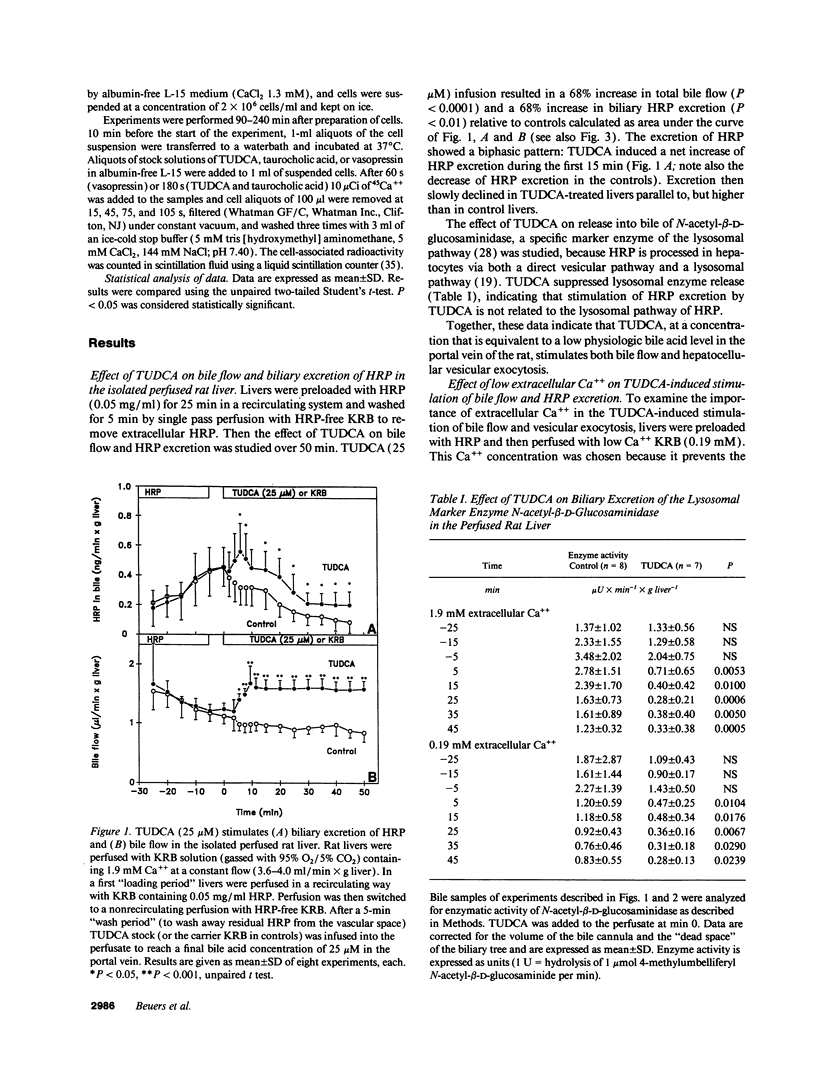
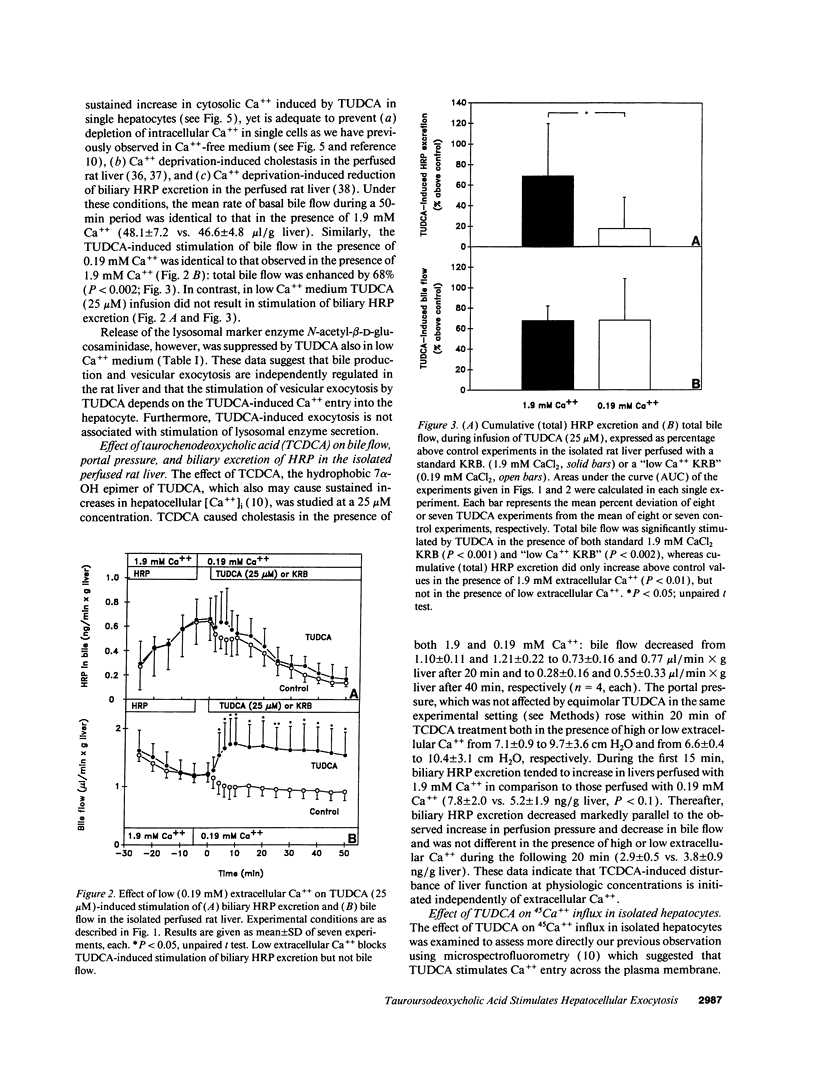
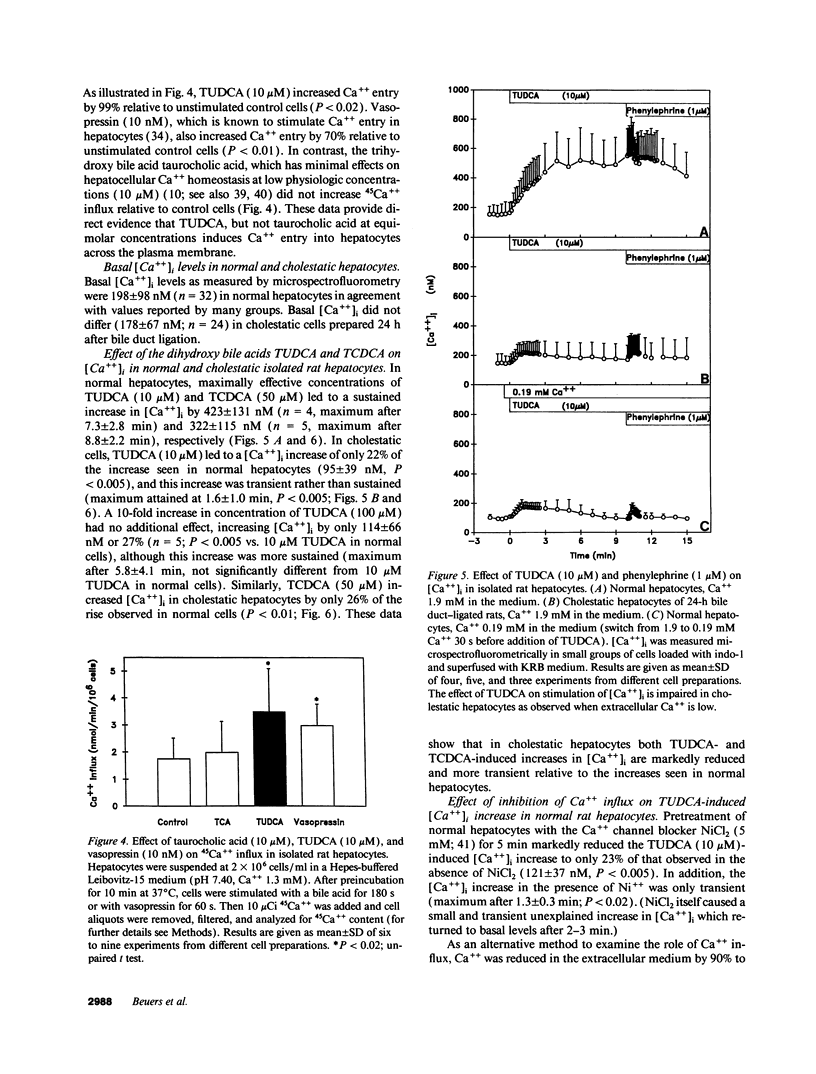
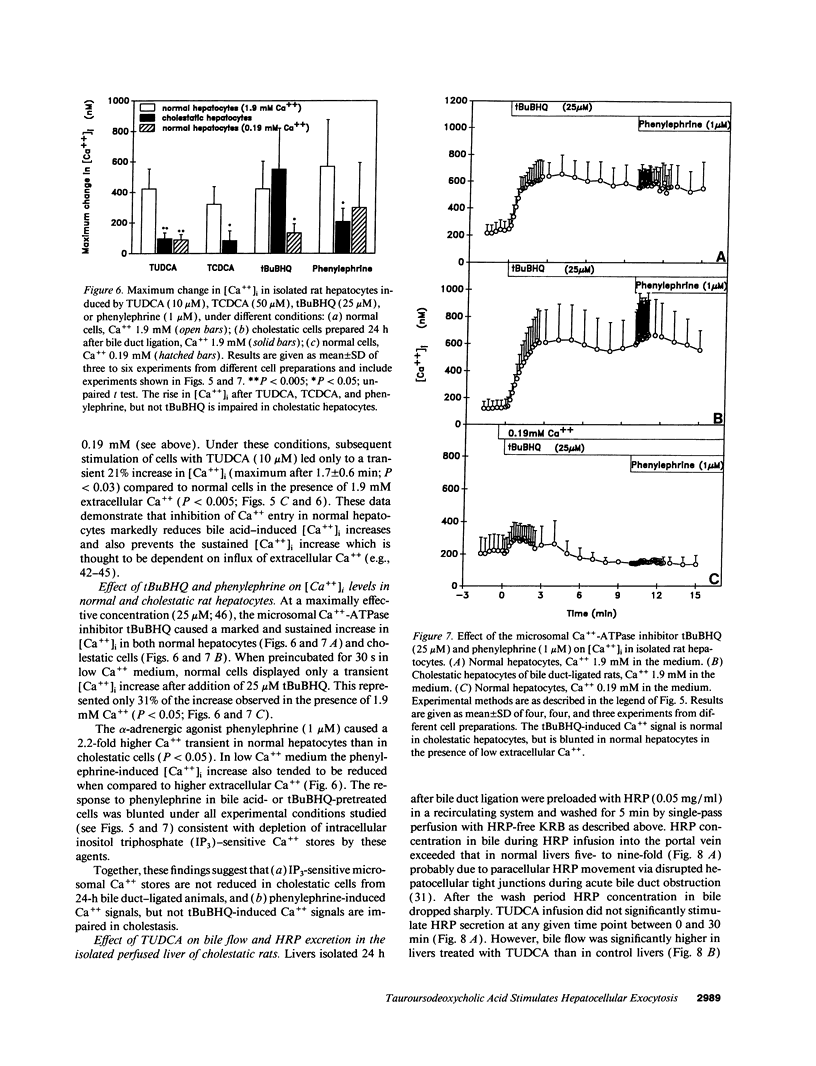
Abstract
To assess the effects of tauroursodeoxycholic acid (TUDCA) on bile excretory function, we examined whether TUDCA modulates vesicular exocytosis in the isolated perfused liver of normal rats in the presence of high (1.9 mM) or low (0.19 mM) extracellular Ca++ and in cholestatic rats 24 h after bile duct ligation. In addition, the effects of TUDCA on Ca++ homeostasis were compared in normal and in cholestatic hepatocytes. In the isolated perfused rat liver, TUDCA (25 microM) stimulated a sustained increase in the biliary excretion of horseradish peroxidase, a marker of the vesicular pathway, in the presence of high, but not low extracellular Ca++ or in the cholestatic liver. In contrast, TUDCA stimulated bile flow to the same extent regardless of the concentration of extracellular Ca++ or the presence of cholestasis. In indo-1-loaded hepatocytes, basal cytosolic free Ca++ ([Ca++]i) levels were not different between normal and cholestatic cells. However, in cholestatic cells [Ca++]i increases induced by TUDCA (10 microM) and its 7 alpha-OH epimer taurochenodeoxycholic acid (50 microM) were reduced to 22% and 26%, respectively, compared to normal cells. The impairment of TUDCA-induced [Ca++]i increase in cholestatic cells could be mimicked by exposing normal cells to low extracellular Ca++ (21%) or to the Ca++ channel blocker NiCl2 (23%). These data indicate that (a) dihydroxy bile acid-induced Ca++ entry may be of functional importance in the regulation of hepatocellular vesicular exocytosis, and (b) this Ca++ entry mechanism across the plasma membrane is impaired in cholestatic hepatocytes. We speculate that the beneficial effect of ursodeoxycholic acid in cholestatic liver diseases may be related to the Ca+(+)-dependent stimulation of vesicular exocytosis by its conjugate.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert P. R., Tashjian A. H., Jr Thyrotropin-releasing hormone-induced spike and plateau in cytosolic free Ca2+ concentrations in pituitary cells. Relation to prolactin release. J Biol Chem. 1984 May 10;259(9):5827–5832. [PubMed] [Google Scholar]
- Anwer M. S., Clayton L. M. Role of extracellular Ca2+ in hepatic bile formation and taurocholate transport. Am J Physiol. 1985 Dec;249(6 Pt 1):G711–G718. doi: 10.1152/ajpgi.1985.249.6.G711. [DOI] [PubMed] [Google Scholar]
- Anwer M. S., Engelking L. R., Nolan K., Sullivan D., Zimniak P., Lester R. Hepatotoxic bile acids increase cytosolic Ca++ activity of isolated rat hepatocytes. Hepatology. 1988 Jul-Aug;8(4):887–891. doi: 10.1002/hep.1840080430. [DOI] [PubMed] [Google Scholar]
- Beuers U., Nathanson M. H., Boyer J. L. Effects of tauroursodeoxycholic acid on cytosolic Ca2+ signals in isolated rat hepatocytes. Gastroenterology. 1993 Feb;104(2):604–612. doi: 10.1016/0016-5085(93)90433-d. [DOI] [PubMed] [Google Scholar]
- Beuers U., Spengler U., Kruis W., Aydemir U., Wiebecke B., Heldwein W., Weinzierl M., Pape G. R., Sauerbruch T., Paumgartner G. Ursodeoxycholic acid for treatment of primary sclerosing cholangitis: a placebo-controlled trial. Hepatology. 1992 Sep;16(3):707–714. doi: 10.1002/hep.1840160315. [DOI] [PubMed] [Google Scholar]
- Beuers U., Spengler U., Zwiebel F. M., Pauletzki J., Fischer S., Paumgartner G. Effect of ursodeoxycholic acid on the kinetics of the major hydrophobic bile acids in health and in chronic cholestatic liver disease. Hepatology. 1992 Apr;15(4):603–608. doi: 10.1002/hep.1840150409. [DOI] [PubMed] [Google Scholar]
- Bruck R., Haddad P., Graf J., Boyer J. L. Regulatory volume decrease stimulates bile flow, bile acid excretion, and exocytosis in isolated perfused rat liver. Am J Physiol. 1992 May;262(5 Pt 1):G806–G812. doi: 10.1152/ajpgi.1992.262.5.G806. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D. Control of exocytosis in adrenal chromaffin cells. Biochim Biophys Acta. 1991 Jul 22;1071(2):174–202. doi: 10.1016/0304-4157(91)90024-q. [DOI] [PubMed] [Google Scholar]
- Combettes L., Berthon B., Doucet E., Erlinger S., Claret M. Characteristics of bile acid-mediated Ca2+ release from permeabilized liver cells and liver microsomes. J Biol Chem. 1989 Jan 5;264(1):157–167. [PubMed] [Google Scholar]
- Corasanti J. G., Smith N. D., Gordon E. R., Boyer J. L. Protein kinase C agonists inhibit bile secretion independently of effects on the microcirculation in the isolated perfused rat liver. Hepatology. 1989 Jul;10(1):8–13. doi: 10.1002/hep.1840100103. [DOI] [PubMed] [Google Scholar]
- Crawford J. M., Berken C. A., Gollan J. L. Role of the hepatocyte microtubular system in the excretion of bile salts and biliary lipid: implications for intracellular vesicular transport. J Lipid Res. 1988 Feb;29(2):144–156. [PubMed] [Google Scholar]
- Crawford J. M., Gollan J. L. Transcellular transport of organic anions in hepatocytes: still a long way to go. Hepatology. 1991 Jul;14(1):192–197. doi: 10.1002/hep.1840140131. [DOI] [PubMed] [Google Scholar]
- Crosignani A., Podda M., Battezzati P. M., Bertolini E., Zuin M., Watson D., Setchell K. D. Changes in bile acid composition in patients with primary biliary cirrhosis induced by ursodeoxycholic acid administration. Hepatology. 1991 Dec;14(6):1000–1007. [PubMed] [Google Scholar]
- Erlinger S. Role of intracellular organelles in the hepatic transport of bile acids. Biomed Pharmacother. 1990;44(8):409–416. doi: 10.1016/0753-3322(90)90045-b. [DOI] [PubMed] [Google Scholar]
- Gall J. A., Bhathal P. S. A quantitative analysis of the liver following ligation of the common bile duct. Liver. 1990 Apr;10(2):116–125. doi: 10.1111/j.1600-0676.1990.tb00444.x. [DOI] [PubMed] [Google Scholar]
- Galle P. R., Theilmann L., Raedsch R., Otto G., Stiehl A. Ursodeoxycholate reduces hepatotoxicity of bile salts in primary human hepatocytes. Hepatology. 1990 Sep;12(3 Pt 1):486–491. doi: 10.1002/hep.1840120307. [DOI] [PubMed] [Google Scholar]
- Gautam A., Ng O. C., Boyer J. L. Isolated rat hepatocyte couplets in short-term culture: structural characteristics and plasma membrane reorganization. Hepatology. 1987 Mar-Apr;7(2):216–223. doi: 10.1002/hep.1840070203. [DOI] [PubMed] [Google Scholar]
- Greim H., Trülzsch D., Roboz J., Dressler K., Czygan P., Hutterer F., Schaffner F., Popper H. Mechanism of cholestasis. 5. Bile acids in normal rat livers and in those after bile duct ligation. Gastroenterology. 1972 Nov;63(5):837–845. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hayakawa T., Bruck R., Ng O. C., Boyer J. L. DBcAMP stimulates vesicle transport and HRP excretion in isolated perfused rat liver. Am J Physiol. 1990 Nov;259(5 Pt 1):G727–G735. doi: 10.1152/ajpgi.1990.259.5.G727. [DOI] [PubMed] [Google Scholar]
- Hayakawa T., Ng O. C., Ma A., Boyer J. L., Cheng O. Taurocholate stimulates transcytotic vesicular pathways labeled by horseradish peroxidase in the isolated perfused rat liver. Gastroenterology. 1990 Jul;99(1):216–228. doi: 10.1016/0016-5085(90)91251-z. [DOI] [PubMed] [Google Scholar]
- Heemskerk J. W., Feijge M. A., Rietman E., Hornstra G. Rat platelets are deficient in internal Ca2+ release and require influx of extracellular Ca2+ for activation. FEBS Lett. 1991 Jun 24;284(2):223–226. doi: 10.1016/0014-5793(91)80690-5. [DOI] [PubMed] [Google Scholar]
- Heuman D. M., Pandak W. M., Hylemon P. B., Vlahcevic Z. R. Conjugates of ursodeoxycholate protect against cytotoxicity of more hydrophobic bile salts: in vitro studies in rat hepatocytes and human erythrocytes. Hepatology. 1991 Nov;14(5):920–926. doi: 10.1002/hep.1840140527. [DOI] [PubMed] [Google Scholar]
- Hughes B. P., Barritt G. J. Inhibition of the liver cell receptor-activated Ca2+ inflow system by metal ion inhibitors of voltage-operated Ca2+ channels but not by other inhibitors of Ca2+ inflow. Biochim Biophys Acta. 1989 Oct 9;1013(3):197–205. doi: 10.1016/0167-4889(89)90135-3. [DOI] [PubMed] [Google Scholar]
- Häcki W., Paumgartner G. Determination of the biliary dead space using 14C-taurocholate as a marker. Experientia. 1973 Sep 15;29(9):1091–1093. doi: 10.1007/BF01946737. [DOI] [PubMed] [Google Scholar]
- Isales C. M., Bollag W. B., Kiernan L. C., Barrett P. Q. Effect of ANP on sustained aldosterone secretion stimulated by angiotensin II. Am J Physiol. 1989 Jan;256(1 Pt 1):C89–C95. doi: 10.1152/ajpcell.1989.256.1.C89. [DOI] [PubMed] [Google Scholar]
- Johnstone J. M., Lee E. G. A quantitative assessment of the structural changes the rat's liver following obstruction of the common bile duct. Br J Exp Pathol. 1976 Feb;57(1):85–94. [PMC free article] [PubMed] [Google Scholar]
- Jones A. L., Schmucker D. L., Mooney J. S., Adler R. D., Ockner R. K. Morphometric analysis of rat hepatocytes after total billary obstruction. Gastroenterology. 1976 Dec;71(6):1050–1060. [PubMed] [Google Scholar]
- Kacich R. L., Renston R. H., Jones A. L. Effects of cytochalasin D and colchicine on the uptake, translocation, and biliary secretion of horseradish peroxidase and [14C]sodium taurocholate in the rat. Gastroenterology. 1983 Aug;85(2):385–394. [PubMed] [Google Scholar]
- Kass G. E., Duddy S. K., Moore G. A., Orrenius S. 2,5-Di-(tert-butyl)-1,4-benzohydroquinone rapidly elevates cytosolic Ca2+ concentration by mobilizing the inositol 1,4,5-trisphosphate-sensitive Ca2+ pool. J Biol Chem. 1989 Sep 15;264(26):15192–15198. [PubMed] [Google Scholar]
- LaRusso N. F., Fowler S. Coordinate secretion of acid hydrolases in rat bile. J Clin Invest. 1979 Oct;64(4):948–954. doi: 10.1172/JCI109561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J. M., Palade G. E. Transcytotic vesicular carriers for polymeric IgA receptors accumulate in rat hepatocytes after bile duct ligation. J Cell Sci. 1991 Feb;98(Pt 2):205–216. doi: 10.1242/jcs.98.2.205. [DOI] [PubMed] [Google Scholar]
- Leuschner U., Fischer H., Kurtz W., Güldütuna S., Hübner K., Hellstern A., Gatzen M., Leuschner M. Ursodeoxycholic acid in primary biliary cirrhosis: results of a controlled double-blind trial. Gastroenterology. 1989 Nov;97(5):1268–1274. doi: 10.1016/0016-5085(89)91698-3. [DOI] [PubMed] [Google Scholar]
- Llopis J., Chow S. B., Kass G. E., Gahm A., Orrenius S. Comparison between the effects of the microsomal Ca(2+)-translocase inhibitors thapsigargin and 2,5-di-(t-butyl)-1,4-benzohydroquinone on cellular calcium fluxes. Biochem J. 1991 Jul 15;277(Pt 2):553–556. doi: 10.1042/bj2770553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe P. J., Kan K. S., Barnwell S. G., Sharma R. K., Coleman R. Transcytosis and paracellular movements of horseradish peroxidase across liver parenchymal tissue from blood to bile. Effects of alpha-naphthylisothiocyanate and colchicine. Biochem J. 1985 Jul 15;229(2):529–537. doi: 10.1042/bj2290529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteau P., Chazouilléres O., Myara A., Jian R., Rambaud J. C., Poupon R. Effect of chronic administration of ursodeoxycholic acid on the ileal absorption of endogenous bile acids in man. Hepatology. 1990 Nov;12(5):1206–1208. doi: 10.1002/hep.1840120521. [DOI] [PubMed] [Google Scholar]
- Mauger J. P., Poggioli J., Guesdon F., Claret M. Noradrenaline, vasopressin and angiotensin increase Ca2+ influx by opening a common pool of Ca2+ channels in isolated rat liver cells. Biochem J. 1984 Jul 1;221(1):121–127. doi: 10.1042/bj2210121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson M. H., Gautam A., Bruck R., Isales C. M., Boyer J. L. Effects of Ca2+ agonists on cytosolic Ca2+ in isolated hepatocytes and on bile secretion in the isolated perfused rat liver. Hepatology. 1992 Jan;15(1):107–116. doi: 10.1002/hep.1840150119. [DOI] [PubMed] [Google Scholar]
- Poupon R. E., Balkau B., Eschwège E., Poupon R. A multicenter, controlled trial of ursodiol for the treatment of primary biliary cirrhosis. UDCA-PBC Study Group. N Engl J Med. 1991 May 30;324(22):1548–1554. doi: 10.1056/NEJM199105303242204. [DOI] [PubMed] [Google Scholar]
- Poupon R., Chrétien Y., Poupon R. E., Ballet F., Calmus Y., Darnis F. Is ursodeoxycholic acid an effective treatment for primary biliary cirrhosis? Lancet. 1987 Apr 11;1(8537):834–836. doi: 10.1016/s0140-6736(87)91610-2. [DOI] [PubMed] [Google Scholar]
- Reichen J., Berr F., Le M., Warren G. H. Characterization of calcium deprivation-induced cholestasis in the perfused rat liver. Am J Physiol. 1985 Jul;249(1 Pt 1):G48–G57. doi: 10.1152/ajpgi.1985.249.1.G48. [DOI] [PubMed] [Google Scholar]
- Renston R. H., Maloney D. G., Jones A. L., Hradek G. T., Wong K. Y., Goldfine I. D. Bile secretory apparatus: evidence for a vesicular transport mechanism for proteins in the rat, using horseradish peroxidase and [125I]insulin. Gastroenterology. 1980 Jun;78(6):1373–1388. [PubMed] [Google Scholar]
- Schölmerich J., Baumgartner U., Miyai K., Gerok W. Tauroursodeoxycholate prevents taurolithocholate-induced cholestasis and toxicity in rat liver. J Hepatol. 1990 May;10(3):280–283. doi: 10.1016/0168-8278(90)90133-c. [DOI] [PubMed] [Google Scholar]
- Stiehl A., Raedsch R., Rudolph G. Acute effects of ursodeoxycholic and chenodeoxycholic acid on the small intestinal absorption of bile acids. Gastroenterology. 1990 Feb;98(2):424–428. doi: 10.1016/0016-5085(90)90834-n. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W., Tsien R. Y. Calcium channels, stores, and oscillations. Annu Rev Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]
- Tsunoda Y., Stuenkel E. L., Williams J. A. Characterization of sustained [Ca2+]i increase in pancreatic acinar cells and its relation to amylase secretion. Am J Physiol. 1990 Nov;259(5 Pt 1):G792–G801. doi: 10.1152/ajpgi.1990.259.5.G792. [DOI] [PubMed] [Google Scholar]
- Zimniak P., Little J. M., Radominska A., Oelberg D. G., Anwer M. S., Lester R. Taurine-conjugated bile acids act as Ca2+ ionophores. Biochemistry. 1991 Sep 3;30(35):8598–8604. doi: 10.1021/bi00099a015. [DOI] [PubMed] [Google Scholar]



