Abstract
Background
Whether locomotor muscle afferent neural activity contributes to exercise hyperpnea and symptoms of dyspnea in heart failure (HF) is controversial. We examined the influence of metaboreceptor stimulation on ventilation with and without maintaining end-exercise end-tidal CO2 levels.
Methods and Results
Eleven patients with HF aged 51±5 years (ejection fraction, 32±3%; New York Heart Association class, 1.6±0.2) and 11 age- and gender-matched healthy control participants aged 43±3 years were studied. Participants underwent 3 steady-state cycling sessions at 60% of peak oxygen consumption for 4 minutes. The first exercise session was a baseline control trial. Bilateral thigh tourniquets were inflated to suprasystolic pressure at end exercise for 2 minutes during 2 of the trials (regional circulatory occlusion) with the addition of inspired CO2 to maintain end-exercise partial pressure of end-tidal CO2 during 1 trial (regional circulatory occlusion+CO2). Minute ventilation was measured continuously throughout each trial. At 2 minutes postexercise during the baseline control trial in patients with HF, minute ventilation was 54% of end exercise, whereas the control group averaged 41% (P=0.11). During regional circulatory occlusion in patients with HF, minute ventilation was 60% of end exercise; however, the control group averaged 35% (P<0.001). During regional circulatory occlusion+CO2, the minute ventilation of patients with HF averaged 67% of end exercise, whereas the control group averaged 44% (P<0.001).
Conclusion
These data suggest that increased afferent neural activity from the large locomotor muscles associated with metabolites generated during exercise contribute to the augmented ventilatory response to exercise in patients with HF.
Keywords: dyspnea, respiration, skeletal muscle, heart failure
Dyspnea is recognized as a leading symptom in patients with chronic heart failure (HF) that often contributes to limitations in activities of daily living. Importantly, a critical link has been established between increased ventilation and worsening prognosis.1 Although a number of potential mechanisms have been proposed to explain the development of dyspnea, the relationship between minute ventilation and the production of carbon dioxide (VE/VCO2 slope) has been shown to be a more powerful predictor of morbidity than measures of exercise capacity and central hemodynamics.2 As such, much attention has been placed on the mechanisms contributing to altered ventilatory drive.3 A number of neurological mediators associated with pulmonary function and hemodynamics are included. These mediators, such as the pulmonary J receptors associated with lung fluid balance, atrial stretch receptors linked with central hemodynamic overload, and central and peripheral chemoreceptors, all have been implicated in the chronic hyperventilation often noted in HF.3–8
Although the specific underlying mechanisms remain unclear, it has been suggested that receptors in the skeletal muscle may contribute to the blood pressure and ventilatory response to exercise in the HF population. This link has spawned the development of the “muscle hypothesis,” which relates afferent neural traffic from the skeletal muscle to the cardiopulmonary responses to exercise in patients with HF.9,10 This theory suggests that neural traffic from types III and IV (myelinated and unmyelinated, respectively) unencapsulated afferent nerve endings originating in skeletal muscle (embedded in the walls of capillaries, venules, and collagen), which are stimulated by the metabolic by-products of skeletal muscle metabolism (metaboreceptors), may be responsible for the overventilation in HF, particularly during exercise when metabolic function is augmented.
However, previous studies examining the influence of the metaboreflex on ventilatory control have demonstrated conflicting results. The disparate results may be due to differences in muscle groups studied (small forearm versus large locomotor muscles), nonphysiological stimuli of the receptors, or failure in controlling for hypocapnic feedback to the central chemoreceptors because of circulatory occlusion and trapping of metabolic by-products in the area of the skeletal muscle.11–13 Thus, controversy remains regarding the role and magnitude of skeletal muscle metaboreceptor action on ventilatory control during exercise in patients with HF.
The purpose of this investigation was to examine the influence of metaboreceptor stimulation on ventilation in patients with HF during exercise with and without controlling for the associated hypocapnia caused by postexercise circulatory occlusion. We hypothesized that patients with moderate HF would demonstrate a greater ventilatory response to metaboreceptor stimulation compared with matched healthy control volunteers after submaximal steady-state cycle ergometry exercise.
Methods
Population Characteristics
Eleven patients with HF from the Mayo Clinic Heart Failure Service and the Cardiovascular Health Clinic (a preventive and rehabilitative center) were recruited (Table 1). Inclusion criteria for the patients with HF included history of ischemic or dilated cardiomyopathy, stable HF symptoms (>3 months), duration of HF symptoms >1 year, left ventricular ejection fraction ≤35%, body mass index <35 kg/m2, and nonsmoker with a smoking history of <15 pack-years. Patients were treated with standard optimized medications for HF at the time of the study.
Table 1.
Participant Characteristics
| Healthy Control | HF | P | |
|---|---|---|---|
| Demographics | |||
| Age, y | 43±3 | 51±5 | 0.21 |
| Gender, male/female | 7/4 | 7/4 | 1.00 |
| Height, m | 1.76±0.02 | 1.73±0.02 | 0.35 |
| Weight, kg | 78.3±3.3 | 87.4±5.6 | 0.18 |
| BMI, kg/m2 | 25.2±1.1 | 29.1±1.8 | 0.10 |
| BSA, m2 | 2.0±0.1 | 2.0±0.1 | 0.33 |
| VO2peak, mL · kg−1 · min−1 | 36.0±3.0 | 17.5±1.4 | <0.001 |
| LVEF, % | 32.1±2.8 | ||
| CHF etiology (ischemic/idiopathic) | 4/7 | ||
| NYHA class | |||
| I | 4 | ||
| II | 7 | ||
| Medications | |||
| ACE inhibitors | 6 (55) | ||
| Angiotensin II receptor blockers | 4 (36) | ||
| Aspirin | 7 (64) | ||
| β-blockers | 10 (91) | ||
| Digitalis | 4 (36) | ||
| Diuretics | 7 (64) | ||
Data are presented as mean±SEM or as n (%). ACE indicates angiotensin-converting enzyme; BMI, body mass index; BSA, body surface area; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Eleven healthy control participants (CTL) were recruited through advertisement in the surrounding community with attempts to match the HF group for age and gender. Control participants had normal cardiac function without evidence of exercise-induced ischemia and were without history of hypertension, lung disease, or coronary artery disease.
All participants gave written informed consent after being provided a description of study requirements. This study was conducted in accordance with the Declaration of Helsinki and approved by the Mayo Clinic Institutional Review Board; all procedures followed institutional and Health Insurance Portability and Accountability Act guidelines.
Overview of Protocol
All participants underwent 2 days of exercise testing procedures separated by a minimum of 48 hours in an environmentally controlled physiology laboratory. The first day of testing consisted of a maximal exercise test to volitional fatigue (peak oxygen consumption [VO2peak]). The second testing day consisted of 3 separate submaximal exercise sessions at 60% of the previously determined maximal oxygen consumption. For both testing days, the participants were asked to avoid strenuous physical activity for 24 hours and refrain from eating or consuming caffeine for 3 hours before arriving at the laboratory for testing. All ventilatory, gas exchange, heart rate (HR), and oxygen saturation data were measured continuously during the maximal exercise test and submaximal exercise sessions. Blood pressure was measured with a manual sphygmomanometer. Before all exercise testing, participants were fitted with a nose clip and standard mouthpiece attached to a PreVent Pneumotach and worn throughout the testing procedure. For maximal exercise testing, all participants were encouraged verbally to continue the exercise protocol to maximal exertion, which was identified as a rating of perceived exertion ≥17 (Borg scale, 6 to 20) or respiratory exchange ratio of ≥ 1.10.
During the second testing day, all participants completed 3 submaximal exercise sessions of recumbent cycling at an intensity of 60% of the previously determined VO2peak. Session 1 was the baseline control trial (BL) consisting of 3 minutes of resting data collection followed by 4 minutes of steady-state submaximal cycle ergometry and 5 minutes of passive recovery data collection. Session 2 was the same rest and exercise protocol followed immediately on cessation of exercise by regional circulatory occlusion (RCO) through inflation of bilateral upper-thigh tourniquets to ≈;20 mm Hg above peak exercise arm systolic blood pressure (SBP) measured during the maximal exercise test. Session 3 was the same rest and exercise protocol followed immediately on cessation of exercise by RCO through inflation of bilateral upper-thigh tourniquets to ≈20 mm Hg above peak exercise arm SBP measured during the maximal exercise test with the addition of CO2 to the inspired air to match end-exercise end-tidal partial pressure of CO2 (RCO+CO2). After the BL session, the remaining 2 sessions were conducted in random order (determined by coin flip) to minimize the confounding influence of cumulative exercise. For all resting values, data were averaged over the entire 3-minute period. During exercise, data were averaged over the last 30 seconds of each minute. During the recovery period, data are reported as the average of 10-second intervals.
Measurement of Ventilation and Gas Exchange
VO2, VCO2, VE, tidal volume, respiratory rate, and partial pressure of end-tidal carbon dioxide (PETCO2) were measured with a metabolic measurement system through a mouth piece and pneumotach while wearing a nose clip for the entire measurement period (MedGraphics CPX/D). Respiratory exchange ratio was calculated as the VCO2divided by the VO2. Manual volume calibration was performed with a 3-L syringe, and gas calibration was performed with manufacturer-recommended gases of known concentration. All calibration procedures were accomplished immediately before each testing protocol.
Statistical Analysis
Statistical analysis and graphic presentation were accomplished with SPSS version 12.0 and GraphPad Prism version 4.0, respectively. A priori, a sample size estimate was conducted, an α level of 0.05, means and SD from the existing literature to calculate an effect size (Cohen d), and an estimate of 25% additional participants to account for dropout and test failure.14,15 These data indicated a sample size need of 11 participants in each group, based on a 2-tailed hypothesis. No dropouts or test failures occurred during this study, and all data were included in the analyses. Matched-pair analysis (2-tailed paired Student t tests) was used for comparisons between the HF and CTL groups. ANOVA with repeated measures was used to determine statistically significant differences within groups between submaximal exercise sessions (exercise session×time). Two-way ANOVA with repeated measures also was also used to determine between-group differences in the percentage of end-exercise ventilation during postexercise recovery (group×time). Bonferroni post hoc analysis was applied when the F ratio was significant. All data are presented as mean±SEM.
Results
Population Characteristics
The clinical characteristics of each study group and the medications in use by the participants at the time of the study are shown in Table 1. There were no significant differences between the groups for age, gender, height, weight, body mass index, or body surface area, although the CTL group tended to be slightly younger. An expected difference between the groups included a lower VO2peak in the HF patient population (P<0.001).
Resting Comparisons
There were no differences in VE (L/min) at rest before the exercise sessions between or within the 2 groups (HF, 13.4±1.0 versus 14.0±1.2 versus 13.0±0.8; CTL, 12.0±0.6 versus 13.0±1.9 versus 13.1±1.2 for BL, RCO, and RCO+CO2, respectively). Similarly, there were no differences between or within the 2 groups for SBP (mm Hg) [HF, 118.8±6.2 versus 118.7±6.1 versus 121.3±5.8; CTL, 118.6±5.6 versus 120.4±5.9 versus 118.2±4.4 for BL, RCO, and RCO+CO2, respectively] or diastolic blood pressure (HF, 76.9±3.9 versus 76.9±4.1 versus 77.3±4.3; CTL, 76.6±3.2 versus 79.6±3.1 versus 79.6±3.3 for BL, RCO, and RCO+CO2, respectively). However, the resting HR (bpm) was significantly increased before all 3 exercise sessions in the HF group compared with the CTL group (P<0.05 for all; HF, 75.8±4.4 versus 76.6±4.4 versus 77.1±4.6; CTL, 64.0±4.0 versus 68.4±3.4 versus 68.1±3.4 for BL, RCO, and RCO+CO2, respectively) with no differences within groups across exercise sessions.
End-Exercise Comparisons
Ventilation for both groups at end exercise for each exercise session is shown in Table 2. Because of the normalization of workload to individual peak work capacities across participants, the HF group had lower exercise VE for all sessions compared with the CTL group. However, the HF group demonstrated a higher VE/VCO2 with a lower PETCO2 for all exercise sessions than did the CTL group, suggesting augmented ventilation for a given level of metabolic work. Within the HF group, tidal volume was lower during the RCO exercise session compared with the BL session (P<0.05), and respiratory rate was increased during the RCO+CO2 session compared with the BL session (P<0.05). End-exercise HR and blood pressure are also shown in Table 2. The submaximal HR at end exercise was significantly lower in the HF group than in the CTL group for all 3 exercise sessions; however, there was no difference between these measures within either group across the exercise sessions. Similarly, the SBP was significantly lower in the HF group than in the CTL group for all 3 exercise sessions, with no differences within groups across exercise sessions. There were no differences in diastolic blood pressure between and within groups.
Table 2.
End-Exercise Ventilation and Gas Exchange Measures in CTL Participants and Patients With HF
| Baseline | RCO | RCO+CO2 | |
|---|---|---|---|
| VO2, mL/min | |||
| CTL | 1734.5±128.2 | 1801.6±148.2 | 1777.5±139.6 |
| HF | 1038.9±78.0* | 1014.1±79.2* | 1059.9±82.2* |
| HR, bpm | |||
| CTL | 116.1±3.1 | 115.4±3.3 | 116.6±3.8 |
| HF | 104.8±4.8* | 104.0±4.5* | 106.1±4.8* |
| SBP, mm Hg | |||
| CTL | 163.6±8.6 | 166.3±8.2 | 168.1±7.3 |
| HF | 140.0±6.6* | 139.5±5.2* | 141.8±6.6* |
| DBP, mm Hg | |||
| CTL | 85.0±3.3 | 84.8±3.0 | 83.3±3.8 |
| HF | 79.3±3.2 | 80.9±3.6 | 81.5±4.1 |
| VE, L/min | |||
| CTL | 48.4±3.6 | 47.2±1.3 | 45.6±3.9 |
| HF | 36.1±2.8* | 33.8±2.8* | 35.4±2.5* |
| VT, mL/min | |||
| CTL | 2032.7±155.8 | 1845.5±124.4 | 1762.7±130.6† |
| HF | 1351.8±131.0* | 1199.1±107.1*† | 1215.5±132.5* |
| RR, breaths/min | |||
| CTL | 24.2±1.4 | 25.6±1.6 | 26.2±1.6 |
| HF | 27.6±1.3 | 28.7±1.6 | 30.5±1.6† |
| PETCO2, mm Hg | |||
| CTL | 41.4±1.6 | 40.6±1.3 | 40.3±1.2 |
| HF | 35.4±1.4* | 35.3±1.4* | 34.7±1.5* |
| VE/VCO2 | |||
| CTL | 27.3±1.2 | 27.7±1.1 | 27.7±1.1 |
| HF | 35.4±1.6* | 36.0±1.8* | 36.6±1.6* |
Data are presented as mean±SEM. DBP indicates diastolic blood pressure; VT, tidal volume; RR, respiratory rate.
P<0.05 compared with CTL.
P<0.05 compared with baseline after Bonferroni correction.
Postexercise Comparisons
Table 3 shows ventilatory data for 2 minutes postexercise normalized to individual end-exercise values for each exercise session. The HF group exhibit increased VE during the RCO+CO2 session compared with the BL session (P<0.05). This increase in VE contributed to a higher VE/VCO2 ratio during the RCO+CO2 session compared with the BL session (P<0.05). In contrast, the CTL group demonstrated slightly lower VE, tidal volume, and respiratory rate during the RCO session than during the BL session but were not statistically different. The HF group demonstrated a greater VE compared with the CTL group during the RCO and RCO+CO2 sessions, which was mediated by a higher tidal volume (P<0.05). The PETCO2 was lower during the RCO session than during the BL session for both groups, whereas it was increased during the RCO+CO2 session compared with the RCO session for both groups (P<0.05 for all). At the end of the postexercise recovery period, the HF group had a significantly increased HR compared with the CTL group during both RCO and RCO+CO2 sessions. In addition, within the HF group, HR was significantly increased during these same 2 sessions compared with the BL session. There were no differences between and within groups for SBP. There was also no difference between groups for diastolic blood pressure; however, diastolic blood pressure was significantly increased during the RCO and RCO+CO2 sessions in both HF and CTL groups compared with the BL session.
Table 3.
Ventilation and Gas Exchange Measures at the Second Minute of Recovery for CTL Participants and Patients With HF
| Baseline | RCO | RCO+CO2 | |
|---|---|---|---|
| HR, bpm | |||
| CTL | 78.8±4.3 | 77.2±3.3 | 75.5±3.3 |
| HF | 79.6±4.8 | 87.0±5.5*† | 85.3±5.0*† |
| SBP, mm Hg | |||
| CTL | 139.3±6.3 | 145.5±3.8 | 145.3±5.1 |
| HF | 132.0±6.3 | 144.7±6.1 | 146.7±7.8 |
| DBP, mm Hg | |||
| CTL | 77.7±2.7 | 88.3±3.8† | 89.1±4.1† |
| HF | 77.8±4.2 | 88.0±3.3† | 88.7±3.6† |
| VE, L/min | |||
| CTL | 21.8±1.7 | 15.2±1.1† | 20.0±2.2‡ |
| HF | 19.6±1.1* | 20.1±1.5* | 23.1±1.5*† |
| VT, mL/min | |||
| CTL | 1221.8±94.2 | 958.2±90.5 | 1095±51.1 |
| HF | 1111.8±136.3* | 947.3±57.4* | 1123.6±85.3* |
| RR, breaths/min | |||
| CTL | 16.2±1.1 | 15.3±1.8 | 18.18±1.7 |
| HF | 18.9±1.3 | 21.0±1.8 | 20.6±1.0 |
| PETCO2, mm Hg | |||
| CTL | 36.4±1.2 | 33.2±1.5† | 38.4±1.2‡ |
| HF | 33.8±1.4* | 30.2±1.0† | 34.8±1.2*‡ |
| VE/VCO2 | |||
| CTL | 32.2±1.5 | 40.8±2.9 | 72.8±7.0†‡ |
| HF | 36.5±1.6* | 43.6±2.9* | 65.8±6.9*†‡ |
| VT/TI | |||
| CTL | 0.85±0.1 | 0.62±0.1 | 0.84±0.1 |
| HF | 0.87±0.08* | 0.92±0.08* | 0.97±0.08* |
Data are presented as mean±SEM. DBP indicates diastolic blood pressure; VT, tidal volume; RR, respiratory rate; TI, inspiratory time. VT/TI represents an index of ventilatory drive.
P<0.05 compared with CTL.
P<0.05 compared with baseline after Bonferroni correction.
P<0.05 compared with RCO after Bonferroni correction.
Absolute and Relative Changes Postexercise
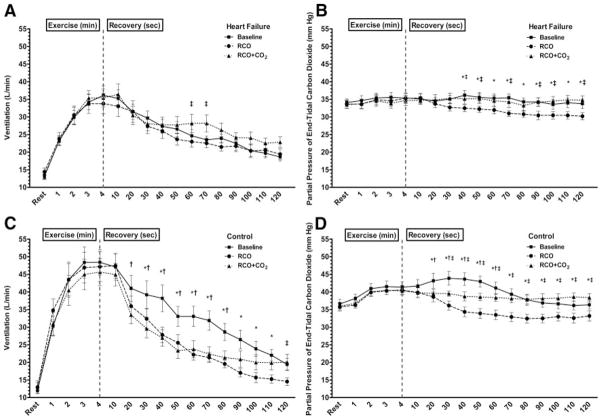
The HF group had an increased VE at the 60- and 70-second postexercise time points during the RCO+CO2 session compared with the RCO session (Figure 1A). Interestingly, there were no other differences in absolute VE among the 3 exercise sessions in the HF group during the postexercise period, despite the significant reduction in PETCO2 during the RCO session (Figure 1B). This finding is in contrast to the CTL group, which demonstrated significantly lower VE between the RCO and BL sessions at nearly all time points during the postexercise measurement period (Figure 1C). Furthermore, in the CTL group, there was a significantly lower VE during the RCO+CO2 session than in the BL session for 10-second segments between 20 and 80 seconds (Figure 1C). The reduced VE during the RCO+CO2 session occurred despite maintenance of PETCO2 at the level of end exercise (Figure 1D).
Figure 1.
VE and PETCO2 for the HF (A and B) and CTL (C and D) groups during 3 exercise conditions. All significance symbols are described after Bonferroni correction. Data are reported as mean±SEM. *Significant difference between the baseline exercise session and the RCO session (P<0.05); †significant difference between the baseline exercise session and the RCO+CO2 session (P<0.05); and ‡significant difference between the RCO and RCO+CO2 session (P<0.05).
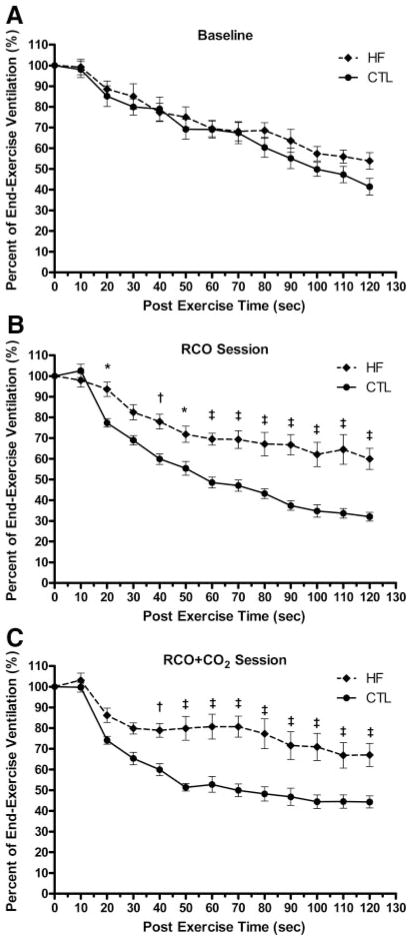
Comparing the VE as a percentage of the end-exercise VE between the HF and CTL groups, there were no differences during the BL exercise session (Figure 2A). During the RCO session, the HF group had significantly increased VE at the 20-second time point and from 40 seconds to the end of the postexercise measurement period (Figure 2B). Similarly, during the RCO+CO2 exercise session, the HF group demonstrated significantly increased VE beginning at 40 seconds and continuing throughout the postexercise measurement period (Figure 2C).
Figure 2.
A, Percent end-exercise ventilation (VE) during the 2-minute postexercise measurement period during the baseline exercise session between the HF and CTL groups. B, Percent end-exercise ventilation during the 2-minute postexercise measurement period during the exercise session with postexercise RCO between the HF and CTL groups. C, Percent end-exercise ventilation during the 2-minute postexercise measurement period during the exercise session with postexercise RCO with the addition of CO2 to the inspirate (RCO+CO2) between the HF and CTL groups. All significance symbols are described after Bonferroni correction. Data are reported as mean±SEM. *P<0.05; †P<0.01; and ‡P<0.001.
Discussion
The purpose of this study was to examine the influence of skeletal muscle metaboreceptors on ventilation independent of central chemoreceptor control. The results of this study suggest that compared with a control population (1) patients with HF have a similar decline in postexercise ventilation under normal conditions; (2) during RCO, patients with HF have an attenuated return of ventilation to baseline, suggesting a significant metaboreceptor contribution to ventilation; and (3) during RCO+CO2, when controlling for changes in central chemoreceptor stimulation through maintenance of end-exercise-PETCO2, patients with HF have a greater attenuation of the postexercise return of ventilation to baseline. These results confirm the presence of the metaboreceptor and highlight the significance of their contribution to ventilation in patients with HF.
Patients with HF often display an exaggerated ventilatory response relative to skeletal muscle metabolic production (ie, VE/VCO2 slope) during physical activity. This overventilation in HF has been linked to poor prognosis,1,16 although the precise mechanisms leading to the augmented ventilation remain controversial.3 Often cited are peripheral neurological mediators acting through heightened neural feedback to the central respiratory centers.4–6,17 Recently, the muscle hypothesis has been proposed to suggest that the metaboreceptor is a primary mediator of altered ventilatory control in HF.18 This hypothesis states that neural traffic from metaboreceptors, types III and IV (myelinated and unmyelinated, respectively) unencapsulated nerve endings originating in the vicinity of skeletal muscle and stimulated by metabolic by-products, is in large part responsible for augmented ventilatory drive in patients with HF.18
Evidence for Metaboreceptor Contribution to Ventilation
The contribution of a muscle metaboreflex to exercise hyperpnea remains controversial. Two primary approaches have been implemented to study the metaboreflex—reduced arterial inflow during exercise and complete circulatory arrest on the cessation of exercise. The advantages and disadvantages of these techniques have been discussed elsewhere.19 It is important to note that the technique used in this study allowed for the determination of the influence of metaboreflex activity, by trapping the metabolic byproducts in the vicinity of the metaboreceptors, independent of the central command or the mechanoreflex, despite its use in the postexercise state. In this light, the use of postexercise circulatory occlusion has been used in both animal and human models. Early studies in healthy animals have suggested a significant involvement of metabolic by-products in both the ventilatory and the blood pressure response to exercise.20–22 For example, McCloskey and Mitchell22 stimulated ventral roots of the central nervous system in anesthetized cats, causing tetanic contraction of the hind limb. As expected, this stimulation resulted in increased ventilation and blood pressure. On cessation of contractions and occlusion of the arterial and venous circulation to and from the exercising muscle bed, ventilation and blood pressure remained increased for the duration of the circulatory arrest, despite reduced input from the mechanoreceptor. Furthermore, after severing the dorsal roots, the ventilatory response to the exercise stimulus was reduced by ≈50%. These results suggest that metabolites produced during muscular work stimulate afferent sensory nerves and contribute to ventilatory control independent of the mechanoreceptor contribution. Interestingly, further study examining the metaboreflex in healthy humans has demonstrated less consistent results, including increased ventilation,23,24 no change in ventilation,25 and a decrease in ventilation during circulatory occlusion26–29; however, much of these differences have been attributed to differences in study populations and methodology.
In this study, the healthy CTL participants had a markedly faster return of ventilation to preexercise levels during circulatory occlusion compared with BL (nonocclusion) postexercise recovery. Although the mechanisms remain unclear, a reduction of circulating metabolic byproducts back to the traditional chemoreceptors during occlusion may result in a lack of central stimulation. The magnitude of the reduction in central chemoreceptor stimulation compared with the modest influence of the metaboreflex in healthy individuals may have overshadowed the effect of the metaboreceptor in this population. However, with the addition of CO2 to the inspirate to clamp end-exercise circulating CO2 levels, our results show that the reduction in ventilation was not abated, suggesting that the metaboreflex plays a less critical role in ventilatory control in healthy humans.
Metaboreceptor Stimulation in Human HF
The relative contribution of the metaboreceptor and its role in mediating the heightened ventilatory response during exercise in patients with HF also remain controversial. However, the results of this study provide clear evidence for a contributory influence of metaboreflex on ventilation in this population. Specifically, our results suggest that despite the slight reduction in ventilation during circulatory occlusion, the return of ventilation toward baseline was significantly attenuated compared with the CTL participants. Moreover, with the addition of CO2 to the inspirate to minimize the effect of reduced venous return to the central chemoreceptors during the circulatory occlusion, the participants with HF demonstrated greater abatement in the return of ventilation to baseline compared with the CTL participants. Figure 2 clearly shows these findings by comparing the ventilatory responses during postexercise recovery normalized to the values immediately before the cessation of exercise. Figure 2B illustrates ventilation during circulatory occlusion, whereas Figure 2C demonstrates ventilation during circulatory occlusion with the addition of CO2 to the inspirate.
Our findings are consistent with earlier studies that have examined the metaboreflex contribution to ventilation in HF by using isolated muscle beds. Specifically, we found a greater ventilatory response with the isolated forearm model of exercise in participants with HF than in matched CTL participants.30 Using the isolated lower-limb dorsiflexion model, Grieve et al12 found similar results, with the patients with HF exhibiting a greater ventilatory response to circulatory occlusion than the CTL participants. However, Francis et al11 did not confirm these results with an upright cycle ergometry model in patients with HF. They found no difference in ventilation during the postexercise circulatory occlusion in patients with HF compared with control exercise; however, they used a technique in which the occlusion cuffs were placed on the distal portion of the thighs and, thus, may have failed to engage the entirety of the exercising muscle. This is confirmed by the levels of end-tidal CO2 being similar between the study conditions, suggesting that the metabolic by-products were not trapped in the vicinity of the metaboreceptor but rather circulated back to the central chemoreceptors and were dissipated as would normally occur. Subsequent work in this area has demonstrated the reproducibility of the postexercise RCO technique both in the isolated muscle group and in locomotor exercise in the HF population and further delineated the contribution of specific metabolites in stimulating the metaboreceptor.13,31,32 As a whole, our results would strongly suggest a clear role for the metaboreceptor in the heightened ventilatory response in patients with HF.
Future Directions
This study examined the influence of metaboreceptor stimulation on ventilation in patients with HF. As such, we did not examine the influence of these receptors on other systems such as the cardiac or vascular systems. Thus, the results of this study are specific to ventilatory control in patients with HF, and future research may be warranted to examine the interaction or similarity between cardiovascular and ventilatory control mechanisms (eg, baroreceptor-metaboreceptor interactions). Despite the modestly reduced ejection fraction in this cohort, the patients in this study were of New York Heart Association classes I to II and thus may slightly underrepresent the impact of the influence of metaboreceptor stimulation on ventilation compared with that which would be observed in sicker patients. Therefore, further research examining the influence of disease severity on metaboreceptor activity is important. In addition, the work intensity chosen was relatively modest in an effort to mimic intensities encountered during activities of daily living. As such, this level of intensity also may tend to underestimate the contribution of this receptor compared with more intense levels of exercise. Moreover, a potential limitation of this study is the relatively small sample size with a slight, but nonsignificant difference in age between the groups; thus, future studies with larger samples with groups more closely matched for age are encouraged to ensure that the underlying assumptions of the analyses hold true. Finally, although the postexercise circulatory occlusion technique allows for the specific determination of the metaboreceptor influence on ventilatory control by excluding the potentially confounding influence of central command and mechanoreceptor effect on the outcome variables, this technique precludes the ability to determine which specific metabolic by-products are responsible for the stimulation of the metaboreceptor. Therefore, there remains a paucity of literature in this area, and it remains important to delineate the specific contributors to metaboreceptor stimulation in an effort to provide more structured targets for individualized therapeutic intervention.
Summary
The results of this study suggest that metaboreceptors located in the interstitial tissue of locomotor muscles contribute to augmented ventilation in the HF population. This resultant increased ventilation may ultimately contribute to the increased work and cost of breathing and further accentuate perceptions of dyspnea and fatigue. Future research in this area is critical because the metaboreflex may represent a novel therapeutic target for individualized patient treatment strategies.
CLINICAL PERSPECTIVE
Patients with heart failure often are limited in their activities of daily living by symptoms of dyspnea and fatigue. The cause of these symptoms has been studied extensively and yet the underlying mechanisms remain unclear. Recently, attention has been placed on metabolically sensitive neurological receptors originating in skeletal muscles as potential contributors to altered hemodynamic status and ventilatory control in this population. The results of this study suggest that these metaboreceptors contribute to augmented ventilation in patients with heart failure. The resultant hyperventilation may subsequently contribute to an increased work and oxygen cost of breathing and may further accentuate perceptions of dyspnea and fatigue and perpetuate the downward spiral of disease progression. Thus, continued research in this area is critical because the metaboreceptor may represent a novel therapeutic target for developing treatment strategies.
Acknowledgments
We thank the participants who took part in this research. We also thank Kathy O’Malley, Minelle Hulsebus, and Andy Miller for their help with study recruitment and technical assistance.
Source of Funding
This work was supported by American Heart Association grant 0725715Z (Dr Olson), National Center for Research Resources grant 1KL2RR024151 (Dr Olson), National Institutes of Health grants HL71478 (Dr Johnson) and HL46493 (Dr Joyner), and the Frank R. and Shari Caywood Professorship (Dr Joyner).
Footnotes
Disclosures
None.
References
- 1.Guazzi M, Reina G, Tumminello G, Guazzi MD. Exercise ventilation inefficiency and cardiovascular mortality in heart failure: the critical independent prognostic value of the arterial CO2 partial pressure. Eur Heart J. 2005;26:472–480. doi: 10.1093/eurheartj/ehi060. [DOI] [PubMed] [Google Scholar]
- 2.Kleber FX, Vietzke G, Wernecke KD, Bauer U, Opitz C, Wensel R, Sperfeld A, Glaser S. Impairment of ventilatory efficiency in heart failure: prognostic impact. Circulation. 2000;101:2803–2809. doi: 10.1161/01.cir.101.24.2803. [DOI] [PubMed] [Google Scholar]
- 3.Johnson RL. Gas exchange efficiency in congestive heart failure. Circulation. 2000;101:2774–2776. doi: 10.1161/01.cir.101.24.2774. [DOI] [PubMed] [Google Scholar]
- 4.Roberts AM, Bhattacharya J, Schultz HD, Coleridge HM, Coleridge JC. Stimulation of pulmonary vagal afferent C-fibers by lung edema in dogs. Circ Res. 1986;58:512–522. doi: 10.1161/01.res.58.4.512. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd TC., Jr Effect of increased left atrial pressure on breathing frequency in anesthetized dog. J Appl Physiol. 1990;69:1973–1980. doi: 10.1152/jappl.1990.69.6.1973. [DOI] [PubMed] [Google Scholar]
- 6.Dempsey J. Crossing the apnoeic threshold: causes and consequences. Exp Physiol. 2004;90:13–24. doi: 10.1113/expphysiol.2004.028985. [DOI] [PubMed] [Google Scholar]
- 7.Olson LJ, Arruda-Olson AM, Somers VK, Scott CG, Johnson BD. Exercise oscillatory ventilation: instability of breathing control associated with advanced heart failure. Chest. 2008;133:474–481. doi: 10.1378/chest.07-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olson TP, Frantz RP, Snyder EM, O’Malley KA, Beck KC, Johnson BD. Effects of acute changes in pulmonary wedge pressure on periodic breathing at rest in heart failure patients. Am Heart J. 2007;153:104.e101–104.e107. doi: 10.1016/j.ahj.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piepoli M. Central role of peripheral mechanisms in exercise intolerance in chronic heart failure: the muscle hypothesis. Cardiologia. 1998;43:909–917. [PubMed] [Google Scholar]
- 10.Schmidt H, Francis DP, Rauchhaus KW, Piepoli MF. Chemo- and ergoreflexes in health, disease and ageing. Int J Cardiol. 2005;98:369–378. doi: 10.1016/j.ijcard.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Francis N, Cohen-Solal A, Logeart D. Peripheral muscle ergoreceptors and ventilatory response during exercise recovery in heart failure. Am J Physiol. 1999;45:H913–H917. doi: 10.1152/ajpheart.1999.276.3.H913. [DOI] [PubMed] [Google Scholar]
- 12.Grieve DA, Clark AL, McCann GP, Hillis WS. The ergoreflex in patients with chronic stable heart failure. Int J Cardiol. 1999;68:157–164. doi: 10.1016/s0167-5273(98)00349-0. [DOI] [PubMed] [Google Scholar]
- 13.Scott AC, Francis DP, Coats AJS, Piepoli MF. Reproducibility of the measurement of the muscle ergoreflex activity in chronic heart failure. Eur J Heart Fail. 2003;5:453–461. doi: 10.1016/s1388-9842(03)00012-6. [DOI] [PubMed] [Google Scholar]
- 14.Scott AC, Davies LC, Coats AJS, Piepole M. Relationship of skeletal muscle metaboreceptors in the upper and lower limbs with the respiratory control in patients with heart failure. Clin Sci. 2002;102:23–30. [PubMed] [Google Scholar]
- 15.Scott AC, Francis DP, Davies LC, Ponikowski P, Coats AJS, Piepoli MF. Contribution of skeletal muscle ‘ergoreceptors’ in the human leg to respiratory control in chronic heart failure. J Physiol. 2000;529:863–870. doi: 10.1111/j.1469-7793.2000.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Francis DP, Shamim W, Davies LC, Piepole M, Ponikowski P, Anker SD, Coats AJ. Cardiopulmonary exercise testing for prognosis in chronic heart failure: continuous and independent prognostic value from VE/VCO2 slope and peak VO2. Eur Heart J. 2000;21:154–161. doi: 10.1053/euhj.1999.1863. [DOI] [PubMed] [Google Scholar]
- 17.Michel CC, Kao FF. Use of a cross-circulation technique in studying respiratory responses to CO2. J Appl Physiol. 1964;19:1070–1074. doi: 10.1152/jappl.1964.19.6.1070. [DOI] [PubMed] [Google Scholar]
- 18.Piepoli M, Ponikowski P, Clark AL, Banasiak W, Alessandro C, Coats AJS. A neural link to explain the “muscle hypothesis” of exercise intolerance in chronic heart failure. Am Heart J. 1999;137:1050–1060. doi: 10.1016/s0002-8703(99)70361-3. [DOI] [PubMed] [Google Scholar]
- 19.Piepoli MF, Dimopoulos K, Concu A, Crisafulli A. Cardiovascular and ventilatory control during exercise in chronic heart failure: role of muscle reflexes. Int J Cardiol. 2008;130:3–10. doi: 10.1016/j.ijcard.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 20.Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol. 1971;215:789–804. doi: 10.1113/jphysiol.1971.sp009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kao FF, Ray LH. Respiratory and circulatory responses of anaesthetized dogs to induced muscular work. Am J Physiol. 1954;179:249–254. doi: 10.1152/ajplegacy.1954.179.2.249. [DOI] [PubMed] [Google Scholar]
- 22.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol. 1972;224:173–187. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sargeant AJ, Rouleau MY, Sutton JR, Jones NL. Ventilation in exercise studied with circulatory occlusion. J Appl Physiol. 1981;50:718–723. doi: 10.1152/jappl.1981.50.4.718. [DOI] [PubMed] [Google Scholar]
- 24.Comroe JH, Schmidt CF. Reflexes from the limbs as a factor in the hyperpnea of muscular exercise. Am J Physiol. 1943;138:536–547. [Google Scholar]
- 25.Asmussen EA, Christiansen H, Nielsen M. Humoral or nervous control of respiration during muscular work? Acta Physiol Scand. 1943;6:160–167. [Google Scholar]
- 26.Haouzi P, Huszczuk A, Porszasz J, Chalon B, Wasserman K, Whipp BJ. Femoral vascular occlusion and ventilation during recovery from heavy exercise. Respir Physiol. 1993;94:137–150. doi: 10.1016/0034-5687(93)90043-a. [DOI] [PubMed] [Google Scholar]
- 27.Innes JA, Solarte AI, Huszcuk A, Yeh E, Whipp BJ, Wasserman K. Respiration during recovery from exercise: effects of trapping and release of femoral blood flow. J Appl Physiol. 1989;67:2608–2613. doi: 10.1152/jappl.1989.67.6.2608. [DOI] [PubMed] [Google Scholar]
- 28.Barman JM, Moreira MF, Consolazio F. The effective stimulus for increased pulmonary ventilation during muscular exertion. J Clin Invest. 1943;22:53–56. doi: 10.1172/JCI101368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freund PR, Hobbs SF, Rowell LB. Cardiovascular responses to muscle ischemia in man—dependency on muscle mass. J Appl Physiol. 1978;45:762–767. doi: 10.1152/jappl.1978.45.5.762. [DOI] [PubMed] [Google Scholar]
- 30.Piepoli M, Clark AL, Coats AJ. Muscle metaboreceptors in hemodynamic, autonomic, and ventilatory responses to exercise in men. Am J Physiol. 1995;269:H1428–H1436. doi: 10.1152/ajpheart.1995.269.4.H1428. [DOI] [PubMed] [Google Scholar]
- 31.Scott AC, Wensel R, Davos CH, Georgiandou P, Kemp M, Hooper J, Coats AJS, Piepoli MF. Skeletal muscle reflex in heart failure patients: role of hydrogen. Circulation. 2003;107:300–306. doi: 10.1161/01.cir.0000042704.37387.29. [DOI] [PubMed] [Google Scholar]
- 32.Scott AC, Wensel R, Davos CH, Kemp M, Kaczmarek A, Hooper J, Coats AJS, Piepole M. Chemical mediators of the muscle ergoreflex in chronic heart failure: a putative role for prostaglandins in reflex ventilatory control. Circulation. 2002;106:214–220. doi: 10.1161/01.cir.0000021603.36744.5e. [DOI] [PubMed] [Google Scholar]