Abstract
The organic nitrite, amyl of nitrite, was initially used as a therapeutic agent in the treatment of angina pectoris in 1867, but was replaced over a decade later by the organic nitrate, nitroglycerin (NTG), due to the ease of administration and longer duration of action. The administration of organic nitrate esters, such as NTG, continues to be used in the treatment of angina pectoris and heart failure during the birth of modern pharmacology. The clinical effectiveness is due to vasodilator activity in large veins and arteries through an as yet unidentified method of delivering nitric oxide (NO), or a NO-like compound to vascular smooth muscle cells. The major drawback with NTG administration is the rapid development of tolerance; and with amyl of nitrite, the duration and route of administration. Although amyl of nitrite are no longer used in the treatments of hypertension or ischemic heart disease, the nitrite anion has recently been discovered to possess novel pharmacologic actions such as modulating hypoxic vasodilation and providing cytoprotection in ischemia-reperfusion injury. Although the actions of these two similar chemical classes (nitrites and organic nitrates) have often been considered to be alike, we still do not understand their mechanism of action. However, the recent discovery that the nitrite anion, derived from either sodium nitrite or an intermediate NTG form, may act as a storage form for NO and provides support for investigating the use of these agents in the treatment of ischemic cardiovascular states. We review what is presently known about the use of nitrites and nitrates, the potential uses of these agents, and their mechanisms of action.
Keywords: Nitrites, Nitrates, Nitroglycerin, Nitric oxide, Ischemia-Reperfusion Injury, Hypoxia, Xanthine oxidoreductase, Mitochondrial aldehyde dehydrogenase
Cardiovascular diseases (CVD) are a group of disorders including congestive heart failure (CHF) and ischemic heart disease that are becoming the leading cause of morbidity and mortality in the world [1, 2]. Initial treatment of both disorders began during the latter 1800s with organic nitrates or with organic nitrites following animal and human experiments of Brunton and Murrell [3, 4]. Ischemic myocardium results when the supply of oxygenated blood through the coronary arteries becomes in adequate to meet the demands of cardiacmuscle, leading to myocardial anoxia [5]. CVD can result from a quantitive or functional nitric oxide (NO) deficiency that can limit NO-dependent signal transduction pathways to the detriment of normal cellular function, and as such, delivery of low concentrations of exogenous NO would be an attractive therapeutic option [6]. Current indications for nitrate therapy include chronic, stable angina pectoris, unstable angina pectoris, complications of acute myocardial infarction, and ventricular unloading in acute and chronic CHF [7]. Nitrates are also used in the perioperative period by anesthesiologists and intensivists to control systemic and pulmonary arterial pressure [7–10]. Due to similar historical introductions of the organic nitrate ester, nitroglycerin (NTG) and the organic nitrite, nitrite of amyl, both with comparable therapeutic actions, we review these therapeutic introductions and the novel mechanisms of action.
History
Sir Thomas Lauder Brunton first used amyl nitrite in the treatment of angina pectoris in 1867 [3]. Asa medical student, Brunton had became aware of prior clinical findings of Benjamin Ward Richardson that inhaled amyl nitrite rapidly increased the action of the heart [11], and also the unpublished observations of Arthur Gamgee demonstrating that amyl nitrite greatly lessened ‘arterial tension’ in both animals and man [3]. During the same period in which Brunton used amyl nitrite, another British physician, William Murrell, began using the organic nitrate, GTN, in the treatment of angina pectoris [4]. With NTG therapy, patients would obtain relief from angina with some patients also reporting that their angina could be aborted by taking the drug at the onset of symptoms [4]. Murrell also worked with Fancourt Barnes and compared the effects of amyl nitrite with NTG, where it was observed that the actions of these drugs differed in the time of onset and duration. It was concluded that NTG would be more clinically useful than amyl nitrite [12]. Murrell’s use of NTG, the world’s first synthesized drug, in the treatment of angina pectoris is still in use 140 years later [12, 13].
In the 1920s, Richard Bodo used the Starling’s heart–lung preparation to determine the effects of two nitrite preparations on coronary flow [14] as earlier work in myocardial studies demonstrated that nitrite preparations produced vasorelaxation [15–17]. Titrated doses of sodium nitrite would produce dose-dependent increases in total coronary flow without a return to control levels between doses [14]. In contrast, amyl nitrite would produce greater responses in coronary flow, but with complete recovery between doses [14], findings that confirmed the earlier observations of Cow [17]. These findings suggested that although amyl nitrite could produce a greater response in flow, a more prolonged effect could be obtained with the inorganic nitrite, sodium nitrite.
The use of nitrates, especially NTG, suggested that coronary vasodilation [18–20] might be the mechanism to improve the symptoms and electrocardiographic (EKG) changes of angina pectoris [21, 22]. However, contemporary studies reported a high incidence of ‘placebo effect’ when assessing symptom relief [23], or that NTG -induced vasodilation could not be demonstrated [24, 25], nor significant changes in measured coronary sinus oxygen content [24, 26, 27].
By the 1930s, nitrites and nitrate esters were established treatments for angina and hypertension [28–31]. Weiss and Ellis performed detailed clinical studies of the cardiovascular effects of sodium nitrite in 1933 in normal individuals, in patients with hypertension, and in patients with renal disorders [29]. In these studies, the degree of systolic pressure decrease following administration of sodium nitrite was dependent upon the level of arterial tone, but administration of sodium nitrite did not improve urinary output or creatinine clearance in patients with renal disorders. Moreover, Weiss suggested that the use of sodium nitrite in the treatment of hypertension would not be efficacious and may be dangerous [29]. Although nitrites generally were beneficial in patients with angina, in some patients the use of nitrites was not beneficial and caused untoward effects [3, 23, 32]. Contro and colleagues observed a paradoxical action following inhalation of amyl nitrite in patients with coronary artery disease, in that EKG changes resembling myocardial ischemia would occur after administration of amyl nitrite due to a sudden drop in blood pressure followed by coronary insufficiency [5]. Finally, the authors suggested that the nitrite could be used as a diagnostic test in patients with borderline or questionable history of coronary artery disease [5]. Interestingly, NTG was also reported to induce EKG changes following administration of this organic nitrate [33]. However, subsequent studies by Kerber and Harrison were unable to reproduce the reported EKG changes with NTG, but were able to confirm with coronary angiography that EKG changes with amyl nitrite were due to myocardial ischemia [34]. Amyl nitrite and NTG have been evaluated in patients with essential hypertension [35]. NTG was able to reduce systolic and mean arterial pressures and cardiac output, but had little effect on diastolic pressure and total peripheral resistance [35]. Amyl nitrite decreased systolic, diastolic, and mean arterial pressures and peripheral vascular resistance, and increased heart rate and cardiac output [35]. By the 1970s, nitrite preparations were infrequently used in the medical management of angina pectoris or hypertension [36]. Moreover, amyl nitrite has been found to have abuse potential resulting in significant methemoglobinemia, blindness, and death [37–40]. Although nitrite is no longer used for the treatment of ischemic heart disease or systemic hypertension [41], it still has benefit in the treatment of cyanide toxicity [42]. All clinically active nitrites and nitrates are esters of nitric or nitrous acid with the exception of sodium nitrite. The nitrites/nitrates are classified as agents that directly relax vascular smooth muscle, but can also relax other smooth muscles such as bronchial, ureteral, and uterine smooth muscle [43–46]. These similar historical descriptions of NTG, amyl nitrite, and sodium nitrite, in their ability to produce direct vasorelaxation, were subsequently found to induce direct vasorelaxation via vascular NO formation [47].
Vascular NO formation
NO formation by endothelial NO synthase (NOS) plays an important role in the regulation of vasomotor tone in the pulmonary and systemic vascular beds (Fig. 1) [48–51]. The importance of NO in the regulation of vasomotor tone has been demonstrated in experimental animals and in human subjects by the use of NOS inhibitors [48, 50–52]. NO is also produced by NOS-independent mechanisms [53–55]. Nitrite can be produced by oxidation of NO under physiologic conditions (Fig. 1) [56–58]. However, NO generation by NOS may be rapidly depleted in ischemic conditions, since NOS is dependent upon the availability of oxygen [59, 60]. Once released from the endothelium, NO diffuses into vascular smooth muscle cells inducing vasodilatation and into the blood stream where it inhibits platelet aggregation and inflammation (Fig. 1) [61–63]. NO is an important paracrine molecule for the maintenance of a healthy endothelium [64, 65]. Loss of endogenous NO activity has a number of detrimental actions, most notably, vasoconstriction, increased activity and adherence of platelets, and accumulation of inflammatory cells at sites of endothelial damage. When the endothelium deteriorates or becomes dysfunctional, blood flow is disturbed and vessels become obstructed, leading to ischemia of downstream organs including the heart [66–71]. Endothelial damage is associated with most forms of CVD, including hypertension, coronary artery disease, CHF, peripheral artery disease, diabetes, chronic renal failure, and pulmonary hypertension [72–76]. Endothelial function is altered in the presence of cardiovascular risk factors, suggesting that endothelial dysfunction is likely to be an important first step in CVD [77–86]. Endothelial dysfunction arises from down-regulation of endothelial NOS expression and activity, and uncoupling of NOS generating free radicals [87]. The delivery of exogenous NO initially would be an attractive therapeutic option if this intervention could slow down the progression of endothelial dysfunction and reduce the risk of CD [6, 87, 88], as the administration of organic nitrates and nitrites have been shown to have beneficial effects in CVD [3, 4, 89–94]. The pharmacologic actions of these two classes (nitrites and organic nitrates) have been considered to be similar [95], however recent interest in the chemical classes of agents that liberate NO have been shown to have important differences [96, 97]. Historical work by Krantz and colleagues demonstrated that the rapid-acting nitrites and organic nitrates, amyl nitrite, NTG, and isobutylglycollate nitrate, could inhibit the ATPase activity in the rabbit aorta, whereas sodium nitrite had little or no effect [98]. It was reported that oxygen uptake was inhibited by sodium nitrite and NTG, whereas amyl nitrite, erythritol tetranitrate, and octyl nitrite did not significantly interfere with oxygen uptake in arterial tissue when these agents were administered in therapeutic levels [99]. These early studies suggested that these two classes of nitrosovasodilators have different mechanisms of action. Additional studies have demonstrated different enzymatic requirements for NO generation by organic nitrates when compared to nitrites [100]. Moreover, studies have shown that a reduced endothelial NO production following administration of organic nitrates, such as NTG, may be a mechanism of nitrate tolerance [101–103], but that amyl nitrite has different vascular actions and hemodynamic effects and produces less tolerance when compared to NTG [97]. These findings were subsequently confirmed in other studies [34], and although NTG was reported to exacerbate EKG changes during exercise [33], Kerber and Harrison were unable to reproduce this effect with NTG [34]. These findings suggest that the different results observed with NTG and amyl nitrite are probably due to the findings that NTG has a direct action on veins to reduce preload, whereas amyl nitrite reduces after load that decreases coronary flow [34]. Although the literature during this time suggests that the cardio-circulatory actions of the nitrites and organic nitrates were similar, important differences in the route of administration could have influenced these circulatory actions. Rapid introduction of amyl nitrite by inhalation or of NTG by intravenous administration could result in profound arteriolar dilation and a marked fall in systemic arterial pressure. The dilation of systemic arterial and venous beds leading to baroreceptor stimulation results in marked reflex increases in heart rate [104, 105]. However, slower introduction of these agents using sublingual NTG or oral sodium nitrite administration would result in smaller decreases in arterial pressure and reductions in central venous pressure producing peripheral venous pooling, a reduction in stroke volume and in cardiac output [105–107]. Although the fundamental action of these two classes is generalized as direct vasodilation, relief of angina pectoris with sublingual NTG appears to be more related to direct venodilation, whereas inhalation of amyl nitrite abates angina from direct and marked arteriolar dilation, and that reduction of myocardial oxygen consumption, rather than augmentation of diminished coronary blood flow, is the common mechanism by which anti-anginal measures can relieve ischemic pain [108]. To further confirm the beneficial venodilatory effects of NTG, Ganz and Marcusused pacing-induced tachycardia to induce angina and observed that only administration of intravenous, but not intracoronary, NTG was effective in relieving ischemia, suggesting that the direct action of NTG on the coronary vascular bed has little role in the anti-anginal effect of the drug but requires an action on the systemic circulation [109]. Although nitrite is no longer used in the treatment of ischemic heart disease or in systemic hypertension [36, 41], it still has benefit in the treatment of cyanide toxicity [42], along with recently discovered actions in mediating or modulating hypoxic vasodilation [54, 110, 111], and the ability to induce cytoprotection in ischemia-reperfusion injury in a number of organ systems [112–114].
Figure 1.
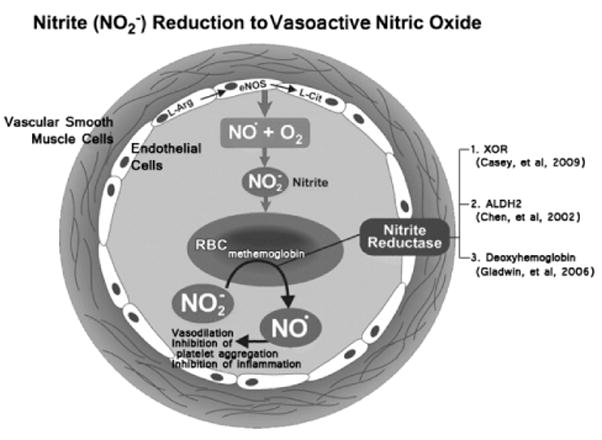
Endothelial nitric oxide synthase (eNOS) generates nitric oxide (NO•) and L-citrulline from L-arginine. Circulating NO• in the presence of oxygen (O2) can be oxidized to form nitrite (NO2−). Circulating NO2− can be reduced by deoxyhemoglobin to NO• and methemoglobin under ischemic conditions. Modified from Lefer, D. (2006). Nitrite therapy for protection against ischemia-reperfusion injury.
Mechanisms of Action for the Nitrates and Nitrites
NTG and amyl nitrite have been used in the treatment of angina pectoris and HF for 140 years [3, 4], and it is generally believed that the therapeutic effect of these drugs involves the release of NO from nitrite (Fig. 1), the activation of guanylyl cyclase, and relaxation of blood vessels [4, 87, 115–117]. Studies have shown that bioactivation of NTG requires thiols or sulfhydryl-containing compounds and that NO or NO-like compounds are believed to be the biologically active species [47, 118–120, 164]. Interactions with NTG and sulfhydryl-containing receptors are necessary for vascular smooth muscle relaxation and repeated administration of NTG produces sulfhydryl depletion and the development of tolerance [101, 120, 121]. Subsequent studies have demonstrated the release of NO following the decomposition of an intermediate S-nitrosothiol [47]. Additional studies suggest that an enzymatic mechanism may be responsible for the bioactivation of NTG. However, these enzymatic systems could not catalyze the selective formation of 1,2-glyceryl dinitrate and nitrite from NTG, and moreover, no association was observed between the development of enzymatic tolerance and tolerance to NTG [122–128]. However, the discovery that mitochondrial aldehyde dehydrogenase (mtALDH) generates 1,2-glyceryl dinitrate and nitrite from NTG, that this reaction requires a reducing thiol cofactor, and that the activity of this enzyme is reduced in NTG tolerance, suggest that this pathway is responsible for NTG bioactivation in vascular smooth muscle [129, 130]. However, one difficulty with these studies has been an inability to detect NO as a byproduct of NTG metabolism [131]. Moreover, the generation of NO was observed when the concentrations of NTG exceeded therapeutic values [103, 132–136]. A potential solution has been proposed that once NTG is bioactivated within the mitochondria, nitrite or an additional action of mtALDH generates the vasodilatory NO bioactivity [13]. One suggested mechanism for this vasodilatory activity is that S-nitrosoglutathione is formed by the reaction of reduced glutathione and nitrite [137, 138]. This molecule subsequently undergoes biotransformation to S-nitrosocysteine [130, 139] that can release NO [140]. However, excessive amounts of NTG or S-nitrosothiols can dysregulate protein S-nitrosylation and contribute to cellular dysfunction and disease [141]. Chronic NTG administration has been shown to result in acetylcholine-induced coronary vasoconstriction rather than relaxation [142, 143] and induce endothelial dysfunction [144]. An early event in the pathophysiology of atherosclerosis are the impairment of endothelial function or endothelial dysfunction that develops before structural changes of intimal hyperplasia or lipid deposition occur [145]. Moreover, reduced bioavailability of NO occurs through reduced endothelial NOS expression and activity [145], which can be evidenced by abnormal vascular responses to an acetylcholine challenge [146, 147]. Therefore, NO deficiency is linked to CVD processes and provides justification for the use of effective NO replacement therapy.
Nitrates
The most commonly used agents at this time include isosorbide dinitrate, isosorbide-5-mononitrate, and NTG which are effective in reducing ventricular preload by increasing peripheral venous capacitance [148–151]. These drugs can also decrease pulmonary and systemic vascular resistances, but require higher doses than those needed for the increase in venous capacitance [152–157]. These agents can reduce ventricular filling pressure, wall stress and myocardial oxygen consumption [158], and may also improve systolic and diastolic ventricular function by improving coronary flow in patients with ischemic cardiomyopathy. However, there is, as yet, no convincing evidence that organic nitrates improve mortality in patients with acute myocardial infarction [159, 160]. The limitations of this class of agents are well known and potentially include adverse hemodynamic effects, drug tolerance, lack of selectivity, and limited bioavailability [87].
Studies in the literature provide evidence that vasorelaxant responses to NTG are mediated by the formation of NO or a closely related molecule [47, 161–163]. However, the mechanism of this vasorelaxant response to NTG is uncertain. Although studies in the literature indicate that NO contributes to the activation of guanylate cyclase and vascular smooth muscle relaxation [164, 165], other studies suggest that vasorelaxant responses to NTG may be independent of NO release and cyclic guanosine monophosphate (cGMP) formation [129].
Role of Mitochondrial Aldehyde Dehydrogenase
It has been reported that ALDH2 catalyzes the formation of glyceryl dinitrate and nitrite from NTG, leading to the production of cGMP and vasorelaxation [129, 164, 166–171]. Moreover, it has been suggested that the nitrite formed from NTG metabolism may be further metabolized to NO and/or converted to a S-nitrosothiol [172]. Although it has been reported that ALDH2 plays an important role in the bioactivation of NTG, the role of this enzyme in the reduction of nitrite to vasoactive NO has only recently been determined. We have shown in rats treated with L-NAME to block NOS, that decreases in systemic arterial pressure in response to intravenous injections of sodium nitrite were attenuated by cyanamide, an inhibitor of ALDH2 [173]. Moreover, these data were consistent with the results of Ohtake and colleagues [172]. The results of these studies provide support for the hypothesis that ALDH2 plays an important role in the bioactivation of NTG [129, 166–171]. In summary, ALDH2 plays an important role in the bioactivation of NTG and nitrite.
Nitrites
Recent studies provide support for the concept that the nitrite anion represents a storage form of NO that can have therapeutic effects [174–176]. Although nitrite was believed to be an inactive end-product of NO metabolism that reflects endothelial function, the vasodilator actions of nitrite were recognized as early as 1867 by Brunton who used amyl nitrite to treat angina [3]. Cardiovascular responses to sodium nitrite have been investigated in both the last century and in this century [29, 106, 110, 168,176, 177]. The ability of sodium nitrite to relax isolated arteries was first reported in 1953 and the effects of nitrite on guanylyl cyclase activity and cGMP levels were documented by Ignarro et al and others [47, 118, 164, 178–181]. The mechanism by which nitrite is reduced to NO is uncertain but the reduction of inorganic nitrite to NO can be mediated by enzymatic mechanisms and by nonenzymatic disproportionation at low pH (Fig. 1) [110, 132, 173]. Moreover, it has also been reported that vasodilator responses to sodium nitrite can be attenuated by allopurinol, suggesting that xanthine oxidoreductase (XOR) can play a role in bioactivation of nitrite (Fig. 1) [117, 172, 173].
Role of Xanthine Oxidoreductase
The mechanism by which NTG activates guanylyl cyclase is uncertain [167–169]. The nitrite that is formed from the reaction of NTG with ALDH2 can be reduced to vasoactive NO or a NO-like compound that activates guanylyl cyclase [167, 168]. XOR is a ubiquitous enzyme that can reduce nitrite to NO, and under severe conditions in the rat heart, large amounts of NO can be generated (Fig. 1) [53, 182, 183]. XOR is widely distributed in mammalian tissues and is a key enzyme in purine metabolism. Although XOR can reduce nitrite to NO, and that nitrite reduction is greatly enhanced under hypoxic and ischemic conditions in the rat [184], the effects of XOR inhibitors on vasodilator responses to sodium nitrite are uncertain, and studies in the literature show no inhibitory effect [167, 168]. XOR has been shown to reduce nitrite to NO and it has been reported that allopurinol inhibits responses to sodium nitrite in the rat (Fig. 1) [54, 185–187]. We have shown that systemic vascular responses to the nitrite anion were inhibited by allopurinol in a dose that did not alter responses to sodium nitroprusside and these findings support the hypothesis that XOR contributes to the activation of nitrite in the rat and that the nitrite anion represents a storage form of NO (Fig. 1) [184]. Moreover, in a recent study presented at the Second International Meeting on the Role of Nitrite in Physiology, Pathophysiology and Therapeutics, National Institutes of Health, Bethesda, Maryland, allopurinol was shown to attenuate decreases in systemic arterial pressure in response to systemic administration of sodium nitrite in L-NAME-treated rats [172] and are consistent with the hypothesis that XOR plays an important role in mediating vasodilator responses to sodium nitrite [47, 119, 188]. Furthermore, the inhibitory effects of cyanamide and of allopurinol on responses to sodium nitrite were shown to not be additive, suggesting that XOR and ALDH2 act in a parallel manner to reduce nitrite to vasoactive NO in the systemic vascular bed of the rat. These data are in agreement with previous studies and provide support for the hypothesis that ALDH2 plays a role in the bioactivation of nitrite (Fig. 1) [167–169].
Role of Xanthine Oxidoreductase and Mitochondrial Aldehyde Dehydrogenase
The hypothesis that XOR contributes to the bioactivation of NTG by reducing nitrite derived from the activity of ALDH2 was recently examined in studies with allopurinol and cyanamide. We observed that responses to NTG were not attenuated by allopurinol in doses that significantly decrease responses to sodium nitrite [173]. The observation that responses to NTG are not altered at a time when responses to sodium nitrite were attenuated suggests that the reduction of the nitrite anion to vasoactive NO did not contribute to the vasodilator responses to NTG in the pulmonary and systemic vascular beds of the intact chest rat. Moreover, when comparison of vasodilator responses to NTG and sodium nitrite was plotted, NTG was ~1000 fold more potent than sodium nitrite in decreasing pulmonary and systemic arterial pressures in the rat [173]. These findings suggested that the conversion of nitrite to vasoactive NO, even if all three-nitrate groups of NTG were reduced to NO, would not be able to account for the vasodilator activity of NTG in the intact chest rat [173]. These findings are consistent with the results of studies showing that vasorelaxant responses to NTG can occur independently of its NO-releasing properties in isolated vessels [189]. These observations are consistent with this hypothesis, and that the identity of the chemical species formed from NTG that activates guanylyl cyclase in the intact vascular bed continue to be uncertain [47, 131, 164].
Conclusion
In this review we discussed the history and actions of organic nitrates and organic/inorganic nitrites in clinical medicine for the treatment of ischemic heart disease. Although the nitrosovasodilators can substitute for endogenous NO, the pathway of bioactivation substantially differs with nitrites and organic nitrates. In the case of organic nitrates such as NTG, NO is only formed if thiols are present as a cofactor. Nitrites such as amyl nitrite and sodium nitrite require reduction of nitrite anion to vasoactive NO and that this bioactivation can be mediated by XOR or ALDH2. The observation that responses to NTG were not altered at a time when responses to sodium nitrite were attenuated, suggests that the reduction of the nitrite anion formed from NTG to NO does not contribute to vasodilator responses to NTG in the pulmonary and systemic vascular beds of the intact chest rat. Finally, recent findings suggest that both XOR and ALDH2 can act in a parallel manner to reduce nitrite to vasoactive NO [172, 173]. Nitrates are still the most widely used drugs for the treatment of ischemic heart disease and recent evidence now suggests that nitrites have a beneficial effect in protecting the heart and other organs from ischemia.
Acknowledgments
NIH Grant HL 62000 and HL 77421
References
- 1.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 2.Yach D, Hawkes C, Gould CL, et al. The global burden of chronic diseases: overcoming impediments to prevention and control. JAMA. 2004;291:2616–2622. doi: 10.1001/jama.291.21.2616. [DOI] [PubMed] [Google Scholar]
- 3.Brunton TL. On the use of nitrite of amyl in angina pectoris. Lancet. 1867;2:97–98. [Google Scholar]
- 4.Murrell W. Nitro-Glycerine in angina pectoris. Lancet. 1879;1:80–81. [Google Scholar]
- 5.Contro S, Haring OM, Goldstein W. Paradoxic action of amyl nitrite in coronary patients. Circulation. 1952;6:250–256. doi: 10.1161/01.cir.6.2.250. [DOI] [PubMed] [Google Scholar]
- 6.Napoli C, Ignarro LJ. Nitric oxide-releasing drugs. Annu Rev Pharmacol Toxicol. 2003;43:97–123. doi: 10.1146/annurev.pharmtox.43.100901.140226. [DOI] [PubMed] [Google Scholar]
- 7.Abrams J. Glyceryl trinitrate (nitroglycerin) and the organic nitrates. Choosing the method of administration. Drugs. 1987;34:391–403. doi: 10.2165/00003495-198734030-00005. [DOI] [PubMed] [Google Scholar]
- 8.Fahmy NR. Nitroglycerin as a hypotensive drug during general anesthesia. Anesthesiology. 1978;49:17–20. doi: 10.1097/00000542-197807000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan JA, Dunbar RW, Jones EL. Nitroglycerin infusion during coronary-artery surgery. Anesthesiology. 1976;45:14–21. doi: 10.1097/00000542-197607000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Rasch DK, Lancaster L. Successful use of nitroglycerin to treat postoperative pulmonary hypertension. Crit Care Med. 1987;15:616–617. doi: 10.1097/00003246-198706000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Richardson BW. Report on the physiological action of nitrite of amyl. Rep Br Assoc Adv Sci. 1864;34:120. [Google Scholar]
- 12.Murrell W. Nitro-Glycerin in Angina Pectoris. Detroit: George S. Davis, Medical Publisher; 1883. [Google Scholar]
- 13.Mayer B, Beretta M. The enigma of nitroglycerin bioactivation and nitrate tolerance: news, views and troubles. Br J Pharmacol. 2008;155:170–184. doi: 10.1038/bjp.2008.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bodo R. The effect of the ‘heart tonics’ and other drugs upon heart-tone and coronary circulation. J Physiol. 1928;64:356–387. doi: 10.1113/jphysiol.1928.sp002447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francois-Frank CA. Effect vasodilatateur du nitrite d’amyle sur les vaisseaux de l’corce crebrale et sur les vaisseaux du myocarde. Compt Rend Soc Biol. 1903;55:1448. [Google Scholar]
- 16.Schloss K. Uber die XWirkung der Nitrite auf die Durchblutung des Herzens (Versuche am Herzen in situ) Deutsches Arch Klin Med. 1913;111:310. [Google Scholar]
- 17.Cow D. Some reactions of surviving arteries. J Physiol. 1910;42:125–143. doi: 10.1113/jphysiol.1911.sp001428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wegria R, Essex HE, Herrick JF, et al. The simultaneous action of certain drugs on the blood pressure and on the flow in the right and left coronary arteries. Am Heart J. 1940;20:557. [Google Scholar]
- 19.Cohen MV, Kirk ES. Differential response of large and small coronary arteries to nitroglycerin and angiotensin. Autoregulation and tachyphylaxis. Circ Res. 1973;33:445–453. doi: 10.1161/01.res.33.4.445. [DOI] [PubMed] [Google Scholar]
- 20.Forman R, Kirk ES. Comparative effects of vasodilator drugs on large and small coronary resistance vessels in the dog. Cardiovasc Res. 1980;14:601–606. doi: 10.1093/cvr/14.10.601. [DOI] [PubMed] [Google Scholar]
- 21.Kinsella D, Troup W, McGregor M. Studies with a new coronary vasodilator drug: Persantin. Am Heart J. 1962;63:146–151. doi: 10.1016/0002-8703(62)90190-4. [DOI] [PubMed] [Google Scholar]
- 22.Sosa JA, McGregor M. Prenylamine in angina pectoris. Can Med Ass. 1963;89:248–251. [PMC free article] [PubMed] [Google Scholar]
- 23.Master AM, Jaffe HL, Dack S. The drug treatment of angina pectoris due to coronary artery disease. Am J M Sc. 1939;197:774. [Google Scholar]
- 24.Gorlin R, Brachfeld N, MacLeod C, et al. The effect of nitroglycerin on the coronary circulation in patients with coronary artery disease or increased left ventricular work. Circulation. 1959;19:705–718. doi: 10.1161/01.cir.19.5.705. [DOI] [PubMed] [Google Scholar]
- 25.Ross RS, Ueda K, Lichtlen PR, et al. Measurement of Myocardial Blood Flow in Animals and Man by Selective Injection of Radioactive Inert Gas into the Coronary Arteries. Circ Res. 1964;15:28–41. doi: 10.1161/01.res.15.1.28. [DOI] [PubMed] [Google Scholar]
- 26.Brachfeld N, Bozer J, Gorin R. Action of nitroglycerin on the coronary circulation in normal and in mild cardiac subjects. Circulation. 1959;19:697–704. doi: 10.1161/01.cir.19.5.697. [DOI] [PubMed] [Google Scholar]
- 27.Rowe GG, Chelius CJ, Afonso S, et al. Systemic and coronary hemodynamic effects of erythrol tetranitrate. J Clin Invest. 1961;40:1217–1222. doi: 10.1172/JCI104352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradbury JB. Some new vasodilators. Br Med J. 1895;2:1213–1218. doi: 10.1136/bmj.2.1820.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiss S, Ellis LB. Influence of sodium nitrite on the cardiovascular system and on renal activity. Arch Intern Med. 1933;52:105–119. [Google Scholar]
- 30.Mathew E. Vasodilators in high blood pressure. Quart J Med. 1909;2:261–268. [Google Scholar]
- 31.Wallace GB, Ringer AI. The lowering of blood pressure by the nitrite group. JAMA. 1909;53:1629–1630. [Google Scholar]
- 32.Evans W, Hoyle C. Comparative value of drugs used in continuous treatment of angina pectoris. Quart J Med. 1933:2. [Google Scholar]
- 33.Russek HI, Urbach KF, Zohman BL. Paradoxical action of glyceryl trinitrate (nitroglycerin) in coronary patients. J Am Med Assoc. 1955;158:1017–1021. doi: 10.1001/jama.1955.02960120017006. [DOI] [PubMed] [Google Scholar]
- 34.Kerber RE, Harrison DC. Paradoxical electrocardiographic effects of amyl nitrite in coronary artery disease. Br Heart J. 1972;34:851–857. doi: 10.1136/hrt.34.8.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chrysant SG, Dunn FG, De Carvalho JG, et al. Action of nitroglycerin and amyl nitrite in labile and essential hypertension: hemodynamic differences. Arch Intern Med. 1977;137:1702–1705. [PubMed] [Google Scholar]
- 36.Paul O. The medical management of angina pectoris. JAMA. 1977;238:1847–1848. [PubMed] [Google Scholar]
- 37.Wu LT, Schlenger WE, Ringwalt CL. Use of nitrite inhalants (“poppers”) among American youth. J Adolesc Health. 2005;37:52–60. doi: 10.1016/j.jadohealth.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 38.Fledelius HC. Irreversible blindness after amyl nitrite inhalation. Acta Ophthalmol Scand. 1999;77:719–721. doi: 10.1034/j.1600-0420.1999.770625.x. [DOI] [PubMed] [Google Scholar]
- 39.Haverkos HW, Dougherty J. Health hazards of nitrite inhalants. Am J Med. 1988;84:479–482. doi: 10.1016/0002-9343(88)90269-0. [DOI] [PubMed] [Google Scholar]
- 40.Sarvesvaran ER, Fysh R, Bowen DA. Amyl nitrite related deaths. Med Sci Law. 1992;32:267–269. doi: 10.1177/002580249203200316. [DOI] [PubMed] [Google Scholar]
- 41.Marsh N, Marsh A. A short history of nitroglycerine and nitric oxide in pharmacology and physiology. Clin Exp Pharmacol Physiol. 2000;27:313–319. doi: 10.1046/j.1440-1681.2000.03240.x. [DOI] [PubMed] [Google Scholar]
- 42.Gracia R, Shepherd G. Cyanide poisoning and its treatment. Pharmacotherapy. 2004;24:1358–1365. doi: 10.1592/phco.24.14.1358.43149. [DOI] [PubMed] [Google Scholar]
- 43.Chen CF, Yeh SU, Chien CT, et al. Renal response during acute unilateral ureteral obstruction in rats. Neurourol Urodyn. 2001;20:125–137. doi: 10.1002/1520-6777(2001)20:1<125::aid-nau14>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 44.Dong YL, Gangula PR, Fang L, et al. Uterine relaxation responses to calcitonin gene-related peptide and calcitonin gene-related peptide receptors decreased during labor in rats. Am J Obstet Gynecol. 1998;179:497–506. doi: 10.1016/s0002-9378(98)70386-2. [DOI] [PubMed] [Google Scholar]
- 45.Facchinetti F, Neri I, Genazzani AR. L-arginine infusion reduces preterm uterine contractions. J Perinat Med. 1996;24:283–285. doi: 10.1515/jpme.1996.24.3.283. [DOI] [PubMed] [Google Scholar]
- 46.Yallampalli C, Garfield RE, Byam-Smith M. Nitric oxide inhibits uterine contractility during pregnancy but not during delivery. Endocrinology. 1993;133:1899–1902. doi: 10.1210/endo.133.4.8404632. [DOI] [PubMed] [Google Scholar]
- 47.Ignarro LJ, Lippton H, Edwards JC, et al. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981;218:739–749. [PubMed] [Google Scholar]
- 48.McMahon TJ, Hood JS, Bellan JA, et al. N omega-nitro-L-arginine methyl ester selectively inhibits pulmonary vasodilator responses to acetylcholine and bradykinin. J Appl Physiol. 1991;71:2026–2031. doi: 10.1152/jappl.1991.71.5.2026. [DOI] [PubMed] [Google Scholar]
- 49.Rees DD, Palmer RM, Schulz R, et al. Characterization of three inhibitors of endothelial nitricoxide synthase in vitro and in vivo. Br J Pharmacol. 1990;101:746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bellan JA, Minkes RK, McNamara DB, et al. N omega-nitro-L-arginine selectively inhibits vasodilator responses to acetylcholine and bradykinin in cats. Am J Physiol Heart Circ Physiol. 1991;260:H1025–1029. doi: 10.1152/ajpheart.1991.260.3.H1025. [DOI] [PubMed] [Google Scholar]
- 51.McMahon TJ, Hood JS, Kadowitz PJ. Pulmonary vasodilator response to vagal stimulation is blocked by N omega-nitro-L-arginine methyl ester in the cat. Circ Res. 1992;70:364–369. doi: 10.1161/01.res.70.2.364. [DOI] [PubMed] [Google Scholar]
- 52.Hauser B, Bracht H, Matejovic M, et al. Nitric oxide synthase inhibition in sepsis? Lessons learned from large-animal studies. Anesth Analg. 2005;101:488–498. doi: 10.1213/01.ANE.0000177117.80058.4D. [DOI] [PubMed] [Google Scholar]
- 53.Zweier JL, Samouilov A, Kuppusamy P. Non-enzymatic nitric oxide synthesis in biological systems. Biochim Biophys Acta. 1999;1411:250–262. doi: 10.1016/s0005-2728(99)00018-3. [DOI] [PubMed] [Google Scholar]
- 54.Cosby K, Partovi KS, Crawford JH, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nature Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 55.Jansson EA, Petersson J, Reinders C, et al. Protection from nonsteroidal anti-inflammatory drug (NSAID)-induced gastric ulcers by dietary nitrate. Free Radic Biol Med. 2007;42:510–518. doi: 10.1016/j.freeradbiomed.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 56.Lauer T, Preik M, Rassaf T, et al. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci U S A. 2001;98:12814–12819. doi: 10.1073/pnas.221381098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heiss C, Lauer T, Dejam A, et al. Plasma nitroso compounds are decreased in patients with endothelial dysfunction. J Am Coll Cardiol. 2006;47:573–579. doi: 10.1016/j.jacc.2005.06.089. [DOI] [PubMed] [Google Scholar]
- 58.Kleinbongard P, Dejam A, Lauer T, et al. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic Biol Med. 2006;40:295–302. doi: 10.1016/j.freeradbiomed.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 59.North AJ, Lau KS, Brannon TS, et al. Oxygen upregulates nitric oxide synthase gene expression in ovine fetal pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 1996;270:L643–649. doi: 10.1152/ajplung.1996.270.4.L643. [DOI] [PubMed] [Google Scholar]
- 60.Kim N, Vardi Y, Padma-Nathan H, et al. Oxygen tension regulates the nitric oxide pathway. Physiological role in penile erection. J Clin Invest. 1993;91:437–442. doi: 10.1172/JCI116220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moncada S, Higgs EA. Nitric oxide and the vascular endothelium. Handb Exp Pharmacol. 2006:213–254. doi: 10.1007/3-540-32967-6_7. [DOI] [PubMed] [Google Scholar]
- 62.Mellion BT, Ignarro LJ, Myers CB, et al. Inhibition of human platelet aggregation by S-nitrosothiols. Heme-dependent activation of soluble guanylate cyclase and stimulation of cyclic GMP accumulation. Mol Pharmacol. 1983;23:653–664. [PubMed] [Google Scholar]
- 63.Mellion BT, Ignarro LJ, Ohlstein EH, et al. Evidence for the inhibitory role of guanosine 3′, 5′-monophosphate in ADP-induced human platelet aggregation in the presence of nitric oxide and related vasodilators. Blood. 1981;57:946–955. [PubMed] [Google Scholar]
- 64.Nossaman BD, Gur S, Kadowitz PJ. Gene and stem cell therapy in the treatment of erectile dysfunction and pulmonary hypertension; potential treatments for the common problem of endothelial dysfunction. Curr Gene Ther. 2007;7:131–153. doi: 10.2174/156652307780363161. [DOI] [PubMed] [Google Scholar]
- 65.Ignarro LJ. Endothelium-derived nitric oxide: pharmacology and relationship to the actions of organic nitrate esters. Pharm Res. 1989;6:651–659. doi: 10.1023/a:1015926119947. [DOI] [PubMed] [Google Scholar]
- 66.Earl GL, Stanek EJ, Spinler SA. Intravenous nitroglycerin tolerance in patients with ischemic cardiomyopathy and congestive heart failure. Pharmacotherapy. 1998;18:203–209. [PubMed] [Google Scholar]
- 67.Abrams J. Therapy of angina pectoris with long-acting nitrates: which agent and when? Can J Cardiol. 1996;12 (Suppl C):9C–16C. [PubMed] [Google Scholar]
- 68.Bauer JA, Fung HL. Pharmacodynamic models of nitroglycerin-induced hemodynamic tolerance in experimental heart failure. Pharm Res. 1994;11:816–823. doi: 10.1023/a:1018917522072. [DOI] [PubMed] [Google Scholar]
- 69.Bauer JA, Fung HL, Zheng W, et al. Continuous versus intermittent nitroglycerin administration in experimental heart failure: vascular relaxation and radioligand binding to adrenoceptors and ion channels. J Cardiovasc Pharmacol. 1993;22:600–608. doi: 10.1097/00005344-199310000-00014. [DOI] [PubMed] [Google Scholar]
- 70.Drexler H, Hayoz D, Munzel T, et al. Endothelial function in chronic congestive heart failure. Am J Cardiol. 1992;69:1596–1601. doi: 10.1016/0002-9149(92)90710-g. [DOI] [PubMed] [Google Scholar]
- 71.Borzak S. Intravenous nitroglycerin for acute myocardial infarction. Henry Ford Hosp Med J. 1991;39:206–209. [PubMed] [Google Scholar]
- 72.Michel RP, Langleben D, Dupuis J. The endothelin system in pulmonary hypertension. Can J Physiol Pharmacol. 2003;81:542–554. doi: 10.1139/y03-008. [DOI] [PubMed] [Google Scholar]
- 73.Stewart DJ. Endothelial dysfunction in pulmonary vascular disorders. Arzneimittel-Forschung. 1994;44:451–454. [PubMed] [Google Scholar]
- 74.Brett SJ, Simon J, Gibbs R, et al. Impairment of endothelium-dependent pulmonary vasodilation in patients with primary pulmonary hypertension. Thorax. 1996;51:89–91. doi: 10.1136/thx.51.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rich S. Clinical insights into the pathogenesis of primary pulmonary hypertension. Chest. 1998;114:237S–241S. doi: 10.1378/chest.114.3_supplement.237s. [DOI] [PubMed] [Google Scholar]
- 76.Butany JW, Verma S, Leask RL, et al. Genetic abnormalities of the endothelium. Microscopy Research & Technique. 2003;60:30–37. doi: 10.1002/jemt.10240. [DOI] [PubMed] [Google Scholar]
- 77.Drexler H, Hornig B. Endothelial dysfunction in human disease. J Molec Cell Cadiol. 1999;31:51–60. doi: 10.1006/jmcc.1998.0843. [DOI] [PubMed] [Google Scholar]
- 78.Michelakis ED, Weir EK. The pathobiology of pulmonary hypertension. Smooth muscle cells and ion channels. Clinics in Chest Medicine. 2001;22:419–432. doi: 10.1016/s0272-5231(05)70281-1. [DOI] [PubMed] [Google Scholar]
- 79.Sattar N. Inflammation and endothelial dysfunction: intimate companions in the pathogenesis of vascular disease? [comment] Clin Sci. 2004;106:443–445. doi: 10.1042/CS20040019. [DOI] [PubMed] [Google Scholar]
- 80.De Meyer GRY, d Herman AG. Vascular endothelial dysfunction. Prog Cardiovasc Dis. 1997;39:325–342. doi: 10.1016/s0033-0620(97)80031-x. [DOI] [PubMed] [Google Scholar]
- 81.Bonora E. The metabolic syndrome and cardiovascular disease. Ann Med. 2006;38:64–80. doi: 10.1080/07853890500401234. [DOI] [PubMed] [Google Scholar]
- 82.Yavuzgil O, Altay B, Zoghi M, et al. Endothelial function in patients with vasculogenic erectile dysfunction. Intl J Cardiol. 2005;103:19–26. doi: 10.1016/j.ijcard.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 83.Collados MT, Velazquez B, Borbolla JR, et al. Endothelin-1 and functional tissue factor: a possible relationship with severity in primary pulmonary hypertension. Heart & Vessels. 2003;18:12–17. doi: 10.1007/s003800300002. [DOI] [PubMed] [Google Scholar]
- 84.Papatsoris AG, Korantzopoulos PG. Hypertension, antihypertensive therapy, and erectile dysfunction. Angiology. 2006;57:47–52. doi: 10.1177/000331970605700107. [DOI] [PubMed] [Google Scholar]
- 85.Kostis JB, Jackson G, Rosen R, et al. Sexual dysfunction and cardiac risk (the Second Princeton Consensus Conference) Am J Cardiol. 2005;96:313–321. doi: 10.1016/j.amjcard.2005.03.065. [DOI] [PubMed] [Google Scholar]
- 86.Azadzoi KM, Goldstein I, Siroky MB, et al. Mechanisms of ischemia-induced cavernosal smooth muscle relaxation impairment in a rabbit model of vasculogenic erectile dysfunction. J Urol. 1998;160:2216–2222. doi: 10.1097/00005392-199812010-00089. [DOI] [PubMed] [Google Scholar]
- 87.Ignarro LJ, Napoli C, Loscalzo J. Nitric oxide donors and cardiovascular agents modulating the bioactivity of nitric oxide: an overview. Circ Res. 2002;90:21–28. doi: 10.1161/hh0102.102330. [DOI] [PubMed] [Google Scholar]
- 88.Napoli C, de Nigris F, Williams-Ignarro S, et al. Nitric oxide and atherosclerosis: an update. Nitric Oxide. 2006;15:265–279. doi: 10.1016/j.niox.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 89.Parker JD, Parker JO. Nitrate therapy for stable angina pectoris. N Engl J Med. 1998;338:520–531. doi: 10.1056/NEJM199802193380807. [DOI] [PubMed] [Google Scholar]
- 90.Hord NG, Tang Y, Bryan NS. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am J Clin Nutr. 2009;90:1–10. doi: 10.3945/ajcn.2008.27131. [DOI] [PubMed] [Google Scholar]
- 91.Webb AJ, Patel N, Loukogeorgakis S, et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Butler AR, Feelisch M. Therapeutic uses of inorganic nitrite and nitrate: from the past to the future. Circulation. 2008;117:2151–2159. doi: 10.1161/CIRCULATIONAHA.107.753814. [DOI] [PubMed] [Google Scholar]
- 93.Dipalma JR. The nitrites and nitrates. Am Fam Physician. 1982;25:216–218. [PubMed] [Google Scholar]
- 94.Anonymous Nitrites and nitrates in the treatment of ischaemic heart disease. Br Med J. 1967;1:617–618. doi: 10.1136/bmj.1.5540.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fisch S, Degraff AC. Coronary Vasodilators. Dis Chest. 1963;44:533–537. doi: 10.1378/chest.44.5.533. [DOI] [PubMed] [Google Scholar]
- 96.Bauer JA, Booth BP, Fung HL. Nitric oxide donors: biochemical pharmacology and therapeutics. Adv Pharmacol. 1995;34:361–381. doi: 10.1016/s1054-3589(08)61098-4. [DOI] [PubMed] [Google Scholar]
- 97.Bauer JA, Nolan T, Fung HL. Vascular and hemodynamic differences between organic nitrates and nitrites. J Pharmacol Exp Ther. 1997;280:326–331. [PubMed] [Google Scholar]
- 98.Krantz JC, Jr, Carr CJ, Bryant HH. Alkyl nitrites. XIV. The effect of nitrites and nitrates on arterial adenosine triphosphatase. J Pharmacol Exp Ther. 1951;102:16–21. [PubMed] [Google Scholar]
- 99.Krantz JC, Jr, Carr CJ, Knapp MJ. Alkylnitrites, XV. The effect of nitrites and nitrates on the oxygen uptake of arterial tissue. J Pharmacol Exp Ther. 1951;102:258–260. [PubMed] [Google Scholar]
- 100.Kowaluk EA, Fung HL. Vascular nitric oxide-generating activities for organic nitrites and organic nitrates are distinct. J Pharmacol Exp Ther. 1991;259:519–525. [PubMed] [Google Scholar]
- 101.Needleman P, Johnson EM., Jr Mechanism of tolerance development to organic nitrates. J Pharmacol Exp Ther. 1973;184:709–715. [PubMed] [Google Scholar]
- 102.Forster S, Woditsch I, Schroder H, et al. Reduced nitric oxide release causes nitrate tolerance in the intact coronary circulation. J Cardiovasc Pharmacol. 1991;17:867–872. doi: 10.1097/00005344-199106000-00002. [DOI] [PubMed] [Google Scholar]
- 103.Chung SJ, Fung HL. Relationship between nitroglycerin-induced vascular relaxation and nitric oxide production. Probes with inhibitors and tolerance development. Biochem Pharmacol. 1993;45:157–163. doi: 10.1016/0006-2952(93)90388-d. [DOI] [PubMed] [Google Scholar]
- 104.Honig CR, Tenney SM, Gabel PV. The mechanism of cardiovascular action of nitroglycerine. An example of integrated response during the unsteady state. Am J Med. 1960;29:910–923. doi: 10.1016/0002-9343(60)90073-5. [DOI] [PubMed] [Google Scholar]
- 105.Mason DT, Braunwald E. The effects of nitroglycerin and amyl nitrite on arteriolar and venous tone in the human forearm. Circulation. 1965;32:755–766. doi: 10.1161/01.cir.32.5.755. [DOI] [PubMed] [Google Scholar]
- 106.Wilkins RW, Haynes FW, Weiss S. The role of the venous system in circulatory collapse induced by sodium nitrite. J Clin Invest. 1937;16:85–91. doi: 10.1172/JCI100841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ablad B, Johnsson G. Comparative effects of intra-arterially administered hydralazine and sodium nitrite on blood flow and volume of forearm. Acta Pharmacol Toxicol (Copenh) 1963;20:1–15. doi: 10.1111/j.1600-0773.1963.tb01713.x. [DOI] [PubMed] [Google Scholar]
- 108.Mason DT, Zelis R, Amsterdam EA. Actions of the nitrites on the peripheral circulation and myocardial oxygen consumption: significance in the relief of angina pectoris. Chest. 1971;59:296–305. doi: 10.1378/chest.59.3.296. [DOI] [PubMed] [Google Scholar]
- 109.Ganz W, Marcus HS. Failure of Intracoronary Nitroglycerin to Alleviate Pacing-Induced Angina. Circulation. 1972;46:880–889. doi: 10.1161/01.cir.46.5.880. [DOI] [PubMed] [Google Scholar]
- 110.Gladwin MT, Raat NJ, Shiva S, et al. Nitrite as a vascular endocrine nitric oxide reservoir that contributes to hypoxic signaling, cytoprotection, and vasodilation. Am J Physiol Heart Circ Physiol. 2006;291:H2026–2035. doi: 10.1152/ajpheart.00407.2006. [DOI] [PubMed] [Google Scholar]
- 111.Crawford JH, Isbell TS, Huang Z, et al. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood. 2006;107:566–574. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shiva S, Sack MN, Greer JJ, et al. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med. 2007;204:2089–2102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lu P, Liu F, Yao Z, et al. Nitrite-derived nitric oxide by xanthine oxidoreductase protects the liver against ischemia-reperfusion injury. Hepatobiliary Pancreat Dis Int. 2005;4:350–355. [PubMed] [Google Scholar]
- 114.Baker JE, Su J, Fu X, et al. Nitrite confers protection against myocardial infarction: role of xanthine oxidoreductase, NADPH oxidase and K(ATP) channels. J Mol Cell Cardiol. 2007;43:437–444. doi: 10.1016/j.yjmcc.2007.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Artz JD, Schmidt B, McCracken JL, et al. Effects of nitroglycerin on soluble guanylate cyclase: implications for nitrate tolerance. J Biol Chem. 2002;277:18253–18256. doi: 10.1074/jbc.C200170200. [DOI] [PubMed] [Google Scholar]
- 116.Beretta M, Gruber K, Kollau A, et al. Bioactivation of nitroglycerin by purified mitochondrial and cytosolic aldehyde dehydrogenases. J Biol Chem. 2008;283:17873–17880. doi: 10.1074/jbc.M801182200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Casey DB, Badejo AM, Jr, Dhaliwal JS, et al. Pulmonary vasodilator responses to sodium nitrite are mediated by an allopurinol-sensitive mechanism in the rat. Am J Physiol Heart Circ Physiol. 2009;296:H524–533. doi: 10.1152/ajpheart.00543.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Arnold WP, Mittal CK, Katsuki S, et al. Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci U S A. 1977;74:3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Murad F, Mittal CK, Arnold WP, et al. Guanylate cyclase: activation by azide, nitro compounds, nitric oxide, and hydroxyl radical and inhibition by hemoglobin and myoglobin. Adv Cyclic Nucleotide Res. 1978;9:145–158. [PubMed] [Google Scholar]
- 120.Needleman P, Jakschik B, Johnson EM., Jr Sulfhydryl requirement for relaxation of vascular smooth muscle. J Pharmacol Exp Ther. 1973;187:324–331. [PubMed] [Google Scholar]
- 121.Needleman P. Organic nitrate metabolism. Annu Rev Pharmacol Toxicol. 1976;16:81–93. doi: 10.1146/annurev.pa.16.040176.000501. [DOI] [PubMed] [Google Scholar]
- 122.Tsuchida S, Maki T, Sato K. Purification and characterization of glutathione transferases with an activity toward nitroglycerin from human aorta and heart. Multiplicity of the human class Mu forms. J Biol Chem. 1990;265:7150–7157. [PubMed] [Google Scholar]
- 123.Yeates RA, Schmid M, Leitold M. Antagonism of glycerol trinitrate activity by an inhibitor of glutathione S-transferase. Biochem Pharmacol. 1989;38:1749–1753. doi: 10.1016/0006-2952(89)90408-5. [DOI] [PubMed] [Google Scholar]
- 124.Millar TM, Stevens CR, Benjamin N, et al. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett. 1998;427:225–228. doi: 10.1016/s0014-5793(98)00430-x. [DOI] [PubMed] [Google Scholar]
- 125.McDonald BJ, Bennett BM. Cytochrome P-450 mediated biotransformation of organic nitrates. Can J Physiol Pharmacol. 1990;68:1552–1557. doi: 10.1139/y90-236. [DOI] [PubMed] [Google Scholar]
- 126.McDonald BJ, Bennett BM. Biotransformation of glyceryl trinitrate by rat aortic cytochrome P450. Biochem Pharmacol. 1993;45:268–270. doi: 10.1016/0006-2952(93)90403-j. [DOI] [PubMed] [Google Scholar]
- 127.Seth P, Fung HL. Biochemical characterization of a membrane-bound enzyme responsible for generating nitric oxide from nitroglycerin in vascular smooth muscle cells. Biochem Pharmacol. 1993;46:1481–1486. doi: 10.1016/0006-2952(93)90115-d. [DOI] [PubMed] [Google Scholar]
- 128.McGuire JJ, Anderson DJ, McDonald BJ, et al. Inhibition of NADPH-cytochrome P450 reductase and glyceryl trinitrate biotransformation by diphenyleneiodonium sulfate. Biochem Pharmacol. 1998;56:881–893. doi: 10.1016/s0006-2952(98)00216-0. [DOI] [PubMed] [Google Scholar]
- 129.Chen Z, Zhang J, Stamler JS. Identification of the enzymatic mechanism of nitroglycerin bioactivation. Proc Natl Acad Sci U S A. 2002;99:8306–8311. doi: 10.1073/pnas.122225199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen Z, Foster MW, Zhang J, et al. An essential role for mitochondrial aldehyde dehydrogenase in nitroglycerin bioactivation. Proc Natl Acad Sci U S A. 2005;102:12159–12164. doi: 10.1073/pnas.0503723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nunez C, Victor VM, Tur R, et al. Discrepancies between nitroglycerin and NO-releasing drugs on mitochondrial oxygen consumption, vasoactivity, and the release of NO. Circ Res. 2005;97:1063–1069. doi: 10.1161/01.RES.0000190588.84680.34. [DOI] [PubMed] [Google Scholar]
- 132.Feelisch M, Kelm M. Biotransformation of organic nitrates to nitric oxide by vascular smooth muscle and endothelial cells. Biochem Biophys Res Commun. 1991;180:286–293. doi: 10.1016/s0006-291x(05)81290-2. [DOI] [PubMed] [Google Scholar]
- 133.Schror K, Forster S, Woditsch I. On-line measurement of nitric oxide release from organic nitrates in the intact coronary circulation. Naunyn Schmiedebergs Arch Pharmacol. 1991;344:240–246. doi: 10.1007/BF00167225. [DOI] [PubMed] [Google Scholar]
- 134.Marks GS, McLaughlin BE, Nakatsu K, et al. Direct evidence for nitric oxide formation from glyceryl trinitrate during incubation with intact bovine pulmonary artery. Can J Physiol Pharmacol. 1992;70:308–311. doi: 10.1139/y92-039. [DOI] [PubMed] [Google Scholar]
- 135.Feelisch M, Brands F, Kelm M. Human endothelial cells bioactivate organic nitrates to nitric oxide: implications for the reinforcement of endothelial defence mechanisms. Eur J Clin Invest. 1995;25:737–745. doi: 10.1111/j.1365-2362.1995.tb01952.x. [DOI] [PubMed] [Google Scholar]
- 136.Mulsch A, Bara A, Mordvintcev P, et al. Specificity of different organic nitrates to elicit NO formation in rabbit vascular tissues and organs in vivo. Br J Pharmacol. 1995;116:2743–2749. doi: 10.1111/j.1476-5381.1995.tb17236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kuo WN, Kocis JM, Robinson MJ, et al. Further study on S-nitrosation by nitrite. Front Biosci. 2003;8:a143–147. doi: 10.2741/1120. [DOI] [PubMed] [Google Scholar]
- 138.Meyer DJ, Kramer H, Ketterer B. Human glutathione transferase catalysis of the formation of S-nitrosoglutathione from organic nitrites plus glutathione. FEBS Lett. 1994;351:427–428. doi: 10.1016/0014-5793(94)00904-x. [DOI] [PubMed] [Google Scholar]
- 139.Zeng H, Spencer NY, Hogg N. Metabolism of S-nitrosoglutathione by endothelial cells. Am J Physiol Heart Circ Physiol. 2001;281:H432–439. doi: 10.1152/ajpheart.2001.281.1.H432. [DOI] [PubMed] [Google Scholar]
- 140.Sarr M, Lobysheva I, Diallo AS, et al. Formation of releasable NO stores by S-nitrosoglutathione in arteries exhibiting toleranceto glyceryl-trinitrate. Eur J Pharmacol. 2005;513:119–123. doi: 10.1016/j.ejphar.2005.02.037. [DOI] [PubMed] [Google Scholar]
- 141.Foster MW, McMahon TJ, Stamler JS. S-nitrosylation in health and disease. Trends Mol Med. 2003;9:160–168. doi: 10.1016/s1471-4914(03)00028-5. [DOI] [PubMed] [Google Scholar]
- 142.Caramori PR, Adelman AG, Azevedo ER, et al. Therapy with nitroglycerin increases coronary vasoconstriction in response to acetylcholine. J Am Coll Cardiol. 1998;32:1969–1974. doi: 10.1016/s0735-1097(98)00456-2. [DOI] [PubMed] [Google Scholar]
- 143.Azevedo ER, Schofield AM, Kelly S, et al. Nitroglycerin withdrawal increases endothelium-dependent vasomotor response to acetylcholine. J Am Coll Cardiol. 2001;37:505–509. doi: 10.1016/s0735-1097(00)01140-2. [DOI] [PubMed] [Google Scholar]
- 144.Schulz E, Tsilimingas N, Rinze R, et al. Functional and biochemical analysis of endothelial (dys)function and NO/cGMP signaling in human blood vessels with and without nitroglycerin pretreatment. Circulation. 2002;105:1170–1175. doi: 10.1161/hc1002.105186. [DOI] [PubMed] [Google Scholar]
- 145.Napoli C, Ignarro LJ. Nitric oxide and pathogenic mechanisms involved in the development of vascular diseases. Arch Pharm Res. 2009;32:1103–1108. doi: 10.1007/s12272-009-1801-1. [DOI] [PubMed] [Google Scholar]
- 146.Berkenboom G, Unger P, Fontaine J. Atherosclerosis and responses of human isolated coronary arteries to endothelium-dependent and -independent vasodilators. J Cardiovasc Pharmacol. 1989;14 (Suppl 11):S35–39. [PubMed] [Google Scholar]
- 147.Fish RD, Nabel EG, Selwyn AP, et al. Responses of coronary arteries of cardiac transplant patients to acetylcholine. J Clin Invest. 1988;81:21–31. doi: 10.1172/JCI113297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bogaert MG. Organic nitrates in angina pectoris. Arch Int Pharmacodyn Ther. 1972;196(Suppl 196):125. [PubMed] [Google Scholar]
- 149.Battock DJ, Levitt PW, Steele PP. Effects of isosorbide dinitrate and nitroglycerin on central circulatory dynamics in coronary artery disease. Am Heart J. 1976;92:455–458. doi: 10.1016/s0002-8703(76)80044-0. [DOI] [PubMed] [Google Scholar]
- 150.Silber S. Nitrates: why and how should they be used today? Current status of the clinical usefulness of nitroglycerin, isosorbide dinitrate and isosorbide-5-mononitrate. Eur J Clin Pharmacol. 1990;38 (Suppl 1):S35–51. doi: 10.1007/BF01417564. [DOI] [PubMed] [Google Scholar]
- 151.Parker JA. Organic nitrates: new formulations and their clinical advantages. Am J Cardiol. 1996;77:38C–40C. doi: 10.1016/s0002-9149(96)00187-7. [DOI] [PubMed] [Google Scholar]
- 152.Johnson JB, Fairley A, Carter C. Effects of sublingual nitroglycerin on pulmonary arterial pressure in patients with left ventricular failure. Ann Intern Med. 1959;50:34–42. doi: 10.7326/0003-4819-50-1-34. [DOI] [PubMed] [Google Scholar]
- 153.Fremont RE. The actions of organic nitrates on the cardiopulmonary and peripheral circulations. Angiology. 1961;12:391–400. doi: 10.1177/000331976101200901. [DOI] [PubMed] [Google Scholar]
- 154.Mikulic E, Franciosa JA, Cohn JN. Comparative hemodynamic effects of chewable isosorbide dinitrate and nitroglycerin in patients with congestive heart failure. Circulation. 1975;52:477–482. doi: 10.1161/01.cir.52.3.477. [DOI] [PubMed] [Google Scholar]
- 155.Charuzi Y. Use of nitroglycerin ointment in acute pulmonary edema and hypertension. Chest. 1982;82:800. doi: 10.1378/chest.82.6.800a. [DOI] [PubMed] [Google Scholar]
- 156.Palevsky HI, Fishman AP. Vasodilator therapy for primary pulmonary hypertension. Annu Rev Med. 1985;36:563–578. doi: 10.1146/annurev.me.36.020185.003023. [DOI] [PubMed] [Google Scholar]
- 157.Bundgaard H, Boesgaard S, Mortensen SA, et al. Effect of nitroglycerin in patients with increased pulmonary vascular resistance undergoing cardiac transplantation. Scand Cardiovasc J. 1997;31:339–342. doi: 10.3109/14017439709075950. [DOI] [PubMed] [Google Scholar]
- 158.Pearlman AS, Engler RL, Goldstein RA, et al. Relative effects of nitroglycerin and nitroprusside during experimental acute myocardial ischemia. Eur J Cardiol. 1980;11:295–313. [PubMed] [Google Scholar]
- 159.GISSI-3Investigators. GISSI-3: effects of lisinopril and transdermal glyceryl trinitrate singly and together on 6-week mortality and ventricular function after acute myocardial infarction. Gruppo Italiano per lo Studio della Sopravvivenza nell’infarto Miocardico. Lancet. 1994;343:1115–1122. [PubMed] [Google Scholar]
- 160.Jugdutt BI. Nitrates in myocardial infarction. Cardiovasc Drugs Ther. 1994;8:635–646. doi: 10.1007/BF00877417. [DOI] [PubMed] [Google Scholar]
- 161.Lippton HL, Gruetter CA, Ignarro LJ, et al. Vasodilator actions of several N-nitroso compounds. Can J Physiol Pharmacol. 1982;60:68–75. doi: 10.1139/y82-009. [DOI] [PubMed] [Google Scholar]
- 162.Gruetter CA, Kadowitz PJ, Ignarro LJ. Methylene blue inhibits coronary arterial relaxation and guanylate cyclase activation by nitroglycerin, sodium nitrite, and amyl nitrite. Can J Physiol Pharmacol. 1981;59:150–156. doi: 10.1139/y81-025. [DOI] [PubMed] [Google Scholar]
- 163.Gruetter CA, Barry BK, McNamara DB, et al. Relaxation of bovine coronary artery and activation of coronary arterial guanylate cyclase by nitric oxide, nitroprusside and a carcinogenic nitrosoamine. J Cyclic Nucleotide Res. 1979;5:211–224. [PubMed] [Google Scholar]
- 164.Ignarro LJ, Gruetter CA. Requirement of thiols for activation of coronary arterial guanylate cyclase by glyceryl trinitrate and sodium nitrite: possible involvement of S-nitrosothiols. Biochim Biophys Acta. 1980;631:221–231. doi: 10.1016/0304-4165(80)90297-4. [DOI] [PubMed] [Google Scholar]
- 165.Kojda G, Patzner M, Hacker A, et al. Nitric oxide inhibits vascular bioactivation of glyceryl trinitrate: a novel mechanism to explain preferential venodilation of organic nitrates. Mol Pharmacol. 1998;53:547–554. doi: 10.1124/mol.53.3.547. [DOI] [PubMed] [Google Scholar]
- 166.Chen Z, Stamler JS. Bioactivation of nitroglycerin by the mitochondrial aldehyde dehydrogenase. Trends Cardiovasc Med. 2006;16:259–265. doi: 10.1016/j.tcm.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 167.Dalsgaard T, Simonsen U, Fago A. Nitrite-dependent vasodilation is facilitated by hypoxia and is independent of known NO-generating nitrite reductase activities. Am J Physiol Heart Circ Physiol. 2007;292:H3072–3078. doi: 10.1152/ajpheart.01298.2006. [DOI] [PubMed] [Google Scholar]
- 168.Dejam A, Hunter CJ, Tremonti C, et al. Nitrite infusion in humans and nonhuman primates: endocrine effects, pharmacokinetics, and tolerance formation. Circulation. 2007;116:1821–1831. doi: 10.1161/CIRCULATIONAHA.107.712133. [DOI] [PubMed] [Google Scholar]
- 169.Duranski MR, Greer JJ, Dejam A, et al. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wenzel P, Hink U, Oelze M, et al. Number of nitrate groups determines reactivity and potency of organic nitrates: a proof of concept study in ALDH-2−/−mice. Br J Pharmacol. 2007;150:526–533. doi: 10.1038/sj.bjp.0707116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hink U, Daiber A, Kayhan N, et al. Oxidative inhibition of the mitochondrial aldehyde dehydrogenase promotes nitroglycerin tolerance in human blood vessels. J Am Coll Cardiol. 2007;50:2226–2232. doi: 10.1016/j.jacc.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 172.Ohtake K, Nakaniski K, Uchida H, et al. Hypotensive effect of nitrite on experimental rat hypertension is mediated through multiple oxidoreductase-involved pathways. Second International Meeting on the Role of Nitrite in Physiology, Pathophysiology, and Therapeutics; September 6–7, 2007; Bethesda, Maryland: Natcher Conference Center, National Institutes of Health; [Google Scholar]
- 173.Golwala NH, Hodenette C, Murthy SN, et al. Vascular responses to nitrite are mediated by xanthine oxidoreductase and mitochondrial aldehyde dehydrogenase in the rat. Can J Physiol Pharmacol. 2009;87:1095–1101. doi: 10.1139/Y09-101. [DOI] [PubMed] [Google Scholar]
- 174.Li H, Cui H, Kundu TK, et al. Nitric oxide production from nitrite occurs primarily in tissues not in the blood: critical role of xanthine oxidase and aldehyde oxidase. J Biol Chem. 2008;283:17855–17863. doi: 10.1074/jbc.M801785200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Manukhina EB, Malyshev IY, Smirin BV, et al. Production and storage of nitric oxide in adaptation to hypoxia. Nitric Oxide. 1999;3:393–401. doi: 10.1006/niox.1999.0244. [DOI] [PubMed] [Google Scholar]
- 176.Cosby K, Partovi KS, Crawford JH, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 177.Weiss S, Wilkins RW, Haynes FW. Nature of circulatory collapse induced by sodium nitrite. J Clin Invest. 1937;16:73–84. doi: 10.1172/JCI100840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Furchgott RF, Bhadrakom S. Reactions of strips of rabbit aorta to epinephrine, isopropylarterenol, sodium nitrite and other drugs. J Pharmacol Exp Ther. 1953;108:129–143. [PubMed] [Google Scholar]
- 179.Matsunaga K, Furchgott RF. Interactions of light and sodium nitrite in producing relaxation of rabbit aorta. J Pharmacol Exp Ther. 1989;248:687–695. [PubMed] [Google Scholar]
- 180.Gruetter CA, Gruetter DY, Lyon JE, et al. Relationship between cyclic guanosine 3′:5′-monophosphate formation and relaxation of coronary arterial smooth muscle by glyceryl trinitrate, nitroprusside, nitrite and nitric oxide: effects of methylene blue and methemoglobin. J Pharmacol Exp Ther. 1981;219:181–186. [PubMed] [Google Scholar]
- 181.Mittal CK, Braughler JM, Ichihara K, et al. Synthesis of adenosine 3′,5′-monophosphate by guanylate cyclase, a new pathway for its formation. Biochim Biophys Acta. 1979;585:333–342. doi: 10.1016/0304-4165(79)90078-3. [DOI] [PubMed] [Google Scholar]
- 182.Li H, Samouilov A, Liu X, et al. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrite reduction. Evaluation of its role in nitric oxide generation in anoxic tissues. J Biol Chem. 2001;276:24482–24489. doi: 10.1074/jbc.M011648200. [DOI] [PubMed] [Google Scholar]
- 183.Li H, Cui H, Liu X, et al. Xanthine oxidase catalyzes anaerobic transformation of organic nitrates to nitric oxide and nitrosothiols: characterization of this mechanism and the link between organic nitrate and guanylyl cyclase activation. J Biol Chem. 2005;280:16594–16600. doi: 10.1074/jbc.M411905200. [DOI] [PubMed] [Google Scholar]
- 184.Casey DB, Badejo AM, Jr, Dhaliwal JS, et al. Pulmonary vasodilator responses to sodium nitrite are mediated by an allopurinol-sensitive mechanism in the rat. Am J Physiol Heart Circ Physiol. 2009;296:H524–533. doi: 10.1152/ajpheart.00543.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dejam A, Hunter CJ, Pelletier MM, et al. Erythrocytes are the major intra-vascular storage sites of nitrite in human blood. Blood. 2005;106:734–739. doi: 10.1182/blood-2005-02-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lefer DJ. Nitrite therapy for protection against ischemia-reperfusion injury. Am J Physiol -Renal Physiol. 2006;290:F777–778. doi: 10.1152/ajprenal.00470.2005. [DOI] [PubMed] [Google Scholar]
- 187.Gladwin MT, Shelhamer JH, Schechter AN, et al. Role of circulating nitrite and S-nitrosohemoglobin in the regulation of regional blood flow in humans. Proc Nat Acad Sci USA. 2000;97:11482–11487. doi: 10.1073/pnas.97.21.11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Katsuki S, Arnold W, Mittal C, et al. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J Cyclic Nucleotide Res. 1977;3:23–35. [PubMed] [Google Scholar]
- 189.Chung SJ, Fung HL. Identification of the subcellular site for nitroglycerin metabolism to nitric oxide in bovine coronary smooth muscle cells. J Pharmacol Exp Ther. 1990;253:614–619. [PubMed] [Google Scholar]


