Abstract
Afferent inputs are known to modulate the activity of locomotor central pattern generators, but their role in the generation of locomotor patterns remains uncertain. This study sought to investigate the importance of afferent input for producing bilateral, coordinated hindlimb stepping in adult cats. Following complete spinal transection, animals were trained to step on the moving belt of a treadmill until proficient, weight-bearing stepping of the hindlimbs was established. Selective dorsal rhizotomies of roots reaching various segments of the lumbosacral enlargement were then conducted, and hindlimb stepping capacity was reassessed. Depending on the deafferented lumbosacral segments, stepping was either abolished or unaffected. Deafferentation of mid-lumbar (L3/L4) or lower-lumbar (L5-S1) segments abolished locomotion. Locomotor capacity in these animals could not be restored with the administration of serotonergic or adrenergic agonists. Deafferentation of L3, L6, or S1 had mild effects on locomotion. This suggested that critical afferent inputs pertaining to hip position (mid-lumbar) and limb loading (lower-lumbar) play an important role in the generation of locomotor patterns after spinal cord injury.
Keywords: central pattern generation, hip position, limb loading, afferent input, locomotion
Introduction
The interplay between the locomotor central pattern generator (CPG) and afferent inputs in the formation of locomotor patterns remains a topic of debate. The ability to train an adult spinal cat to step on a moving treadmill belt has been known for many years,1,2 yet the underlying mechanisms are uncertain. The works of Sherrington3 and Brown4 provide two opposing views in explaining these observations: that the activity seen is reflex-driven or is the result of intrinsic properties of the spinal cord, respectively. The latter view has dominated our thinking in recent years and the intrinsic factors are now known as the CPG.5,6
Evidence in favor of a CPG comes from studies in which the spinal cord is isolated from descending and/or peripheral connections, either anatomically or pharmacologically.7 In these preparations, no motor activity is observed in the absence of stimulation. The application of excitatory neuro-transmitters such as l-DOPA or serotonin leads to a pattern of reciprocal activity in flexor and extensor nerves.8,9 This activity is also reciprocal across the spinal cord so that ipsilateral flexors and contralateral extensors are activated simultaneously.10 The similarity of this pattern to that observed in walking has led to the view that these patterns are the output of the locomotor CPG.11,12
Treadmill stepping patterns in the awake, spinalized animal, nevertheless, are very different from the patterns seen in fictive preparations. Stepping on a moving treadmill belt provides sensory inputs to the spinal cord, which play a major role in phase transitions.13 For example, transitions from stance (extensor phase) to swing (flexor phase) occur only when sensory information pertaining to hip position and limb loading is permissive. Stretch of the flexor muscles resets the locomotor pattern in decerebrate cats,14 and hindrance of hip extension delays or prevents the initiation of swing while prolonging stance.14 Hip position is also important for the swing to stance transition.15
The locomotor activity patterns in the presence of sensory inputs are also more robust, involving shorter flexor than extensor bursts and a period of double stance.16 Importantly, they also contain a distinct burst of activity in the semitendinosus muscle of the hindlimbs that appears before the onset of stance and is not seen in fictive preparations.17 It therefore appears that sensory inputs not only modulate the timing and intensity of CPG generated motor patterns and adapt them to the environment,18–20 but also contribute to the generation of locomotor bursts.17–21
Further insights regarding the interplay between the CPG and afferent inputs in locomotion are gained from neuro-mechanical models. Yakovenko et al.22 showed that in instances with low central drive (i.e., a weak CPG) the contribution of the stretch reflex is crucial in maintaining stable gait patterns. Ekeberg and Pearson23 further demonstrated that gait can be established through sensory inputs and independently of a CPG. They also examined the relative importance of hip position and limb loading for locomotion, and were able to generate a stable gait using either a combination of hip angle (relayed through inputs to mid-lumbar segments) and ankle force (relayed through inputs to lower-lumbar segments), or ankle force alone.23
The goal of the present study was to assess directly the role of afferent inputs in locomotor pattern generation. We specifically asked the question: could a chronically spinalized cat, previously trained to step on a moving treadmill belt, cope with sensory disturbances? Stepping capacity was evaluated after removal of sensory inputs reaching various spinal segments. We found that sensory information is a necessary component for locomotor pattern generation. Afferent inputs pertaining to hip position and limb loading are both critically needed for stepping in the chronically spinalized cat. The findings further demonstrate that the mid-lumbar segments in cats (equivalent to L1/L2 upper-lumbar segments in rodents) are incapable of producing a potent locomotor pattern in the absence of afferent inputs.
Some of this work was previously presented in abstract form.24
General methods
Experiments were performed on nine adult cats (1 male) weighing 2.8–4.5 kg. All experiments were approved by the University of Alberta Animal Welfare Committee.
Spinalization
A laminectomy was performed at the T11 vertebral level under isoflourane anaesthesia and aseptic surgical conditions. The dura mater was opened and the spinal cord exposed. A complete transection of the spinal cord was made under microscope vision using micro-dissection scissors, and the completeness of the lesion verified visually. The lesion space was filled with absorbable haemostat (Surgicel) and the dura mater, muscle and skin sutured shut in layers. Analgesia (Ketoprofen) and antibiotics (Cefazolin) were administered. Following recovery from surgery, animals were transferred to individual cages and attended twice daily for bladder expression. Antibiotics and analgesics were continued as required.
Treadmill Training
Treadmill training was initiated on the third postoperative day. The animals were placed in a harness suspended over a custom-made, split-belt treadmill with independent force plates under each belt. The forelimbs were supported on a stationary platform and the hindlimbs were placed over the moving belts. A plexiglass divider prevented excessive limb adduction. Training intensity increased to three 10 min sessions a day over the first week. The treadmill belts were set at a speed of 0.25 ms−1. Training involved manual placement of the hindlimbs and perineal stimulation until weight bearing and toe clearance were achieved. Initially, training was also supplemented with quipazine (0.5 mg/kg i.p.), a non-specific serotonergic precursor. Subsequently, only perineal stimulation and later the moving treadmill belts alone were needed to stimulate a stepping behavior.
Deafferentation
Once stable, proficient stepping was achieved (typically after 3 months of training) a baseline record of the stepping was obtained (see section Testing further). In a subsequent surgical procedure the dura mater was opened to expose the dorsal rootlets innervating various lumbosacral spinal segments. Dorsal rootlets to: (1) S1, (2) L6 and the rostral third of L7 (hereafter referred to as L6), (3) L4, (4) L3, L4 and the rostral third of L5 (hereafter referred to as L3/4), or (5) L5-S1 were identified, tied with suture and cut bilaterally. The size of the rhizotomy in the L3/4 and the L6 deafferentations was approximately the same. The dura mater, muscle layers and skin were then sutured and the animal recovered as previously described.
Two days after surgery training recommenced on the treadmill at the same speed and intensity as before. Perineal stimulation was applied as needed to facilitate stepping.
Testing
A total of three testing sessions were performed for each cat. Two, full-length tests were performed prior to the deafferentation and two weeks after the deafferentation, and one shorter test was conducted one week postdeafferentation. The ability of the cat to stand and to step spontaneously at a variety of treadmill speeds ranging from 0.1 to 0.6 m/s and the cat’s pawshake and flexor withdrawal reflexes were assessed. Kinematic, kinetic, and electromyographical (EMG) recordings were acquired to quantify the hindlimb responses of the cat. To quantify the kinematics, reflective markers were bilaterally placed on the iliac crest, hip, knee, ankle, and metatarso-phalengeal joints and the tip of the third toe. The limbs were then filmed using two high-speed, digital camcorders placed orthogonally to the sagittal plane of each limb. To record EMG activity, pairs of fine wires (9-strand stainless steel Cooner wire, insulated except for 2–3 mm at the tip) were transcutaneously inserted into the major hip, knee, and ankle flexor and extensor muscles under isoflourane anaesthesia prior to the testing session. After the acquisition of a complete set of data, quipazine (0.5 mg/kg i.p.) was administered and the tests repeated 20 min later. Clonidine (an α2 noradrenergic agonist) was then administered (75 µg/kg i.p.) and the tests repeated when the paw shake reflex had disappeared, typically 15–20 min following administration of the drug. The time from the administration of the quipazine to the completion of the clonidine and quipazine testing was less than 1 h.
One week after the deafferentation a shorter test was conducted in which kinematic, kinetic, and EMG data were recorded but no pharmacological agents were administered.
Data acquisition and analysis
The EMG recordings were band-pass filtered (30–1000 Hz) and amplified 1000× using custom-built amplifiers and a Neurolog system (Digitimer, Welyen Garden City, UK). Data were sampled (4000 samples/sec) using a CED 1401Power analog-to-digital card and Signal2 or Spike2 software (Cambridge Electronic Design, Cambridge, UK). Video clips of the hindlimbs (120 frames/sec) were transferred to a computer and limb movements were extracted using MotionTracker2D (Dr. Douglas Webber, University of Pittsburgh). Data analyses were performed in the MATLAB environment (Mathworks, Natick, USA) using custom-written routines.
Ground reaction forces (GRFs) generated during stepping in each testing session were normalized to the amount of force generated by each leg during periods of full weight-bearing standing obtained in the predeafferentation testing session for each animal. A 95% confidence level was deemed to be significant.
Results
Following 2–4 months of intensive treadmill training all spinal cats were able to step independently on the moving treadmill belt with only lateral, balance support provided, and without the need for pharmacological or perineal stimulation. Animals were able to step at a wide variety of speeds and under “taxing” circumstances in which the split belts were set to different speeds. Once the animals stepped proficiently, no instances of double flexion were encountered, that is, at least one hindlimb provided weight-support at any one time during stepping.
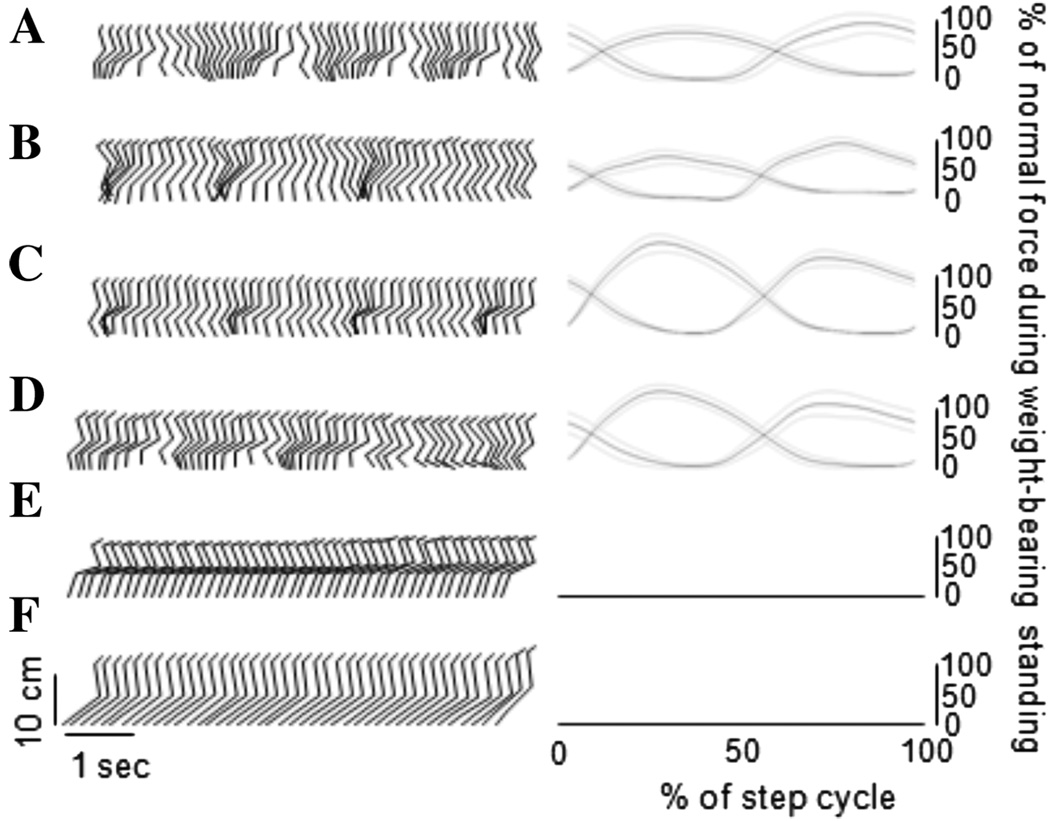
The location of the deafferentation dramatically affected spinal stepping. Reconstructed stick figures representing the spontaneous stepping of the hindlimbs on a moving treadmill belt at 0.25 m/s from a spinalized, but sensory intact animal and one example animal from each of the rhizotomized groups are shown in Figure 1. Also shown are the generated GRFs averaged over all the steps (mean ± standard deviation, 50 ± 15 steps) taken by the animal in each condition. Rhizotomies of single spinal segments (L4, L6, or S1) had little effect on the stepping pattern. In stark contrast, the L3/4 and L5-S1 deafferentations removed the ability of the animal to step spontaneously on the moving treadmill belt.
Figure 1.
Stick-figure representation of the effect of various deafferentations on hindlimb walking movements in the completely spinalized cat. (A) No deafferentation, (B) S1 rhizotomy, (C) L6 rhizotomy, (D) L4 rhizotomy, (E) L3/4 rhizotomy, and (F) L5-S1 rhizotomy. To the right of the stick figures are the GRFs averaged over a step cycle and normalized to the full load taken by the hindlimb during full weight-bearing standing. The upper four plots are qualitatively similar indicating a lack of major effect of the deafferentation on the evoked stepping pattern. The L3/4 and L5-S1 rhizotomies abolished spontaneous stepping activity of the hindlimbs as evidenced by the lack of modulation of joint angles in the stick-figures and the flat GRF traces.
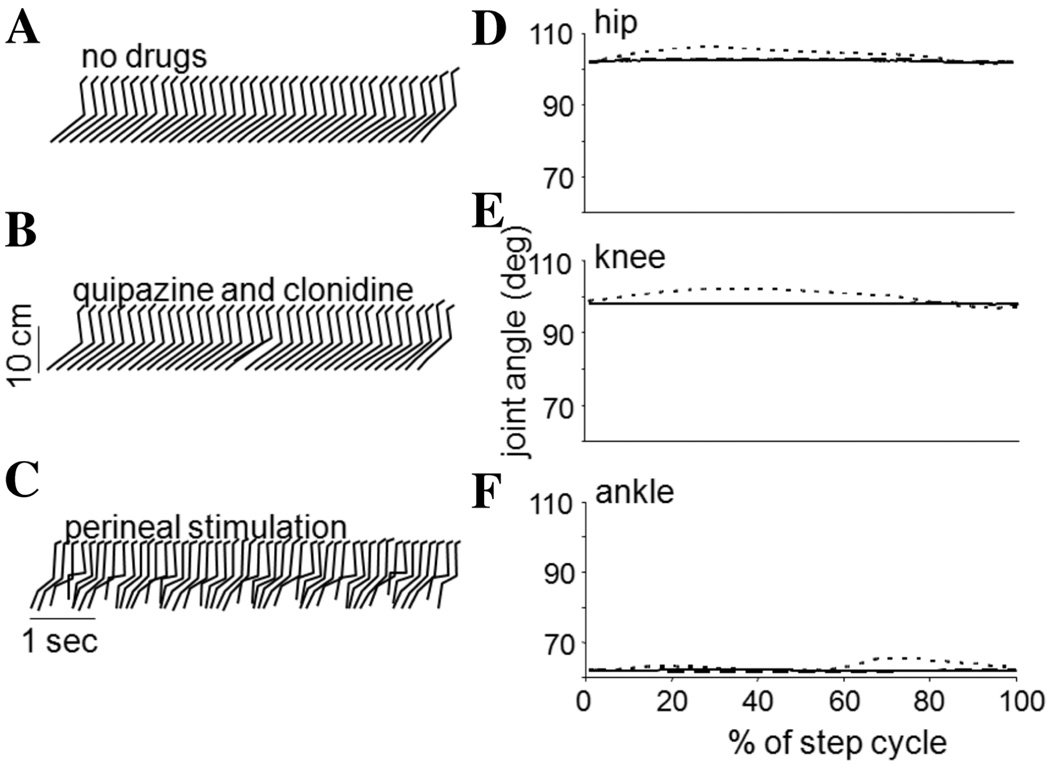
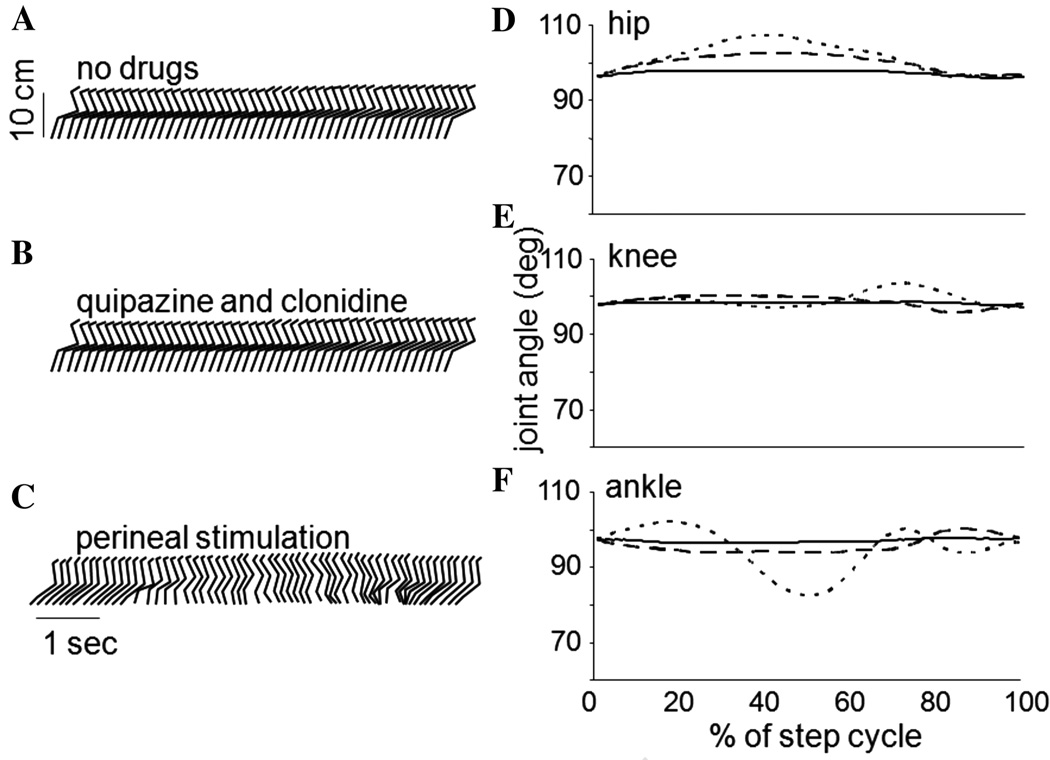
The effects of the L3/4 and L5-S1 deafferentations which led to the loss of sensory information pertaining to hip position and limb loading, respectively, are further exemplified in Figures 2 and 3. Stick figure representations from one cat in each group and joint angles averaged over a step cycle (35 ± 12 steps) are presented for three stepping conditions. With no stimulation other than the moving treadmill belt, neither the L3/4 nor L5-S1 deafferented cat could spontaneously step (Figs. 2A, 3A, respectively). Addition of the serotonergic and noradrenergic agonists quipazine and clonidine, failed to elicit a functional stepping pattern on the moving treadmill belt (Figs. 2A, 3A). In both deafferentations the addition of the pharmacological agents led to some increase in the phasic modulation of load force. Perineal stimulation in both cases restored some semblance of rhythmic oscillations in the legs (Figs. 2C, 3C); however, neither pharmacological nor perineal stimulation was capable of eliciting a stepping behavior similar to that obtained with intact sensory inputs (Fig. 1A), and significant abnormalities were noted. Moreover, the effects of the L3/4 and L5-S1 deafferentations were not the same.
Figure 2.
Stick figure representations and joint angle plots of the stepping evoked with various forms of stimulation in a cat with an L3/4 deafferentation. (A) shows the stepping on a moving treadmill belt with no pharmacological or perineal stimulation, and (B) with the addition of quipazine followed by clonidine. No appreciable stepping is demonstrated in each of these plots. In (C), perineal stimulation is applied to an animal with no pharmacological agents. In this case, rhythmic oscillations in the hindlimbs are evoked. Joint angle modulations are shown on the right for the hip (D), knee (E), and ankle (F). The solid line corresponds to no stimulation, the dashed to the quipazine followed by clonidine, and the dotted to the perineal stimulation. Note that in all cases, the hips only traversed a comparatively small range of angles and the hindlimb is not placed forward of the hip.
Figure 3.
Stick figure representations and joint angle plots of the stepping evoked with various forms of stimulation in a cat with an L5-S1 deafferentation. (A) shows the stepping on a moving treadmill belt with no pharmacological or perineal stimulation, and (B) with the addition of quipazine followed by clonidine. No appreciable stepping is demonstrated in each of these plots. In (C), perineal stimulation is applied to an animal with no pharmacological agents. In this case, disorganized elements of a locomotor pattern are evoked. Joint angle modulations are shown on the right for the hip (D), knee (E), and ankle (F). The solid line corresponds to no stimulation, the dashed to the quipazine followed by clonidine, and the dotted to the perineal stimulation.
The range of angles that the hip traversed during a step following the deafferentation compared to predeafferentation ranges provided an indicator of the deficit generated by the loss of the sensory information. The S1, L6, and L4 deafferenations produced a small, non-significant decrease in the range of hip motion (a decrease of ~5%). The L5-S1 deafferenation also produced only a small decrease in the range of hip angle that was not statistically significant during perineal stimulation. In contrast, the L3/4 deafferentation significantly reduced the range of hip angle by 45% (P < 0.05, paired t-test).
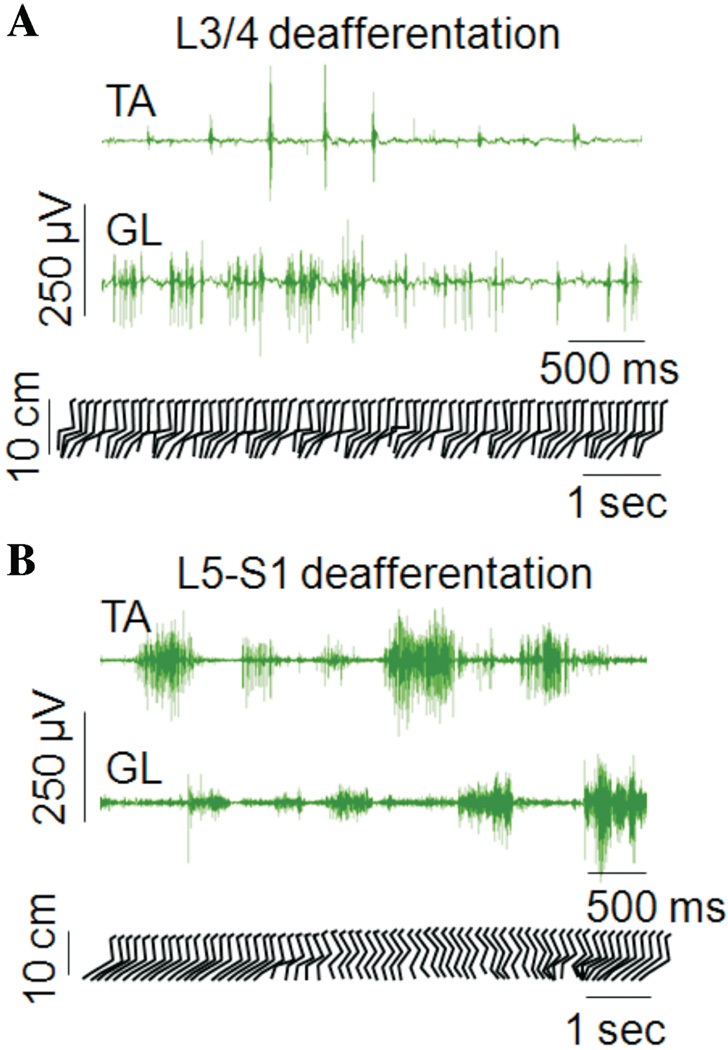
The differences in hindlimb stepping in cats with L3/4 and L5-S1 deafferentations are further highlighted in Figure 4 in which the best stepping trials obtained in these animals are shown. Stick figure representations along with EMG activity are shown in which stepping was evoked by a combination of the moving treadmill belt, perineal stimulation, quipazine and clonidine. The L3/4 cat was unable to bring the leg forward of the hip, the stepping movements were rapid and limb placement was on the dorsum of the paw. In contrast, the L5-S1 cat performed each phase of the step cycle and placed the foot on the plantar surface of the paw, but exhibited deficits in the transitions between the phases of the step cycle.
Figure 4.
Evoked stepping during a prolonged period of perineal stimulation in an L3/4 and an L5-S1 deafferentated cat that had received quipazine and clonidine. Differences in the locomotor deficits associated with each of the deafferentations are illustrated. (A) Alternating, rhythmic-like stepping movements of the hindlimbs could be elicited following the L3/4 deafferentation, but with deficits in limb placement in space: the paw was not placed in front of the hip and the cat stepped on the dorsum of the paw. (B) Normal range of limb placement could be achieved with proper stepping on the plantar surface of the paw following the L5-S1 deafferentation; however, the hindlimbs were unable to switch regularly between the swing and stance phases and pauses occurred at these transition points.
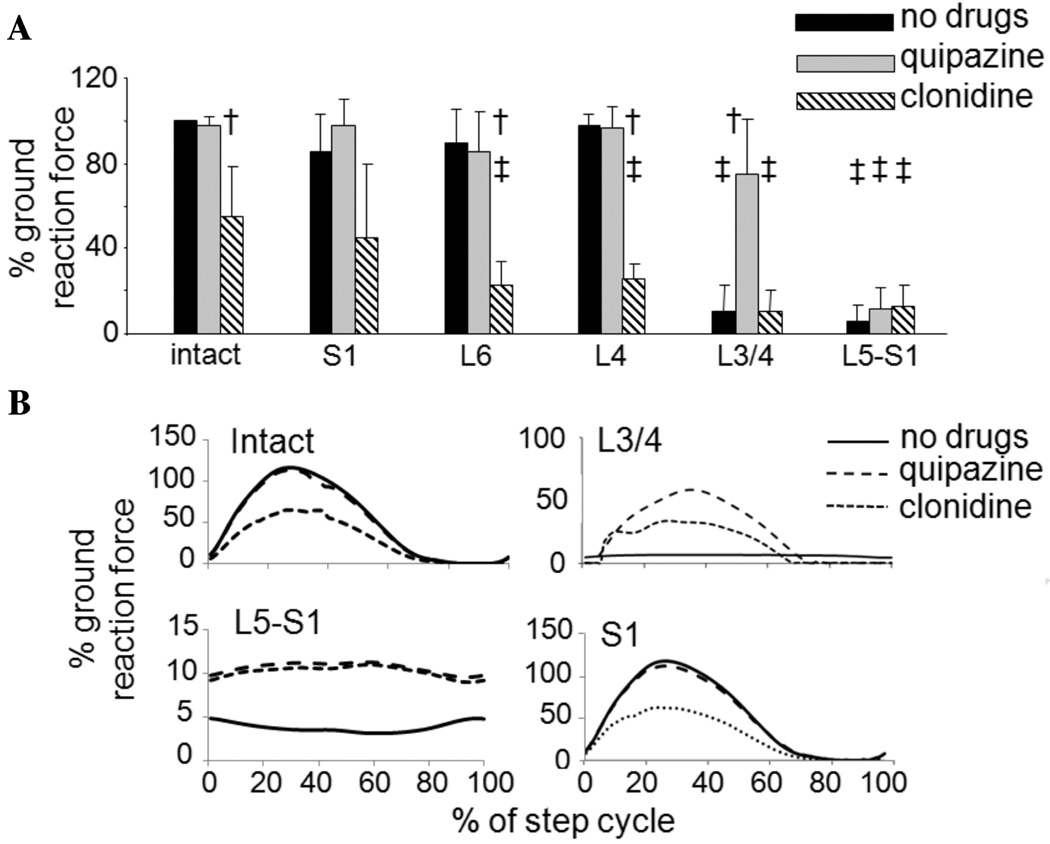
A summary of the average peak GRFs (loading) produced by the hindlimbs during stepping following each deafferentation is shown in Figure 5A. Data are normalized to each cat’s GRF produced during weight-bearing standing prior to the deafferentation. On average, the L3/4 and L5-S1 deafferentations significantly reduced the GRF to ~10% of baseline values (Fig. 5A black bars; P < 0.05, ANOVA, Tukey HSD post hoc comparisons). All other deafferentations produced changes of less than 10%.
Figure 5.
GRF generated during hindlimb stepping following the various rhizotomies. The forces are normalized to the load carried by the limb during full weight-bearing standing prior to the deafferentation. (A) Mean GRF and standard deviation bars are shown for all animals in the study. No decreases in force were seen with the S1, L6, and L4 deafferentations relative to predeafferentation levels (black bars), while large decreases were seen following the L3/4 and L5-S1 deafferentations. Quipazine generally increased the peak GRF, especially following the L3/4 deafferentation (gray bars), while clonidine generally decreased it (hashed bars). Cross symbols (†) indicate the significant changes in GRF induced by the application of pharmacological agents within a condition (sensory intact or a particular deafferentation). Double cross symbols (‡) indicate the significant changes in GRF relative to predeafferentation levels. (B) GRF averaged over a step cycle for a spinal animal with intact dorsal roots, an animal with L3/4, L5-S1, or S1 rhizotomy. In each plot the no-drugs (solid), quipazine (dashed), and clonidine (dotted) traces are shown. Note that the y-axis range is different for the different rhizotomy conditions.
Clonidine significantly decreased GRF in animals with intact sensory inputs as well as in those with L6 and L4 rhizotomies (Fig. 5A hashed bars; P < 0.05, ANOVA, Tukey HSD post hoc comparisons). While clonidine had no effect on GRF in animals with L3/4 or L5-S1 deafferentations, quipazine significantly increased GRF in animals with L3/4 deafferentation relative to pre-drug levels (Fig. 5A gray bars). The effects of clonidine, the α2-noradrenergic agonist, are not surprising in light of its inhibitory actions. Clonidine is used as an antispasmodic agent in individuals with spinal cord injury,25 and the hallmark indicator of its action in spinal cats is the loss of the paw shake reflex. The effectiveness of clonidine in facilitating locomotor-like patterns in spinal cats has been attributed to the localization of its receptors to the mid-lumbar segments of the cord, segments previously thought to contain the locomotor CPG networks.26 In this study, clonidine was ineffective in producing locomotor-like stepping patterns in the absence of sensory inputs to the L3/4 or L5-S1 segments, and significantly reduced the level of GRF produced during stepping. In contrast to clonidine, receptors for the serotonergic agonist, quipazine, are widely distributed throughout the spinal cord (Cowley and Schmidt 1997). Because quipazine enhances the excitability of spinal motoneurons,27,28 its effects on increasing GRF were anticipated. Interestingly, however, quipazine had no effect on GRF after the L5-S1 deafferentation, suggesting that its effects may be primarily presynaptic.29
Representative GRF traces averaged over a step cycle from a spinal, sensory-intact animal, and an animal in each of the L3/4, L5-S1, and S1 deafferentation groups are shown in Figure 5B. Whereas the sensory-intact and S1 deafferented cats showed a normal pattern of modulation of step force during all stimulation conditions (less with clonidine), there was little or no modulation in the GRF generated in the cats with the L3/4 or L5-S1 deafferentations.
Discussion
The ability of the spinal cat to step on a moving treadmill belt under a variety of conditions is remarkable. Devoid of descending supraspinal control, and with only sensory inputs, the cord is able to generate a motor pattern that adapts to changing and novel environmental demands. Moreover, proficient spinal stepping continues to be “safe,” in that at least one leg is always in contact with the ground, bearing the weight of the hindquarters. The present study demonstrated that the spinal cord is also able to adapt to changes in sensory inputs. Removal of the sensory inputs to an entire spinal segment had only minor consequences on the motor output. This would suggest that after learning to walk, the disconnected spinal cord retains plastic capacity even after some sensory disruption. Carrier and colleagues examined the effect of neuroectomy on the locomotion of spinal cats.30 They demonstrated that spinal animals that underwent a neuroectomy after learning to walk were able to maintain a symmetrical locomotor pattern, despite a unilateral absence of dorsiflexion, again suggesting that the spinal cord is able to make further plastic changes after learning how to generate spinal locomotion.
Both hip angle and load information are necessary for spinal stepping
Throughout this study successful stepping consisted of both weight-bearing of the hindquarters through the hindlimbs and forward movement of the limbs with paw placement occurring in front of the hip. The most striking finding is the effect of both the L3/4 and the L5-S1 deafferentations in abolishing the treadmill-evoked stepping behavior in the spinal cats. Removal of the sensory inputs to the L3/4 spinal segments removes the information concerning the hip position whilst the L5-S1 deafferentation leaves that inputs intact but removes all of the load information. The “rules” of locomotion as outlined by Prochazka13 and with experimental evidence from Pearson and coworkers14,15,31 indicate that the phase transitions within the stepping pattern rely upon hip position or load information. In this study we demonstrated that both of these components are required for the generation of spinal stepping. This is somewhat in contrast to the recent modeling result from Ekeburg and Pearson23 who showed that only loading information was required to generate adequate stepping in their coupled hindlimb model of a cat. An earlier model by Yakovenko et al.22 highlighted the role of sensory feedback (stretch reflexes) in the presence of weak central drive. However, this is the first study that experimentally examined the role of bilateral sensory feedback to spinal locomotion. The results herein will further refine studies focused on modeling the neural control of locomotion.
Differences between mid-lumbar and lower-lumbar deafferentations
The L3/4 and the L5-S1 deafferentations abolished the spinal cats’ ability to step spontaneously on a moving treadmill belt. In both conditions additional stimulation (primarily perineal) generated some oscillations in the hindlimbs but the patterns were missing critical elements that define locomotion. Moreover, the evoked oscillations were characteristically different in the two deafferentations. The animals without information concerning the position of their hip (L3/4 rhizotomy) were unable to bring their paws forward of their hips, even when the spinal circuits were activated through several forms of stimulation. This suggests that the hip sensory information is critical for enabling proper activation of the flexor muscles and in turn, forward placement of the leg in space. In contrast, the removal of all load information (L5-S1) did not alter the placement of the limb during each individual phase of the gait cycle. Rather, the primary deficit was in the switching between the phases. The animal was unable to switch easily and fluidly from the stance to swing phase with either leg. Presumably, this is a result of the lack of loading information leading to uncertainty about whether it is “safe” to take one leg off the ground. Furthermore, the amount of loading produced by the legs was severely reduced.
Deficits in stepping induced by deafferentation of a single spinal segment were comparatively minor, even when the same size deafferentations as the L3/4 rhizotomies was performed in another part of the spinal cord (e.g., the L6 deafferentation). This leads us to believe that the effects we see from the L3/4 and L5-S1 rhizotomies are not due to the size of the sensory disruption, but to the nature of the sensory inputs removed. In the L4, L6, and S1 deafferentations, adequate information pertaining to hip position and limb loading was preserved, allowing for proper generation of the locomotor pattern.
Comparison with fictive and in vitro preparations
Previous studies in fictive preparations demonstrated that in the absence of any sensory feedback, the spinal cord is capable of generating a rhythmic, locomotor-like pattern. In the present study we showed that removal of sensory information concerning just one of the primary inputs for spinal locomotion disrupts and inhibits the stepping ability of the chronically spinalized cat. It is likely that the rhythmic oscillations induced via a combination of stimuli (treadmill belt, pharmacological agents, and perineal stimulation) were produced by the CPG networks in the spinal cord. Such oscillations may represent the patterns seen during fictive locomotion that is commonly induced by pharmacological agents or electrical stimulation of the mesencephalic locomotor centre or the dorsal columns. However, this study demonstrates that such oscillations are inadequate for locomotion in spinal animals.
Interestingly, the application of perineal stimulation was much more efficacious in eliciting alternating oscillations of the hindlimbs following the L3/4 and L5-S1 rhizotomies than the serotonergic and noradrenergic agonists.32 This suggests a more prominent role for lower segments of the lumbar enlargement in the production of locomotion than previously suggested.33,34
Previous in vitro studies in neonatal preparations of rodent spinal cords suggested that the CPG networks involved in locomotion reside within the lumbar segments of the spinal cord.35,36 Similarly, studies in adult cat preparations33,37–39 highlighted the importance of the lumbar segments for the generation of stepping-like locomotor patterns. However, findings from both the rodent and cat preparations have diverged in their interpretation of the organization and distribution of these CPG networks. While some studies suggest that the locomotor CPG is localized to the upper- and mid-lumbar segments in rodents,35 and cats38 respectively, others demonstrated that the CPG networks are more distributed throughout the lumbar cord.33,36,37,39 Furthermore, some researchers within the latter camp proposed that the locomotor rhythmogenic capacity of the lumbar segments decreases caudally (i.e., within the lower-lumbar segments).36,39
The findings from this study do not address the distribution or organization of the CPG locomotor networks. Nonetheless, they strongly highlight the importance of sensory inputs to pattern generation. Most importantly, the findings demonstrate that the L3/4 segments in the spinal cats are incapable of generating stepping locomotor patterns in the absence of sensory information pertaining to hip position. Similarly, these same segments are incapable of generating appropriate stepping patterns in the absence of loading information relayed to lower-lumbar segments. Furthermore, lower-lumbar segments with intact sensory inputs are incapable of generating full locomotor patterns in the absence of sensory information pertaining to hip position relayed to the mid-lumbar segments. Therefore, the concepts of localized or distributed CPG with a rhythmogenic gradient in the lumbar cord do not appear to be prominent in the absence of sensory inputs to either the L3/L4 or L5-S1 segments. After chronic spinalization, sensory inputs pertaining to hip position and limb loading appear to play a primary role in the rhythmogenesis of locomotor stepping.
Functional implications
This study demonstrated the importance of the previously hypothesized rules for locomotion (Prochazka13) which depend on hip position and limb loading. Indeed these rules not only appear to safeguard against unsafe stepping, but are a prerequisite for the initiation and maintenance of stepping in the adult spinalized cat. The results present sensory inputs as critical components for locomotor pattern generation in the spinalized cat.
Based on the present findings which demonstrated the critical dependence of the CPG on sensory inputs, one may refute the argument that the locomotor CPG is a necessary element for stepping. However, previous work from our laboratory and the laboratory of others strongly suggests that both the CPG and sensory inputs are necessary for stepping, and each alone is incapable of producing robust locomotion.40,41
Therefore, when developing rehabilitation programs or control paradigms for devices to restore stepping after spinal cord injury in humans, it may be advantageous to utilize spinal CPG and sensory-driven rules of locomotion as guidelines.40,42–44
Conclusions
In this study we demonstrated the critical importance of both hip position and limb loading for the generation of locomotor patterns that enable spinal stepping. Removal of either leads to an inability to step spontaneously on a moving treadmill belt. With additional stimulation animals with either sensory loss can produce rhythmic oscillations; however, these oscillations are incomplete locomotor patterns. Both the CPG and afferent inputs are necessary for locomotor pattern generation.
Acknowledgments
We wish to express our sincere appreciation to Enid Pehowich, Lisa Stirling, Roger Calixto, and Bernice Lau for their assistance with this study, and the Health Science Laboratory Animal Service at the University of Alberta for their excellent care of the animals. This study was funded by the Alberta Heritage Foundation for Health Research (AHFMR), the Canadian Institutes of Health Research (CIHR), and the National Institute of Health (NIH).
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 1987;412:84–95. doi: 10.1016/0006-8993(87)91442-9. [DOI] [PubMed] [Google Scholar]
- 2.Shurrager PS, Dykman RA. Walking spinal carnivores. J. Comp. Physiol. Pyschol. 1951;44:252–262. doi: 10.1037/h0059889. [DOI] [PubMed] [Google Scholar]
- 3.Sherrington CS. On the spinal animal and the nature of spinal reflex activity. Phil. Trans. R. Soc. Lond. (B) 1898;190 [Google Scholar]
- 4.Brown TG. The intrinsic factors in the act of progression in the mammal. Proc. R. Soc. Lond. 1911;84:308–319. [Google Scholar]
- 5.Grillner S, Zangger P. On the central generation of locomotion in the low spinal cat. Exp. Brain Res. 1979;34:241–261. doi: 10.1007/BF00235671. [DOI] [PubMed] [Google Scholar]
- 6.Kiehn O, Katz PS. Making circuits dance: neuromodulation of motor systems. Beyond Neurotransmission, Neuromodulation. 1990 [Google Scholar]
- 7.Duysens J, van de Crommert HWAA. Neural control of locomotion. Part 1. The central pattern generator from cats to humans. Gait Posture. 1998;7:131–141. doi: 10.1016/s0966-6362(97)00042-8. [DOI] [PubMed] [Google Scholar]
- 8.Jankowska E, Jukes MGM, Lund S, Lundberg A. The effect of DOPA on the spinal cord. 6. Half-centre organization of interneurones transmitting effects from the flexor reflex afferents. Acta Physiol. Scand. 1967;70:389–402. doi: 10.1111/j.1748-1716.1967.tb03637.x. [DOI] [PubMed] [Google Scholar]
- 9.Jankowska E, Jukes MGM, Lund S, Lundberg A. The effect of DOPA on the spinal cord. 5. Reciprocal organization of pathways transmitting excitatory action to alpha motoneurones of flexors and extensors. Acta Physiol. Scand. 1967;70:369–388. doi: 10.1111/j.1748-1716.1967.tb03636.x. [DOI] [PubMed] [Google Scholar]
- 10.Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu. Rev. Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- 11.Kremer E, Lev-Tov A. Localization of the spinal network associated with generation of hindlimb locomotion in the neonatal rat and organization of its transverse coupling system. J. Neurophysiol. 1997;77:1155–1170. doi: 10.1152/jn.1997.77.3.1155. [DOI] [PubMed] [Google Scholar]
- 12.Burke RE, Degtyarenko M, Simon ES. Patterns of locomotor drive to motoneurons and last-order interneurons: clues to the structure of the CPG. J. Neurophysiol. 2001;86:447–462. doi: 10.1152/jn.2001.86.1.447. [DOI] [PubMed] [Google Scholar]
- 13.Prochazka A. Proprioceptive feedback and movement regulation. Neural Control of Movement. 1996:89–127. [Google Scholar]
- 14.Hiebert GW, Whelan PJ, Prochazka A, Pearson KG. Contribution of hind limb flexor muscle afferents to the timing of phase transitions in the cat step cycle. J. Neurophysiol. 1996;75:1126–1137. doi: 10.1152/jn.1996.75.3.1126. [DOI] [PubMed] [Google Scholar]
- 15.McVea DA, Donelan JM, Tachibana A, Pearson KG. A role for hip position in initiating the swing-to-stance transition in walking cats. J. Neurophysiol. 2005;94:3497–3508. doi: 10.1152/jn.00511.2005. [DOI] [PubMed] [Google Scholar]
- 16.Yakovenko S, McCrea DA, Stecina K, Prochazka A. Control of locomotor cycle durations. J. Neurophysiol. 2005;94:1057–1065. doi: 10.1152/jn.00991.2004. [DOI] [PubMed] [Google Scholar]
- 17.Smith JL, Chung SH, Zernicke RF. Gait-related motor patterns and hindlimb kinetics for the cat trot and gallop. Exp. Brain Res. 1993;94:308–322. doi: 10.1007/BF00230301. [DOI] [PubMed] [Google Scholar]
- 18.van de Crommert HWAA, Mulder T, Duysens J. Neural control of locomtion. Part 2. Sensory control of the central pattern generator and its relation to treadmill training. Gait Posture. 1998;7:251–263. doi: 10.1016/s0966-6362(98)00010-1. [DOI] [PubMed] [Google Scholar]
- 19.Buschges A, El Manira A. Sensory pathways and their modulation in the control of locomotion. Curr. Opin. Neurobiol. 1998;8:733–739. doi: 10.1016/s0959-4388(98)80115-3. [DOI] [PubMed] [Google Scholar]
- 20.Pearson KG. Generating the walking gait: role of sensory feedback. Progr. Brain Res. 2004;143:123–129. doi: 10.1016/S0079-6123(03)43012-4. [DOI] [PubMed] [Google Scholar]
- 21.Samuel ADT, Sengupta P. Sensorimotor integration: locating locomotion in neural circuits. Curr. Biol. 2005;15:341–343. doi: 10.1016/j.cub.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 22.Yakovenko S, Gritsenko V, Prochazka A. Contribution of stretch reflexes to locomotor control: a modelling study. Biol. Cybern. 2004;90:146–155. doi: 10.1007/s00422-003-0449-z. [DOI] [PubMed] [Google Scholar]
- 23.Ekeberg O, Pearson KG. Computer simulation of stepping in the hind legs of the cat: an examination of mechanisms regulating the stance-to-swing transition. J. Neurophysiol. 2005;94:4256–4268. doi: 10.1152/jn.00065.2005. [DOI] [PubMed] [Google Scholar]
- 24.Norton JA, Guevremont L, Mushahwar VK. Significance of segmental sensory input for stepping in the adult chronically spinalised cat. Soc. Neurosci. 2005 [Google Scholar]
- 25.Barbeau H, Norman KE. The effect of noradrenergic drugs on the recovery of walking after spinal cord injury. Spinal Cord. 2003;41:137–143. doi: 10.1038/sj.sc.3101374. [DOI] [PubMed] [Google Scholar]
- 26.Chau CW, Giroux N, Senecal J, et al. Spinal Cord Trauma; Neural Repair and Functional Recovery. Faculty of Medicine, Universite de Montreal; 2001. Topology and coupling of alpha-2-adrenergic receptors in cat lumbosacral spinal cord following complete lesions. [Google Scholar]
- 27.Jackson DA, White SR. Receptor subtypes mediating facilitation by serotonin of excitability of spinal motoneurons. Neuropharmacology. 1990;29:787–797. doi: 10.1016/0028-3908(90)90151-g. [DOI] [PubMed] [Google Scholar]
- 28.Myslinki NR, Anderson EG. The effect of serotonin precursors on Alpha- and Gamma-motoneuron activity. J. Pharmacol. Exp. Ther. 1983;204:19–26. [PubMed] [Google Scholar]
- 29.Curtins DR, Leah JD, Peet MJ. Effects of noradrenaline and 5-hydroxytryptamine on spinal Ia afferent terminations. Brain Res. 1983;258:328–332. doi: 10.1016/0006-8993(83)91160-5. [DOI] [PubMed] [Google Scholar]
- 30.Carrier L, Brustein E, Rossignol S. Locomotion of the hindlimbs after neurectomy of ankle flexors in intact and spinal cats: model for the study of locomotor plasticity. J. Neurophysiol. 1997;77:1979–1993. doi: 10.1152/jn.1997.77.4.1979. [DOI] [PubMed] [Google Scholar]
- 31.Lam T, Pearson KG. Proprioceptive modulation of hip flexor activity during the swing phase of locomotion in decerebrate cats. J. Neurophysiol. 2001;86:1321–1332. doi: 10.1152/jn.2001.86.3.1321. [DOI] [PubMed] [Google Scholar]
- 32.Barbeau H, Chau C, Rossignol S. Noradrenergic agonists and locomotor training affect locomotor recovery after cord transection in adult cats. Brain Res. Bull. 1993;30:387–393. doi: 10.1016/0361-9230(93)90270-l. [DOI] [PubMed] [Google Scholar]
- 33.Guevremont L, Renzi C, Norton JA, et al. Locomotor-related networks in the lumbosacral enlargement of the adult spinal cat: activation through intraspinal microstimulation. IEEE Trans. Neural Syst. Rehabil. Eng. 2006;14:266–272. doi: 10.1109/TNSRE.2006.881592. [DOI] [PubMed] [Google Scholar]
- 34.Strauss I, Lev-Tov A. Neural pathways between sacrocaudal afferents and lumbar pattern generators in neonatal rats. J. Neurophysiol. 2003;89:773–784. doi: 10.1152/jn.00716.2002. [DOI] [PubMed] [Google Scholar]
- 35.Cazalets J-R, Borde M, Clarac F. Localization and organization of the central pattern generator for hindlimb locomotion in newborn rat. J. Neurosci. 1995;15:4943–4951. doi: 10.1523/JNEUROSCI.15-07-04943.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kjaerulff O, Kiehn O. Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. J. Neurosci. 1996;16:5777–5794. doi: 10.1523/JNEUROSCI.16-18-05777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dai X, Noga BR, Douglas JR, Jordan LM. Localisation of spinal neurons activated during locomotion using the C-fos immunohistochemical method. J. Neurophysiol. 2005 doi: 10.1152/jn.00578.2004. [DOI] [PubMed] [Google Scholar]
- 38.Langlet C, Leblond H, Rossignol S. The mid-lumbar segments are needed for the expression of locomotion in chronic spinal cats. J. Neurophysiol. 2005;93:2474–2488. doi: 10.1152/jn.00909.2004. [DOI] [PubMed] [Google Scholar]
- 39.Deliagina TG, Orlovsky GN, Pavlova GA. The capacity for generation of rhythmic oscillations is distributed in the lumbosacral spinal cord of the cat. Exp. Brain Res. 1983;53:81–90. doi: 10.1007/BF00239400. [DOI] [PubMed] [Google Scholar]
- 40.Guevremont L, Norton JA, Mushahwar VK. A physiologically-based controller for generating overground locomotion using functional electrical stimulation. J. Neurophysiol. 2007;97:2499–2510. doi: 10.1152/jn.01177.2006. [DOI] [PubMed] [Google Scholar]
- 41.Musienko PE, Bogacheva IN, Gerasimenko YP. Significance of peripheral feedback in the generation of stepping movements during epidural stimulation of the spinal cord. Neurosci. Behav. Physiol. 2007;37:181–190. doi: 10.1007/s11055-007-0166-5. [DOI] [PubMed] [Google Scholar]
- 42.Guevremont L, Norton JA, Lau B, Mushahwar VK. An open- and closed-loop control system for generating over-ground locomotion using functional electrical stimulation. NIH Neural Prosthesis Workshop. 2005 [Google Scholar]
- 43.Veltnik PH, de Vries W, Hermens H, et al. A comprehensive FES control system for mobility restoration in paraplegics. In: Pedotti A, Ferrarin M, Quintern J, Riener R, editors. Neuroprosthetics; from Basic Research to Clinical Applications. Springer; 1990. [Google Scholar]
- 44.Courtine G, Gerasimenko YP, Van Den Brand R, et al. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat. Neurosci. 2009;12:1333–1342. doi: 10.1038/nn.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]