Summary
NKG2D (natural-killer group 2, member D) is an activating receptor present on the surface of natural killer (NK) cells, some NKT cells, CD8+ cytotoxic T cells, γδ T cells, and under certain conditions CD4+ T cells. Present in both humans and mice, this highly conserved receptor binds to a surprisingly diverse family of ligands that are distant relatives of MHC-class-I molecules. There is increasing evidence that ligand expression can result in both immune activation (tumor clearance, viral immunity, autoimmunity, and transplantation) and immune silencing (tumor evasion). In this review, we describe this family of NKG2D ligands and the various mechanisms that control their expression in stressed and normal cells. We also discuss the host response to both membrane-bound and secreted NKG2D ligands and summarize the models proposed to explain the consequences of this differential expression.
Keywords: natural killer cell, NKG2D, NK cells, MIC, cytotoxicity, cancer, soluble ligands
Introduction
Cytotoxic lymphocytes include cytotoxic T lymphocytes (CTLs), γδ T cells, and NK cells. NK cells are a component of the innate immune system and use multiple receptors to recognize their targets. NK cell cytotoxicity is determined by a balance of signals (1) emanating from the recognition of “missing-self” (2) and “induced-self” on target cells. “Missing-self” recognition has been extensively studied and involves the activation of NK cells when they encounter cells with low or absent expression of major histocompatibility complex class I molecules. In this circumstance, the inhibitory receptors for MHC class I, which have immunoreceptor tyrosine-based inhibition motifs (ITIM) in their cytoplasmic domains, are not engaged by MHC ligands on the target cells, thereby allowing an immune response to proceed. Such inhibitory receptors include the human killer cell immunoglobulin-like receptors (KIR), members of the mouse Ly49 receptor family, and the CD94/NKG2A receptor. Their MHC ligands are well characterized and have been reviewed elsewhere (1). In contrast, NK cell recognition of “induced-self”, or self-proteins upregulated in infected or transformed cells, is only partially understood. Although many NK cell activating receptors have been proposed to mediate “induced-self” recognition, the exact ligands recognized remain unknown in many cases. An exception to this is NKG2D, an invariant receptor shared by NK cells and T cells that can potently induce killing. NKG2D recognizes a family of “induced-self” ligands that has been extensively studied and remains a field of active investigation. Here, we review the discovery of the NKG2D ligands and their striking diversity in structure, expression patterns, and regulation. In addition, we discuss the host response to both membrane-bound and secreted NKG2D ligands and summarize the models proposed to explain the consequences of this differential expression.
NKG2D receptor complex and signaling
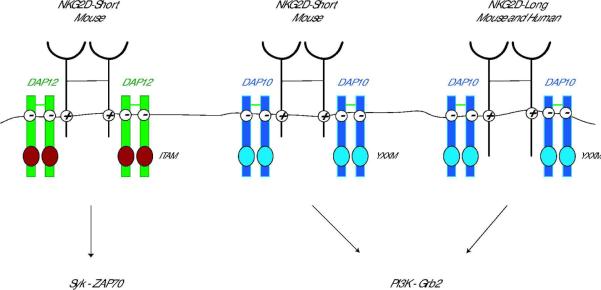
NKG2D is a type II transmembrane-anchored C-type lectin-like receptor expressed as a disulfide-linked homodimer on the cell surface and is encoded by the KLRK1 (killer cell lectin-like receptor subfamily member 1) gene. KLRK1 is highly evolutionarily conserved and is located on chromosome 6 in mice and on a syntenic region of human chromosome 12. Essentially all NK cells, most NKT cells, subsets of γδ T cells, all human CD8+ T cells, activated mouse CD8+ T cells, and a subset of CD4+ T cells express NKG2D. NKG2D-deficient mice have recently been generated. These mice exhibit normal lymphocyte numbers, including NK cells, in all organs analyzed (3). In addition, NKG2D-deficient NK cells express normal levels of maturation markers, as well as an intact repertoire of activating and inhibitory receptors (3), although subtle effects on NK cell development have been reported (4). NKG2D-deficient mice are impaired in the killing of certain tumors in vitro and Guerra et al. (3) also showed that they had an increased susceptibility to primary tumorigenesis in vivo, thus confirming the important role of NKG2D in tumor immunosurveillance. NKG2D has no signaling motif within its short intracellular domain (5), and similarly to many other activating receptors, associates with signal-transducing proteins via charged residues in its transmembrane domain (Fig. 1). In mice, there are two isoforms of NKG2D generated by alternative splicing that differ in the presence or absence of 13 amino acids at the N-terminus in the cytoplasmic domain (6). The long form of NKG2D (NKG2D-L) associates exclusively with the DAP10 adapter protein. In contrast, the short form of NKG2D (NKG2D-S) can pair with either DAP10 or another signal-transducing protein, DAP12 (6, 7). This differential adapter pairing has functional consequences, as the different adapters trigger distinct signaling cascades. The cytoplasmic YINM motif of DAP10 recruits the p85 subunit of phosphoinositide kinase-3 (PI3K) and growth factor receptor-bound protein 2 (Grb2) (8, 9). DAP12 carries an immunoreceptor tyrosine-based activation motif (ITAM) whose phosphorylation leads to the recruitment of the zeta-chain associated protein kinase 70 (Zap70) and spleen tyrosine kinase (Syk) (10). Thus, NKG2D engagement can result in both the PI3K and Grb2 and Syk and Zap70 signaling cascades. Each NKG2D homodimer associates with two homodimers of DAP10, hence forming a hexameric structure (11). Whether a single NKG2D homodimer can pair with both DAP10 and DAP12 homodimers has not yet been determined, but one could imagine this scenario to be beneficial to induce both signaling cascades upon triggering a single receptor. Crystal structures of both mouse and human NKG2D receptors in the soluble form and bound to ligands have been reported and suggest that NKG2D binds to its ligands through “rigid adaptation” recognition, allowing binding to a wide variety of ligands (12–14).
Fig 1. Schematic representation of the NKG2D receptor complex.
NKG2D is a type-2 transmembrane homodimer that signals via association with adapter molecules through charged residues in the transmembrane domain. Mouse NKG2D associates with both DAP10 and DAP12 signaling molecules, whereas human NKG2D associates with DAP10 only. Pairing with DAP12 results in the phosphorylation of the immunoreceptor tyrosine-based activation motif (ITAM) and triggering of the Syk and/or Zap70 cascade. Association with DAP10 leads to tyrosine phosphorylation on the YINM motif and triggering of the PI3K and Grb2 signaling cascade.
Discovery of NKG2D ligands
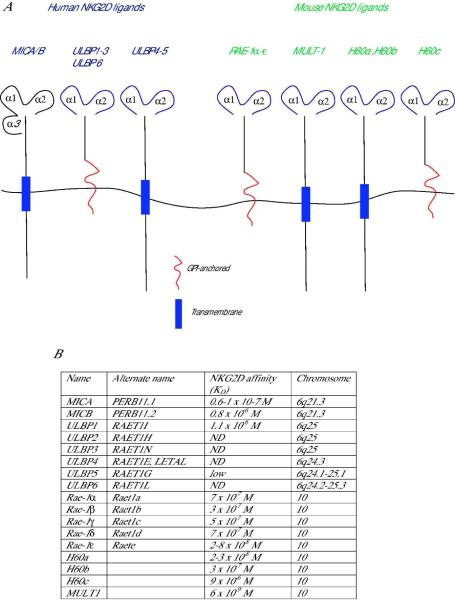
The most remarkable trait of the NKG2D receptor system is the diversity of ligands that can bind to this single invariant receptor. NKG2D ligands are distantly related homologues of MHC class I proteins and new members of this family continue to be discovered in both mice and humans (Fig. 2).
Fig 2. Structures and affinities of mouse and human NKG2D ligands.
(A) MHC class-I-chain-related protein A (MICA) and MICB are the only ligands known to date to contain an α3-like domain. All other ligands contain only α1 and α2 domains. Both mouse and human NKG2D ligands can be either transmembrane-anchored proteins (shown in blue rectangle) or glycosylphosphatidylinositol (GPI)- anchored (shown in red). (B) The affinities are expressed as the equilibrium dissociation constant (KD) and given for interactions between NKG2D receptor and ligands of the same species. Mouse NKG2D binds some human ligands (at lower affinity than mouse ligands), but human NKG2D does not bind mouse ligands.
MHC class-I-chain-related protein A (MICA) and B (MICB), which are encoded by genes within the human MHC and are genetically linked to HLA-B, were first described as cell-stress-induced proteins expressed in gastrointestinal epithelium (15). Using a soluble form of MICA, Bauer et al. identified its receptor as NKG2D (16). Subsequently, two human cell surface glycoproteins that bound to the human cytomegalovirus (HCMV) UL16 glycoprotein were described and named UL16-binding protein 1 and 2 (ULBP1 and ULBP2) (17). Similar to MICA and MICB, ULBP1 and ULBP2 also bound to the NKG2D receptor and stimulated human NK cells. Based on sequence homology with ULBP1 and ULBP2, four additional human ULBP family members were described and named ULBP3, ULBP4, RAET1G (or ULBP5), and RAET1L (or ULBP6) (18–20). All six ULPB family members, officially named RAET1 genes, are encoded in a gene cluster on chromosome 6 (6q24.2–6q25.3), which is syntenic to a region on mouse chromosome 10 that contains the mouse Raet1 genes which are orthologs of the human RAET1 genes.
The prototype member of Raet1 gene family was first discovered as retinoic acid early inducible cDNA clone-1 (Rae-1), which was rapidly induced on F9 teratocarcinoma cells in response to treatment with retinoic acid (21, 22). Subsequently, two groups detected binding of a soluble form of mouse NKG2D to mouse transformed cell lines and used expression cloning techniques to identify the NKG2D ligands (23, 24), which included Rae-1 and a related protein name histocompatibility antigen 60 (H60) (25). Presently, there are five known members of the Rae-1 family, named Rae-1α, Rae-1β, Rae-1γ, Rae-1δ, and Rae-1ε, which are differentially expressed in various mouse strains and highly related to each other (>85% identity). The H60 family comprises 3 members. H60a, the first ligand of the family to be described, was initially identified as a minor histocompatibility antigen by immunizing C57BL/6 mice with MHC-identical BALB.B cells (25). Recently, using the amino sequence of H60a as a query, Takeda et al. and Whang et al. identified two novel members of this family, named H60b and H60c (26, 27). Finally, Murine UL-16-binding protein-like transcript 1 (MULT1) is the unique member of the third family of mouse NKG2D ligands and was found by database searching for mouse sequences with similarities to human ULBP (28, 29).
Structural nature of membrane-bound ligands
Mouse and human NKG2D ligands are structural homologs of MHC class I molecules but remain a relatively distantly related family. The NKG2D ligands differ widely in sequence, domain structure, and affinity for the NKG2D receptor (Fig. 2). MICA and MICB are encoded within the human MHC, with which they share 28–35% sequence homology. Similarly to MHC class I molecules, MICA and MICB possess three immunoglobulin (Ig)-like domains (α1, α2, and α3) and have a short cytoplasmic tail. Unlike MHC molecules, MICA and MICB do not associate with β2-microglobulin or bind peptides. Indeed, the α1 and α2 domains lack the critical residues in conventional MHC class I molecules that have been shown to interact with antigenic peptides. The other mouse and human NKG2D ligands are structurally similar to MIC, but lack the α3 domain (Fig. 2).
NKG2D ligands differ in the way they are attached to the membrane. Human ULBP1, ULBP2, ULBP3, and ULBP6 and mouse Rae-1α-ε and H60c are attached to the cell surface membrane via glycosylphosphatidylinositol (GPI) anchors. Human MICA, MICB, ULPB4, and ULBP5 and mouse H60a and H60b are transmembrane proteins and have cytoplasmic tails of varying length and sequences. It has been suggested that the membrane anchorage of NKG2D ligands might impact their affinity for lipid rafts (30). Specifically, the GPI-anchored ULBP1, ULBP2, and ULBP3 glycoproteins are constitutively present in lipid rafts, whereas the transmembrane domain-containing MICA is not (30).
NKG2D ligands are highly polymorphic, particularly MICA and MICB genes for which 70 and 31 alleles have been described, respectively (http://www.ebi.ac.uk/imgt/hla/align.html). There is also evidence for some degree of polymorphism in the mouse Raet1 and H60 genes, as well as the human RAET1 genes and promoter sequences (31, 32). Interestingly, allelic variants of these ligands have been shown to bind with variable affinity to NKG2D (33, 34).
Diversity of ligands driven by viral pressure
There is ample evidence of pathogens driving the diversity of NKG2D ligands. Viruses have evolved numerous mechanisms to evade NK cells (35), and in particular NKG2D-mediated viral surveillance. Most examples of NKG2D evasion mechanisms come from the study of human and mouse cytomegalovirus (HCVM and MCMV, respectively). Both HCMV and MCMV upregulate transcription of the ligands for NKG2D, which would potentially result in NKG2D-mediated lysis of infected cells by NK cells (35). As a result, viruses have deployed evasive maneuvers to prevent expression of these NKG2D ligands on the cell surface. The HCMV protein UL16 binds to ULBP1, ULBP2, ULBP6 (RAET1L), and MICB and retains these ligands intracellularly (36–42). However, UL16 is unable to bind to MICA, ULBP3, and ULBP4. Therefore, these host genes may have evolved to counter the action of UL16 and permit expression of NKG2D ligands on the surface of the HCMV-infected cell. In response, HCMV likely evolved another immunoevasin, UL142, which binds to MICA and prevents its expression via retention of full-length MICA in the cis-Golgi (43, 44). Interestingly, UL142 does not bind to the MICA*008 allele, which lacks a cytoplasmic tail, thereby making it resistant to the action of UL142. The MICA*008 allele is frequently found in the human population, suggesting selective pressure has been exerted by HCMV on humans. Similarly to HCMV, MCMV encodes immunoevasins that prevent accessibility of mouse NKG2D ligands to the cell surface. The MCMV gene products m145, m152, and m155 selectively retain in the cytoplasm MULT1, Rae-1, and H60, respectively (45–48). In addition, m138 also downregulates H60, MULT1, and Rae-1ε (49, 50). These findings highlight the benefit for ligands to exhibit diversity and polymorphism in order to maintain proper recognition of infected cells by NK cells.
Expression
Normal cells
Despite the widespread agreement that NKG2D ligands are upregulated in “stressed cells”, ligand transcripts and sometimes protein can in some cases be found in normal cells. Raet1 transcripts have been described in the embryonic brain of 129/J mice, but are absent post-birth (51). Whether Rae-1 plays a role in the embryo during development remains unknown. H60a mRNA is found in multiple tissues, including spleen, cardiac and skeletal muscle, thymus, and skin, and H60b mRNA is limited to cardiac and skeletal muscles. The most recent addition to the H60 family, H60c, is transcribed largely in the skin (26, 27). Interestingly, H60a is productively expressed in BALB/c mice, but not C57BL/6 mice (hence it serves as a minor transplantation antigen), whereas H60b and H60c transcripts are detected in both C57BL/6 and BALB/c mice. MULT1 mRNA is found in the heart, thymus, lung, and kidney across most mice strains (28). The transcription of human NKG2D ligands exhibits a similar broad pattern of expression. MICA protein is expressed constitutively in intestinal epithelial cells (15). In healthy individuals, low levels of constitutive MICA expression does not result in immune cell attack of the gut; however in Crohn's and celiac autoimmune diseases, NKG2D+ intraepithelial lymphocytes (IELs) can attack the gut epithelium, presumably through elevated expression of MICA (52). MICA proteins and ULBP molecules are also found on both primary bronchial epithelial cells and epithelial cell lines (53). Transcripts of both MICA and MICB have recently been shown through a total body scan to be widely distributed, except in the central nervous system (54). Similarly to mouse MULT1, human ULBP transcripts appear widely expressed in humans, but lack of good staining reagents has prevented a thorough analysis of protein expression (17, 18).
NKG2D ligands are also upregulated on rapidly proliferating cells. Zwirner et al. first reported that phytohemagglutinin (PHA) induced the expression of MICA protein in CD4+ and CD8+ T cells (55). TCR/CD3 engagement and costimulation via CD28 induced a sustained increased expression of MICA on activated allogeneic CD4+ and CD8+ T cells (56). In addition, Cerboni et al. described the induction of MICA and ULBP1-3 on a fraction of dividing CD4+ and CD8+ T cells activated with the superantigen Staphylococcus aureus enterotoxin B (57). In mice, expression of Rae-1, and in lower amounts H60a, was detected on BALB/c, but not C57BL/6, bone marrow cells repopulating lethally irradiated recipients (58). Ovalbumin (OVA)-specific T cells activated with OVA antigen were also shown to upregulate H60 in BALB/c mice (59).
In summary, transcription of the genes encoding the human and mouse NKG2D ligands have been detected in numerous normal healthy tissues in the adult and in the normal mouse embryo. However, as discussed below post-transcriptional mechanisms exist to prevent translation and expression of these ligands in the healthy individual, presumably to avoid autoimmunity. There are conflicting reports in the literature regarding the expression of NKG2D ligand proteins in healthy, adult tissues, and some reports of protein expression in healthy tissues might be due to non-specific staining in immunohistochemistry studies. In general, there is consensus that if NKG2D ligands are expressed in normal adult tissues, it is in low amounts, possibly below the levels needed to activate immune cells expressing NKG2D receptors.
Virally infected cells
Viral infection induces the expression of NKG2D ligands, but the exact mechanism by which this occurs is for the most part unknown. Viral products could directly affect the transcriptional control of NKG2D ligands. Alternatively, infection could indirectly promote ligand expression through the induction of interferons or cytokines. As described above, the role of NKG2D has been most extensively studied following infection with mouse and human cytomegalovirus. As noted before, the MCMV and HCMV genomes encode proteins that prevent the expression of NKG2D ligands on the surface of virally infected cells. NKG2D is also involved in the control of other viral infections such as ectromelia (mousepox) virus (ECTV). In the mousepox-resistant C57BL/6 strain, depletion of NK cells results in increased viral titers and death (60). Recently, Fang et al. determined that NKG2D is important in the early resistance to mousepox (61). Infection of mouse embryonic fibroblasts (MEFs) in vitro with ECTV increased the expression of MULT1 at 18 h post-infection. In addition, in vivo infection with ECTV resulted in increased Raet1 transcripts in the draining lymph nodes of infected mice, as compared with uninfected controls. Infection with a neurotropic JHM strain of mouse hepatitis virus (MHV) also lead to increased transcription of H60, MULT1, and Raet1 in the brain of BALB/c mice (62). Furthermore, blocking NKG2D during acute MHV infection increased mortality (62). By contrast, in a mouse model of human hepatitis B virus (HBV) infection, blocking NKG2D diminished hepatitis and liver pathology (63, 64). In addition, human immunodeficiency virus (HIV) infection of primary CD4+ T cell blasts induced the expression of ULBP1, ULBP2, and ULBP3 (65). Recently, Ward et al. attributed this ligand induction to the action of the HIV vpr gene product (66). Vpr was sufficient to induce ligand expression and acted through the activation of the DNA damage pathway to do so (66). Interestingly, there is evidence that the HIV protein Nef can downregulate NKG2D ligands upon infection (67). Finally, in vitro infection of human dendritic cells with influenza virus leads to the upregulation of ULBP1, ULBP2, and ULBP3 (68). Collectively, these findings indicate that several viruses can induce NKG2D ligands and that the NKG2D receptor on NK cells or T cells is important in the control of these infections.
Transformed cells
Many tumor cell lines and freshly extracted primary tumors express NKG2D ligands constitutively. Prior studies have established that expression of NKG2D ligands on tumors render them susceptible to killing by NK cells in vitro (16, 69, 70) and result in the in vivo rejection of transplantable tumors expressing these ligands (71, 72). Moreover, NKG2D ligands have been reported on a wide variety of human and mouse tumors. MICA and MICB are expressed on a subset of human hepatocellular carcinoma tissues and are involved in hepatoma cell sensitivity to NK cells (73). Tumor cells extracted from patients with different types of leukemia, including AML (acute myeloid leukemia), ALL (acute lymphatic leukemia), CML (chronic myeloid leukemia), and CLL (chronic lymphatic leukemia), express heterogeneous levels of NKG2D ligands, MICA being the most highly expressed of all (74). In addition, using the human NK cell line NKL as effector cells, Salih et al. showed that expression of NKG2D ligands on patient AML and CML cells rendered them susceptible to NK cell-mediated lysis in an NKG2D-dependent manner (74). Glioblastoma cells express the NKG2D ligands MICA, MICB, and ULBP1-3 mRNA (75). Interestingly, high expression of MHC class I on glioblastoma cells prevented efficient NKG2D-mediated killing of tumor cells. Killing could be restored by adenovirus-mediated over-expression of MICA in metastatic glioma cells (75). In colorectal cancer patients, high levels of MICA were associated with a good prognosis (76). Human melanoma cells express high amounts of NKG2D ligands (77), and NKG2D ligands expression was lost during the progression of uveal melanoma (78).
In mice, NKG2D ligand expression in primary tumorigenesis has not been extensively analyzed. Using the well-studied transgenic adenocarcinoma of the mouse prostate (79) (79) model of prostate cancer (79), Guerra et al. observed sporadic expression of NKG2D ligands on tumors (3). Interestingly, higher levels of ligands were observed on late-arising small tumors, as compared with early-arising large tumors. In addition, tumors induced in NKG2D-deficient mice expressed higher amounts of NKG2D ligands; strongly supporting the hypothesis that immunoselection by NKG2D favors the loss of NKG2D ligand expression by primary tumors. These investigators also investigated the role of NKG2D in a mouse model of lymphoid tumorigenesis, in which the Eμ-myc transgene drives c-myc oncogene expression in the B cell lineage. Again, NKG2D ligands MULT1 and Rae-1 were detected at variable amounts on primary tumors, but their expression was not increased in NKG2D-deficient mice, suggesting that NKG2D is not involved in tumor progression in this setting. In summary, NKG2D ligands are expressed on the majority of tumors from essentially all cell and tissue types, and in some cases can elicit a productive immune response.
Regulation of ligands
Transcriptional regulation
The three main mechanisms by which NKG2D ligand transcription can be induced are DNA damage, TLR stimulation, and cytokine exposure. The DNA damage response pathway is involved in maintaining the integrity of the genome. The PI3K-related protein kinases ATM (ataxia telangiectasia, mutated) and ATR (ATM and Rad3 related) sense DNA lesions, specifically double-strand breaks and stalled DNA replication, respectively. This sensing results in cell-cycle arrest and DNA repair, or cell apoptosis if the DNA damage is too extensive to be repaired. This pathway has been shown to be constitutively active in human cancer cells (80–82). Gasser et al. provided evidence that this pathway actively regulates NKG2D ligand transcription (83). Both mouse and human cells upregulated NKG2D ligands following treatment with DNA-damaging agents. This effect was dependent on ATR function, as inhibitors of ATR and ATM kinases prevented ligand upregulation in a dose-dependent fashion. These findings provide a link between the constitutive activity of the DNA damage response in tumors (80, 81) and the frequent upregulation of NKG2D ligands by these transformed cells. The exact molecular events linking the ATR/ATM-dependent recognition of DNA damage and the transcription of NKG2D ligands remain elusive.
Toll-like receptor (TLR) signaling also results in NKG2D ligand transcription. Treatment of peritoneal macrophages with TLR agonists in vitro, and injection of LPS in vivo both resulted in Rae-1 upregulation on peritoneal macrophages (84). TLR agonists increased the transcription of Raet1 genes but not MULT1 or H60, in a Myd88-dependent fashion. Subsequently, various groups have observed a similar effect of TLR agonists on human cells (85, 86). TLR signaling on dendritic cells (DCs) also results in NKG2D ligand expression. Specifically, two groups showed the differential upregulation of NKG2D ligands, particularly ULBP1 and ULBP2, by TLR agonists such as poly(I:C) and LPS (68, 87).
Cytokines can also influence NKG2D ligand expression. In particular, interferons have pleiotropic effects on NKG2D ligand expression. In humans, IFN-α leads to the expression of MICA on dendritic cells (88). By contrast, Bui et al. showed that IFN-α and IFN-γ treatment led to the selective downregulation of H60 on certain mouse sarcoma cells. This STAT-1-dependent effect occurred at the transcript level (89). In accordance with this study, treatment of human melanoma cells with IFN-γ resulted in decreased MICA message levels, also in a STAT-1-dependent fashion (90). Finally, transforming growth factor (TGF-β) also decreases the transcription of MICA, ULBP2, and ULPB4 on human malignant gliomas (91, 92). Therefore, cytokines and interferons can differentially affect NKG2D ligand expression in different cell types and environments.
Other stimuli have also been reported to induce NKG2D ligand transcription. The Raet1 genes were discovered because they were induced on F9 teratocarcinoma cell lines following treatment with retinoic acid (21). A retinoic acid- responsive element was mapped in the promoter of Raet1, suggesting a direct regulation of Rae-1 expression by retinoic acid (RA) (93). Subsequently, treatment of hepatocellular carcinoma cells with RA was also found to induce the expression of MICA and MICB (73). In addition, the promoters of MICA and MICB contain heat shock response elements, and MIC transcripts can be induced in stressed cells (94). Adenovirus E1A oncogene was also shown to upregulate NKG2D ligands on mouse and human cell lines (95). Finally, the transcription factor AP-1, which is involved in tumorigenesis and cellular stress responses, was found to regulate Raet1 through the JunB subunit (96). Presently, transcriptional regulation of the genes encoding NKG2D ligands in humans and mice are poorly understood and this represents an important area for future investigation.
Post-transcriptional and post-translational regulation
Various mechanisms are responsible for the post-transcriptional regulation of NKG2D ligands. Stern-Ginossar et al. identified a group of endogenous cellular microRNAs (miRNAs) that bound to the 3'-UTR (untranslated region) of MICA and MICB (97) and repressed their translation. In addition, Yadav et al. identified four miRNAs that suppressed MICA expression (98). In accordance with these findings, silencing of Dicer, a key protein in the miRNA processing pathway, leads to the upregulation of MICA and MICB (99). However, in this study, upregulation of NKG2D ligands was found to be dependent on the DNA damage sensor ATM, thus suggesting that upregulation of NKG2D ligands in the absence of Dicer might be due to genotoxic stress in addition to the absence of regulatory miRNAs. In mouse cells lacking Dicer, upregulation of Rae-1 is frequently observed on splenocytes (N. Bezman, unpublished observation). Interestingly, HCMV was found to encode a viral miRNA, hcmv-miR-UL112, that competed with endogenous miRNA for binding to MICA and MICB 3'-UTR, thus repressing the translation of these ligands (100).
Recently, Nice et al. elegantly showed that MULT1 protein undergoes ubiquitination dependent on the lysines in its cytoplasmic tail, resulting in its rapid degradation (101). Ubiquitination was reduced in response to heat shock or UV irradiation, thus allowing cell surface expression of MULT1. Thus, heat shock operates on two levels: it increases the transcription of human MICA and MICB ligands, and it increases mouse MULT1 protein expression by decreasing its ubiquitination. Genotoxic stress did not affect MULT1 ubiquitination, illustrating the fact that different stimuli regulate NKG2D ligands differently. Whether other ligands with long cytoplasmic tails are similarly regulated has not yet been investigated. The presence of multiple lysines in the cytoplasmic tail of H60a, H60b, MICA, MICB, and RAET-1G suggests that this translational control mechanism might be used by other NKG2D ligands. Interestingly, Thomas et al. have recently described the capacity of the KSHV (Kaposi's sarcoma-associated herpesvirus)-encoded E3 ubiquitin ligase K5 to down-regulate cell surface expression of MICA and MICB (102). In this case, ubiquitination resulted in the redistribution of MICA to the plasma membrane, rather than its targeting to degradation as observed with MULT1. Because ubiquitination is dependent on motifs in the cytoplasmic domains of the ligands, it is likely that additional posttranscriptional mechanisms are responsible for controlling the surface expression of the GPI-anchored NKG2D ligands proteins that lack cytoplasmic regions.
In addition, multiple putative regulatory motifs in the cytoplasmic domains of H60a, H60b, and MULT1 warrant further investigation. These include regulatory motifs such as the sorting/internalization motif in H60a (103). Another important mechanism of NKG2D ligand expression is through shedding from the cell surface. We will review this evasion mechanism in more detail later in this review.
Host response to membrane-bound ligands
Normal acute response to membrane ligands by immune cells
Expression of NKG2D ligands can lead to a very rapid immune response, in particular by NK cells for which NKG2D is a primary activating receptor. Ectopic expression of NKG2D ligands on tumors renders them susceptible to NK cell lysis in vitro (71, 72). In addition, tumors bearing NKG2D ligands are rejected in vivo, or progress less rapidly than parental tumors (71, 104). This acute rejection by NK cells is not restricted to transformed cells, as NK cells can also potently reject Rae-1-expressing splenocytes in vivo ((105) and our unpublished observation). In addition to cell lysis, activating NK cells via NKG2D can trigger the production of cytokines, including IFN-γ, GM-CSF, and MIP-1β (106).
NKG2D-bearing T cells also respond to cells expressing NKG2D ligands. Most studies have suggested that NKG2D plays co-stimulatory role on CD8+ T cells, whereas it is usually insufficient to generate a T cell response when triggered alone (107–109). However, the ability of NKG2D to costimulate T cells is dependent upon the activation state of the T cells, because in many situations engaging NKG2D on CD8+ T cells does not induce activation or augment TcR-induced responses (110). In accordance with these findings, we have recently investigated the response of CD8+ T cell isolated from mice infected with MCMV to dendritic cells expressing Rae-1 and have observed no enhancement of the T cell response (our unpublished observation). Therefore, why T cells can be costimulated by NKG2D in some situations, but not others, is presently unknown.
Cutaneous TCRγδ+ intraepithelial lymphocytes (IELs), also known as dendritic epidermal T cells (DETCs), express NKG2D (15, 16). Using various mouse models of cutaneous malignancy, Girardi et al. showed a critical role of NKG2D+ DETCs for tumor recognition (111). TCRγδ+ T cells efficiently killed PDV tumor cells (mouse keratinocytes transformed with the carcinogen DMBA) in a NKG2D- and TCR-dependent fashion in vitro. Moreover, TCRδ-deficient mice exhibited increased susceptibility to PDV tumor challenge and chemically induced carcinogenesis. Recently, Whang et al. defined a novel NKG2D ligand named H60c, which is expressed in mouse skin (27), and observed efficient cytolysis of H60c-expressing keratinocytes by DETCs. This effect was dependent on NKG2D, as NKG2D-deficient DETCs were severely impaired in their ability to kill keratinocytes.
Strid et al. demonstrated rapid pleiotropic effects in the skin after Rae-1 expression (112). Using an elegantly designed “bi-transgenic” mouse in which Rae-1 can be induced in the epidermis following doxycycline treatment, they showed that in the absence of any inflammation, acute expression of Rae-1 resulted in a local immune reorganization. Within 120 h of doxycycline treatment, both Langerhans cells and DETCs exhibited changes in morphology and activation markers. The effect on DETCs morphology is unsurprising given that these cells express NKG2D and that engagement of NKG2D leads to the downstream activation of Vav-1, which has a critical role in controlling NK cell cytoskeletal polarization (113). However, Langerhans cells do not express NKG2D, and their response to Rae-1 expression is likely indirect, probably due to cytokines induced by NKG2D-mediated activation of DETCs in the tissue.
Importantly, transient NKG2D ligand expression does not always induce effector cell-mediated cytotoxicity. NKG2D-mediated crosstalk between NK cells and dendritic cells (DC) during viral infection has been suggested to augment NK cell responses (68, 114, 115). To investigate the effect of NKG2D ligand expression in various cell populations, we have recently generated a knock-in mouse in which a LoxP-stop-LoxP Rae-1ε cassette was inserted into the Rosa26 locus. Crossing this mouse to various tissue- or cell-restricted Cre recombinase will enable restricted Rae-1 expression into a location of interest. In particular, we are currently investigating the effects of DC-restricted expression of Rae-1 to explore the crosstalk between DC and NK cells during viral infections.
Chronic response to membrane-bound NKG2D ligands
Despite the efficient eradication of cells that express NKG2D ligands transiently, constitutive ligand expression has been shown to impair NKG2D function in humans and mice. This observation was first reported by Groh et al. who analyzed tumor-infiltrating lymphocytes (TILs) from human epithelial tumors (116). Presence of MICA on tumor cells consistently correlated with decreased NKG2D levels on NK cells and CD8+ T cells and impaired NKG2D-mediated IFN-γ production by CD8+ T cells. Subsequently, various groups described similarly impaired NKG2D function in patients with NKG2D ligand-expressing tumors (117–119).
Ligand-induced downregulation of the NKG2D receptor was also described by Ogasawara et al. in NOD mice (120). When NK cells from NOD mice were activated by IL-2, the NK cells then themselves expressed NKG2D ligands, which in turn downregulated their expression of NKG2D receptor and impaired its function. Moreover, when NK cells from C57BL/6 mice were co-cultured in vitro with tumor cells expressing NKG2D ligands, this again resulted in the down-modulation of NKG2D on the NK cells and impaired their NKG2D-dependent functions (120, 121). Subsequently, several mouse models have been constructed to gain further understanding on the effect of sustained NKG2D engagement on receptor function (Table 1). To separate ligand expression from any other aspect of tumorigenesis or inflammation, most models developed have expressed a NKG2D ligand under a ubiquitous promoter in an otherwise normal mouse. In the majority of cases, these transgenic mice developed normally, and exhibited no sign of autoimmunity. One exception comes from a study in which a MICB transgene was driven by a ubiquitous promoter (122). These mice exhibited a 50% increase in the number of white blood cells, and a 10 to 20% reduction in body weight compared to their littermate control. In addition, transient exfoliation of the skin was observed at a young age. This study suggests an involvement of human MICB in skin inflammation, but it did not investigate the effect of MICB on lymphocyte populations and receptor expression, notably NKG2D on NK and CD8+ T cells. Moreover, off-target effects of insertion of MICB into the mouse genome might explain these unexpected results. In a separate study, Wiemann et al. created a mouse expressing human MICA under the mouse MHC class I H-2Kb promoter on the C57BL/6 background (123). These mice did not display overt signs of autoimmunity. Recapitulating earlier in vitro observations, constitutive MICA expression resulted in the down-modulation of NKG2D on the surface of NK cells. As a result, constitutive MICA expression impaired the ability of NK cells to reject MICA-transfected RMA tumors. These investigators also investigated the effect of MICA expression on the CD8+ T cell response to L. monocytogenes. Infection of mice expressing MICA driven by the H2-Kb promoter with a L. monocytogenes strain recombinant for a secreted form of OVA resulted in lower percentages of IFN-γ- secreting OVA-specific CD8+ T cells compared to wildtype mice. Together these results showed that constitutive MICA expression results in NKG2D dysfunction on NK cells and L. monocytogenes-activated CD8+ T cells. Park et al. expressed human MICA under the control of the T3b promoter, leading to restricted expression of MICA in the intestine (124). This resulted in the clonal expansion of CD4+, CD8αα-double positive IELs in the small intestine. T3b-MICA transgenic mice developed less severe DSS-induced colitis compared to wildtype mice. These data suggest that tissue-restricted expression of MICA might lead to the development of a regulatory subset of immune cells that prevents intestinal inflammation or to the down-modulation of NKG2D, which would suppress effector T cell function. Whether NKG2D levels on lymphocytes were affected in these T3b-MICA transgenic mice was not analyzed. In another mouse model, Oppenheim et al. expressed Rae-1ε in FVB mice either under the involucrin promoter (inducing squamous epithelium expression) or the chicken β-actin promoter (ubiquitous expression) (105). Similarly to the findings of Wiemann et al., these studies also revealed a defect in NKG2D-mediated cytotoxicity in vivo and an increased susceptibility to tumorigenesis. However, Rae-1 transgenic (Tg) mice generated anti-HY-specific memory T cells as effectively as wildtype controls and CD8+ T cells had a normal response to lymphocytic choriomeningitis virus (LCMV) at day 7 post-infection. We have generated transgenic mice in the C57BL/6 strain expressing Rae-1ε under the control of the human β-actin promoter (58, 110). These mice are also impaired in NKG2D-dependent functions and have increased susceptibility to Rae-1-transduced RMA tumors (our unpublished data). However, upon MCMV infection, Rae-1 Tg mice could efficiently generate MCMV-specific CD8+ T cells, despite reduced NKG2D levels on these cells (manuscript in preparation). Together with observations from Oppenheim et al., our findings suggest that CD8+ T cells from Rae-1 Tg mice do not require NKG2D for the generation of effector and memory T cell functions. The defect in IFN-γ production by CD8+ T cells in MICA-Tg mice observed by Wiemann et al. in the context of L. monocytogenes infection might be due to impaired NKG2D function on another lymphocyte subset that influences the CD8+ T cell response, or simply to differential requirement for NKG2D on CD8+ T cells in combating viral versus bacterial infections.
Table 1.
Mouse models of NKG2D ligand expression
| Ligand | Promoter | Expression | Strain | Observation | Ref. |
|---|---|---|---|---|---|
| MICA | H-2Kb | All cells | C57BL/6 |
|
123 |
| MICA | T3b | Intestinal epithelium | C57BL/6 |
|
124 |
| MICB | CAG | All cells | C57BL/6 |
|
122 |
| Rae-1α | Human β-actin | All cells | FVB |
|
Our unpub.data |
| Rae-1ε | Involucrin | Epithelium | FVB |
|
105 |
| Rae-1ε | Ubiquitin | All cells | FVB |
|
105 |
| Rae-1ε | Human β-actin | All cells | C57BL/6 |
|
Our unpub.data |
| Rae-1ε | Human β-actin | All cells | BALB/c |
|
58 |
| Rae-1ε | Involucrin | Tet-inducible in epithelium |
|
112 |
Abbreviations: MCMV, mouse cytomegalovirus; DSS, dextran sulfate sodium; CTL, cytotoxic T lymphocytes; DETC, dendritic epidermal T cells, IEL, intraepithelial lymphocytes.
Interestingly, both in vitro (125) and in vivo (105) studies have suggested that constitutive NKG2D engagement also impairs NKG2D-independent pathways, particularly missing-self recognition by NK cells. In contrast to these observations, using our Rae-1 Tg mouse models, we have observed normal NKG2D-independent responses of NK cells having been exposed to Rae-1 constitutively (manuscript in preparation), indicating that disrupting the NKG2D signaling pathways in NK cells and T cells does not have “off-target” effects on immune responses.
Host response to soluble ligands
Discovery of soluble NKG2D ligands in humans
In addition to membrane-bound NKG2D ligands, secreted forms of the ligands have been described in humans. In 2002, two independent groups reported secretion of MICA proteins from tumor cells and the presence of high amounts of soluble MICA (sMICA) in cancer patient sera (116, 126). Secreted NKG2D ligands have been detected in a variety of cancers. Elevated amounts of soluble MICA are found in many cancers, including hematopoietic malignancies (74, 126, 127), epithelial cancers (116), colorectal cancer (128), liver cancer (129), prostate cancer (130), and melanoma (131). Subsequently, MICB secretion was also described in a variety of tumors (117). Cancer cells can also shed proteins of the ULBP family. ULBP-2 is secreted both from tumor cell lines and primary tumor cells from some patients with hematopoietic malignancies (132, 133). Recently, soluble ULBP2 in the sera of melanoma patients was also shown to be a strong indicator of poor prognosis (134). Although no evidence exists so far for shedding of additional ligands in human cancers, recent studies have shown that other NKG2D can be secreted from cells in vitro. Cerboni et al. developed an ELISA to measure soluble ULBP3 and found that it was secreted from mouse BaF/3 cells transfected with human ULBP3 (135). Finally, Song et al. also provided evidence for the existence of secreted ULBP1 protein, as detected by Western blot analysis of supernatants from gastric tumor cell lines (136).
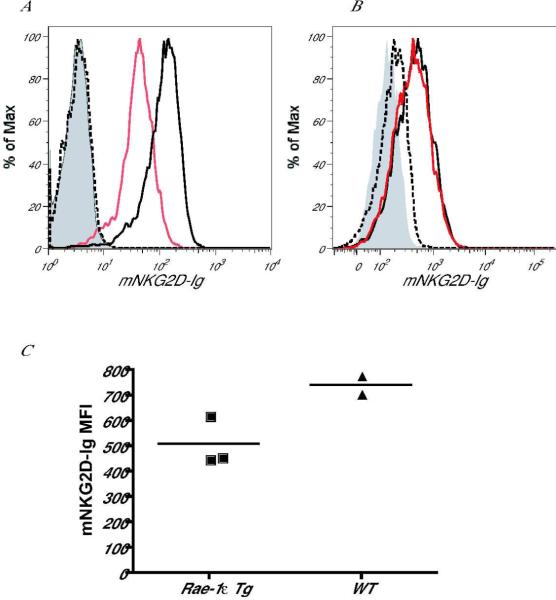
In mice, no soluble NKG2D ligands have previously been described. We found that the mouse ligand Rae-1 could be shed from Rae-1ε-transduced B16 melanoma tumor cells and from normal, healthy cells in Rae-1ε Tg mice. In order to detect soluble Rae-1, we incubated cell supernatants from Rae-1ε-transduced B16 melanoma cells with soluble recombinant mouse NKG2D-Ig (mNKG2D-Ig) fusion protein (or control Ig) for 30 minutes. We then used this reagent to stain human MICA-transduced BaF/3 cells. Decreased staining of MICA- BaF/3 cells indicated the presence of soluble ligand in the supernatant of the Rae-1-transduced B16 tumors. Using this assay, we found that supernatant from Rae-1ε-transduced B16 melanoma cells contained a soluble protein capable of binding to mNKG2D-Ig, which is absent from the supernatant of parental B16 cells (Fig. 3A). This indicates that Rae-1ε can be shed from the surface of tumor cells or secreted by the tumor cells. This effect was specific to B16 melanoma cells, because Rae-1ε-transduced RMA T lymphoma cells did not exhibit the same characteristic (Fig. 3B). In addition, to determine whether Rae-1 can be secreted from cells in Rae-1ε Tg mice, we compared the ability of sera from Rae-1 Tg or littermate control mice to bind to mNKG2D-Ig. We found that incubation of Rae-1ε Tg sera with mNKG2D-Ig resulted in a marked decrease of mouse NKG2D-Fc binding to human MICA-transduced BaF/3 cells, as compared to serum from healthy, wildtype mice (Fig. 3C). These results suggest that Rae-1ε can be shed in vivo in the absence of tumorigenesis. These findings are in accordance with data published on the secretion of NKG2D ligands by benign cells, such as in the context of autoimmunity (137, 138), pregnancy (139, 140) or SEB-activated T cells (135).
Fig. 3. NKG2D ligands can be secreted from tumor cell lines and Rae-1 Tg mice.
(A, B) Supernatant harvested from (A) B16 (black line) or Rae-1ε-transduced melanoma tumor cells (red line) or (B) RMA (black line) or Rae-1ε-transduced (red line) lymphoma cells was incubated with mouse NKG2D-Ig (mNKG2D-Ig) for 30 min on ice. This mixture was then used to stain MICA-transduced BaF/3 cell transfectants on ice for 30 min. A secondary goat anti-human IgG antibody was used to measure binding of mNKG2D-Ig to the transfectants. Controls included cells stained with control human Ig (grey filling) and cells stained with mNKG2D-Ig pre-incubated with soluble MULT1-secreting transfectants (dashed line). (C) Serum from Rae-1 Tg or littermate control mice was incubated with mNKG2D-Ig for 30 min on ice. This mixture was then used to stain MICA-BaF/3 cell transfectants on ice for 30 min. A secondary goat anti-human IgG antibody was used to measure binding of mNKG2D-Ig to the transfectants. Mean fluorescence intensity (MFI) of mNKG2D-Ig binding to MICA-BaF/3 cells is shown following incubation with WT or Rae-1 Tg sera.
Mechanisms of generating soluble ligands
Two distinct mechanisms of generating soluble NKG2D ligands have been described. The first mechanism involves the cleavage of ligands from the cell surface by proteases. Prior studies reported that a broad-range metalloprotease inhibitor (MPI) reduced the levels of soluble MICA (sMICA) detected in tumor cell supernatants and increased the levels of surface MICA on these tumors (126). Subsequently, metalloproteases were also found to be responsible for the shedding of both soluble MICB (sMICB) and soluble ULBP2 (sULBP2) (117, 133). One group reported that an inhibitor to phosphatidylinositol-specific phospholipase C (PI-PLC) increased the surface expression of GPI-anchored ULBP1 and ULBP2 on gastric tumor cell lines (136). Although these data suggest that PI-PLC might also be involved in cleaving NKG2D ligands, it is noteworthy that the investigators did not measure soluble ULBP in this assay. Thus, the increase in surface expression of NKG2D ligands might have been independent of their secretion. Recently, two groups have reported the involvement of members of the “a disintegrin and metalloproteinase” (ADAM) family in the shedding of NKG2D ligands (141, 142). Many ADAM members are membrane-tethered proteases, best known for their ability to cleave ectodomains of transmembrane proteins (143). Inhibitors of ADAM10 and ADAM17 (also known as TNFα-converting enzyme, or TACE) (114) suppressed MICA and ULBP2 shedding (141). In agreement with these findings, Kohga et al. recently showed that chemotherapy treatment of hepatocellular carcinoma cell lines downregulated ADAM10, which led to decreased amounts of soluble MICA in the circulation (144). In addition, ADAM17 silencing by siRNA significantly reduced shedding of MICB (142). Adding to our understanding of shedding mechanisms, Groh et al. showed that MICA is associated with endoplasmic reticulum protein 5 (ERp5) on the cell surface. ERp5 promotes shedding by forming transitory disulfide bonds with MICA, inducing a conformational change in the α3 domain of MICA (145). Interestingly, in search for metastatic-promoting factors by a forward genetic screen, Gumireddy et al. identified ERp5 as a protein promoting in vivo metastasis of breast cancer cells (146). Whether this ERp5-dependent tumor growth advantage was dependent on cleavage of NKG2D ligands from breast cancer cells was not investigated. Blocking ERp5 isomerase or ADAM protease activity might provide a therapeutic strategy to reduce secretion of NKG2D ligands by tumors.
A second mechanism to generate soluble NKG2D ligands is by alternative RNA splicing. Two groups have demonstrated the existence of alternative RNA splicing of the ULBP family of human ligands (19, 147). Splice variant transcripts of ULBP5 (RAET1G) encoded by the RAET1G2 gene, was detected in a T cell leukemia line, although in this study the presence of soluble protein in the cell supernatant was not analyzed (19). Similar to ULBP5, ULBP4 (RAET1E) can also be alternatively spliced to generate the soluble RAET1E2 form (147). These studies highlight that in addition to proteolytic cleavage at the protein level, alternative splicing at the RNA level might play an important role in NKG2D immune evasion.
Mouse models to understand the consequences of soluble ligands
Until recently, most studies investigating the role of soluble NKG2D in tumorigenesis have been solely correlative. Defining the role of soluble ligands in human cancer progression is complicated by the fact that tumors secrete a variety of factors that might influence NKG2D function autonomously, such as TGF-β (148–150). The initial study suggesting an immunomodulatory role of soluble MICA (sMICA) in cancer patients showed a correlation between the presence of soluble MICA in sera of patients with MICA+ epithelial tumors and the level of NKG2D down-regulation on tumor-infiltrating and peripheral blood CD8+ T cells (116). In addition, incubation of CD8+ T cells with sera from patients with MICA+ tumors decreased the level of NKG2D on CD8+ T cells. However, these sera might have contained other NKG2D-modulating factors such as TGF-β. Of note however, incubation of human lymphocytes in high amounts of recombinant sMICA (100 ng/mL) did lead to a decrease in surface NKG2D expression. Using a mouse model in which human MICA was expressed under the H-2Kb promoter, Wiemann et al. also detected secreted MICA in the sera of the mice; however, sMICA could not downregulate NKG2D. Incubation of wildtype splenocytes with MICA-transgenic (Tg) splenocytes modulated surface NKG2D levels on wildtype splenocytes, but soluble MICA (sMICA) from MICA-Tg mice sera did not. This difference might be due to differential binding affinities of MICA to mouse and human NKG2D. In additional studies, neither sULBP2 nor sMICA/B could downregulate NKG2D levels on the human NK cell lines NKL (117, 133). In this scenario, NKG2D affinity to human NKG2D ligands is not an issue. Altogether, these findings raise the important question of the physiological role of soluble NKG2D ligands during tumorigenesis.
Is tumor shedding of NKG2D ligands an efficient mechanism by which tumors evade NK cell immunosurveillance? A recent study investigated this exact question by designing a set of constructs encoding different variants of MICB. MICB was expressed either as a full-length protein (MIC), a shedding-resistant protein (MICA-A2), or a soluble protein (rsMIC). MICA-A2 contained an amino acid substitution in the α3 domain of MICB making it resistant to protease action. sMIC was generated by deleting the transmembrane and cytoplasmic regions of MICB. The authors transduced a prostate tumor model TRAMP-C2 (TC2) cell line with the different constructs and showed that shedding-resistant MIC-A2 prevented TC2 tumor formation, whereas sMIC allowed for faster TC2 tumor growth. These findings support the hypothesis that soluble NKG2D ligands secreted by tumor cells can enhance tumor growth in vivo. Following these findings, the authors hypothesized that a feasible way of inhibiting tumor growth would be to prevent MICA shedding in vivo. Because blocking ERp5 or the ADAM proteases would have pleiotropic effects, the authors suggested blocking the site on MICA that is recognized by the ERp5 isomerase. In a recent paper, the authors identified a 6 amino acid motif in the α3 domain of MICA that is critical for its interaction with ERp5, but dispensable for MICA recognition by NKG2D (151). Future efforts should be placed in developing small molecules inhibitors or blocking antibodies to prevent MICA shedding.
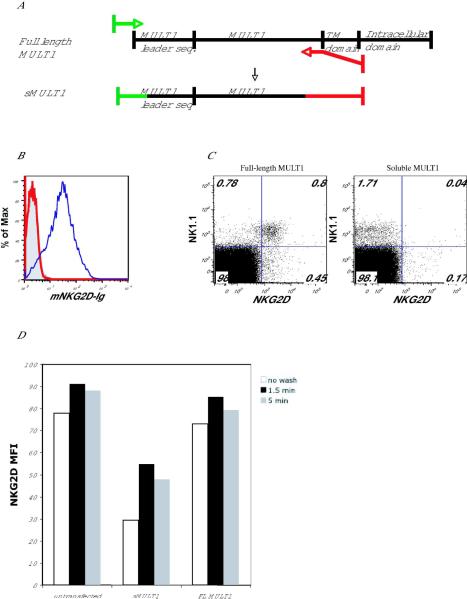
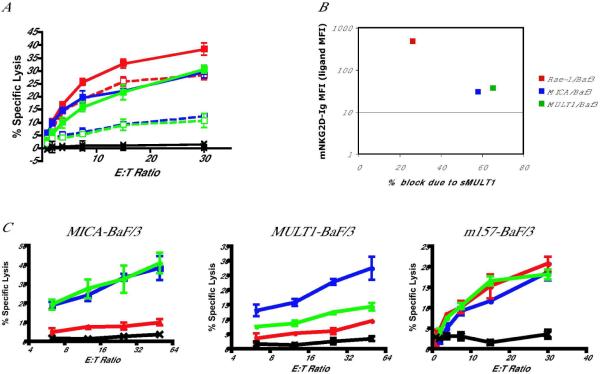
To investigate whether antibody blocking of secreted ligands might restore NKG2D function, we developed a model in which MULT1 would be in the soluble form, while tumors would express a membrane-bound Rae-1 ligand. That way, blocking of the soluble MULT1 using neutralizing antibodies against MULT1 would not impair recognition of tumors expressing cell surface-bound Rae-1. We created a truncated MULT1 construct by adding a STOP codon before the transmembrane and cytoplasmic domains (Fig. 4A). The resulting construct (sMULT1) was compared with the full-length construct (FL MULT1) in all studies. We transfected 293T cells with either sMULT1 or FL MULT1 constructs. After 48 h, we harvested the supernatant and removed cell debris by centrifugation. To test for the presence of sMULT1, we incubated supernatants with mouse NKG2D-Ig fusion protein and then used this reagent to stain human MICA-transduced BaF/3 cells (mouse NKG2D binds to human MICA ligands). Soluble MULT1 in the supernatant effectively bound mNKG2D-Ig and thus prevented staining of the MICA-transduced BaF/3 cells (Fig. 4B). In addition, we found that culturing mouse splenocytes with sMULT1 down-regulated NKG2D on NK cells, as well as αδ+ T cells and CD8+ T cells (Fig. 4C and data not shown). These results indicate that soluble MULT1 can effectively decrease NKG2D surface expression on lymphocytes. Reduction of NKG2D staining of NK cells and T cells cultured in the presence of sMULT1 was due to both NKG2D receptor internalization and receptor masking as shown with acid-washing experiments to remove bound sMULT1 from the cells (Fig. 4D). Acid washing of NKG2D-bearing NK cells and T cells pre-incubated with sMULT1 resulted in increased receptor expression, but not back to the level of control cells not exposed to sMULT1. We also asked whether sMULT1 could impair NKG2D-dependent cytotoxicity. We performed a standard chromium release cytotoxicity assay using as effectors IL-2 grown mouse NK cells pre-incubated with supernatant from 293T cells transfect with sMULT1 or FL MULT1. As targets, we used Rae-1-BaF/3, MICA-BaF/3, and MULT1-BaF/3 cells, which express varying amounts of NKG2D ligands, which bind to mouse NKG2D-Ig with different affinities (Fig. 5A). We found that sMULT1 decreased NK cell killing of these targets in a manner proportional to the amount of ligand present on the target cells (Fig. 5B), whereas supernatants from 293T cells transfected with a FL MULT1 construct did not affect NK cell killing due to the absence of soluble MULT1 in these cultures.
Fig. 4. Soluble MULT1 construct is efficiently secreted and downregulates NKG2D on splenocytes.
(A) A soluble MULT1 (sMULT1) construct was created by inserting a STOP codon at a site between the extracellular domain and the transmembrane domain of full-length MULT1 (FL MULT1) and cloning the PCR product into pEF-bOS vector. Constructs encoding sMULT1 or FL MULT1 were transfected into 293T cells. 48 h post-transfection, supernatant from sMULT1 or FL MULT1 transfectants was collected. (B) sMULT1(red line) or FL MULT1(blue line) supernatant was incubated with 10 μg/mL of mNKG2D-Ig for 30 min on ice. This mixture was then used to stain MICA-BaF/3 cell transfectants. Control human Ig staining is shown (grey filling). (C) sMULT1 (right plot) or FL MULT1(left plot) supernatant was added to splenocytes overnight. NKG2D expression on NK cells was detected by using anti-NKG2D (CX5) antibody. (D) Mouse NKG2D-transfected BaF/3 cells were incubated with supernatant from untransfected 293T cells or 293T cells transfected with sMULT-1 or FL MULT1. After 5 h, cells were spun and acid-washed (pH=2.5) for 1.5 and 5 min. Cells were then washed and stained with anti-NKG2D (CX5) and anti-NK1.1 to detect NK cells.
Fig. 5. Soluble MULT1 impairment of NKG2D cytotoxicity is reversed by the addition of an anti-MULT1 antibody.
(A–C) NK cells were isolated from spleen by removing CD4+ and CD8+ cells using antibody-coated magnetic beads, and then positively selecting for DX5+ cells. Cells were grown in IL-2-containing medium (4000 IU/ml IL-2) for 5 days. (A) On day 5, NK cells were harvested, incubated with FL MULT1 (solid line) or sMULT1 (dashed line) - containing supernatant for 2 h and then used in a standard 51Cr release assay against various NKG2D-ligand bearing target cells. MICA-BaF/3 (blue line), MULT1-BaF/3 (green line), and Rae1-BaF/3 (red line) cells were blocked to varying degree by the addition of sMULT1. (B) Blocking effect of sMULT1 is proportional to the amount of ligand detected on the cell surface of targets, and measured by staining with mNKG2D-Ig. (C) NK cells cultured in IL-2 for 5 days were incubated with FL MULT1 (blue line), sMULT1 alone (red line), or sMULT1 in the presence of 20 μ/ml anti-MULT1 antibody (green line) for 2 h. NK cells were then used in a standard 51Cr release assay against various NKG2D-ligand bearing target cells. Black line shows background killing of untransfected BaF/3 parental cells.
Finally, we tested the ability of anti-MULT1 monoclonal antibodies to reverse the block in NKG2D-dependent cytotoxicity mediated by sMULT1. Addition of an anti-MULT1 antibody during the cytotoxicity assay fully reversed the impaired killing of MICA-BaF/3 cells and partially reversed the impaired killing of MULT1-BaF/3 cells, presumably due to binding of the antibody to cell surface MULT1 on these MULT1-Ba/F3 target cells. Interestingly, sMULT1 had no effect on killing of BaF/3 cells transduced with MCMV m157, the ligand for the activating Ly49H receptor on mouse NK cells, suggesting that NKG2D engagement in this model does not cross-tolerize other NK cell activating receptors such as Ly49H (Fig. 5C).
Concluding remarks
Despite being one of the most extensively studied activating NK receptors, NKG2D maintains many elusive aspects. Not only are new MHC-class-I-related ligands and ligand polymorphisms regularly being described, but there is now evidence for new ligand isoforms, such as RAET1E2 and RAET1G2. The list of stimuli that induce NKG2D ligand expression is also large and growing. The specific molecular players linking the actual stimuli to the transcription of these ligands is not well understood. For example, despite strong evidence that the ATM/ATR DNA damage pathway leads to transcription of human and mouse NKG2D ligands (83), the transcriptional regulators that control the promoter of NKG2D ligands are unknown. A detailed characterization of the promoter regions of NKG2D ligands will be critical to advance our understanding of the transcriptional mechanisms controlling their expression.
Probably best understood is the signaling mechanism of the NKG2D receptor. We know a lot about the molecular players that link receptor triggering to downstream effector functions, namely cytotoxicity and cytokine production. However, it has become increasingly apparent that this cytotoxic receptor is under very stringent control, and that that exposure to too much ligand or too long exposure to ligands can have detrimental effects on NKG2D-mediated signaling. This leaves us with the challenge of understanding the tipping point between immune activation and immune suppression.
Once this transition point is better defined, the manipulation of ligand expression shows many promises therapeutically. Patients that lack ligand expression altogether in their tumors or pathogen-infected cells, as a result of viral immunoevasins or tumor escape variants, will benefit from ligand-inducing treatments, such as TLR agonists, DNA-damaging agents (for example in the setting of chemotherapy in tumor patients), or treatment with TGF-β antagonists (TGF-β is a known downmodulator of both NKG2D ligands and the NKG2D receptor). On the other hand, patients with constitutively high expression of NKG2D ligands that inactivates the NKG2D receptor on NK cells and T cells, as it occurs in certain cancer patients, might benefit from drugs that reduce ligand expression or restore normal levels of NKG2D on effector cytotoxic lymphocytes. For this purpose, one could conceive the use of blocking antibodies against these NKG2D ligands. Finally, for those patients with elevated soluble NKG2D ligands in the sera, a recent growing understanding of the mechanism of ligand shedding (141, 142, 144, 145) and of the detrimental role of soluble ligands (Fig. 5 and (151)) show great promises for future therapies. These therapies might conceivably include the blocking of ERp5 binding to ligand (152) or blocking ERp5 isomerase function. Therefore, selectively modulating NKG2D and its ligands, and thereby the function of cytotoxic lymphocytes, may provide many opportunities to influence the outcome of infectious diseases, cancer, and certain autoimmune diseases.
Acknowledgements
This study was supported by NIH grants AI066897 and CA095137. L.L.L. is an American Cancer Society Research Professor.
Footnotes
Disclosure L.L.L. and the University of California (San Francisco, CA) have licensed intellectual property rights relative to NKG2D for commercial applications.
References
- 1.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–74. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 2.Ljunggren H-G, Kärre K. In search of the “missing self”: MHC molecules and NK cell recognition. Immunology Today. 1990;11:237–44. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 3.Guerra N, et al. NKG2D-Deficient Mice Are Defective in Tumor Surveillance in Models of Spontaneous Malignancy. 2008;28:571–80. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zafirova B, et al. Altered NK Cell Development and Enhanced NK Cell-Mediated Resistance to Mouse Cytomegalovirus in NKG2D-Deficient Mice. 2009;31:270–82. doi: 10.1016/j.immuni.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houchins JP, Yabe T, McSherry C, Bach FH. DNA sequence analysis of NKG2, a family of related cDNA clones encoding type II integral membrane proteins on human natural killer cells. J Exp Med. 1991;173:1017–20. doi: 10.1084/jem.173.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diefenbach A, et al. Selective associations with signaling proteins determine stimulatory versus costimulatory activity of NKG2D. Nat Immunol. 2002;3:1142–9. doi: 10.1038/ni858. [DOI] [PubMed] [Google Scholar]
- 7.Gilfillan S, Ho EL, Cella M, Yokoyama WM, Colonna M. NKG2D recruits two distinct adapters to trigger NK cell activation and costimulation. Nat Immunol. 2002;3:1150–5. doi: 10.1038/ni857. [DOI] [PubMed] [Google Scholar]
- 8.Chang C, et al. Cutting edge: KAP10, a novel transmembrane adapter protein genetically linked to DAP12 but with unique signaling properties. J Immunol. 1999;163:4651–4. [PubMed] [Google Scholar]
- 9.Wu J. An activating immunoreceptor complex formed by NKG2D and DAP10. Science. 1999;285:730–2. doi: 10.1126/science.285.5428.730. [DOI] [PubMed] [Google Scholar]
- 10.Lanier LL, Corliss BC, Wu J, Leong C, Phillips JH. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature. 1998;391:703–7. doi: 10.1038/35642. [DOI] [PubMed] [Google Scholar]
- 11.Garrity D, Call ME, Feng J, Wucherpfennig KW. The activating NKG2D receptor assembles in the membrane with two signaling dimers into a hexameric structure. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:7641–6. doi: 10.1073/pnas.0502439102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McFarland BJ, Strong RK. Thermodynamic Analysis of Degenerate Recognition by the NKG2D Immunoreceptor: Not Induced Fit but Rigid Adaptation. Immunity. 2003;19:803–12. doi: 10.1016/s1074-7613(03)00320-0. [DOI] [PubMed] [Google Scholar]
- 13.Wolan DW, et al. Crystal structure of the murine NK cell-activating receptor NKG2D at 1.95 A. Nat Immunol. 2001;2:248–54. doi: 10.1038/85311. [DOI] [PubMed] [Google Scholar]
- 14.McFarland BJ, Kortemme T, Yu SF, Baker D, Strong RK. Symmetry Recognizing Asymmetry: Analysis of the Interactions between the C-Type Lectin-like Immunoreceptor NKG2D and MHC Class I-like Ligands. Structure. 2003;11:411–22. doi: 10.1016/s0969-2126(03)00047-9. [DOI] [PubMed] [Google Scholar]
- 15.Groh V, Bahram S, Bauer S, Herman A, Beauchamp M, Spies T. Cell stress-regulated human major histocompatibility complex class I gene expressed in gastrointestinal epithelium. Proc Natl Acad Sci USA. 1996;93:12445–50. doi: 10.1073/pnas.93.22.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bauer S, et al. Activation of natural killer cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–30. [PubMed] [Google Scholar]
- 17.Cosman D, et al. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123–33. doi: 10.1016/s1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- 18.Radosavljevic M, et al. A cluster of ten novel MHC class I related genes on human chromosome 6q24.2–q25.3. Genomics. 2002;79:114–23. doi: 10.1006/geno.2001.6673. [DOI] [PubMed] [Google Scholar]
- 19.Bacon L, Eagle RA, Meyer M, Easom N, Young NT, Trowsdale J. Two Human ULBP/RAET1 Molecules with Transmembrane Regions Are Ligands for NKG2D. J Immunol. 2004;173:1078–84. doi: 10.4049/jimmunol.173.2.1078. [DOI] [PubMed] [Google Scholar]
- 20.Jan Chalupny N, Sutherland CL, Lawrence WA, Rein-Weston A, Cosman D. ULBP4 is a novel ligand for human NKG2D. Biochem Biophys Res Commun. 2003;305:129–35. doi: 10.1016/s0006-291x(03)00714-9. [DOI] [PubMed] [Google Scholar]
- 21.Nomura M, Takihara Y, Shimada K. Isolation and characterization of retinoic acid-inducible cDNA clones in F9 cells: one of the early inducible clones encodes a novel protein sharing several highly homologous regions with a Drosophila polyhomeotic protein. Differentiation. 1994;57:39–50. doi: 10.1046/j.1432-0436.1994.5710039.x. [DOI] [PubMed] [Google Scholar]
- 22.Zou Z, Nomura M, Takihara Y, Yasunaga T, Shimada K. Isolation and characterization of retinoic acid-inducible cDNA clones in F9 cells: a novel cDNA family encodes cell surface proteins sharing partial homology with MHC class I molecules. J Biochem (Tokyo) 1996;119:319–28. doi: 10.1093/oxfordjournals.jbchem.a021242. [DOI] [PubMed] [Google Scholar]
- 23.Cerwenka A, et al. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity. 2000;12:721–7. doi: 10.1016/s1074-7613(00)80222-8. [DOI] [PubMed] [Google Scholar]
- 24.Diefenbach A, Jamieson AM, Liu SD, Shastri N, Raulet DH. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nature Immunology. 2000;1:119–26. doi: 10.1038/77793. [DOI] [PubMed] [Google Scholar]
- 25.Malarkannan S, et al. The molecular and functional characterization of a dominant minor H antigen, H60. J Immunol. 1998;161:3501–9. [PubMed] [Google Scholar]
- 26.Takada A, et al. Two Novel NKG2D Ligands of the Mouse H60 Family with Differential Expression Patterns and Binding Affinities to NKG2D. J Immunol. 2008;180:1678–85. doi: 10.4049/jimmunol.180.3.1678. [DOI] [PubMed] [Google Scholar]
- 27.Whang MI, Guerra N, Raulet DH. Costimulation of Dendritic Epidermal γδ T Cells by a New NKG2D Ligand Expressed Specifically in the Skin. J Immunol. 2009;182:4557–64. doi: 10.4049/jimmunol.0802439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carayannopoulos LN, Naidenko OV, Fremont DH, Yokoyama WM. Cutting Edge: Murine UL16-Binding Protein-Like Transcript 1: A Newly Described Transcript Encoding a High-Affinity Ligand for Murine NKG2D. J Immunol. 2002;169:4079–83. doi: 10.4049/jimmunol.169.8.4079. [DOI] [PubMed] [Google Scholar]
- 29.Diefenbach A, Hsia JK, Hsiung M, Raulet DH. A novel ligand for the NKG2D receptor activates NK cells and macrophages and induces tumor immunity. European Journal of Immunology. 2003;33:381–91. doi: 10.1002/immu.200310012. [DOI] [PubMed] [Google Scholar]
- 30.Eleme K, et al. Cell Surface Organization of Stress-inducible Proteins ULBP and MICA That Stimulate Human NK Cells and T Cells via NKG2D. J Exp Med. 2004;199:1005–10. doi: 10.1084/jem.20032194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romphruk A, et al. Polymorphisms of NKG2D ligands: diverse RAET1/ULBP genes in Northeastern Thais. Immunogenetics. 2009;61:611–7. doi: 10.1007/s00251-009-0394-7. [DOI] [PubMed] [Google Scholar]
- 32.Eagle RA, Traherne JA, Ashiru O, Wills MR, Trowsdale J. Regulation of NKG2D Ligand Gene Expression. Human Immunology. 2006;67:159–69. doi: 10.1016/j.humimm.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 33.Steinle A, et al. Interactions of human NKG2D with its ligands MICA, MICB, and homologs of the mouse RAE-1 protein family. Immunogenetics. 2001;53:279–87. doi: 10.1007/s002510100325. [DOI] [PubMed] [Google Scholar]
- 34.Li Z, Groh V, Strong RK, Spies T. A single amino acid substitution causes loss of expression of a MICA allele. Immunogenetics. 2000;51:246–8. doi: 10.1007/s002510050039. [DOI] [PubMed] [Google Scholar]
- 35.Lodoen MB, Lanier LL. Viral modulation of NK cell immunity. Nat Rev Microbiol. 2005;3:59–69. doi: 10.1038/nrmicro1066. [DOI] [PubMed] [Google Scholar]
- 36.Cosman D. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123–33. doi: 10.1016/s1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- 37.Welte SA, et al. Selective intracellular retention of virally induced NKG2D ligands by the human cytomegalovirus UL16 glycoprotein. European Journal of Immunology. 2003;33:194–203. doi: 10.1002/immu.200390022. [DOI] [PubMed] [Google Scholar]
- 38.Dunn C, et al. Human Cytomegalovirus Glycoprotein UL16 Causes Intracellular Sequestration of NKG2D Ligands, Protecting Against Natural Killer Cell Cytotoxicity. J Exp Med. 2003;197:1427–39. doi: 10.1084/jem.20022059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu J, Chalupny NJ, Manley TJ, Riddell SR, Cosman D, Spies T. Intracellular Retention of the MHC Class I-Related Chain B Ligand of NKG2D by the Human Cytomegalovirus UL16 Glycoprotein. J Immunol. 2003;170:4196–200. doi: 10.4049/jimmunol.170.8.4196. [DOI] [PubMed] [Google Scholar]
- 40.Rolle A, et al. Effects of human cytomegalovirus infection on ligands for the activating NKG2D receptor of NK cells: up-regulation of UL16-binding protein (ULBP)1 and ULBP2 is counteracted by the viral UL16 protein. J Immunol. 2003;171:902–8. doi: 10.4049/jimmunol.171.2.902. [DOI] [PubMed] [Google Scholar]
- 41.Robert AE, James AT, James RH, Insiya J, John T. ULBP6/RAET1L is an additional human NKG2D ligand. European Journal of Immunology. 2009;9999:NA. [Google Scholar]
- 42.Vales-Gomez M, Browne H, Reyburn HT. Expression of the UL16 glycoprotein of Human Cytomegalovirus protects the virus-infected cell from attack by natural killer cells. BMC Immunol. 2003;4:4. doi: 10.1186/1471-2172-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chalupny NJ, Rein-Weston A, Dosch S, Cosman D. Down-regulation of the NKG2D ligand MICA by the human cytomegalovirus glycoprotein UL142. Biochemical and Biophysical Research Communications. 2006;346:175–81. doi: 10.1016/j.bbrc.2006.05.092. [DOI] [PubMed] [Google Scholar]
- 44.Ashiru O, Bennett NJ, Boyle LH, Thomas M, Trowsdale J, Wills MR. NKG2D ligand MICA is retained in the Golgi by the human Cytomegalovirus protein UL142. J Virol. 2009:JVI.01175–09. doi: 10.1128/JVI.01175-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krmpotic A, et al. NK cell activation through the NKG2D ligand MULT-1 is selectively prevented by the glycoprotein encoded by mouse cytomegalovirus gene m145. J Exp Med. 2005;201:211–20. doi: 10.1084/jem.20041617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hasan M, et al. Selective Down-Regulation of the NKG2D Ligand H60 by Mouse Cytomegalovirus m155 Glycoprotein. J Virol. 2005;79:2920–30. doi: 10.1128/JVI.79.5.2920-2930.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lodoen MB, Abenes G, Umamoto S, Houchins JP, Liu F, Lanier LL. The Cytomegalovirus m155 Gene Product Subverts Natural Killer Cell Antiviral Protection by Disruption of H60-NKG2D Interactions. J Exp Med. 2004;200:1075–81. doi: 10.1084/jem.20040583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lodoen M, et al. NKG2D-mediated Natural Killer Cell Protection Against Cytomegalovirus Is Impaired by Viral gp40 Modulation of Retinoic Acid Early Inducible 1 Gene Molecules. J Exp Med. 2003;197:1245–53. doi: 10.1084/jem.20021973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lenac T, et al. The herpesviral Fc receptor fcr-1 down-regulates the NKG2D ligands MULT-1 and H60. J Exp Med. 2006;203:1843–50. doi: 10.1084/jem.20060514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arapovic J, Lenac Rovis T, Reddy AB, Krmpotic A, Jonjic S. Promiscuity of MCMV immunoevasin of NKG2D: m138/fcr-1 down-modulates RAE-1ε in addition to MULT-1 and H60. Molecular Immunology. 2009;47:114–22. doi: 10.1016/j.molimm.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 51.Takayanagi H, et al. T-cell-mediated regulation of osteoclastogenesis by signalling crosstalk between RANKL and IFN-γ. Nature. 2000;408:600–95. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- 52.Matthieu A, et al. CD4+NKG2D+ T Cells in Crohn,Äôs Disease Mediate Inflammatory and Cytotoxic Responses Through MICA Interactions. Gastroenterology. 2007;132:2346–58. doi: 10.1053/j.gastro.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 53.Kraetzel K, et al. NKG2D-dependent effector function of bronchial epithelium-activated alloreactive T-cells. Eur Respir J. 2008;32:563–70. doi: 10.1183/09031936.00096407. [DOI] [PubMed] [Google Scholar]
- 54.Schrambach S, Ardizzone M, Leymarie V, Sibilia J, Bahram S. In Vivo Expression Pattern of MICA and MICB and Its Relevance to Auto-Immunity and Cancer. PLoS ONE. 2007;2:518. doi: 10.1371/journal.pone.0000518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zwirner NW, Fernández-Viña MA, Stastny P. MICA, a new polymorphic HLA-related antigen, is expressed mainly by keratinocytes, endothelial cells, and monocytes. Immunogenetics. 1997;47:139–48. doi: 10.1007/s002510050339. [DOI] [PubMed] [Google Scholar]
- 56.Molinero LL, Fuertes MB, Rabinovich GA, Fainboim L, Zwirner NW. Activation-induced expression of MICA on T lymphocytes involves engagement of CD3 and CD28. J Leukoc Biol. 2002;71:791–7. [PubMed] [Google Scholar]
- 57.Cerboni C, Zingoni A, Cippitelli M, Piccoli M, Frati L, Santoni A. Antigen-activated human T lymphocytes express cell-surface NKG2D ligands via an ATM/ATR-dependent mechanism and become susceptible to autologous NK- cell lysis. Blood. 2007;110:606–15. doi: 10.1182/blood-2006-10-052720. [DOI] [PubMed] [Google Scholar]
- 58.Ogasawara K, Benjamin J, Takaki R, Phillips JH, Lanier LL. Function of NKG2D in natural killer cell-mediated rejection of mouse bone marrow grafts. Nat Immunol. 2005;6:938–45. doi: 10.1038/ni1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rabinovich BA, et al. Activated, But Not Resting, T Cells Can Be Recognized and Killed by Syngeneic NK Cells. J Immunol. 2003;170:3572–6. doi: 10.4049/jimmunol.170.7.3572. [DOI] [PubMed] [Google Scholar]
- 60.Jacoby RO, Bhatt PN, Brownstein DG. Evidence that NK cells and interferon are required for genetic resistance to lethal infection with ectromelia virus. Archives of Virology. 1989;108:49–58. doi: 10.1007/BF01313742. [DOI] [PubMed] [Google Scholar]
- 61.Fang M, Lanier LL, Sigal LJ. A Role for NKG2D in NK Cell-Mediated Resistance to Poxvirus Disease. PLoS Pathogens. 2008;4:e30. doi: 10.1371/journal.ppat.0040030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walsh KB, Lanier LL, Lane TE. NKG2D Receptor Signaling Enhances Cytolytic Activity by Virus-Specific CD8+ T Cells: Evidence for a Protective Role in Virus-Induced Encephalitis. J Virol. 2008;82:3031–44. doi: 10.1128/JVI.02033-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vilarinho Sl, Ogasawara K, Nishimura S, Lanier LL, Baron JL. Blockade of NKG2D on NKT cells prevents hepatitis and the acute immune response to hepatitis B virus. Proceedings of the National Academy of Sciences. 2007;104:18187–92. doi: 10.1073/pnas.0708968104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yongyan C, Haiming W, Rui S, Zhongjun D, Jian Z, Zhigang T. Increased susceptibility to liver injury in hepatitis B virus transgenic mice involves NKG2D-ligand interaction and natural killer cells. Hepatology. 2007;46:706–15. doi: 10.1002/hep.21872. [DOI] [PubMed] [Google Scholar]
- 65.Ward J, et al. HIV modulates the expression of ligands important in triggering natural killer cell cytotoxic responses on infected primary T-cell blasts. Blood. 2007;110:1207–14. doi: 10.1182/blood-2006-06-028175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ward J, et al. HIV-1 Vpr Triggers Natural Killer Cell-Mediated Lysis of Infected Cells through Activation of the ATR-Mediated DNA Damage Response. PLoS Pathog. 2009;5:e1000613. doi: 10.1371/journal.ppat.1000613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cerboni C, et al. Human immunodeficiency virus 1 Nef protein downmodulates the ligands of the activating receptor NKG2D and inhibits natural killer cell-mediated cytotoxicity. J Gen Virol. 2007;88:242–50. doi: 10.1099/vir.0.82125-0. [DOI] [PubMed] [Google Scholar]
- 68.Draghi M, et al. NKp46 and NKG2D Recognition of Infected Dendritic Cells Is Necessary for NK Cell Activation in the Human Response to Influenza Infection. J Immunol. 2007;178:2688–98. doi: 10.4049/jimmunol.178.5.2688. [DOI] [PubMed] [Google Scholar]
- 69.Cerwenka A. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity. 2000;12:721–7. doi: 10.1016/s1074-7613(00)80222-8. [DOI] [PubMed] [Google Scholar]
- 70.Diefenbach A, Jamieson AM, Liu SD, Shastri N, Raulet DH. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nature Immunol. 2000;1:119–26. doi: 10.1038/77793. [DOI] [PubMed] [Google Scholar]
- 71.Cerwenka A, Baron JL, Lanier LL. Ectopic expression of retinoic acid early inducible-1 gene (RAE-1) permits natural killer cell-mediated rejection of a MHC class I-bearing tumor in vivo. Proc Natl Acad Sci U S A. 2001;98:11521–6. doi: 10.1073/pnas.201238598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413:165–71. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jinushi M, et al. Expression and role of MICA and MICB in human hepatocellular carcinomas and their regulation by retinoic acid. Int J Cancer. 2003;104:354–61. doi: 10.1002/ijc.10966. [DOI] [PubMed] [Google Scholar]
- 74.Salih HR, et al. Functional expression and release of ligands for the activating immunoreceptor NKG2D in leukemia. Blood. 2003;102:1389–96. doi: 10.1182/blood-2003-01-0019. [DOI] [PubMed] [Google Scholar]
- 75.Friese MA, et al. MICA/NKG2D-Mediated Immunogene Therapy of Experimental Gliomas. Cancer Res. 2003;63:8996–9006. [PubMed] [Google Scholar]
- 76.Nicholas FSW, et al. Expression of the stress-related MHC class I chain-related protein MICA is an indicator of good prognosis in colorectal cancer patients. International Journal of Cancer. 2006;118:1445–52. doi: 10.1002/ijc.21510. [DOI] [PubMed] [Google Scholar]
- 77.Vetter CS, Groh V, Straten Pt P, Spies T, Brocker EB, Becker JC. Expression of Stress-induced MHC Class I Related Chain Molecules on Human Melanoma. J Invest Dermatol. 2002;118:600–5. doi: 10.1046/j.1523-1747.2002.01700.x. [DOI] [PubMed] [Google Scholar]
- 78.Vetter CS, Lieb W, Brocker EB, Becker JC. Loss of nonclassical MHC molecules MICA//B expression during progression of uveal melanoma. Br J Cancer. 2004;91:1495–9. doi: 10.1038/sj.bjc.6602123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aman MJ, Tosello-Trampont AC, Ravichandran K. FcgRIIB1/SHIP-mediated inhibitory signaling in B cells involves lipid rafts. J Biol Chem. 2001;24:24. doi: 10.1074/jbc.M104069200. [DOI] [PubMed] [Google Scholar]
- 80.Bartkova J, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–70. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- 81.Gorgoulis VG, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–13. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- 82.Stephan G, David HR. Activation and self-tolerance of natural killer cells. Immunological Reviews. 2006;214:130–42. doi: 10.1111/j.1600-065X.2006.00460.x. [DOI] [PubMed] [Google Scholar]
- 83.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–90. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hamerman JA, Ogasawara K, Lanier LL. Cutting Edge: Toll-Like Receptor Signaling in Macrophages Induces Ligands for the NKG2D Receptor. J Immunol. 2004;172:2001–5. doi: 10.4049/jimmunol.172.4.2001. [DOI] [PubMed] [Google Scholar]
- 85.Kloss M, et al. Interaction of Monocytes with NK Cells upon Toll-Like Receptor-Induced Expression of the NKG2D Ligand MICA. J Immunol. 2008;181:6711–9. doi: 10.4049/jimmunol.181.10.6711. [DOI] [PubMed] [Google Scholar]
- 86.Nedvetzki S, et al. Reciprocal regulation of human natural killer cells and macrophages associated with distinct immune synapses. Blood. 2007;109:3776–85. doi: 10.1182/blood-2006-10-052977. [DOI] [PubMed] [Google Scholar]
- 87.Ebihara T, et al. Induction of NKG2D ligands on human dendritic cells by TLR ligand stimulation and RNA virus infection. Int Immunol. 2007;19:1145–55. doi: 10.1093/intimm/dxm073. [DOI] [PubMed] [Google Scholar]
- 88.Jinushi M, et al. Critical Role of MHC Class I-Related Chain A and B Expression on IFN-α-Stimulated Dendritic Cells in NK Cell Activation: Impairment in Chronic Hepatitis C Virus Infection. J Immunol. 2003;170:1249–56. doi: 10.4049/jimmunol.170.3.1249. [DOI] [PubMed] [Google Scholar]
- 89.Bui JD, Carayannopoulos LN, Lanier LL, Yokoyama WM, Schreiber RD. IFN-Dependent Down-Regulation of the NKG2D Ligand H60 on Tumors. J Immunol. 2006;176:905–13. doi: 10.4049/jimmunol.176.2.905. [DOI] [PubMed] [Google Scholar]
- 90.Schwinn N, et al. Interferon-gamma down-regulates NKG2D ligand expression and impairs the NKG2D-mediated cytolysis of MHC class I-deficient melanoma by natural killer cells. International Journal of Cancer. 2009;124:1594–604. doi: 10.1002/ijc.24098. [DOI] [PubMed] [Google Scholar]
- 91.Friese MA, et al. RNA Interference Targeting Transforming Growth Factor-β Enhances NKG2D-Mediated Antiglioma Immune Response, Inhibits Glioma Cell Migration and Invasiveness, and Abrogates Tumorigenicity In vivo. Cancer Res. 2004;64:7596–603. doi: 10.1158/0008-5472.CAN-04-1627. [DOI] [PubMed] [Google Scholar]
- 92.Eisele G, et al. TGF-β and metalloproteinases differentially suppress NKG2D ligand surface expression on malignant glioma cells. Brain. 2006;129:2416–25. doi: 10.1093/brain/awl205. [DOI] [PubMed] [Google Scholar]
- 93.Nomura M, Zou Z, Joh T, Takihara Y, Matsuda Y, Shimada K. Genomic structures and characterization of Rae1 family members encoding GPI-anchored cell surface proteins and expressed predominantly in embryonic mouse brain. J Biochem (Tokyo) 1996;120:987–95. doi: 10.1093/oxfordjournals.jbchem.a021517. [DOI] [PubMed] [Google Scholar]
- 94.Venkataraman GM, Suciu D, Groh V, Boss JM, Spies T. Promoter Region Architecture and Transcriptional Regulation of the Genes for the MHC Class I-Related Chain A and B Ligands of NKG2D. J Immunol. 2007;178:961–9. doi: 10.4049/jimmunol.178.2.961. [DOI] [PubMed] [Google Scholar]
- 95.Routes JM, Ryan S, Morris K, Takaki R, Cerwenka A, Lanier LL. Adenovirus serotype 5 E1A sensitizes tumor cells to NKG2D-dependent NK cell lysis and tumor rejection. J Exp Med. 2005;202:1477–82. doi: 10.1084/jem.20050240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nausch N, Florin L, Hartenstein B, Angel P, Schorpp-Kistner M, Cerwenka A. Cutting Edge: The AP-1 Subunit JunB Determines NK Cell-Mediated Target Cell Killing by Regulation of the NKG2D-Ligand RAE-□. J Immunol. 2006;176:7–11. doi: 10.4049/jimmunol.176.1.7. [DOI] [PubMed] [Google Scholar]
- 97.Stern-Ginossar N, et al. Human microRNAs regulate stress-induced immune responses mediated by the receptor NKG2D. Nat Immunol. 2008;9:1065–73. doi: 10.1038/ni.1642. [DOI] [PubMed] [Google Scholar]
- 98.Yadav D, Ngolab J, Lim RS-H, Krishnamurthy S, Bui JD. Cutting Edge: Down-Regulation of MHC Class I-Related Chain A on Tumor Cells by IFN-•-Induced MicroRNA. J Immunol. 2009;182:39–43. doi: 10.4049/jimmunol.182.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tang KF, et al. Decreased Dicer expression elicits DNA damage and up-regulation of MICA and MICB. J Cell Biol. 2008;182:233–9. doi: 10.1083/jcb.200801169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stern-Ginossar N, et al. Host Immune System Gene Targeting by a Viral miRNA. Science. 2007;317:376–81. doi: 10.1126/science.1140956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nice TJ, Coscoy L, Raulet DH. Posttranslational regulation of the NKG2D ligand Mult1 in response to cell stress. J Exp Med. 2009;206:287–98. doi: 10.1084/jem.20081335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thomas M, Wills M, Lehner PJ. Natural killer cell evasion by an E3 ubiquitin ligase from Kaposi's sarcoma-associated herpesvirus. Biochemical Society Transactions. 2008;036:459–63. doi: 10.1042/BST0360459. [DOI] [PubMed] [Google Scholar]
- 103.Samarakoon A, Chu H, Malarkannan S. Murine NKG2D ligands: “Double, double toil and trouble”. Molecular Immunology. 2009;46:1011–9. doi: 10.1016/j.molimm.2008.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413:165–71. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Oppenheim DE, et al. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat Immunol. 2005;6:928–37. doi: 10.1038/ni1239. [DOI] [PubMed] [Google Scholar]
- 106.Sutherland CL, Chalupny NJ, Schooley K, VandenBos T, Kubin M, Cosman D. UL16-Binding Proteins, Novel MHC Class I-Related Proteins, Bind to NKG2D and Activate Multiple Signaling Pathways in Primary NK Cells. J Immunol. 2002;168:671–9. doi: 10.4049/jimmunol.168.2.671. [DOI] [PubMed] [Google Scholar]
- 107.Roberts AI, et al. Cutting edge: NKG2D receptors induced by IL-15 costimulate CD28-negative effector CTL in the tissue microenvironment. J Immunol. 2001;167:5527–30. doi: 10.4049/jimmunol.167.10.5527. [DOI] [PubMed] [Google Scholar]
- 108.Groh V, Rhinehart R, Randolph-Habecker J, Topp MS, Riddell SR, Spies T. Costimulation of CD8αβ T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat Immunol. 2001;2:255–60. doi: 10.1038/85321. [DOI] [PubMed] [Google Scholar]
- 109.Maasho K, Opoku-Anane J, Marusina AI, Coligan JE, Borrego F. Cutting Edge: NKG2D Is a Costimulatory Receptor for Human Naive CD8+ T Cells. J Immunol. 2005;174:4480–4. doi: 10.4049/jimmunol.174.8.4480. [DOI] [PubMed] [Google Scholar]
- 110.Ehrlich LI, et al. Engagement of NKG2D by cognate ligand or antibody alone is insufficient to mediate costimulation of human and mouse CD8+ T cells. J Immunol. 2005;174:1922–31. doi: 10.4049/jimmunol.174.4.1922. [DOI] [PubMed] [Google Scholar]
- 111.Girardi M, et al. Regulation of Cutaneous Malignancy by γδ T Cells. Science. 2001 [PubMed] [Google Scholar]
- 112.Strid J, et al. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat Immunol. 2008;9:146–54. doi: 10.1038/ni1556. [DOI] [PubMed] [Google Scholar]
- 113.Graham DB, et al. Vav1 Controls DAP10-Mediated Natural Cytotoxicity by Regulating Actin and Microtubule Dynamics. J Immunol. 2006;177:2349–55. doi: 10.4049/jimmunol.177.4.2349. [DOI] [PubMed] [Google Scholar]
- 114.Andoniou CE, et al. Interaction between conventional dendritic cells and natural killer cells is integral to the activation of effective antiviral immunity. Nat Immunol. 2005;6:1011–9. doi: 10.1038/ni1244. [DOI] [PubMed] [Google Scholar]
- 115.Jinushi M. Critical role of MHC class I-related chain A and B expression on IFN-α-stimulated dendritic cells in NK cell activation: impairment in chronic hepatitis C virus infection. J Immunol. 2003;170:1249–56. doi: 10.4049/jimmunol.170.3.1249. [DOI] [PubMed] [Google Scholar]
- 116.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–8. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 117.Salih HR, Goehlsdorf D, Steinle A. Release of MICB Molecules by Tumor Cells: Mechanism and Soluble MICB in Sera of Cancer Patients. Human Immunology. 2006;67:188–95. doi: 10.1016/j.humimm.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 118.Nowbakht P, et al. Ligands for natural killer cell-activating receptors are expressed upon the maturation of normal myelomonocytic cells but at low levels in acute myeloid leukemias. Blood. 2005;105:3615–22. doi: 10.1182/blood-2004-07-2585. [DOI] [PubMed] [Google Scholar]
- 119.Konjević G, et al. Low expression of CD161 and NKG2D activating NK receptor is associated with impaired NK cell cytotoxicity in metastatic melanoma patients. Clinical and Experimental Metastasis. 2007;24:1–11. doi: 10.1007/s10585-006-9043-9. [DOI] [PubMed] [Google Scholar]
- 120.Ogasawara K, et al. Impairment of NK Cell Function by NKG2D Modulation in NOD Mice. Immunity. 2003;18:41–51. doi: 10.1016/s1074-7613(02)00505-8. [DOI] [PubMed] [Google Scholar]
- 121.Coudert JD, et al. Altered NKG2D function in NK cells induced by chronic exposure to NKG2D ligand-expressing tumor cells. Blood. 2005;106:1711–7. doi: 10.1182/blood-2005-03-0918. [DOI] [PubMed] [Google Scholar]
- 122.Nomura E, et al. Hyperkeratosis and leukocytosis in transgenic mice carrying MHC class I chain-related gene B (MICB) Tissue Antigens. 2003;61:300–7. doi: 10.1034/j.1399-0039.2003.00014.x. [DOI] [PubMed] [Google Scholar]
- 123.Wiemann K, et al. Systemic NKG2D Down-Regulation Impairs NK and CD8 T Cell Responses In Vivo. J Immunol. 2005;175:720–9. doi: 10.4049/jimmunol.175.2.720. [DOI] [PubMed] [Google Scholar]
- 124.Park EJ, et al. Clonal expansion of double-positive intraepithelial lymphocytes by MHC class I-related chain a expressed in mouse small intestinal epithelium. J Immunol. 2003;171:4131–9. doi: 10.4049/jimmunol.171.8.4131. [DOI] [PubMed] [Google Scholar]
- 125.Coudert JD, Scarpellino L, Gros F, Vivier E, Held W. Sustained NKG2D engagement induces cross-tolerance of multiple distinct NK cell activation pathways. Blood. 2008;111:3571–8. doi: 10.1182/blood-2007-07-100057. [DOI] [PubMed] [Google Scholar]
- 126.Salih HR, Rammensee HG, Steinle A. Cutting Edge: Down-Regulation of MICA on Human Tumors by Proteolytic Shedding. J Immunol. 2002;169:4098–102. doi: 10.4049/jimmunol.169.8.4098. [DOI] [PubMed] [Google Scholar]
- 127.Boissel N, et al. BCR/ABL Oncogene Directly Controls MHC Class I Chain-Related Molecule A Expression in Chronic Myelogenous Leukemia. J Immunol. 2006;176:5108–16. doi: 10.4049/jimmunol.176.8.5108. [DOI] [PubMed] [Google Scholar]
- 128.Doubrovina ES, et al. Evasion from NK Cell Immunity by MHC Class I Chain-Related Molecules Expressing Colon Adenocarcinoma. J Immunol. 2003;171:6891–9. doi: 10.4049/jimmunol.171.12.6891. [DOI] [PubMed] [Google Scholar]
- 129.Masahisa J, et al. Impairment of natural killer cell and dendritic cell functions by the soluble form of MHC class I-related chain A in advanced human hepatocellular carcinomas. Journal of hepatology. 2005;43:1013–20. doi: 10.1016/j.jhep.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 130.Wu JD, Higgins LM, Steinle A, Cosman D, Haugk K, Plymate SR. Prevalent expression of the immunostimulatory MHC class I chain- related molecule is counteracted by shedding in prostate cancer. The Journal of Clinical Investigation. 2004;114:560–8. doi: 10.1172/JCI22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jinushi M, Hodi FS, Dranoff G. Therapy-induced antibodies to MHC class I chain-related protein A antagonize immune suppression and stimulate antitumor cytotoxicity. Proceedings of the National Academy of Sciences. 2006;103:9190–5. doi: 10.1073/pnas.0603503103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Onda H, et al. A novel secreted tumor antigen with a glycosylphosphatidylinositol-anchored structure ubiquitously expressed in human cancers. Biochem Biophys Res Commun. 2001;285:235–43. doi: 10.1006/bbrc.2001.5149. [DOI] [PubMed] [Google Scholar]
- 133.Waldhauer I, Steinle A. Proteolytic Release of Soluble UL16-Binding Protein 2 from Tumor Cells. Cancer Res. 2006;66:2520–6. doi: 10.1158/0008-5472.CAN-05-2520. [DOI] [PubMed] [Google Scholar]
- 134.Paschen A, et al. Differential Clinical Significance of Individual NKG2D Ligands in Melanoma: Soluble ULBP2 as an Indicator of Poor Prognosis Superior to S100B. Clinical Cancer Research. 2009;15:5208–15. doi: 10.1158/1078-0432.CCR-09-0886. [DOI] [PubMed] [Google Scholar]
- 135.Cerboni C, Ardolino M, Santoni A, Zingoni A. Detuning CD8+ T lymphocytes by down-regulation of the activating receptor NKG2D: role of NKG2D ligands released by activated T cells. Blood. 2009;113:2955–64. doi: 10.1182/blood-2008-06-165944. [DOI] [PubMed] [Google Scholar]
- 136.Song H, Kim J, Cosman D, Choi I. Soluble ULBP suppresses natural killer cell activity via down-regulating NKG2D expression. Cellular Immunology. 2006;239:22–30. doi: 10.1016/j.cellimm.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 137.Hüe S, et al. A Direct Role for NKG2D/MICA Interaction in Villous Atrophy during Celiac Disease. 2004;21:367–77. doi: 10.1016/j.immuni.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 138.Groh V, Bruhl A, El-Gabalawy H, Nelson JL, Spies T. Stimulation of T cell autoreactivity by anomalous expression of NKG2D and its MIC ligands in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2003 doi: 10.1073/pnas.1632807100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mincheva-Nilsson L, et al. Placenta-Derived Soluble MHC Class I Chain-Related Molecules Down-Regulate NKG2D Receptor on Peripheral Blood Mononuclear Cells during Human Pregnancy: A Possible Novel Immune Escape Mechanism for Fetal Survival. J Immunol. 2006;176:3585–92. doi: 10.4049/jimmunol.176.6.3585. [DOI] [PubMed] [Google Scholar]
- 140.Hedlund M, et al. Human Placenta Expresses and Secretes NKG2D Ligands via Exosomes that Down-Modulate the Cognate Receptor Expression: Evidence for Immunosuppressive Function. J Immunol. 2009;183:340–51. doi: 10.4049/jimmunol.0803477. [DOI] [PubMed] [Google Scholar]
- 141.Waldhauer I, et al. Tumor-Associated MICA Is Shed by ADAM Proteases. Cancer Res. 2008;68:6368–76. doi: 10.1158/0008-5472.CAN-07-6768. [DOI] [PubMed] [Google Scholar]
- 142.Boutet P, et al. Cutting Edge: The Metalloproteinase ADAM17/TNF-α-Converting Enzyme Regulates Proteolytic Shedding of the MHC Class I-Related Chain B Protein. J Immunol. 2009;182:49–53. doi: 10.4049/jimmunol.182.1.49. [DOI] [PubMed] [Google Scholar]
- 143.White JM. ADAMs: modulators of cell-cell and cell-matrix interactions. Current Opinion in Cell Biology. 2003;15:598–606. doi: 10.1016/j.ceb.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 144.Kohga K, et al. Anticancer Chemotherapy Inhibits MHC Class I-Related Chain A Ectodomain Shedding by Downregulating ADAM10 Expression in Hepatocellular Carcinoma. Cancer Res. 2009;69:8050–7. doi: 10.1158/0008-5472.CAN-09-0789. [DOI] [PubMed] [Google Scholar]
- 145.Kaiser BK, et al. Disulphide-isomerase-enabled shedding of tumour-associated NKG2D ligands. Nature. 2007;447:482–6. doi: 10.1038/nature05768. [DOI] [PubMed] [Google Scholar]
- 146.Gumireddy K, et al. In vivo selection for metastasis promoting genes in the mouse. Proceedings of the National Academy of Sciences. 2007;104:6696–701. doi: 10.1073/pnas.0701145104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cao W, et al. RAET1E2, a Soluble Isoform of the UL16-binding Protein RAET1E Produced by Tumor Cells, Inhibits NKG2D-mediated NK Cytotoxicity. Journal of Biological Chemistry. 2007;282:18922–8. doi: 10.1074/jbc.M702504200. [DOI] [PubMed] [Google Scholar]
- 148.Kopp H-G, Placke T, Salih HR. Platelet-Derived Transforming Growth Factor-β Down-Regulates NKG2D Thereby Inhibiting Natural Killer Cell Antitumor Reactivity. Cancer Res. 2009:0008–5472. doi: 10.1158/0008-5472.CAN-09-2123. CAN-09-2123. [DOI] [PubMed] [Google Scholar]
- 149.Lee J-C, Lee K-M, Kim D-W, Heo DS. Elevated TGF-{beta}1 Secretion and Down-Modulation of NKG2D Underlies Impaired NK Cytotoxicity in Cancer Patients. J Immunol. 2004;172:7335–40. doi: 10.4049/jimmunol.172.12.7335. [DOI] [PubMed] [Google Scholar]
- 150.Dasgupta S, Bhattacharya-Chatterjee M, O'Malley BW, Jr., Chatterjee SK. Inhibition of NK Cell Activity through TGF-β by Down-Regulation of NKG2D in a Murine Model of Head and Neck Cancer. J Immunol. 2005;175:5541–50. doi: 10.4049/jimmunol.175.8.5541. [DOI] [PubMed] [Google Scholar]
- 151.Wu JD, Atteridge CL, Wang X, Seya T, Plymate SR. Obstructing Shedding of the Immunostimulatory MHC Class I Chain-Related Gene B Prevents Tumor Formation. Clinical Cancer Research. 2009;15:632–40. doi: 10.1158/1078-0432.CCR-08-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang X, Lundgren AD, Singh P, Goodlett DR, Plymate SR, Wu JD. An six-amino acid motif in the α3 domain of MICA is the cancer therapeutic target to inhibit shedding. Biochemical and Biophysical Research Communications. 2009;387:476–81. doi: 10.1016/j.bbrc.2009.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]