, but these findings suggest that self-efficacy to perform functional tasks is important in explaining the link between disease severity and physical functioning. Self-efficacy for pain control accounted for 32% of disease severity's total effect on RA pain. Although this is lower than that for self-efficacy to perform functional tasks it remains substantial and suggests that one does need to consider the role of self-efficacy for pain control in explaining the association of disease severity to pain. Contrary to our hypotheses there was no relationship between disease severity and affective and social function. Problems in these areas (i.e., affective and social functioning) may not be related to disease severity in RA patients. Self-efficacy appears to be a key variable impacting these areas of functioning. Future work should examine what other factors may be impacting these areas of function.
Our findings regarding the positive relationships between disease severity and self-efficacy could lead to the erroneous conclusion that to decrease pain and improve functioning, one only needs to focus on decreasing disease severity to improve both self-efficacy and pain and functioning. However, another key finding of our study was disease severity only accounts for a portion of the variability in self-efficacy, suggesting that levels of self-efficacy can vary independent of disease severity. This means that, even among patients with high disease severity those with higher self-efficacy are much more likely to experience less pain and better physical functioning. Furthermore, among patients with high disease severity, one may be able to enhance self-efficacy through interventions such as self-management or coping skills training and thereby produce improvements in pain and function. In short, to optimize patient outcomes it is critical to focus not only on reducing disease severity, but also on increasing RA patients' levels of self-efficacy.
What makes the results of this study particularly noteworthy is that they were obtained after controlling for medication use (opioids). This suggests that understanding the additional mediating role of self-efficacy can improve our understanding of the influence of disease severity on pain and function above and beyond what might be explained on the basis of disease severity and use of RA medications alone. The statistical approach used in this study represents a methodological improvement over many prior studies of self-efficacy in RA. The use of SEM allowed for the inclusion of a latent disease severity variable (CRP, physicians rating of disease severity, abnormal joints). Further, it allowed us to simultaneously consider each of the three domains of self-efficacy, pain, and each area of functioning (physical, affective, social).
The results of this study suggest that an increased focus on self-efficacy may be particularly valuable to clinicians with patients who continue to experience problems with pain and function despite optimal medical management. In such cases, one may want to assess arthritis self-efficacy since it may identify an additional route for improving RA outcomes (i.e. educational or psychosocial interventions to increase arthritis self-efficacy). Short measures of self-efficacy, some with as few as two items, that are valid and reliable have been developed (39,40) and can be integrated into clinical practice with minimal additional burden on patient or provider (40).
RA patients who have low levels of self-efficacy may benefit from a referral for psychosocial intervention that can increase arthritis self-efficacy. Past work in samples of patients with arthritis also has demonstrated that psychosocial treatments can lead to increased self-efficacy in arthritic samples (41,42). Smarr et al. (11) found that stress management training in RA patients resulted in higher levels of self-efficacy which were associated with decreased depression, pain, and disease activity. Another study found that pain coping skills training led to increases in self-efficacy that were related to better pain coping, increased physical functioning, and less psychological disability (43). Lorig et al. (42) recently reported that an internet-based self-management program led to increased self-efficacy which was subsequently related to improvements in important indicators of health.
This study has some limitations. First, the cross-sectional design of this study precludes our ability to make causal attributions regarding the studied relationships. This design makes it impossible to know whether disease severity influences pain and functioning through self-efficacy or whether disease severity influences self-efficacy through pain and functioning. Longitudinal designs should be designed to more closely evaluate these potential cause and effect relationships. These relationships may actually be cyclic in nature such that high disease severity leads to more pain which leads to less self-efficacy for pain control thus increasing both disease severity and pain.
Second, these findings were obtained in a sample of patients with RA. Future studies need to test whether these findings can be replicated in patients with other rheumatic diseases (e.g., osteoarthritis), other disease-related pain conditions (e.g., sickle cell disease), and in other settings (e.g., community clinics, primary care). Participants in this study were volunteers for a psychosocial intervention trial and findings may not generalize to a broader sample of RA patients (e.g., those not interested in psychosocial interventions). Generalizability of study results may also be compromised by the large percentage of female participants (81%).
This study design allowed us to examine the relationships between disease severity, self-efficacy, and RA outcomes. Our proposed model fit the data well and study results suggest that disease severity has a direct relationship with RA outcomes and also significantly impacts these RA outcomes via its relationship with arthritis self-efficacy. These findings suggest that self-efficacy is a variable that both clinicians and researchers should consider when attempting to understand variations in pain and function in RA patients.
Acknowledgments
This study was supported by NIH Grant # AR049059.
References
- 1.Brekke M, Hjortdahl P, Kvien TK. Changes in self-efficacy and health status over 5 years: a longitudinal observational study of 306 patients with rheumatoid arthritis. Arthritis Rheum. 2003;49(3):342–8. doi: 10.1002/art.11112. [DOI] [PubMed] [Google Scholar]
- 2.Hammond A, Bryan J, Hardy A. Effects of a modular behavioural arthritis education programme: a pragmatic parallel-group randomized controlled trial. Rheumatology (Oxford) 2008;47(11):1712–8. doi: 10.1093/rheumatology/ken380. [DOI] [PubMed] [Google Scholar]
- 3.Lorig KR, Ritter PL, Dost A, Plant K, Laurent DD, McNeil I. The expert patients programme online, a 1-year study of an Internet-based self-management programme for people with long-term conditions. Chronic Illn. 2008;4(4):247–56. doi: 10.1177/1742395308098886. [DOI] [PubMed] [Google Scholar]
- 4.Taal E, Rasker JJ, Seydel ER, Wiegman O. Health status, adherence with health recommendations, self-efficacy and social support in patients with rheumatoid arthritis. Patient Educ Couns. 1993;20(2-3):63–76. doi: 10.1016/0738-3991(93)90122-d. [DOI] [PubMed] [Google Scholar]
- 5.Bandura A, Wood R. Effect of perceived controllability and performance standards on self-regulation of complex decision making. J Pers Soc Psychol. 1989;56(5):805–14. doi: 10.1037//0022-3514.56.5.805. [DOI] [PubMed] [Google Scholar]
- 6.Leventhal H, Cameron L. Behavioral theories and the problem of compliance. Patient Educ Couns. 1987;10(2):117–38. [Google Scholar]
- 7.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84(2):191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 8.Lorig K, Chastain RL, Ung E, Shoor S, Holman HR. Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis Rheum. 1989;32(1):37–44. doi: 10.1002/anr.1780320107. [DOI] [PubMed] [Google Scholar]
- 9.Barlow JH, Shaw KL, Wright CC. Development and preliminary validation of a self-efficacy measure for use among parents of children with juvenile idiopathic arthritis. Arthritis Care Res. 2000;13(4):227–36. doi: 10.1002/1529-0131(200008)13:4<227::aid-anr7>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 10.Lefebvre JC, Keefe FJ, Affleck G, Raezer LB, Starr K, Caldwell DS, et al. The relationship of arthritis self-efficacy to daily pain, daily mood, and daily pain coping in rheumatoid arthritis patients. Pain. 1999;80(1-2):425–35. doi: 10.1016/s0304-3959(98)00242-5. [DOI] [PubMed] [Google Scholar]
- 11.Smarr KL, Parker JC, Wright GE, Stucky-Ropp RC, Buckelew SP, Hoffman RW, et al. The importance of enhancing self-efficacy in rheumatoid arthritis. Arthritis Care Res. 1997;10(1):18–26. doi: 10.1002/art.1790100104. [DOI] [PubMed] [Google Scholar]
- 12.Strahl C, Kleinknecht RA, Dinnel DL. The role of pain anxiety, coping, and pain self-efficacy in rheumatoid arthritis patient functioning. Behav Res Ther. 2000;38(9):863–73. doi: 10.1016/s0005-7967(99)00102-3. [DOI] [PubMed] [Google Scholar]
- 13.Barlow JH, Williams RB, Wright C. The Arthritis Self-Efficacy Scale in a UK context. Psychol Health Med. 1997;2:5–19. [Google Scholar]
- 14.Shelby RA, Somers TJ, Keefe FJ, Pells JJ, Dixon KE, Blumenthal JA. Domain specific self-efficacy mediates the impact of pain catastrophizing on pain and disability in overweight and obese osteoarthritis patients. J Pain. 2008 Oct;9(10):912–9. doi: 10.1016/j.jpain.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barlow JH, Cullen LA, Rowe IF. Educational preferences, psychological well-being and self-efficacy among people with rheumatoid arthritis. Patient Educ Couns. 2002;46(1):11–9. doi: 10.1016/s0738-3991(01)00146-x. [DOI] [PubMed] [Google Scholar]
- 16.Keefe FJ, Lefebvre JC, Maixner W, Salley AN, Jr, Caldwell DS. Self-efficacy for arthritis pain: relationship to perception of thermal laboratory pain stimuli. Arthritis Care Res. 1997;10(3):177–84. doi: 10.1002/art.1790100305. [DOI] [PubMed] [Google Scholar]
- 17.Riemsma RP, Rasker JJ, Taal E, Griep EN, Wouters JM, Wiegman O. Fatigue in rheumatoid arthritis: the role of self-efficacy and problematic social support. Br J Rheumatol. 1998;37(10):1042–6. doi: 10.1093/rheumatology/37.10.1042. [DOI] [PubMed] [Google Scholar]
- 18.Keefe FJ, Van Horn Y. Cognitive-behavioral treatment of rheumatoid arthritis pain: Understanding and enhancing maintenance of treatment gains. Arthritis Care Res. 1993;6:213–222. doi: 10.1002/art.1790060408. [DOI] [PubMed] [Google Scholar]
- 19.Kelley JE, Lumley MA, Leisen JC. Health effects of emotional disclosure in rheumatoid arthritis patients. Health Psych. 1997;16:1–10. doi: 10.1037//0278-6133.16.4.331. [DOI] [PubMed] [Google Scholar]
- 20.Katz P. Introduction to special patient outcomes in rheumatology issue of arthritis care and research. Arthritis Rheum. 2003;49:S1–S4. doi: 10.1002/acr.20585. [DOI] [PubMed] [Google Scholar]
- 21.Meenan RF, Mason JH, Anderson JJ, Guccione AA, Kazis LE. AIMS2: The content and properties of a revised and expanded Arthritis Impact Measurement Scales Health Status Questionnaire. Arthritis Rheum. 1992;35:1–10. doi: 10.1002/art.1780350102. [DOI] [PubMed] [Google Scholar]
- 22.Merkel PA, Herlyn K, Martin RW, Anderson JJ, Mayes MD, Bell P, et al. Measuring disease activity and functional status in patients with scleroderma and Raynaud's phenomenon. Arthritis Rheum. 2002;46(9):2410–20. doi: 10.1002/art.10486. [DOI] [PubMed] [Google Scholar]
- 23.Ren XS, Kazis L, Meenan RF. Short-form Arthritis Impact Measurement Scales 2: tests of reliability and validity among patients with osteoarthritis. Arthritis Care Res. 1999;12(3):163–71. doi: 10.1002/1529-0131(199906)12:3<163::aid-art3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 24.Schiaffino KM, Revenson TA, Gibofsky A. Assessing the impact of self-efficacy beliefs on adaptation to rheumatoid arthritis. Arthritis Care Res. 1991;4(4):150–7. doi: 10.1002/art.1790040404. [DOI] [PubMed] [Google Scholar]
- 25.Felson DT, Anderson JJ, Boers M, Bombardier C, Chernoff M, Fried B, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. The Committee on Outcome Measures in Rheumatoid Arthritis Clinical Trials. Arthritis Rheum. 1993;36(6):729–40. doi: 10.1002/art.1780360601. [DOI] [PubMed] [Google Scholar]
- 26.Anderson JJ, Felson DT, Meenan RF, Williams HJ. Which traditional measures should be used in rheumatoid arthritis clinical trials? Arthritis Rheum. 1989;32(9):1093–9. doi: 10.1002/anr.1780320907. [DOI] [PubMed] [Google Scholar]
- 27.Connelly M, Keefe FJ, Affleck G, Lumley MA, Anderson T, Waters S. Effects of day-to-day affect regulation on the pain experience of patients with rheumatoid arthritis. Pain. 2007;131(1-2):162–70. doi: 10.1016/j.pain.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuchs HA, Pincus T. Reduced joint counts in controlled clinical trials in rheumatoid arthritis. Arthritis Rheum. 1994;37(4):470–5. doi: 10.1002/art.1780370406. [DOI] [PubMed] [Google Scholar]
- 29.Keefe FJ. Behavioral assessment and treatment of chronic pain: current status and future directions. J Consult Clin Psychol. 1982;50(6):896–911. doi: 10.1037//0022-006x.50.6.896. [DOI] [PubMed] [Google Scholar]
- 30.Hoyle RH. Structural Equation Modeling. Thousand Oaks (CA): SAGE Publications, Inc.; 1995. [Google Scholar]
- 31.Muthen LK, Muthen BO. Mplus: Statistical Analysis with Latent Variables: User's Guide. Los Angeles (CA): Muthen & Muthen; 2005. [Google Scholar]
- 32.Enders CK, Bandalos DL. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural Equation Modeling. 2001;8(3):430–457. [Google Scholar]
- 33.Sobel M. Asymptotic intervals for indirect effects in structural equation models. Social Methodol. 1982;13:290–312. [Google Scholar]
- 34.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7(1):83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36(4):717–31. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 36.Muthén L, Muthén BO. How to use a monte carlo study to decide on sample size and determine power. Structural Equation Modeling. 2002;9:599–620. [Google Scholar]
- 37.Browne MW, Cudeck R. Alternative Ways of Assessing Model Fit. Social Methods Res. 1992;21(2):230–58. [Google Scholar]
- 38.Kline RB. Principles and practice of structural equation modeling. New York: Guilford Press; 2005. [Google Scholar]
- 39.Jensen MP, Keefe FJ, Lefebvre JC, Romano JM, Turner JA. One- and two-item measures of pain beliefs and coping strategies. Pain. 2003;104(3):453–69. doi: 10.1016/S0304-3959(03)00076-9. [DOI] [PubMed] [Google Scholar]
- 40.Lorig K, Stewart A, Ritter PL, Gonzalez VM, Laurent D, Lynch J. Outcome measures for health education and other health care interventions. Thousand Oaks (CA): Sage Publications; 1996. [Google Scholar]
- 41.Lorig KR, Ritter PL, Laurent DD, Fries JF. Long-term randomized controlled trials of tailored-print and small-group arthritis self-management interventions. Med Care. 2004;42(4):346–54. doi: 10.1097/01.mlr.0000118709.74348.65. [DOI] [PubMed] [Google Scholar]
- 42.Lorig KR, Ritter PL, Laurent DD, Plant K. The internet-based arthritis self-management program: a one-year randomized trial for patients with arthritis or fibromyalgia. Arthritis Rheum. 2008;59(7):1009–17. doi: 10.1002/art.23817. [DOI] [PubMed] [Google Scholar]
- 43.Keefe FJ, Blumenthal J, Baucom D, Affleck G, Waugh R, Caldwell DS, et al. Effects of spouse-assisted coping skills training and exercise training in patients with osteoarthritic knee pain: a randomized controlled study. Pain. 2004;110(3):539–49. doi: 10.1016/j.pain.2004.03.022. [DOI] [PubMed] [Google Scholar]


