Abstract
Human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs) possess potentiality to produce all cell and tissue types of the human body. Under chemically defined culture systems, hESCs and hiPSCs have been efficiently directed to functional spinal motoneurons and astrocytes. The differentiation process faithfully recapitulates the developmental process predicted from studies in vertebrate animals and human specimens, suggesting the usefulness of stem cell differentiation systems in understanding human cellular development. Motoneurons and astrocytes differentiated from genetically altered hESCs or disease hiPSCs exhibit predicted phenotypes. They thus offer a simplified dynamic model for analyzing pathological processes that lead to human motoneuron degeneration, which in turn may serve as a template for pharmaceutical screening. In addition, the human stem cell-derived motoneurons and astrocytes, including those specifically derived from a patient, may become a source for cell therapy.
Keywords: embryonic stem cells, induced pluripotent stem cells, astrocytes, spinal muscular atrophy (SMA), amyotrophic lateral sclerosis (ALS)
Introduction
Motoneurons in the spinal cord innervate skeletal muscles, which is necessary for controlling movements. They originate from neuroepithelial cells in a restricted area of the developing spinal cord (neural tube) at around 4–5 weeks of human gestation. During embryonic development, motoneurons extend their processes (nerves) to the periphery to innervate skeletal muscles that are adjacent to the spinal cord. In an adult human body, however, motoneuron’s axons are projected as far as one meter away from the cell bodies in the spinal cord to reach their target muscles. In order to achieve this, motoneurons contain a high content of structural proteins, like neurofilaments. This geophysical feature of motoneurons demands a high metabolic rate compared to smaller neurons, which potentially renders motoneurons more susceptible to genetic, epigenetic, and/or environmental changes. Since motoneurons are generally non-renewable, degeneration or loss of spinal motoneurons is often associated with debilitating or fatal neurological conditions such as pediatric spinal muscular atrophy (SMA) and adult onset amyotrophic lateral sclerosis (ALS).
Development of therapeutics for motoneuron diseases will depend upon our understanding of how human motoneurons are generated during embryogenesis, functionally maintained during adult life, degenerate under pathological conditions, and how the disease process may be halted or reversed. Human pluripotent stem cells, including human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs), can renew themselves for an extended period and be induced to generate all cell types in the body, including spinal motoneurons and their neighboring glial cells. Because of this, they provide an ideal model to study human motoneurons and glial cells directly. Over the past several years, hESCs have been successfully directed to spinal motoneurons1, 2 and astrocytes.3 What is important to note is that the in vitro differentiation process mirrors in vivo development in terms of temporal time course, response to extrinsic morphogens, activation of transcriptional networks, and functional maturation14. Hence, stem cell differentiation offers a simplified model to understand human motoneuron and astrocyte development that is otherwise inaccessible. The in vitro produced human motoneurons and astrocytes could potentially become a source for cell therapy. In recent years, progress on genetically altered hESCs or disease hiPSCs, including those with ALS4 and SMA,5 would allow for tracing the degenerative process of human motoneurons and may be further modified for drug screening, thus leading to therapeutic development.
Stem cell model for human motoneuron and astrocyte development
Molecular interactions underlying the specification of motoneurons in vertebrate animals have been well defined. During chick embryo development, in response to a specific gradient (concentration) of sonic hedgehog (SHH) diffused from the notochord and floor plate, naïve neuroepithelial cells in the motoneuron progenitor (pMN) domain are specified to motoneuron progenitors by expressing a set of transcription factors including Olig2. During the neurogenic phase, the Olig2-expressing progenitors migrate a short distance ventrally, downregulate Olig2 expression, upregulate neurogenic transcription factors such as Ngn2 and HB9, and become post-mitotic motoneurons.6 Based on this principle, mouse ESCs, after being neuralized by retinoic acid (RA), can be efficiently differentiated to spinal motoneurons in the presence of SHH.7 In light of this, the molecular mechanism underlying motoneuron specification appears to be preserved in vitro.
To determine whether motoneuron differentiation from hESCs follows the same mechanism, we first guide the naïve hESCs to a synchronized population of neuroepithelial cells. Differentiation of neuroepithelia is achieved by plating individual hESC aggregates at a low density under a chemically defined medium.8 This neural induction process is very efficient, yielding over 90% of the differentiated progenies being Pax6+ and Sox1+, which are markers for neuroepithelial cells.9 Despite the absence of any exogenous neural inducers, differentiating hESCs produce molecules at multiple levels to block transforming growth factor β (TGF β) signaling and to activate fibroblast growth factor signaling,10 suggesting a conserved mechanism underlying human neural induction. It was recently suggested that addition of dual inhibitors of bone morphogenetic proteins (BMPs) can significantly increase the neural differentiation from human stem cells.11 We did find that addition of BMP antagonists, such as Noggin, can inhibit BMP signaling in our neural differentiation system. However, such treatment does not significantly alter the neural differentiation efficiency given the already high yield.10 We reason that the dual inhibition of BMPs during differentiation may be helpful if the starting hESCs or other stem cells have poor neural induction due to the presence of a high amount of BMPs in partially differentiated cells.
Besides the conserved molecular mechanisms described above, neural differentiation of hESCs manifests in morphological transformation that is readily identifiable. After 6–8 days of hESC differentiation, the round hESCs become columnar epithelia, which organize into neural tube-like rosettes by 14 days of culture.8 This timeline of in vitro neural differentiation strikingly resembles the temporal course of neural plate and neural tube formation at the end of third gestation week in human embryos, suggesting the preservation of an intrinsic developmental program in the hESC differentiation in vitro. It is in this differentiation process that we discovered a multipotent neural stem cell stage that can be readily patterned to versatile regional progenitors.1 We refer to these precursors as primitive anterior neuroepithelia because they express anterior transcription factors including Otx2, Lhx2, and Pax6, but not the definitive neuroectoderm marker Sox1, and because they can be patterned to progenitors with various regional identities in response to morphogens.9 Indeed, treatment with specific position-inducing morphogens of the primitive, but not the definitive, neuroepithelia results in differentiation of forebrain, midbrain, spinal cord, and dorsal-ventral progenitors.1, 12, 13 This finding sets the foundation for differentiating human stem cells to specific neuronal and glial subtypes, including spinal motoneurons (Fig. 1).14
Figure 1.

Parallels between in vitro stem cell differentiation and human embryo development. hESCs derived from a blastocyst or hiPSCs established from somatic cells are first differentiated to neuroepithelia that organize into neural tube-like rosettes in 2 weeks. This corresponds to the formation of neural plate/tube in a 3-week-old human embryo. In response to SHH and RA the neuroepithelia become ventral spinal progenitors in the next 10 days. This corresponds to the expansion of neural progenitors in a developing spinal cord. These progenitors then give rise to post-mitotic motoneurons in the 4th and 5th week of culture. In the human spinal cord, motoneurons appear at the 5–6th week. Additional 2 months of culture results in generation of astrocytes. It should be noted that hESCs are developmentally equivalent to 1-week-old embryos. Both motoneurons and astrocytes generated from transgenic hESCs or disease hiPSCs may be modified as templates for drug screening whereas those from non-mutated stem cells may be a source for cell therapy. Modified from Krencik and Zhang 2006.42
For motoneuron differentiation, the primitive neuroepithelia are patterned to ventral spinal progenitors by treatment with retinoic acid (RA), a caudalizing morphogen, and sonic hedgehog (SHH), a glycoprotein that induces ventralization. In 2 weeks, a large population of progenitors will express Olig2, a transcription factor specific for motoneuron progenitors. These progenitors then downregulate Olig2, upregulate HB9, a transcription factor specific for spinal motoneurons, exit the cell cycle, and become post-mitotic motoneurons by 4 weeks of hESC differentiation.1 These motoneurons carry additional markers that are normally expressed in those of the spinal cord, including Islet 1/2 and Lhx3. Like mouse ESCs, treatment of hESC-derived neuroepithelia with RA results in differentiation of motoneurons of mainly the cervical and brachial spinal cord, as shown by their expression of HoxC5 and 8.1 Furthermore, the in vitro differentiation of spinal motoneurons corresponds to the appearance of motoneurons in the human spinal cord at around 5 weeks of development. Again, these findings indicate that the in vitro differentiation process follows the same transcriptional program in response to a similar set of morphogens at a predictable time course (Fig. 1). This suggests that the in vitro stem cell differentiation system may be instrumental for understanding how individual subtypes of motoneurons are specified by examining the transcriptional networks in response to specific sets of extracellular factors.
The in vitro generated spinal motoneurons gradually mature over the next several weeks. Shortly after the expression of HB9, the motoneurons express acetylcholine transferase, an enzyme necessary for synthesizing the neurotransmitter acetylcholine.1 It is presently unknown what instructs the motoneurons to adopt this cholinergic fate. In addition, motoneurons express vesicular choline transferase, an enzyme required for storing and releasing the transmitter, suggesting functionality of the in vitro produced motoneurons. Indeed, when co-cultured with myocytes, the human motoneurons induce aggregation of acetylcholine receptors on myocytes, as indicated by bungarotoxin staining, and form neuro-muscular junctions, reminiscent of that which occurs in vivo. They further induce muscular contraction in vitro.1 These findings confirm that the human motoneurons are functional. The stem cell differentiation system will likely provide an ideal platform to address this fundamental question about how to obtain a cholinergic identity.
Along with the cellular and molecular changes, motoneurons become electrophysiologically active after about 7–8 weeks of hESC differentiation. Synaptic currents are more readily detected with additional weeks of cultures. This is likely due to the enhanced synaptogenesis by the presence of differentiating astrocytes.12 Astrocytes have been shown to be critical for synaptogenesis and synaptic transmission in animals15 as well as in hESC-derived neurons.12 Therefore, functional development of motoneurons is critically affected by the timing of differentiation and the presence of surrounding astrocytes.
Astrocytes are involved in virtually every aspect of physiology and pathology of the CNS.16 However, directed differentiation and functional assessment of hESC-derived astrocytes has been surprisingly unexplored. Part of the reason is the paucity of information on the molecular mechanism of astrocytic development and lack of specific markers for astrocyte progenitors.17, 18 In hESC neural differentiation cultures, astrocytes appear after the differentiation of neurons,8, 12 which mirrors the sequence of neurogenesis first and gliogenesis at a later time. Systematic analysis of astrocytic markers, including nuclear factor NF1A, CD44, S100β, and glial fibrillary acidic protein (GFAP) revealed that astrocyte progenitors began to appear as early as one month of hESC differentiation, though the majority emerged at 2–4 months in culture (Fig. 1). These astroglial progenitors were then enriched by dissociation and expansion in the presence of EGF, a condition that minimizes the presence of neurogenic progenitors. By 4 months in culture, the expanded progenitors gave rise to a nearly pure population of astrocytes in the presence of ciliary neurotrophic factor (CNTF), determined by their typical stellate morphology, immunoreactivity to CD44, S-100β, and GFAP, and their ability to take up glutamate and transmit calcium waves.3 In human development, the earliest astrocyte generation, indicated by expression of GFAP, is around 3 months of gestation. Thus, astrocyte differentiation from hESCs in vitro mirrors generation of astrocytes in vivo.
Astrocytes in different regions of the CNS possess differential cellular and functional phenotypes, which may play important roles in the health of neighboring neurons. While it is well established that regional patterning of neuroepithelia determines the identity of neuronal subtypes, it is unknown whether astrocyte subtypes are endowed in a similar way or rather are due to adaptation to the local brain environment. Using the stem cell differentiation model that allows tracing of lineage development (Fig. 1), we discovered that the regional identity of astrocyte subtypes is determined when the neuroepithelia are patterned to regional progenitors, which produce different types of astrocytes during the gliogenic phase. Furthermore, the in vitro generated human astrocyte subtypes exhibit differential functional properties.3 Therefore, early patterning of neuroepithelia not only defines the neuronal subclasses but also determines astrocyte subtypes and at least some of the astrocyte functions. The close relationship between motoneurons and astrocytes during development provides one explanation why the health of motoneurons is tied with astrocytes under homeostatic environment and during pathological conditions.
Modeling human motoneuron degeneration
Most disease processes involve multiple cellular and tissue types, which are best modeled using intact animals. Indeed, animal models have been instrumental to our understanding of human disease pathogenesis. However, many neurodegenerative diseases, including those affecting motoneurons, do not naturally occur in commonly used laboratory animals, though introduction of certain pathogenic aspects of human diseases into animals can often create phenotypes that resemble human diseases. In familial cases of ALS, mutations in the superoxide dismutase 1 (SOD1) gene are associated with motoneuron degeneration. Introduction of mutant SOD1 into mice induces disease phenotypes that resemble those manifested in patients. In these cases, expression of multiple copies of mutant SOD1 is required for provoking phenotypes.19, 20 In human patients, a single copy is sufficient. The most prevalent form of mutant SOD1 in ALS patients, the alanine to valine substitution (A4V), does not appear to generate phenotypes in mice.21 Therefore, differences do exist between humans and mice in supporting the pathogenic potential of the mutant SOD1. In another motoneuron disease, spinal muscular atrophy (SMA), a loss-of-function mutation in the survival of motoneuron SMN1 gene results in progressive motoneuron degeneration in the spinal cord.22, 23 In animals, knockout of SMN1 is lethal. This is because SMN is a housekeeping protein that is expressed in all tissues. In humans, there are two copies of SMNs, SMN1 and SMN2, and the SMN2 gene generates a small proportion of the full-length functional SMN protein. Hence, a human SMN2 gene needs to be introduced to the SMN knockout background in mice in order to create an SMA model. Additionally, astrocytes, which were found to be significantly different between rodents and human 24, are also critical in the pathogenesis of ALS and potentially also SMA. Therefore, model systems with a human background would supplement our current investigations on the pathogenesis of motoneuron degeneration.
There are two major routes to building disease models using human stem cells. One is to genetically alter human stem cells, whereas the other is to derive stem cells from patients with target diseases, especially those with inheritable changes. Genetic alteration of hESCs is essentially the same as the first step in building transgenic animals. Targeting specific gene loci by homologous recombination in mouse ESCs is now a routine technique for most laboratories. This in principle also works in hESCs.25 Nevertheless, repeated cellular cloning required by traditional homologous recombination often renders the established hESC lines unstable. Random gene insertion, by lentiviruses for instance, can reduce the cloning cycles, but the transgenes that are integrated during the stem cell stage are often downregulated along differentiation.26 We therefore first screened gene loci that are resistant to transgene silencing using a lentiviral vector with a built-in Cre-loxP cassette. While the initial selection of clones is time-consuming, the established cell lines are generally resistant to gene silencing after the hESCs are differentiated to cells of the three embryonic germ layers, including functional neurons and astrocytes.27 Because of a built-in Cre-loxP cassette, any gene of interest, including disease-provoking genes, may be introduced through Cre-mediated recombination with high efficiency. These hESCs are therefore referred to as master cell lines. The most recent technological development in the field is the use of zinc fingers which can target specific loci with high efficiency,28 thus potentially alleviating the burden of screening large numbers of cell clones. Nevertheless, whether the transgene expression is sustained following stem cell differentiation remains to be seen.
Human stem cells with disease traits may now be generated directly from human patients using the technology established by Yamanaka and colleagues.29 These induced pluripotent stem cells (hiPSCs), can now be generated from somatic cells such as dermal fibroblasts by expressing pluripotent transcription factors, including Oct3/4 and Sox2 combined with either Klf4 and c-Myc, or Lin28 and nanog.29, 30 hiPSCs exhibit very similar phenotypes of hESCs. Using similar approaches, hiPSCs have also been generated from patients with motoneuron diseases including ALS5 and SMA.4 For hiPSCs to be useful, it is essential that the hiPSCs can differentiate to targeted functional cell types. In parallel comparison with hESCs, we found that hiPSCs, generated with various methods from diverse sources of donor cells, still differentiate to neural precursors albeit at a much lower efficiency.31 Nevertheless, these hiPSC-derived neural precursors can be further differentiated to motoneurons and astrocytes(Fig. 2).31 For example, hiPSCs from ALS and SMA patients can differentiate to motoneurons.4, 5 Preliminary results indicate that some of the disease phenotypes, such as death of motoneurons, appear to occur in those from SMA hiPSCs.4 Therefore, patient hiPSCs could provide a useful model to dissect cellular and molecular mechanisms underlying motoneuron degeneration.
Figure 2.
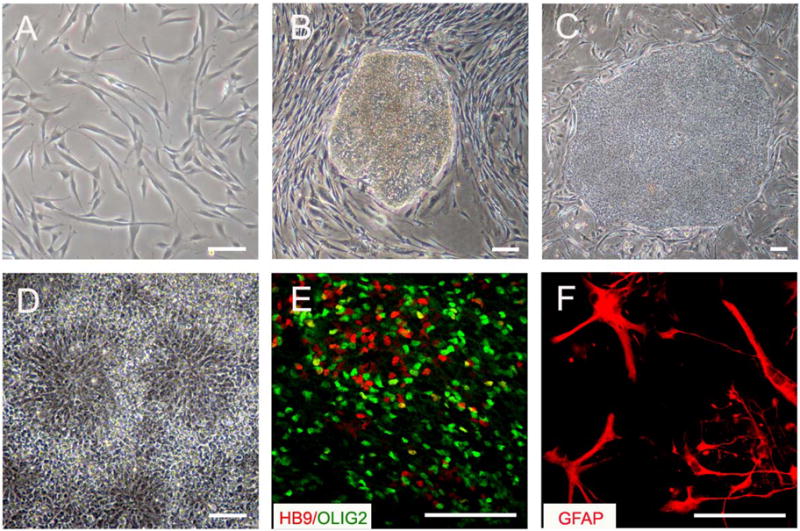
Differentiation of spinal motoneurons and astrocytes from hiPSCs. (A) Human fibroblasts were infected with retrovirus to express Oct3/4, Sox2, Klf4, and c-Myc. (B) Stem cell-like colonies appeared 3–4 weeks following viral infection. (C) The stem cell-like colonies were isolated and expanded, and hiPSC lines were subsequently established. (D) Under a chemically defined culture, the hiPSCs were differentiated to neuroepithelia which form neural tube-like rosettes in 2 weeks. (E) The neuroepithelia further generated Olig2+ (green) motoneuron progenitors and HB9+ (red) postmitotic motoneurons in an additional 3 weeks. (F) GFAP+ astrocytes were generated from hiPSCs after 3 months of stem cell differentiation. Scale bar: 50μm.
Whether transgenic hESCs and disease hiPSCs model more detailed pathological processes besides cell death remains largely untested. In particular, neural degeneration occurs mostly in adult life, suggesting that pathological phenotypes may not manifest in neural cells that are cultured from the transgenic hESCs or hiPSCs. Additional interventions, such as oxidative stress, may be necessary to instigate pathological traits. Alternatively, introduction of multiple copies of disease genes or induction of disease genes at a particular condition (via inducible transgene expression) may accelerate the pathogenic process, which endows transgenic hESCs an advantage. The additional advantage of culture systems is that it permits separation or mixing of different cell types, which helps dissecting cellular contributions to pathological processes. For example, co-culturing hESC-derived motoneurons with human primary astrocytes expressing mutant SOD132 or mouse primary glial cells carrying SOD1 mutation33 results in loss of motoneurons but not other neuronal subtypes, indicating the specific effect of these mutant astrocytes on motoneurons. These findings are consistent with in vivo transgenic studies that mutant SOD1-expressing astrocytes are toxic to motoneurons and accelerate the disease progression,34 validating the usefulness of the culture system to model certain aspects of the disease process. Of course, a simple life or death of motoneurons is far from sufficient to model the slowly progressive nature of degeneration. Furthermore, both transgenic hESCs and disease hiPSCs may be transplanted into animals to follow their pathological process in vivo over a long period. Once certain aspects of neurodegeneration are modeled, they could be modified as a template for screening drugs that potentially slow down or stop the degeneration process (Fig. 1).
Developing stem cell based therapy for motoneuron diseases
Cell based therapy has been tried in a number of neurological diseases with focal pathology, notably Parkinson’s disease.35 Replacement of diseased or lost cells in widespread areas of the brain and spinal cord, such as in the case of ALS and SMA, will likely be very difficult. However, replacement of diseased cells in critical parts of the brain and spinal cord, such as the respiratory centers of the spinal cord, could be life-saving. Progression of motoneuron diseases like ALS, and possibly also SMA, is subject to the dynamic interactions between motoneurons and their neighboring astrocytes. Therefore, replacing diseased or toxic astrocytes at an early stage could rescue motoneurons from further degeneration. By the time most motoneurons are lost, replacement of motoneurons and astrocytes becomes necessary.
Replacement of astrocytes to support the health of motoneurons has been demonstrated in a rat model of ALS in which primary astrocytes not only survive the transplant but also contribute to the increased lifespan of the grafted animals.36 In this case, grafted astrocytes appear to migrate along the spinal cord a certain distance, offering a possibility of supporting motoneurons in the transplant site and neighboring areas. Of course, the human spinal cord is substantially larger and longer than that of a mouse. Nevertheless, it is surgically feasible to inject cells to multiple sites. More importantly, an enriched or pure population of human astrocytes can now be readily differentiated from hESCs and hiPSCs in a chemically defined culture system in large quantities.3 This culture system can be readily adapted to a clean facility for production of a clinical grade of human astrocytes. In particular, astrocytes can be generated from the patient’s own somatic cells through reprogramming to hiPSCs, which can be transplanted back to the patient to avoid immune rejection.
Replacement of motoneurons through transplantation is technically very challenging. First, differentiation of motoneurons from human stem cells, though the most reproducible procedure to date, does not yield a pure population. We have recently modified our original protocol and increased the differentiation efficiency to nearly 50%. Furthermore, we replaced protein growth factors with small molecules in the differentiation procedure so that the motoneuron differentiation process can now be readily adapted to production in a clean facility.37 Second, unlike glial cell transplants, grafted human stem cells-derived postmitotic motoneurons usually have a poor survival rate. In contrast, motoneurons derived from mouse ESCs have been shown to survive well in embryonic and neonatal CNS environment, and their axons innervate muscles.7,38 They also appear to survive in the adult mouse spinal cord. Remarkably, their axons grow to denervated muscles.39 Nevertheless, transplantation of hESC-derived motoneurons into adult mouse brain survived poorly.40 Strategies must be developed to promote the survival of human motoneurons, either by grafting committed motoneuron progenitors or by co-grafting with astrocytes. Perhaps what is even more daunting is that the grafted and survived motoneurons need to travel a long distance in order to reach target muscles and make functional connection with muscles. In an SMA mouse model, HB9-GFP motoneurons transplanted into the neonatal spinal cord not only survive but also grow out nerves and innervate muscles. The grafted animals appear to survive longer.41 This finding raises hopes that motoneuron replacement in the developing nervous system may be possible. In adult mice, co-transplantation of glial cells in the sciatic nerve to attract axonal growth from the motoneurons that are grafted to the spinal cord appears to occur,39 indicative that motoneuron replacement in adults is not impossible.
Conclusion
Human stem cells, including hESCs and hiPSCs, can be efficiently differentiated into motoneurons and astrocytes, two of the main cell types that are targeted in motoneuron diseases. These cellular models offer an unprecedented tool for dissecting molecular mechanisms underlying motoneuron health and disease, thus complementing the existing transgenic animal models. While these cellular models have resulted in discoveries of developmental mechanisms, significant efforts are underway to create novel tools to simulate pathological processes in a dish and to translate findings obtained in the cellular models to treatment options for these devastating motoneuron diseases.
Acknowledgments
Studies from our laboratories described in the article have been supported by the NIH-NINDS (NS045926, NS046587, NS057778, NS061243), the ALS Association, partly from the NICHD (P30 HD03352), Ministry of Science and Technology, China (2006CB94700, 2006AA02A101), and Shanghai Municipality (06dj14001).
Reference List
- 1.Li XJ, et al. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol. 2005;23:215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- 2.Singh RN, et al. Enhancer-specified GFP-based FACS purification of human spinal motor neurons from embryonic stem cells. Exp Neurol. 2005;196:224–234. doi: 10.1016/j.expneurol.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Krencik R, Weick JH, Zhang Z, Zhang SC. Regional and functional specific astrocytes from human embryonic stem cells. Society for Neuroscience Abstract. 2009;808.9 [Google Scholar]
- 4.Ebert AD, et al. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277–280. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dimos JT, et al. Induced Pluripotent Stem Cells Generated from Patients with ALS Can Be Differentiated into Motor Neurons. Science. 2008 doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 6.Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- 7.Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 8.Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 9.Pankratz MT, et al. Directed neural differentiation of human embryonic stem cells via an obligated primitive anterior stage. Stem Cells. 2007;25:1511–1520. doi: 10.1634/stemcells.2006-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lavaute TM, et al. Regulation of neural specification from human embryonic stem cells by BMP and FGF. Stem Cells. 2009;27:1741–1749. doi: 10.1002/stem.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chambers SM, et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson MA, Weick JP, Pearce RA, Zhang SC. Functional neural development from human embryonic stem cells: accelerated synaptic activity via astrocyte coculture. J Neurosci. 2007;27:3069–3077. doi: 10.1523/JNEUROSCI.4562-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan Y, et al. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells. 2005;23:781–790. doi: 10.1634/stemcells.2004-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang SC. Neural subtype specification from embryonic stem cells. Brain Pathol. 2006;16:132–142. doi: 10.1111/j.1750-3639.2006.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- 16.Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Rowitch DH. Glial specification in the vertebrate neural tube. Nat Rev Neurosci. 2004;5:409–419. doi: 10.1038/nrn1389. [DOI] [PubMed] [Google Scholar]
- 18.Zhang SC. Defining glial cells during CNS development. Nat Rev Neurosci. 2001;2:840–843. doi: 10.1038/35097593. [DOI] [PubMed] [Google Scholar]
- 19.Bruijn LI, et al. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327–338. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- 20.Bruijn LI, Miller TM, Cleveland DW. UNRAVELING THE MECHANISMS INVOLVED IN MOTOR NEURON DEGENERATION IN ALS. Annu Rev Neurosci. 2004;27:723–749. doi: 10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
- 21.Furukawa Y, Fu R, Deng HX, Siddique T, O’Halloran TV. Disulfide cross-linked protein represents a significant fraction of ALS-associated Cu, Zn-superoxide dismutase aggregates in spinal cords of model mice. Proc Natl Acad Sci U S A. 2006;103:7148–7153. doi: 10.1073/pnas.0602048103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monani UR, et al. The human centromeric survival motor neuron gene (SMN2) rescues embryonic lethality in Smn(−/−) mice and results in a mouse with spinal muscular atrophy. Hum Mol Genet. 2000;9:333–339. doi: 10.1093/hmg/9.3.333. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh-Li HM, et al. A mouse model for spinal muscular atrophy. Nat Genet. 2000;24:66–70. doi: 10.1038/71709. [DOI] [PubMed] [Google Scholar]
- 24.Oberheim NA, et al. Uniquely hominid features of adult human astrocytes. J Neurosci. 2009;29:3276–3287. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zwaka TP, Thomson JA. Homologous recombination in human embryonic stem cells. Nat Biotechnol. 2003;21:319–321. doi: 10.1038/nbt788. [DOI] [PubMed] [Google Scholar]
- 26.Xia X, Zhang Y, Zieth CR, Zhang SC. Transgenes delivered by lentiviral vector are suppressed in human embryonic stem cells in a promoter-dependent manner. Stem Cells Dev. 2007;16:167–176. doi: 10.1089/scd.2006.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du ZW, Hu BY, Ayala M, Sauer B, Zhang SC. Cre recombination-mediated cassette exchange for building versatile transgenic human embryonic stem cells lines. Stem Cells. 2009;27:1032–1041. doi: 10.1002/stem.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hockemeyer D, et al. Efficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc-finger nucleases. Nat Biotechnol. 2009;27:851–857. doi: 10.1038/nbt.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 30.Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 31.Hu B, Weick JH, Yu J, Thomson JA, Zhang SC. Neural differentiation of human iPS cells is variable and less efficient than hESCs. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.0910012107. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marchetto MC, et al. Non-cell-autonomous effect of human SOD1 G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell. 2008;3:649–657. doi: 10.1016/j.stem.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Di Giorgio FP, Boulting GL, Bobrowicz S, Eggan KC. Human embryonic stem cell-derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS-causing mutation. Cell Stem Cell. 2008;3:637–648. doi: 10.1016/j.stem.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 34.Clement AM, et al. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science. 2003;302:113–117. doi: 10.1126/science.1086071. [DOI] [PubMed] [Google Scholar]
- 35.Bjorklund A, Lindvall O. Cell replacement therapies for central nervous system disorders. Nat Neurosci. 2000;3:537–544. doi: 10.1038/75705. [DOI] [PubMed] [Google Scholar]
- 36.Lepore AC, et al. Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat Neurosci. 2008;11:1294–1301. doi: 10.1038/nn.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li XJ, et al. Directed differentiation of ventral spinal progenitors and motor neurons from human embryonic stem cells by small molecules. Stem Cells. 2008;26:886–893. doi: 10.1634/stemcells.2007-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yohn DC, Miles GB, Rafuse VF, Brownstone RM. Transplanted mouse embryonic stem-cell-derived motoneurons form functional motor units and reduce muscle atrophy. J Neurosci. 2008;28:12409–12418. doi: 10.1523/JNEUROSCI.1761-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deshpande DM, et al. Recovery from paralysis in adult rats using embryonic stem cells. Ann Neurol. 2006;60:32–44. doi: 10.1002/ana.20901. [DOI] [PubMed] [Google Scholar]
- 40.Lee H, et al. Directed differentiation and transplantation of human embryonic stem cell-derived motoneurons. Stem Cells. 2007;25:1931–1939. doi: 10.1634/stemcells.2007-0097. [DOI] [PubMed] [Google Scholar]
- 41.Corti S, et al. Motoneuron transplantation rescues the phenotype of SMARD1 (spinal muscular atrophy with respiratory distress type 1) J Neurosci. 2009;29:11761–11771. doi: 10.1523/JNEUROSCI.2734-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krencik R, Zhang SC. Stem cell neural differentiation: a model for chemical biology. Curr Opin Chem Biol. 2006;10:592–597. doi: 10.1016/j.cbpa.2006.10.002. [DOI] [PubMed] [Google Scholar]


