Abstract
The Jeryl Lynn (JL) vaccine against mumps virus (MuV) contains two components, MuVJL5 and MuVJL2, which differ by over 400 nt. Due to the occurrence of bias in the direction of mutation, these differences and those found in nucleotide sequences of different isolates of the minor component in the vaccine (MuVJL2) might be due to the effect of ADAR-like deaminases on MuV grown in tissue-cultured cells. A molecular clone of MuVJL2 (pMuVJL2) and MuVJL2-specific helper plasmids were constructed in order to investigate molecular interactions between MuVJL5 and MuVJL2, to augment the existing molecular clone of MuVJL5 (pMuVJL5) and MuVJL5-specific helper plasmids. Genome and mRNA termini of MuVJL2 were characterized, and an unusual oligo-G insertion transcriptional editing event was detected near the F mRNA polyadenylation site of MuVJL2, but not of MuVJL5. Genes encoding glycoproteins of rMuVJL2 and rMuVJL5 have been exchanged to characterize the oligo-G insertion, which associated with the specific sequence of the F gene of MuVJL2 and not with any other genes or the RNA-dependent RNA polymerase of strain MuVJL2. The results indicate that a single G-to-A sequence change obliterates the co-transcriptional editing of the F mRNA and that this oligo-G insertion does not affect the growth of the virus.
INTRODUCTION
The Jeryl Lynn (JL) mumps virus (MuV) vaccine contains two different component strains, MuVJL5 and MuVJL2, that differ considerably in their nucleotide sequences (Amexis et al., 2002). The mechanisms that generated the two variants are unclear. MuV belongs to the genus Rubulavirus of the subfamily Paramyxovirinae. The inner core consists of the non-segmented negative-strand viral RNA (length, 15 384 nt) associated with the nucleocapsid (N) protein, lesser amounts of the polymerase associated phospho- (P) protein and small amounts of the large (L) RNA-dependent RNA polymerase (RdRp) protein. In the virion, the ribonucleoprotein (RNP) is surrounded by a host-derived membrane, which is spanned by two glycoproteins: the haemagglutinin–neuraminidase (HN) and fusion (F) proteins. On the inner face of the membrane is a membrane or matrix (M) protein that interacts with the cytoplasmic tails of the HN and F proteins and with the RNP. The virus also expresses three non-structural proteins. Two are derived from the second transcription unit, which encodes the non-structural V protein directly and, after co-transcriptional editing of the transcripts, gives rise to two other proteins, P and W. The W protein is a second non-structural protein consisting of a truncated version of V, which substitutes a short peptide of unknown function, encoded by the third potential reading frame, for the cysteine-rich tail that is characteristic of the V proteins of all members of the Paramyxovirinae. The third non-structural protein is a small hydrophobic (SH) protein that is derived from a transcription unit between those encoding the F and HN proteins (Elliott et al., 1989; Elango et al., 1989). Although some aspects of the replication of MuV have been studied at the molecular level, others have been inferred only by analogy to other members of the Paramyxovirinae. For example, the genome and 5′ transcriptional termini of MuV have been generally inferred from sequence comparisons rather than determined directly. The mRNA 3′ termini have been better characterized by sequencing cDNA clones generated by oligo-dT priming on MuV mRNA (Elango et al., 1988). Furthermore, polyadenylation and polymerase slippage at the 3′ end of the genes and at the editing site have not been subjected to rigorous analysis, and direct proof of the inferred sequence determinants is lacking. The development of reverse-genetics systems (Amexis et al., 2002; Lemon et al., 2007) now allows many of the basic parameters of MuV replication to be studied in detail.
The MuV JL vaccine has been reported to be derived from a single clinical isolate (Afzal et al., 1993), which was converted into a live-attenuated vaccine by passage of virus in non-human host cells. The complete nucleotide sequences of MuVJL5 and MuVJL2 have been reported (Clarke et al., 2000; Amexis et al., 2002) and show 414 nucleotide changes, resulting in 87 amino acid changes, between MuvJL5 and MuVJL2. This level of variation is at the same level as differences between genotypes of MuV. There are biological differences between MuVJL5 and MuVJL2. For example, MuVJL2 grows better in embryonated eggs than MuVJL5, but immunological differences have not been reported (Amexis et al., 2002). Both MuVJL5 and MuVJL2 are non-neurovirulent in the rat neurovirulence test (Rubin et al., 1999, 2000, 2003). Molecular differences other than the nucleotide sequences have not been investigated extensively.
METHODS
Virus strains and cell-culture procedures.
A549 and Vero cells were grown in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) supplemented with 10 % (v/v) fetal calf serum (FCS; Invitrogen). MuV strains MuVJL5, MuVJL2 and MuVKH were obtained from Dr D. Clarke (Wyeth-Lederle Vaccines, Pearl River, NY 10965, USA), Dr M. Afzal (National Institute for Biological Standards and Control, Potters Bar, London, UK) and Dr S. A. Rubin (Center for Biologics Evaluation and Research, Food and Drug Administration, Bethesda, MD, USA), respectively. Modified vaccinia virus Ankara (MVA-T7) was grown as described previously (Duprex et al., 1999). For virus growth curves, Vero cells were grown in 75 cm2 bottles and infected at 50 % confluence at an m.o.i. of 0.01, and maintained in 10 ml DMEM/10 % (v/v) FCS. Virus titres were determined by TCID50 for both cell-associated and supernatant virus. Cells were scraped at the given time points into the growth medium and supernatant was separated by 5 min centrifugation at 3000 r.p.m. (1200 g) (with pelleted cell-associated material resuspended in 10 ml fresh DMEM/10 % FCS). TCID50 titres were determined in triplicate by a 50 % end-point dilution assay in 96-well trays of Vero cells, as described previously (Duprex et al., 1999).
Bacterial strains and plasmids.
Escherichia coli strain DH5αIQ was used for routine transformations; highly competent E. coli strains XL blue and TOP10 were obtained from Stratagene and Invitrogen, respectively, and used where fewer transformants were expected. The full-length MuV clone pMuVFL encoding MuVJL5, helper plasmids encoding MuVJL5 N, P and L proteins and pMuVDICAT were obtained from Dr D. Clarke (Wyeth-Lederle Vaccines, Pearl River, NY 10965, USA). The cytomegalovirus (CMV) promoter-based expression plasmid pCG (Cathomen et al., 1995) was used as the basis for construction of MuVJL2-specific helper plasmids pCG-NJL2, pCG-PJL2 and pCG-LJL2.
Reagents.
Oligonucleotides were obtained from Qiagen. TRIzol reagent was from Invitrogen. Restriction enzymes, reverse transcriptase SuperScript III, high-fidelity Taq DNA polymerase, Pfu polymerase, Phusion DNA polymerase, Klenow fragment of DNA polymerase, exonuclease III and DNA ligase were obtained from New England Biolabs (NEB) or Invitrogen and used according to the manufacturers' instructions.
Recombinant DNA manipulations.
PCR products corresponding to MuV genes were generated with unique terminal restriction-enzyme sites and cloned into suitable intermediate vectors to generate two vectors containing approximately half of the MuVJL2 genome. One half comprised the leader to the HN gene and was constructed in pUC18 by sequential ligations of genes from MuvJL2. The other half, comprising the L gene, was amplified in two overlapping segments and cloned sequentially into a modified form of the pBluescript-derived pMuVJL5 by deletion from N to HN, i.e. from an SphI site near the start of the N gene to an NheI site near the end of the HN gene, thus replacing the L gene of MuVJL5 with that of MuVJL2. In the final stage, the leader to HN region of MuVJL2 from the pUC18-based intermediate was cloned into the pBluescript/MuVJL2 L gene intermediate to generate a full-length clone of strain MuVJL2. Details of oligonucleotides, enzymes etc. can be supplied on request.
The initial full-length clone of MuVJL2 was modified by rounds of in vitro mutagenesis of subgenomic clones, which were transferred to the full-length clone to insert further restriction sites at intergenic locations or to eliminate them elsewhere. Recombinant DNA operations were initially performed by standard RT-PCR, restrictions and ligations according to the manufacturers' protocols, but a simple form of ligation-independent cloning (Aslanidis & de Jong, 1990; Li & Evans, 1997) was used for most procedures after the generation of the first full-length clone of MuVJL2. In brief, 1–200 ng restricted vector and 1–200 ng desalted PCR product (generated using oligonucleotides with termini homologous with vector and a DNA polymerase that generates blunt-ended PCR products) for insert were incubated for 10 min at 37 °C in a final volume of 10 μl of NEB buffer 1 (or the buffer with the lowest ionic strength consistent with digestion of vector) with 10 units exonuclease III. Next 2 μl 1 M NaCl was added and exonuclease III was heat-inactivated at 75 °C for 15 min. The reaction mixture was cooled slowly from approximately 55 to 37 °C in about 1.5 h in an insulated beaker of water and transformed into competent E. coli. The final full-length clone of MuVJL2 was designated pMuVJL2 and has restriction sites with blunt ends or 5′ restriction overhangs engineered in all intergenic positions, internal to all viral genes except SH and spaced evenly throughout L, because exonuclease III is reliable for 3′–5′ digestion of such restriction sites. The clone of the HN gene of MuVJL2 to the pMuVJL5 vector involved cloning to an SgfI site with a 3′ overhang on which exonuclease III is inactive. Klenow fragment of DNA polymerase, which has both 5′–3′ and 3′–5′ exonuclease activities, was used instead of exonuclease III for digestion of DNA during this procedure. In vitro mutagenesis was also performed by exonuclease III digestion of overlapping blunt-ended PCR termini generated with mutagenic oligonucleotides followed by annealing as above. Smaller plasmids (i.e. any gene except L in the initial small plasmid cloning vectors) could be mutated from a single PCR product, but longer DNAs (e.g. of the L gene half-genome clones) were generated as two overlapping pieces from the site of mutation to a site in the ampicillin-resistance gene of the vector.
Determination of MuV RNA termini by rapid amplification of cDNA ends (RACE).
The 5′ termini of all MuV mRNAs and both genomic termini were determined by RACE after PCR using G-tailed cDNAs with a negative-sense gene-specific primer located close to the gene start for each mRNA (that for N generated two termini – that of the N mRNA and that of the antigenome; that for HN generated termini for both HN and SH) or a positive-sense primer located close to the end of the L gene for the 5′ terminus of the genome, and a common oligo-dC tailed primer as described previously (Barr et al., 1994). The 3′ termini of F gene mRNAs were determined in similar fashion after PCR using oligo-dT-primed cDNAs with a positive-sense primer located near the end of the F gene and an oligo-dT-tailed primer. Nucleotide sequences of all RNA termini were determined directly from the PCR products.
Rescue of recombinant viruses from cDNA clones.
Initial rescue by transfection of full-length and helper plasmids to MVA-T7-infected A549 cells, followed by overlay with Vero cells (Clarke et al., 2000), was used for rescue of all recombinant viruses. When MuVJL2 helper plasmids were used, 2 μg pCG-NJL2, 1 μg pCG-P JL2 and 0.1 μg pCG-L JL2 substituted for the MuVJL5 analogues. Rescued recombinant viruses were verified by nucleotide sequence analysis of PCR-amplified viral genes using amplification without reverse transcription as a negative control.
RESULTS
Sequence variation between MuVJL2 and MuVJL5 is extensive
Numerous clone-to-clone variations were detected when the PCR products corresponding to MuVJL2 genes were sequenced and, in most instances, it was difficult to determine whether these were generated in the RT-PCR or whether they reflect genuine quasi-species variations in the virus population. The final consensus full-length sequence is available under GenBank accession no. FN431985. The numbers of differences between our consensus MuVJL2 sequence, the published consensus MuVJL2 sequence and the consensus sequence of strain MuVJL5 (Clarke et al., 2000; Lemon et al., 2007) are shown in Table 1. Three clusters of variations between our MuVJL2 sequences and those published were present and may be the result of hypermutation events, as they were all of the form where T (in the cDNA sequence) in one sequence was substituted by C in the other. One cluster is in the 3′ non-coding region of the L gene (nt 15254–15317) where there are six changes in 64 bases, of which five are T in the published MuVJL2, but are C in our sequence – MuVJL5 has T at all these positions. The second region is in the P gene (nt 2217–2323) where there are seven changes in 107 bases, of which six are C in the published MuVJL2, but are T in our sequence – MuVJL5 has T at these positions. The third region (nt 2459–2582) is also in the P gene (and was variable within our own series of clones) where there are three changes in 126 bases, all of which are T in the published MuVJL2 sequence and in one of our cDNA clones, but are C in two other cDNA clones – MuVJL5 has T at these positions. These three clusters contain 16 changes in 297 bases, a divergence of 5.4 %; this is very high compared with the remainder of these full-length sequences, where there are 35 changes in 14 987 nt, a divergence of 0.2 %, and thus contributes substantially to the overall genomic sum of 51 changes in 15 384 bases, a divergence of 0.3 %. A further potential biased mutation event that may have occurred during passage of MuVJL2 and MuVJL5 is located in the 3′ non-coding region of the N gene (nt 1804–1870) where there are nine changes between MuVJL5 and both MuVJL2 sequences, of which eight are T in both of the MuVJL2 sequences, but C in MuVJL5. A tenth change (nt 1820) in this region is present only in our MuVJL2 sequence, where T replaces C of the other two sequences.
Table 1.
Nucleotide and amino acid differences between MuVJL5 and MuVJL2
Values are shown as number of changes (percentage difference). MuVJL2 db is the sequence from Amexis et al. (2002); MuVJL2 cons is our consensus sequence.
| Sequence | Length (aa) | Comparison | ||
|---|---|---|---|---|
| MuVJL5/MuVJL2 db | MuVJL5/MuVJL2 cons | MuVJL2 db/MuVJL2 cons | ||
| Nucleotide | – | 414 (2.7) | 421 (2.7) | 51 (0.3) |
| Protein | ||||
| N | 550 | 7 (1.3) | 6 (1.1) | 1 (0.2) |
| P | 392 | 12 (3.0) | 10 (2.6) | 6 (1.5) |
| V | 225 | 7 (3.1) | 4 (1.8) | 3 (1.3) |
| M | 376 | 5 (1.3) | 7 (1.9) | 4 (1.1) |
| F | 539 | 13 (2.4) | 13 (2.4) | 2 (0.3) |
| SH | 58 | 6 (10.3) | 5 (8.6) | 1 (1.7) |
| HN | 583 | 14 (2.4) | 16 (2.7) | 2 (0.3) |
| L | 2262 | 13 (0.6) | 10 (0.4) | 3 (0.1) |
Transcription of the MuVJL2 genome
Direct determination of the MuV genome and mRNA 5′ termini by PCR and sequencing was carried out in parallel with PCR and sequencing during construction of pMuVJL2. This is important, as the current basis for the MuV genome annotation is founded primarily on inference. The 5′ termini of all mRNAs and the genomic termini of MuVJL2 were determined precisely by RACE (Fig. 1). All termini were found to be as suggested previously on the basis of sequence homology and conservation (Elango et al., 1988; Okazaki et al., 1992). A non-templated base [predominantly seen as G in the positive sense, as found previously (Barr et al., 1994; Collins et al., 1984)] was present between the mRNA 5′ termini and the cDNA tail for all mRNA starts, and corresponds to the mRNA cap structure, as no such nucleotide is present in either genome-terminal sequence.
Fig. 1.
Location of MuVJL2 genome termini and mRNA 5′ termini. Sequence chromatograms that identify MuVJL2 genome termini and mRNA 5′ termini. In (a–h), the orientation of the chromatograms has been reversed so that all sequences can be read in the standard positive direction. Ten bases of tail/cap artefact and 10 bases of viral sequence are shown for each. (a, i) Genome 5′ and 3′ termini; (b–h) 5′ termini of the N, P/V, M, F, SH, HN and L mRNAs, respectively. Pairs of sequences (a) and (b) or (f) and (g) were taken from the same electrochromatograms. Arrowheads in (b–h) indicate the base inserted complementary to the mRNA methylated guanosine cap structure – this is predominantly C in the cDNA copy and thus appears as G in this orientation. The peak signals in the M mRNA start (d) were higher than optimal, generating artefact peaks under the tail. The F mRNA start (e) also contains a minor signal from the M–F gene junction. The HN mRNA start (g) contains a signal from an abundant SH–HN transcript of approximately equal intensity to the tail itself (Afzal et al., 1990).
In an early study (Elango et al., 1988), the mRNA 3′ termini and polyadenylation signals of all genes except L were identified directly in the SBL1 strain of MuV (MuVSBL) and located in tracts of six and seven adenosine residues (positive-sense sequence). We directed our attention to the 3′ end of the F gene of MuVJL2 where there are two such tracts separated by only seven intervening bases, one of six and one of seven adenosine residues, which are both rather similar to potential polyadenylation-site motifs. There is also sequence divergence between strains MuVJL2 and MuVJL5 with three changes out of eight bases in part of the region comprising the two polyadenylation motifs (Fig. 2). In addition, the Enders strain of MuV (MuVEnders) expresses only F–SH read-through transcripts and has an A-to-G mutation in the region comprising the seven-adenosine tract of the other MuV strains (Takeuchi et al., 1991). We mapped the 3′ end of the F mRNA by RACE to determine which polyadenylation motif (or neither, or both) might be used during mRNA synthesis for MuVJL2. All polyadenylation of the F gene of MuVJL2 occurred at the second motif (Fig. 3) in the seven-adenosine tract at the end of the F gene, which is also used for polyadenylation of the MuVJL5 F mRNA.
Fig. 2.
Potential polyadenylation motifs at the 3′ termini of MuV F gene transcripts. The sequences of the 3′-terminal regions (positive strand) of the F mRNAs from MuVSBL, MuVJL2, MuVJL5, MuVKH and MuVEnders are aligned, with differences from MuVJL5 boxed. The two polyadenosine tracts at the end of the F gene of MuVJL2 are indicated by bars beneath the sequences.
Fig. 3.
Oligo-G insertion event at the 3′ terminus of the F mRNA in MuVJL2. Sequence chromatograms from strains MuVJL2 (a, b), MuVJL5 (c, d) and MuVKH (e) are shown. (a) and (c) show the virion RNA sequence of the F–SH intergenic region determined from PCR products generated with F- and SH-specific primers, where random primers were used to prime cDNA synthesis on virion RNA extracted from centrifuged growth medium (i.e. supernatants) from infected cells (primarily genome/antigenome RNA). (b), (d) and (e) show the 3′-terminal region of the F mRNA determined from PCR products generated with F gene- and poly(A)-specific primers, where oligo-dT was used to prime cDNA synthesis on RNA extracted from infected cells (primarily mRNA). The oligo-G insertion event in (b) is indicated by a black bar and polyadenylation in (b), (d) and (e) by dotted arrows.
An oligo-G insertion transcriptional editing event in the 3′ UTR of the F gene of MuVJL2
Although the first of the two potential polyadenylation signals did not appear to be used, it did allow slippage of the RdRp to occur as, surprisingly, a small number of additional G residues were inserted into the F mRNA in MuVJL2-infected cells at the GG sequence after the first motif (Fig. 3b). This process resembles slippage of the viral polymerase at the P/V editing site in the P gene. The F gene of the Kilham strain of MuV (MuVKH) is similar to MuVJL2 in that it contains tracts of both six and seven adenosine residues at the end of the F gene (Tecle et al., 2000; Lemon et al., 2007), but is similar to MuVJL5 in that it contains GA instead of GG between the two tracts (Fig. 2). When the 3′-terminal region of the MuVKH F mRNA was sequenced, there was no oligo-G insertion, which suggests that the presence of the second G of the GG between the two adenosine tracts is necessary for the oligo-G insertion event to occur (Fig. 3e).
Development of a reverse-genetics system for MuVJL2
It became clear that not only was the sequence of our MuVJL2 component (Afzal et al., 1993) (received as an early passage after isolation and plaque purification of the MuVJL5 and MuVJL2) different from the MuVJL5 sequence, but also that the level of variation between our MuVJL2 sequence and the published MuVJL2 sequence was significant (51 nt). In order to facilitate further molecular studies, to investigate the phenotypes of recombinants between MuVJL2 and MuVJL5, we developed a reverse-genetics system for MuVJL2 that complements the existing system for MuVJL5 (Lemon et al., 2007; Clarke et al., 2000). The plasmid pMuVMPBS (Lemon et al., 2007) derived from MuVJL5 is hereafter referred to as pMuVJL5 for clear differentiation from pMuVJL2. Plasmid pMuVJL2 was constructed by using the pBluescript plasmid, T7 promoter and hepatitis delta ribozyme from the pMuVJL5 system (Clarke et al., 2000) and engineered to fit the consensus sequence described above except (i) where restriction sites were introduced or removed that would not alter the viral proteins, but would allow easy exchange of genes between MuVJL5, MuVJL2 and other MuV strains, and (ii) where a potential minor mutation event was detected in the P gene (three nucleotides, 2459, 2534 and 2582, are all T in the full-length plasmid, but C in most of the P gene clones), because both MuVJL5 and the previously published MuVJL2 sequence have T at these three positions. Restriction-enzyme sites at intergenic locations between genes encoding the envelope proteins of pMuVJL5 have been incorporated during the construction of pMuVJL2 (the unique restriction site between F and SH is, however, AvrII in pMuVJL2 rather than BmgBI as in pMuVJL5, because pMuVJL2 has an additional BmgBI site in the L gene and there are both SgfI and SapI unique restriction sites between SH and HN in pMuVJL2). Additional unique restriction-enzyme sites have been generated between all other genes and internal to all viral genes (except for that of SH) and spaced fairly evenly throughout the L gene to facilitate molecular studies (Fig. 4).
Fig. 4.
Molecular clone of MuVJL2, indicating gene boundaries and restriction sites in pMuVJL2. The bar shows the antigenome of pMuVJL2 and the locations of viral genes (not to scale). Arrows beneath the bar indicate the location of unique restriction sites suitable for ligation-independent cloning using exonuclease III in pMuVJL2. The vector sequence flanking the antigenome contains a NotI site upstream of a T7 RNA polymerase promoter located 5′ to the antigenome (i.e. to the left of N) and a KasI site downstream of the antigenome 3′ terminus (i.e. to the right of L) which is internal to the hepatitis delta ribozyme (these restriction sites are shown in bold). (a) Restriction sites present in the consensus MuVJL2 sequence – these were either already unique in the consensus MuVJL2 sequence or made unique by mutagenesis of sites at other locations in the MuV genome or the plasmid vector. (b) Restriction sites introduced into the final clone by in vitro mutagenesis. Additional SmaI, AvrII, BsrGI and XhoI restriction sites in the MuVJL2 sequence (c) were removed by in vitro mutagenesis. A SapI site and two FspI sites were removed from the vector sequence by in vitro mutagenesis or deletion to render sites in the MuVJL2 sequence unique in the final clone. Restriction-enzyme names are abbreviated for clarity. Details of their position in the MuVJL2 sequence are available on request. The asterisks indicate that these sites are unique in the plasmid DNA which is methylated, as there are two sites at 11408–11413 and 11608–11613 that are also cleavable with StuI and NruI, respectively, in unmethylated plasmid DNA.
Although helper plasmids appear to be interchangeable in the rescue systems for all molecular clones tested, from our direct experience, it appeared advisable to use homologous plasmids in order to avoid inter-strain recombination events driven by vaccinia virus (data not shown). Therefore, MuVJL2-specific helper plasmids encoding the N, P and L genes (pCG-NJL2, pCG-PJL2 and pCG-LJL2, respectively) were constructed in the CMV promoter-driven vector pCG and these were used successfully in the rescue of the pMuVJL2. A mumps minigenome construct that encodes enhanced green fluorescent protein (EGFP) (pMuVEGFP) has been constructed by replacing the chloramphenicol acetyltransferase open reading frame of pMuVDICAT (Clarke et al., 2000) with that for EGFP. pMuVEGFP has been rescued with both the MuVJL2 and MuVJL5 sets of helper plasmids (data not shown). A recombinant virus, rMuVJL2, was rescued successfully from plasmid pMuVJL2. The growth of this virus was very similar to that of the original plaque-purified virus MuVJL2, confirming that the synonymous genetic alterations that we have made do not affect virus growth dynamics in Vero cells (Fig. 5).
Fig. 5.
Growth curves of MuVJL2 and rMuVJL2. Supernatant (a) and cell-associated (b) titres of samples harvested from infected Vero cells at days 0–7 post-infection were determined by TCID50. The stock plaque-purified MuVJL2 supplied by Dr M. Afzal and the final recombinant virus rescued from cDNA are shown as MuVJL2 (▪) and rMuVJL2 (▴), respectively. Error bars represent sd.
Construction of chimaeric rMuVJL2 and rMuVJL5 viruses to characterize the oligo-G insertion at the end of the F mRNA of MuVJL2
In order to determine whether the oligo-G insertion at the end of the F mRNA of MuVJL2 was determined by the nucleotide sequence at the end of the MuVJL2 F gene, by characteristics of the viral polymerase of strain MuVJL2 or by other determinants in the genome of this MuV strain, the F gene of MuVJL2 was transferred into MuVJL5 and the F gene of MuVJL5 into MuVJL2 by using the unique between-gene restriction sites that are present in the plasmids. The corresponding viruses were rescued and the 3′ termini of the F mRNAs were characterized. The recombinant rMuVJL5 (FJL2) displayed the same oligo-G insertion as the MuVJL2 F gene, and the rMuVJL2 (FJL5) displayed no oligo-G insertion (Fig. 6). This indicates that the oligo-G insertion was associated with the F gene sequence of MuVJL2 rather than being a characteristic of the viral polymerase of strain MuVJL2.
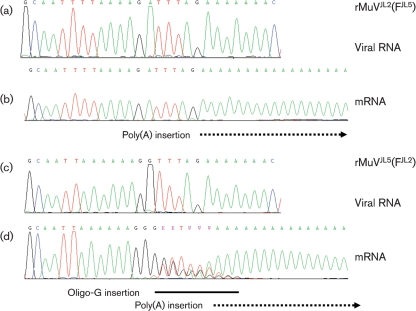
Fig. 6.
3′-Terminal sequences of F genes and transcripts of rMuVJL2(FJL5) and rMuVJL5(FJL2). (a, b) Sequence from rMuVJL2(FJL5); (c, d) sequence from rMuVJL5(FJL2). (a) and (c) show sequence electrochromatograms of the virion sequence of the F polyadenylation region cut to the first nucleotide difference between MuVJL2 and MuVJL5 in the SH gene; (b) and (d) show the 3′-terminal region of the F mRNA 2. rMuVJL2(FJL5) has the F sequence characteristic of rMuVJL5 and the SH sequence characteristic of rMuVJL2, and rMuVJL5(FJL2) has the F sequence characteristic of rMuVJL2 and the SH sequence characteristic of rMuVJL5. The oligo-G insertion event in (d) is indicated by a black bar and polyadenylation in (b) and (d) by a dotted arrow.
DISCUSSION
The origin of the sequence variation between the MuVJL5 and MuVJL2 components of the live-attenuated JL vaccine is unclear. The JL vaccine was derived from a single clinical isolate. These, in general, display a consensus sequence with little detectable variation and, hence, it is likely that some part of the variation between the two vaccine components has been generated after the original isolation and during the attenuation process. Selection of a more neurovirulent variant of MuVJL5 has been shown to be associated with only three amino acid changes and relative sequence stability of this virus (Rubin et al., 2003). The substantial sequence differences between MuVJL2 determined in different laboratories suggest that cytosine or adenine deamination events may play a significant role in the generation of diversity between the MuVJL strains. It is to be noted that Amexis et al. (2002) did not succeed in propagating a MuVJL2 virus, but that the sequence was derived from sequence variations found in the vaccine. Though it was inferred to be preferably propagated in chicken embryo cells, their MuVJL2 could not be propagated on Vero cells and, hence, they suggested that MuVJL2 was a not completely defective satellite virus. Our sequence is derived from a plaque-purified Vero cell-propagated MuVJL2 isolate. Amexis et al. (2002) posed the question of whether the MuVJL2 sequence is a passenger virus of MuVJL5. The fact that we were able to set up a rescue system based on the MuVJL2 consensus sequence with its own helper plasmids indicates that the vaccine is a mixture of two independently replicating viruses. In fact, titres of MuVJL2 are routinely at least 1 log10 higher than those of MuVJL5 in Vero cells in our hands. This, and the number and location of unique restriction-enzyme sites that did not affect the viral protein sequences and the even spacing of unique restriction sites in pMuVJL2, should be of benefit in future molecular studies of MuV and may prove to be advantageous in the use of the MuVJL2 rescue system.
The prevalence of C→U and U→C mutations and the identification of the localized nature of the biased hypermutation indicate that deamination reactions by ADAR- or APOBEC-like enzymes may play a role in the generation of the sequence variation between MuVJL5 and MuVJL2, although this remains to be formally proven. In most cases, biased hypermutation events lead to functional impairment of the affected region of the viral genome, for example in measles virus sequences obtained from cases of subacute sclerosing panencephalitis (Cattaneo et al., 1988). Interestingly, loss of function does not seem to be the case here. The potential events in L and N genes are located in the 3′ non-coding regions. One potential event in the P gene affects an area with low sequence similarity at the N-terminal part of the V/P protein. The other event present in most but not all of our MuVJL2 P clones affects the V but not the P protein and this may alter the interferon sensitivity of the MuVJL2 virus. Biased hypermutation has been described for several other viruses (Bass, 2002) and may reflect the deaminating activity of the ADAR or APOBEC enzymes themselves (Bass, 2002; Bishop et al., 2004) or their ability to bind to RNA (Nie et al., 2007).
This study shows for the first time, to our knowledge, the MuV genome and 5′ termini of the mRNAs and that these had been predicted correctly from the conservation of motifs, and demonstrates the capped nature of these mRNAs in contrast to the uncapped terminal sequences of the genome. Earlier studies were not able to determine the precise mRNA start sites, although polyadenylation signals had been identified correctly (Elango et al., 1988). The polyadenylation signal at the end of the F gene of MuVJL2 required clarification because of the sequence variation amongst strains of MuV at this point. Read-through from the F to SH transcription units is found in MuVEnders (Afzal et al., 1990; Takeuchi et al., 1991), where there is a failure to terminate and polyadenylate. In addition, there was sequence variation amongst our own six PCR-derived clones from virion RNA of strain MuVJL2 at this point – one had a single addition to the six-adenosine tract, another a single deletion from the seven-adenosine tract. It was not clear, therefore, which motif would be used as the polyadenylation signal during F gene mRNA synthesis for strain MuVJL2. The data showed clearly that termination and polyadenylation of the F mRNA of MuVJL2 occurs only at the seven-adenosine tract, as in MuVSBL, MuVJL5 and MuVKH. During the study of the polyadenylation signals of the F gene, an oligo-G insertion event was identified in the 3′ non-coding region of the F gene of MuVJL2 (and also in rMuVJL2). This leads to variable levels of insertion of a number of additional G residues (one to seven, with a mean of approximately four residues) at a site that directly follows the six-adenosine tract and is similar in sequence to a polyadenylation motif. The insertion of variable numbers of G residues does not appear to affect the ability of the virus to grow, and the motif appears only to be recognized during transcription and not replication of the viral genome. Exchanges of the F genes of rMuVJL5 and rMuVJL2 showed clearly that the insertion of G residues is a property of the sequence motif in the F gene of MuVJL2 and not of the RdRp or the other genes of MuVJL2, as it occurred in the F gene of MuVJL2 placed in either the rMuVJL2 or the rMuVJL5 background. However, the oligo-G insertion into the F mRNA prior to polyadenylation was not observed in MuVKH, which also contains the same six- and seven-adenosine tracts as MuVJL2. MuVJL2 has the sequence GG at the oligo-G insertion site, whereas MuVKH has the sequence GA. This suggests that presence of a pair of G residues between the two polyadenosine tracts is necessary for the oligo-G insertion event to occur in MuVJL2. The oligo-G insertion at the 3′ end of the MuVJL2 F mRNA clearly resembles the viral RdRp slippage motif at the P/V editing site (Elliott et al., 1990; Paterson & Lamb, 1990). At present, we do not know whether this oligo-G insertion is a chance event that reflects the conjunction of the pair of G residues between the six- and seven-adenosine tracts so far restricted to MuVJL2, or whether it has a function. It may, for example, regulate transcription of the SH gene by affecting transcription attenuation or the formation of read-through products, which is a frequent occurrence at this site (Afzal et al. 1990).
In conclusion, we have demonstrated significant sequence variation and instability in the MuVJL2 component of the JL vaccine. In part, these sequence changes may be generated by activity of cytosine and/or adenine deaminases in the cell. However, the inter-relationship of MuVJL2 and MuVJL5 is not clear and the sequence diversity even between isolates of MuVJL2 raises further questions about the relationships between the two vaccine components. We have developed a versatile rescue system for MuV based on MuVJL2 and used this and the MuVJL5 rescue system to prove that an oligo-G insertion event at the end of the F gene of MuVJL2 is determined by the sequence at the slippage site.
Acknowledgments
This work was supported by Wellcome Trust grant no. 064263. We thank Dr D. Clarke for the supplying the MuVJL5 rescue system, Dr K. Lemon in our laboratory for many plasmids, oligonucleotides and discussions and Ms Rosie Neeson and Ms Caitriona Byrne for technical assistance.
Footnotes
The GenBank/EMBL/DDBJ accession number for the final consensus full-length sequence of Jeryl Lynn mumps virus sub-strain JL2 genomic RNA is FN431985.
References
- Afzal, M. A., Elliott, G. D., Rima, B. K. & Orvell, C. (1990). Virus and host cell-dependent variation in transcription of the mumps virus genome. J Gen Virol 71, 615–619. [DOI] [PubMed] [Google Scholar]
- Afzal, M. A., Pickford, A. R., Forsey, T., Heath, A. B. & Minor, P. D. (1993). The Jeryl Lynn vaccine strain of mumps virus is a mixture of two distinct isolates. J Gen Virol 74, 917–920. [DOI] [PubMed] [Google Scholar]
- Amexis, G., Rubin, S., Chizhikov, V., Pelloquin, F., Carbone, K. & Chumakov, K. (2002). Sequence diversity of Jeryl Lynn strain of mumps virus: quantitative mutant analysis for vaccine quality control. Virology 300, 171–179. [DOI] [PubMed] [Google Scholar]
- Aslanidis, C. & de Jong, P. J. (1990). Ligation-independent cloning of PCR products (LIC-PCR). Nucleic Acids Res 18, 6069–6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr, J., Chambers, P., Harriott, P., Pringle, C. R. & Easton, A. J. (1994). Sequence of the phosphoprotein gene of pneumonia virus of mice: expression of multiple proteins from two overlapping reading frames. J Virol 68, 5330–5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass, B. L. (2002). RNA editing by adenosine deaminases that act on RNA. Annu Rev Biochem 71, 817–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop, K. N., Holmes, R. K., Sheehy, A. M. & Malim, M. H. (2004). APOBEC-mediated editing of viral RNA. Science 305, 645. [DOI] [PubMed] [Google Scholar]
- Cathomen, T., Buchholz, C. J., Spielhofer, P. & Cattaneo, R. (1995). Preferential initiation at the second AUG of the measles virus F mRNA: a role for the long untranslated region. Virology 214, 628–632. [DOI] [PubMed] [Google Scholar]
- Cattaneo, R., Schmid, A., Eschle, D., Baczko, K., ter Meulen, V. & Billeter, M. A. (1988). Biased hypermutation and other genetic changes in defective measles viruses in human brain infections. Cell 55, 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke, D. K., Sidhu, M. S., Johnson, J. E. & Udem, S. A. (2000). Rescue of mumps virus from cDNA. J Virol 74, 4831–4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, P. L., Huang, Y. T. & Wertz, G. W. (1984). Nucleotide sequence of the gene encoding the fusion (F) glycoprotein of human respiratory syncytial virus. Proc Natl Acad Sci U S A 81, 7683–7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprex, W. P., Duffy, I., McQuaid, S., Hamill, L., Cosby, S. L., Billeter, M. A., Schneider-Schaulies, J., ter Meulen, V. & Rima, B. K. (1999). The H gene of rodent brain-adapted measles virus confers neurovirulence to the Edmonston vaccine strain. J Virol 73, 6916–6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elango, N., Varsanyi, T. M., Kovamees, J. & Norrby, E. (1988). Molecular cloning and characterization of six genes, determination of gene order and intergenic sequences and leader sequence of mumps virus. J Gen Virol 69, 2893–2900. [DOI] [PubMed] [Google Scholar]
- Elango, N., Kovamees, J., Varsanyi, T. M. & Norrby, E. (1989). mRNA sequence and deduced amino acid sequence of the mumps virus small hydrophobic protein gene. J Virol 63, 1413–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott, G. D., Afzal, M. A., Martin, S. J. & Rima, B. K. (1989). Nucleotide sequence of the matrix, fusion and putative SH protein genes of mumps virus and their deduced amino acid sequences. Virus Res 12, 61–75. [DOI] [PubMed] [Google Scholar]
- Elliott, G. D., Yeo, R. P., Afzal, M. A., Simpson, E. J., Curran, J. A. & Rima, B. K. (1990). Strain-variable editing during transcription of the P gene of mumps virus may lead to the generation of non-structural proteins NS1 (V) and NS2. J Gen Virol 71, 1555–1560. [DOI] [PubMed] [Google Scholar]
- Lemon, K., Rima, B. K., McQuaid, S., Allen, I. V. & Duprex, W. P. (2007). The F gene of rodent brain-adapted mumps virus is a major determinant of neurovirulence. J Virol 81, 8293–8302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C. & Evans, R. M. (1997). Ligation independent cloning irrespective of restriction site compatibility. Nucleic Acids Res 25, 4165–4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie, Y., Hammond, G. L. & Yang, J. H. (2007). Double-stranded RNA deaminase ADAR1 increases host susceptibility to virus infection. J Virol 81, 917–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki, K., Tanabayashi, K., Takeuchi, K., Hishiyama, M., Okazaki, K. & Yamada, A. (1992). Molecular cloning and sequence analysis of the mumps virus gene encoding the L protein and the trailer sequence. Virology 188, 926–930. [DOI] [PubMed] [Google Scholar]
- Paterson, R. G. & Lamb, R. A. (1990). RNA editing by G-nucleotide insertion in mumps virus P-gene mRNA transcripts. J Virol 64, 4137–4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin, S. A., Snoy, P. J., Wright, K. E., Brown, E. G., Reeve, P., Beeler, J. A. & Carbone, K. M. (1999). The mumps virus neurovirulence safety test in Rhesus monkeys: a comparison of mumps virus strains. J Infect Dis 180, 521–525. [DOI] [PubMed] [Google Scholar]
- Rubin, S. A., Pletnikov, M., Taffs, R., Snoy, P. J., Kobasa, D., Brown, E. G., Wright, K. E. & Carbone, K. M. (2000). Evaluation of a neonatal rat model for prediction of mumps virus neurovirulence in humans. J Virol 74, 5382–5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin, S. A., Amexis, G., Pletnikov, M., Li, Z., Vanderzanden, J., Mauldin, J., Sauder, C., Malik, T., Chumakov, K. & Carbone, K. M. (2003). Changes in mumps virus gene sequence associated with variability in neurovirulent phenotype. J Virol 77, 11616–11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi, K., Tanabayashi, K., Hishiyama, M., Yamada, A. & Sugiura, A. (1991). Variations of nucleotide sequences and transcription of the SH gene among mumps virus strains. Virology 181, 364–366. [DOI] [PubMed] [Google Scholar]
- Tecle, T., Johansson, B., Yun, Z. & Orvell, C. (2000). Antigenic and genetic characterization of the fusion (F) protein of mumps virus strains. Arch Virol 145, 1199–1210. [DOI] [PubMed] [Google Scholar]