Abstract
Objective
To examine the relationship between markers of vascular dysfunction and neurodevelopmental status in pediatric HIV disease.
Design
Cross-sectional design within a prospective, 15-site cohort study conducted in the United States.
Methods
Nine vascular biomarkers were examined in 89 HIV-infected children: soluble P-selectin (sP-selectin)/sCD62P, fibrinogen, adiponectin, monocyte chemoattractant protein-1/CCL-2, interleukin-6, C-reactive protein, soluble vascular cell adhesion molecule-1/sCD106, sE-selectin/sCD62E, soluble intercellular adhesion molecule-1/sCD54. The Wechsler Intelligence Scale for Children-IV (WISC-IV) was administered yielding indices for Verbal Comprehension, Perceptual Reasoning, Working Memory and Processing Speed, and overall composite score Full Scale IQ (FSIQ). Linear regression models were used to evaluate neurodevelopmental status (measured by WISC-IV scores) as a function of each biomarker while adjusting for demographics, disease severity and receipt of highly active antiretroviral therapy. Biomarker levels were evaluated in quartiles to evaluate trends in WISC-IV responses.
Results
Among the 89 HIV-infected children (median age = 12 years), 56% were female, 71% Black, 16% Hispanic, and 43% had yearly household income <$20,000. Log(sP-selectin) was significantly correlated with all WISC-IV scores; adjusted slopes showed 6 to 11 point average decrease in scores for each one log unit increase in sP-selectin. Final linear regression models for log(fibrinogen) adjusted for sociodemographic and disease characteristics also indicated a negative correlation with all WISC-IV scores (13 to 30 points decrease for each one log unit increase in fibrinogen); these decreases were significant in the Verbal Comprehension, Perceptual Reasoning and FSIQ scores.
Conclusion
Pro-inflammatory microvascular and immunologic mechanisms may be involved in neurodevelopmental impairment in children with perinatally-acquired HIV disease.
Keywords: pediatric HIV, pediatric NeuroAIDS, role of chemokines in NeuroAIDS, vascular dysfunction and NeuroAIDS
Introduction
Children and adolescents with perinatal HIV-1 infection are at risk of developing central nervous system (CNS) disease, the clinical manifestations of which have ranged from frank encephalopathy prior to the advent of the highly active antiretroviral therapy (HAART), to more subtle, but still clinically significant impairments in the HAART era, such as language disorders, cognitive impairment, developmental delays, motor deficits, behavioral problems or psychiatric disorders.[1-5] Recent research has advanced our understanding of the neuropathologic processes that occur in individuals with HIV-1. HIV-1 crosses the blood-brain barrier (BBB) via activated monocytes, which differentiate into perivascular macrophages. In the CNS, HIV-1 infects microglia and, to a more limited degree, astrocytes, but does not infect post-mitotic neurons. Rather, the neuropathology of HIV-1 is primarily characterized by indirect, non-apoptotic mechanisms of neuronal damage.[6] These mechanisms include synaptodendritic injury, which leads to excessive pruning and aberrant sprouting, resulting in neuronal dysfunction.[6] At the systemic level, macrophage activation and endothelial dysfunction have been hypothesized to relate to the development of HIV-associated neurocognitive disease (HAND) in adults,[7] and this process appears to be facilitated by certain chemokines – i.e. low molecular weight cytokines that mediate multiple microvascular and immunologic mechanisms, including inflammation, coagulation and cell-to-cell communication.[6, 8-9]
Studies conducted on non-infected human cell cultures indicate that the chemokine P-selectin/sCD62P facilitates monocyte differentiation into a pro-inflammatory phenotype characterized by the expression of surface antigens CD14+CD16+ and inhibits macrophage maturation.[8] Studies from people with peripheral arterial occlusive disease have demonstrated that soluble P selectin (sP-selectin)/sCD62P can initiate a signaling cascade in neutrophils, allowing them to bind to intercellular adhesion molecule-2 (ICAM-2) and fibrinogen, in turn allowing them to form a complex with platelets and endothelial cells,[10-11] suggesting that not only is sP-selectin/sCD62P a marker for inflammatory vascular disease, but it is actually a mediator as well. Furthermore, during HIV-1 infection, increased numbers of CD14+CD16+ monocytes in the peripheral blood are associated with a high risk of HIV encephalopathy.[12-13] These activated cells preferentially transmigrate through microvascular endothelium into HIV-1 infected CNS and are important sources of secretory neurotoxins upon their arrival in the CNS.[12-13]
Taken together, this evidence suggests that the pathophysiology of neurodevelopmental impairments in children and adolescents with perinatally acquired HIV infection may involve an intricate interplay between multiple microvascular processes involving immune activation such as inflammation, coagulation and transendothelial cell migration. Elucidating these mechanisms may suggest strategies to stage progression of neurologic disease and intervene before irreversible damage to synaptodendritic architecture occurs.
The present study aimed to examine the relationship between neurodevelopmental outcomes and peripheral blood levels of nine selected markers of inflammation, coagulation and endothelial dysfunction (“biomarkers”) in a cohort of children and adolescents with perinatally acquired HIV infection. We hypothesized that peripheral blood levels of the biomarkers would be correlated with levels of neurodevelopmental functioning, with the direction of association dependent on the individual biomarker.
Methods
Participants
The Adolescent Master Protocol (AMP) is a longitudinal, prospective cohort study which opened to enrollment in March 2007 at 15 sites in the United States (US) and Puerto Rico as part of the Pediatric HIV/AIDS Cohort Study (PHACS). AMP is designed to define the impact of HIV infection and antiretroviral therapy (ART) on pre-adolescents and adolescents with perinatal HIV-infection. Study domains include growth and sexual maturation, metabolic risk factors and cardiac function, and neurodevelopmental and behavioral functioning. Children ages 7 to < 16 years and born to HIV-infected mothers were eligible for enrollment into AMP. For this analysis, we included perinatally HIV-1 infected participants who had vascular biomarkers evaluated from blood samples as part of an assessment of vascular dysfunction among those with and without hyperlipidemia,[14] as well as evaluations of neurodevelopmental status at their entry visit.
Procedures
Study Assessments
At study entry, a complete physical exam was conducted with collection of body measurements and blood samples for determination of fasting lipid levels. In addition, measurements of CD4+ T cells and HIV-1 RNA (viral load) were obtained along with a lifetime history of ART use, and a history of HIV disease severity as reflected by CDC disease class, nadir CD4% and peak viral load. Maternal, caregiver, and household characteristics were collected by interview. The study is ongoing and visits are scheduled every 6 months.
Assessment of the Biomarkers
Serum levels of nine biomarkers of vascular dysfunction were assessed, including monocyte chemoattractant protein/CCL-2 (referred to as MCP-1), interleukin 6 (IL-6), C-reactive protein (CRP) (proinflammatory markers),[15-16] soluble vascular cell adhesion molecule-1/sCD106 (referred to as sVCAM-1), soluble E-selectin/sCD62E (referred to as sE-selectin), soluble intercellular adhesion molecule-1/sCD54 (referred to as sICAM-1) (endothelial activation markers),[17] fibrinogen (procoagulant state marker),[18] soluble P-selectin/sCD62P (referred to as sP-selectin) (procoagulant state and endothelial activation marker),[17-18] and adiponectin (modulator of endothelial inflammatory response).[19]
Fibrinogen and CRP were measured by nephelometry on the Dade-Behring (Deerfield, Illinois) auto-analyzer using manufacturer's reagents and instructions. Intra- and inter-assay CVs were 2.6% and 2.7%, respectively for fibrinogen and 4.4% and 5.7%, respectively for CRP. Adiponectin was measured by a double antibody radioimmunoassay (Linco Research, St. Charles, Missouri), with intra- and inter-assay CVs < 5%. IL-6, MCP-1, sVCAM-1, sICAM-1, sE-selectin and sP-selectin were measured by ELISA using reagents manufactured by R&D Systems (Minneapolis, MN). Intra- and inter-assay CVs were, respectively; IL-6, 6.8% and 9.4%; MCP-1, 4.0% and < 7.5%; sVCAM-1, 5.9% and 10.2%; sICAM-1, 4.8% and 10.1%; sE-selectin, 5.0% and 8.8%; sP-selectin, 4.2% and 9.8%.
Assessment of Neurodevelopmental Outcomes
Children enrolled in AMP were administered the Wechsler Intelligence Scale for Children-Fourth edition (WISC-IV)[20] at AMP study entry. Ten subtests of the WISC-IV are combined to yield four index scores: Verbal Comprehension, Perceptual Reasoning, Working Memory, and Processing Speed, as well as an overall composite score, Full Scale IQ (FSIQ). These are standardized scores based on the general US population (mean=100; SD=15). The four index scores served as the neurodevelopmental outcomes in the analysis. The FSIQ is clearly dependent on the four index scores, but was included in the analysis and reported in the results in order to illustrate how the relationship between the biomarkers and cognitive index scores translates into this index of general intellectual functioning. Validity ratings completed by the psychometrist or psychologist administering the tests indicated that all assessments in our sample were valid.
Statistical methods
Our cross-sectional analysis included children with perinatally-acquired HIV infection participating in AMP who had WISC-IV results along with the selected biomarker blood levels taken within 6 months of their WISC-IV evaluation. Participants with CRP values above 10 mg/L were excluded from the analysis due to high likelihood of an acute infection at the time blood was obtained, suggesting a potentially confounding non-specific acute inflammatory process.
For each of the nine biomarkers, we compared the FSIQ score and the four index scores between those with high versus low levels of the biomarker, and evaluated the association between continuous levels of each of the biomarkers with the WISC-IV scores. We calculated Spearman correlation coefficients between each biomarker and the FSIQ score along with each of the WISC-IV index scores. In addition, to understand the relationships between individual biomarkers, as well as between biomarkers and HIV disease, correlations of all 9 biomarkers with one another and with HIV disease characteristics (including current and nadir CD4+, and current and peak viral load measurements) were examined using Spearman correlation coefficients.
We then fit general linear regression models for each of the outcomes, with the biomarker level as the primary predictor, first using univariate models, and then adjusting for selected covariates. When necessary, the biomarker levels were log-transformed to approximate a normal distribution, in order to allow a more robust model that was less sensitive to influential outliers. The following covariates were considered for inclusion in the regression models: participant age, gender, race, Hispanic vs. non-Hispanic ethnicity, English- vs. non-English primary language, caregiver education, household income, relationship of subject and caregiver, hyperlipidemia status, receipt of HAART, CDC HIV disease class, current CD4+ percentage (CD4%), nadir CD4%, log (HIV RNA) and log (peak HIV RNA). The relationship between each covariate and WISC-IV outcome was first examined in simple linear regression models; those with p<0.20 were considered for inclusion in the final multivariable models. Backwards selection with inclusion criteria p<0.10 was used to select final reduced models for each outcome and biomarker combination.
Physiological serum concentrations of adiponectin have been observed to be higher in females than in males, irrespective of body size and fat distribution.[21-23] Thus, regression analyses for adiponectin were adjusted for the potential confounding effect of gender on the relationship between adiponectin and WISC-IV outcomes, regardless of its statistical significance.
Biomarker levels were also grouped in quartiles to explore trends in response. Given the relatively small sample size, only the covariates from the reduced models were considered for adjustment. Due to the large number of combinations of 5 outcomes and 9 biomarkers, consistency of results across biomarkers was examined to help support conclusions. Analyses were conducted using SAS Version 9.1.3, and statistical significance was based on p-values<0.05.
Results
Participants
Among 319 perinatally HIV-infected participants in AMP as of December 2008, all HIV-infected participants with hyperlipidemia (n=51) and a random subset of HIV-infected participants without hyperlipidemia (n=49) had blood samples tested for vascular biomarker levels. Ninety-two of these 100 participants also had neurodevelopmental outcomes measured within 6 months. Three additional participants were excluded due to their high CRP values. Table 1 provides demographic and health characteristics of the 89 participants included in the analysis. The participants’ mean age at the time of neurodevelopmental and biomarker evaluation was 12 years, ranging from 7 to 16 years. Their median CD4+ cell count was 762 cells/ mm3 and median CD4+ cell percentage was 34.2%. Almost all of the participants (91%) were receiving HAART at the time of evaluation. These characteristics were reflective of the 319 participants enrolled in AMP at that time (data not shown).
Table 1.
Demographic and Health Characteristics of 89 Perinatally HIV-infected Children and Adolescents with Vascular Biomarker Measurements and Neurodevelopmental Outcomes
| Characteristic | Total (N=89) |
|---|---|
| Age (years) | 12.30 (10.50-14.10)* |
| Female | 50 (56%) |
| Race | |
| Black or African American | 63 (71%) |
| White | 19 (21%) |
| American Indian | 1 (1%) |
| More than One Race | 2 (2%) |
| Unknown | 4 (4%) |
| Hispanic or Latino | 14 (16%) |
| English Primary Language | 79 (89%) |
| Primary Caregiver is Biological Parent | 36 (40%) |
| Low Annual Household Income: 20K or less | 36 (43%) |
| Caregiver Education (High School or less) | 51 (57%) |
| CDC Class C | 24 (27%) |
| Nadir CD4 Count (cells/mm3) | 366 (184-595) |
| Nadir CD4 % | 20 (12-26) |
| Log(10) Peak Viral Load | 5.39 (4.97-5.78) |
| Antiretroviral (ARV) Regimen | |
| HAART with PI | 70 (79%) |
| HAART without PI | 11 (12%) |
| Non-HAART ARV | 3 (3%) |
| Not on Antiretroviral Drugs | 5 (6%) |
| On HAART | 81 (91%) |
| On PIs | 71 (80%) |
| On NNRTIs | 26 (29%) |
Continuous variables are described as Median (Q1-Q3).
Biomarkers
The distributions of the nine vascular biomarker levels are summarized in Table 2. All biomarkers had right-skewed distributions (and were thus log-transformed for statistical analysis) with the exception of sICAM-1 and sE-selectin. Many of the biomarkers were inter-correlated. A moderate positive association was observed for sP-selectin with sE-selectin (Spearman r=0.37) and sICAM-1 (r=0.32). Stronger associations were observed for CRP with IL-6 (r=0.45) and with fibrinogen (r=0.29), and between sICAM-1 and sVCAM-1 (r=0.39). All other associations were weak, with |r|<0.26. (data not shown)
Table 2.
Distribution of Biomarkers of Vascular Dysfunction
| Biomarker | N | Mean | Standard Deviation | Median | Lower Quartile | Upper Quartile |
|---|---|---|---|---|---|---|
| sP-selectin/sCD62Pa | 89 | 47.4 | 30.3 | 38.9 | 27.8 | 54.1 |
| fibrinogenb | 89 | 392.7 | 71.8 | 386.0 | 345.0 | 428.0 |
| adiponectinc | 89 | 9.5 | 3.8 | 8.8 | 6.4 | 11.5 |
| CRPb | 89 | 0.9 | 1.3 | 0.4 | 0.2 | 0.9 |
| IL-6d | 89 | 1.2 | 1.2 | 0.9 | 0.6 | 1.3 |
| MCP-1/CCL-2d | 89 | 126.4 | 57.6 | 108.4 | 91.2 | 146.7 |
| sICAM-1/sCD54a | 89 | 292.4 | 113.3 | 298.6 | 248.2 | 351.5 |
| sVCAM-1/sCD106a | 89 | 813.3 | 283.2 | 755.7 | 638.5 | 933.3 |
| sE-selectin/sCD62Ea | 89 | 53.9 | 21.2 | 50.5 | 39.9 | 67.1 |
ng/mL
mg/dL
μg/mL
pg/mL.
CRP = C-reactive protein; IL-6 = interleukin-6; MCP-1 = monocyte chemoctatic protein-1; sICAM-1 = soluble intercellular adhesion molecule-1; sVCAM-1 = soluble vascular adhesion molecule-1.
Neurodevelopmental outcomes
Neurodevelopmental outcomes from the WISC-IV were normally distributed, with mean scores in the low average range (87 to 89) for three of four WISC-IV index scores (Verbal Comprehension, Working Memory and Processing Speed), and in the average range for Perceptual Reasoning (mean=94.1). Standard deviations for WISC-IV index scores ranged from 14.3 to 16.6.
Relationship between the neurodevelopmental outcomes and biomarkers
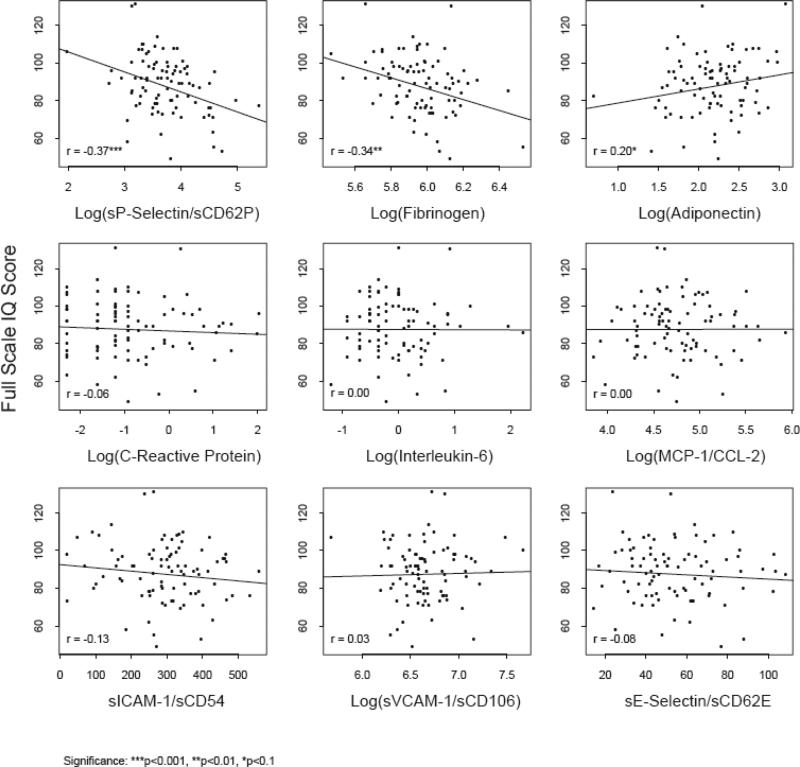
Correlation coefficients between FSIQ and the nine biomarkers are shown in Figure 1. Log(fibrinogen) and log(sP-selectin) had negative correlations with all WISC-IV scores ranging from -0.15 to -0.37; these correlations were statistically significant for FSIQ, Verbal Comprehension and Perceptual Reasoning and marginally significant (P<0.10) for Working Memory. In addition, adiponectin had a marginally significant positive correlation with FSIQ, Processing Speed, and Perceptual Reasoning, while sICAM-1 had a marginal negative association with Processing Speed. Univariate linear regression models for the relationship of all 9 biomarkers, as measured on a continuous scale, versus FSIQ score and each of the 4 WISC IV index scores are shown in Table 3 (upper panel).
Figure 1.
Relationships of Vascular Biomarkers with Full Scale IQ: Simple Linear Regression and Correlation Coefficients
Table 3.
Correlations of Vascular Biomarkers with Neurodevelopmental Outcomes, and Estimated Difference in outcomes for High versus Low levels of Biomarkers
| Biomarker | Full Scale IQ (n=88) | Verbal Comprehension (n=89) | Processing Speed (n=89) | Perceptual Reasoning (n=89) | Working Memory (n=89) |
|---|---|---|---|---|---|
| Continuous Biomarker Levels – Regression Model Results | |||||
| Log(sP-Selectin) | -10.65*** | -8.33** | -6.47* | -9.96*** | -8.02* |
| Log(fibrinogen) | -28.90** | -25.07** | -12.14 | -28.88*** | -21.13* |
| Log(adiponectin) | 7.47* | 6.13 | 7.08* | 6.33* | 1.79 |
| Log(CRP) | -0.90 | 0.54 | -3.35* | -0.54 | -0.03 |
| Log(IL-6) | -0.07 | -0.68 | -1.50 | 0.64 | 1.59 |
| Log(MCP-l) | 0.13 | -1.04 | -1.38 | 3.47 | -0.62 |
| sICAM-1 | -0.02 | -0.01 | -0.02* | -0.01 | -0.01 |
| Log(sVCAM-l) | 1.36 | -0.79 | 1.16 | 1.19 | 3.19 |
| sE-Selectin | -0.06 | -0.06 | -0.05 | -0.03 | -0.07 |
| High vs. Low1 – Regression Model Results | |||||
| sP-Selectin | -7.95* | -6.74* | -5.45* | -6.03* | -6.82* |
| Fibrinogen | -8.34* | -6.36* | -3.76 | -7.51* | -6.95* |
| Adiponectin | 3.24 | 2.69 | 3.14 | 2.60 | 0.45 |
| CRP | -3.16 | -0.40 | -5.91* | -2.19 | -3.19 |
| IL-6 | -0.91 | -0.25 | -3.88 | -0.35 | 1.15 |
| MCP-1 | -4.37 | -4.92 | -2.76 | -2.02 | -3.44 |
| sICAM-1 | -1.27 | -1.33 | -1.67 | -1.52 | 1.02 |
| sVCAM-1 | 2.35 | 2.77 | 3.56 | 0.13 | 0.96 |
| sE-Selectin | -1.16 | -2.14 | -1.43 | 0.67 | -2.32 |
1 – High versus low levels based on dichotomizing at the median level for each biomarker Significance:
p<0.001
p<0.01
p<0.1
Positive values reflect increased WISC-IV scores for increased levels of biomarkers.
CRP = C-reactive protein; IL-6 = interleukin-6; MCP-1 = monocyte chemoctatic protein-1; sICAM-1 = soluble intercellular adhesion molecule-1; sVCAM-1 = soluble vascular adhesion molecule-1.
In addition, linear regression models comparing high versus low biomarker levels indicated that those with higher sP-selectin levels (above the median) had marginally lower scores on the four WISC-IV indices and FSIQ as compared to those with lower sP-selectin levels (see Table 3, bottom panel). Similarly, those with high fibrinogen levels had marginally lower scores on three WISC-IV index scores: Verbal Comprehension, Perceptual Reasoning and Working Memory. The relationship between adiponectin and the WISC-IV outcomes (Table 3) did not change after adjusting for gender.
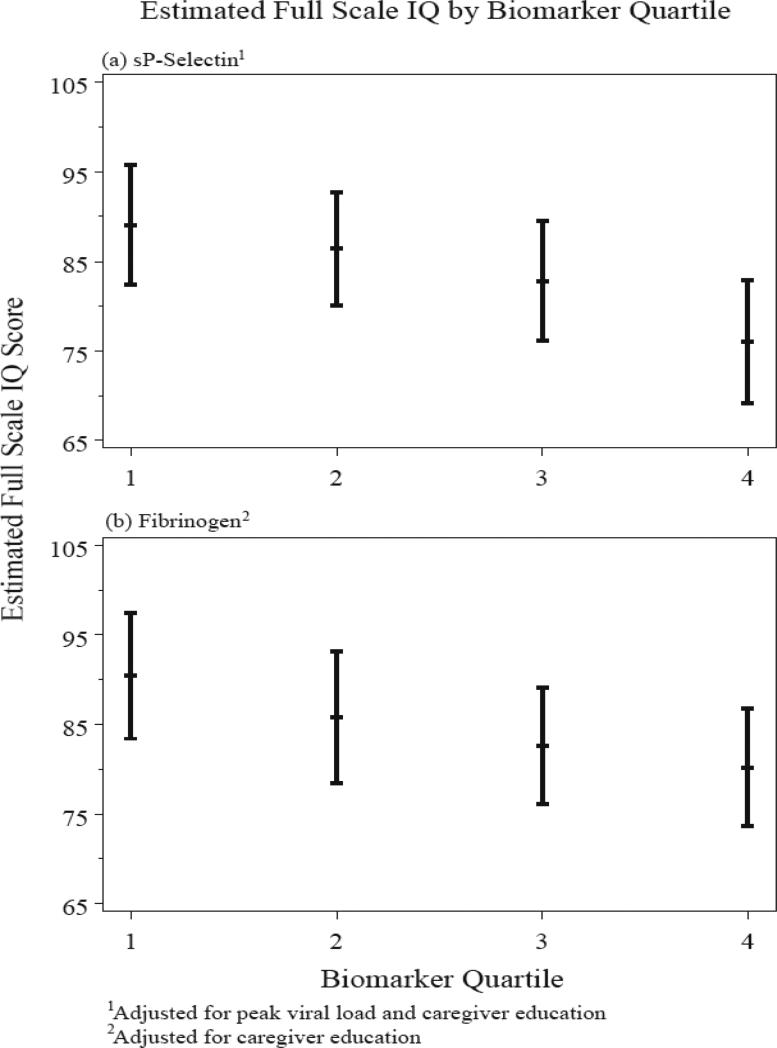
Linear regression models adjusting for selected health and demographic covariates indicated that the effects of sP-selectin and fibrinogen persisted even after accounting for these potential confounders (Table 4). In the final models, log(sP-selectin) was significantly associated with the four WISC-IV index scores and FSIQ, with adjusted slopes indicating a 6 to 11 point average decrease in scores for each one log unit increase in sP-selectin. Final adjusted linear regression models for log(fibrinogen) also indicated a negative association with all scores, with estimated decreases of about 13 to 30 points for each one log unit increase in fibrinogen. These decreases were significant in the Verbal Comprehension (p<0.05), Perceptual Reasoning (p<0.001), and FSIQ scores (p<0.01). Linear regression analysis using biomarker quartiles as the predictor of interest revealed consistently decreasing WISC-IV scores with higher levels of sP-selectin and fibrinogen (Figure 2).
Table 4.
Final linear regression models for effects of log(P-selectin) and log(fibrinogen) on neuropsychiatric outcomes, adjusting for potential confounders.
| Full Model Results* | Reduced Model Results** | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | N | Estimate | SE | P-value | N | Estimate | SE | P-value |
| Log(sP-selectin) | ||||||||
| Full Scale IQ | 87 | -9.70 | 2.94 | 0.001 | 87 | -10.49 | 2.79 | <.001 |
| Verbal Comprehension | 88 | -8.13 | 2.98 | 0.008 | 88 | -8.14 | 2.81 | 0.005 |
| Processing Speed | 88 | -5.83 | 2.96 | 0.05 | 89 | -5.62 | 2.76 | 0.05 |
| Perceptual Reasoning | 88 | -7.81 | 2.69 | 0.005 | 88 | -8.14 | 2.63 | 0.003 |
| Working Memory | 88 | -7.56 | 3.35 | 0.03 | 88 | -7.96 | 3.23 | 0.02 |
| Log(fibrinogen) | ||||||||
| Full Scale IQ | 87 | -24.98 | 9.13 | 0.008 | 88 | -25.90 | 8.73 | 0.004 |
| Verbal Comprehension | 88 | -23.58 | 9.13 | 0.01 | 88 | -21.20 | 8.73 | 0.02 |
| Processing Speed | 88 | -11.05 | 9.18 | 0.23 | 89 | -13.44 | 8.30 | 0.11 |
| Perceptual Reasoning | 88 | -24.23 | 8.19 | 0.004 | 89 | -29.91 | 7.86 | <.001 |
| Working Memory | 88 | -16.49 | 10.40 | 0.12 | 88 | -18.84 | 9.82 | 0.06 |
Each row represents a separate multiple linear regression model, adjusted for age, sex, ethnicity (Hispanic versus non-Hispanic), non-English language, relationship to caregiver, caregiver education, treatment with highly active antiretroviral therapy (HAART versus not on HAART), nadir CD4 percent, and log peak HIV-1 RNA viral load.
Each row represents a separate multiple linear regression model, adjusted for covariates above, which remained significant at p<0.10 in a reduced multivariate model.
Figure 2.
Relationship between WISC-IV overall composite scores (Full Scale IQ scores) with peripheral levels of sP-selectin (a) and fibrinogen (b).
With respect to other covariates included in the models, participants with higher peak viral load levels had significantly lower FSIQ as well as all four WISC-IV index scores. In contrast, nadir CD4% was correlated with Processing Speed only (p<0.01); current CD4%, current HIV RNA serum concentration and CDC class C diagnosis were not correlated with index scores. Subjects on HAART had higher Perceptual Reasoning scores, while subjects with a non-biological primary caregiver had marginally lower scores. Higher caregiver education level was associated with higher FSIQ, Verbal Comprehension, and Perceptual Reasoning scores. Females tended to have lower Perceptual Reasoning scores. Finally, those of Hispanic ethnicity had marginally lower Verbal Comprehension scores. Hyperlipidemia status, age, race, household income, and exposure to a non-English language were not associated with the WISC-IV outcomes.
Participants with higher nadir CD4 count had lower levels of IL-6 (Spearman r=-0.22), sVCAM-1 (r=-0.35), and sP-selectin (-0.20), and higher levels of adiponectin (r=0.23). However, there were no associations between peak viral load and any of the nine biomarkers. Nadir CD4% and peak viral load were retained in adjusted models due to their association with one or more of the WISC-IV index scores.
Discussion
In this study, an exploratory analysis of the peripheral blood levels of nine selected markers of vascular dysfunction and neurodevelopmental outcomes was conducted in 89 children and adolescents with perinatally acquired HIV-1 infection. Socio-demographic characteristics of the cohort were representative of US children and adolescents with perinatal HIV-1 infection. The WISC-IV scores of the cohort were in the low-average to average range and generally consistent with recent studies of children with HIV infection.[2, 24-26] All four WISC-IV index scores showed significant negative correlations with log(sP-selectin). This finding suggests that adverse neurodevelopmental outcomes in children and adolescents with HIV infection might be linked to changes in populations of pro-inflammatory monocytes associated with increased peripheral activity of sP-selectin.
sP-selectin is an adhesion molecule that is expressed on both endothelial cells and platelets.[12] In normal physiological states, ample stores of functional sP-selectin are present in intracellular storage sites, and can be rapidly translocated to the endothelial surface in response to hypoxia or inflammation.[8,27] Data from in vitro studies with non-infected human mononuclear cells suggest that, following its expression by the endothelial cells, sP-selectin facilitates monocyte differentiation into CD14+CD16+ dendritic-like cells and inhibits macrophage maturation.[8] In persons infected with HIV-1, an expanded subset of CD14+CD16+ monocytes in the peripheral blood have been associated with high risk of HAND. These activated cells readily transmigrate through microvascular endothelium and are believed to release neurotoxins upon arrival in the CNS, resulting in neurodegeneration.[12-13] Persistently elevated levels of HIV DNA have been reported in CD14+CD16+ monocytes of HAART-experienced adults with undetectable plasma HIV RNA levels who have HAND.[28] Thus, the correlation found in the present study supports the assertions first made by Li et al,[8] that sP-selectin might relate to disease severity in HIV-infected individuals. The present study provides in vivo data that allow us to build on the conclusions from the in vitro work by Li et al,[8] who hypothesized about the possible dual role of sP-selectin in HIV-related CNS disorders: 1) HIV-infection-related systemic inflammation leads to increased sP-selectin activity in the microvasculature, leading to substantially increased levels of circulating CD14+CD16+ monocytes, which then penetrate the BBB in increased numbers, after which they release neurotoxic factors; 2) by inhibiting the maturation of macrophages, in the environment of already decreased peripheral CD4+ concentrations, sP-selectin additionally weakens the immune system by undermining the ability of the macrophages to effectively kill opportunistic pathogens.[8] Interestingly, in the present study there were no significant covariate effects of the CDC disease severity class, and sP-selectin only showed a marginal association with nadir CD4%. This raises the possibility that in the HAART era, disease severity indicators may need to be refined to include seemingly more subtle, but functionally very important clinical outcomes, such as neurodevelopmental impairment.
Verbal Comprehension and Perceptual Reasoning (as well as FSIQ) were negatively correlated with log(fibrinogen). This correlation may also suggest a role in endothelial dysfunction of cerebral microvasculature and its potential contribution to neurodevelopmental impairment in children and adolescents with perinatally acquired HIV-1 infection. Based on the studies of Woollard et al.[11] of individuals with arterial occlusive disease, our data raise the intriguing possibility that sP-selectin and fibrinogen may participate in immunologic events in cerebral microvasculature that lead to interactions between granulocyte subsets of leukocytes, platelets and endothelial cells that are likely to further compromise the BBB. It has been shown that functional blockade of sP-selectin using a monoclonal antibody directed against murine sP-selectin improves early post-stroke re-flow and stroke outcomes in mice, suggesting that sP-selectin might play an important role in the pathophysiology of stroke.[27] Thus, it is possible that both fibrinogen and sP-selectin participate in a cascade of microvascular events associated with CNS disease in the context of pediatric HIV-1 infection. Indeed, there is now evidence that fibrinogen enhances platelet sP-selectin expression in mice, thus facilitating its role in inflammation, thrombosis and hemostasis.[29]
WISC-IV scores were not significantly correlated with peripheral blood levels of MCP-1. MCP-1 plays a key role in CNS infiltration of HIV-1 infected leukocytes that express CCR2 receptor and in activation of macrophages in the pathogenesis of HAND. Dose-dependent correlation between MCP-1 concentration in the cerebrospinal fluid (CSF) and degree of HAND has been reported.[30-32] Additionally, MCP-1 may affect macrophage migratory movement by altering BBB permeability through regulation of voltage-gated K+ channels.[33] Interestingly, CD14+CD16+ monocytes lack surface expression of CCR2 and thus do not selectively migrate toward MCP-1 in vitro, suggesting that CD14+CD16+ cells may require pre-activation by cytokines on the periphery.[34] This in turn suggests that systemic monocyte activation may fuel progression or worsening clinical status of HAND.[35] Thus, MCP-1-induced changes in permeability of the BBB may be necessary for the transmigration of the CD14+CD16+ monocytes, but not sufficient to facilitate their selective migration towards BBB. In perinatally-HIV infected children, similar “division of labor” between MCP-1 (acts from inside CNS by affecting BBB permeability) and sP-selectin (pre-activates CD14+CD16+ on the periphery) might take place in the process of CD14+CD16+ transmigration into CNS, ultimately affecting neurodevelopmental outcomes.
Findings from early animal models [36-37] and studies conducted with HIV-infected humans in the pre-HAART era suggested that sVCAM-1, sICAM-1 and/or sE-selectin played important facilitating roles in AIDS disease progression as well as in development of CNS disease.[38-39] In the present study, these three biomarkers were not significantly correlated with WISC-IV scores. It is possible that (some of) these three biomarkers are involved in the severe forms of (CNS) disease progression commonly seen in the pre-HAART era, but not in the more subtle forms examined in the present study.
Viral particles may serve as triggers for some of the chemokine-mediated processes described above, or, as hypothesized in a recent review, have a direct neuropathogenic effect.[12] In the present study, current HIV-1 RNA concentration was not correlated with WISC-IV scores, but the peak viral load showed significant covariate effect on the correlation of Working Memory, Perceptual Reasoning and FSIQ scores with sP-selectin. Combined, these two findings suggest that reaching a certain threshold viral RNA concentration in the plasma might play a role in the pathophysiology of adverse neurodevelopmental outcomes. Since peak viral load did not show any association with sP-selectin, fibrinogen (or any of the remaining 7 biomarkers), the observed effect of the peak VL may be independent from the pro-inflammatory and microvascular immunologic mechanisms discussed above.
Several covariate effects (i.e., sex, age, nadir CD4%) were inconsistently significant in the analysis; these associations may have been artifacts of multiple comparisons conducted in the analysis, and should be interpreted with caution. Likewise, the weak positive correlation of adiponectin levels with two of the four WISC-IV index scores requires cautious interpretation.
This study has several limitations. First, due to the cross-sectional design, the causal direction of the observed correlations cannot be ascertained. Thus, we cannot conclude there is a causal role between elevated levels of sP-selectin and/or fibrinogen and lower neurodevelopmental outcomes. Further, due to the cross-sectional design, we can only speculate about the chronicity or tempo of this inverse relationship. In order to minimize confounding effects of an acute, non-specific inflammatory process, participants with high CRP were excluded from the analysis. Still, it remains unclear at which point(s) along the developmental trajectory, including gestation or critical postnatal periods of both white and grey matter maturation in the CNS, that these hypothetical mechanisms might be initiated. Finally, there was no control group of demographically matched non-HIV-infected children and adolescents, which limits the specificity of the negative correlation between the WISC-IV scores and peripheral blood levels of sP-selectin and fibrinogen for perinatally-HIV-infected study participants. For example, the observed correlations may be attributed to pediatric HIV-1 infection in general (regardless of the mode of transmission), to chronic inflammatory phenomena unrelated to HIV-1 infection, or to other factors related to risk for below-average neurodevelopmental outcomes. While these two biomarkers may also be inversely correlated with WISC-IV scores in HIV-uninfected children, previous studies have noted that both sP-selectin and fibrinogen are elevated in HIV-infected children as compared to HIV-uninfected children,[14] suggesting that the clinical relevance of elevated sP-selectin and fibrinogen in HIV-infected children may be greater. Clinical and preclinical data [8,11] mandate a need for longitudinal studies to evaluate whether sP-selectin and fibrinogen inversely correlate with the progression of decline in neurodevelopmental outcome for HIV-1 infected children.
In conclusion, this exploratory cross-sectional analysis demonstrates a highly significant correlation of two inflammatory markers of immune activation and vascular pathology, sP-selectin and fibrinogen, with adverse neurodevelopmental outcomes in perinatally HIV-infected children. Future studies of this type should employ longitudinal measurements of both biomarkers and neurodevelopmental scores, in order to examine the direction and chronicity of their relationships and, hopefully, developmental windows of opportunity to intervene and optimize neurodevelopmental outcomes in these youth now expected to survive into the third decade of life and beyond.
ACKNOWLEDGEMENTS
We thank the children and families for their participation in the PHACS Adolescent Master Protocol (AMP), and the individuals and institutions involved in the conduct of PHACS AMP. The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute of Allergy and Infectious Diseases, the National Institute on Drug Abuse, the National Institute of Mental Health, the National Heart Lung and Blood Institute, and the National Institute of Deafness and Other Communication Disorders through cooperative agreements with the Harvard University School of Public Health (U01 HD052102-04) (Principal Investigator: George Seage; Project Director: Julie Alperen) and the Tulane University School of Medicine (U01 HD052104-01) (Principal Investigator: Russell Van Dyke; Co-Principal Investigator: Kenneth Rich; Project Director: Patrick Davis). Data management services were provided by Frontier Science and Technology Research Foundation (PI: Suzanne Siminski), and regulatory services and logistical support were provided by Westat, Inc (PI: Mercy Swatson).
The following institutions, clinical site investigators and staff participated in conducting PHACS AMP in 2009, in alphabetical order: Baylor College of Medicine: William Shearer, Norma Cooper, Lynette Harris; Bronx Lebanon Hospital Center: Murli Purswani, Mahboobullah Baig, Anna Cintron; Children's Diagnostic & Treatment Center: Ana Puga, Sandra Navarro, Doyle Patton; Children's Hospital, Boston: Sandra Burchett, Nancy Karthas, Betsy Kammerer; Children's Memorial Hospital: Kathleen Malee, Scott Hunter, Eric Cagwin; Jacobi Medical Center: Andrew Wiznia, Marlene Burey, Molly Noycze; St. Christopher's Hospital for Children: Janet Chen, Elizabeth Gobs, Mitzie Grant; St. Jude Children's Research Hospital: Katherine Knapp, Kim Allison, Patricia Garvie; San Juan Hospital/Department of Pediatrics: Midnela Acevedo-Flores, Heida Rios, Vivian Olivera; Tulane University Health Sciences Center: Margarita Silio, Cheryl Borne, Patricia Sirois; University of California, San Diego: Stephen Spector, Kim Norris, Sharon Nichols; University of Colorado Denver Health Sciences Center: Elizabeth McFarland, Emily Barr, Robin McEvoy; University of Maryland, Baltimore: Douglas Watson, Nicole Messenger, Rose Belanger; University of Medicine and Dentistry of New Jersey: Arry Dieudonne, Linda Bettica, Susan Adubato; University of Miami: Gwendolyn Scott, Lisa Himic, Elizabeth Willen
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tardieu M, Le CJ, Persoz A, Meyer L, Blanche S, Mayaux MJ. HIV-1-related encephalopathy in infants compared with children and adults. French Pediatric HIV Infection Study and the SEROCO Group. Neurology. 2000 Mar 14;54(5):1089–95. doi: 10.1212/wnl.54.5.1089. [DOI] [PubMed] [Google Scholar]
- 2.Nozyce ML, Lee SS, Wiznia A, Nachman S, Mofenson LM, Smith ME, Yogev R, McIntosh K, Stanley K, Pelton S. A Behavioral and Cognitive Profile of Clinically Stable HIV-Infected Children. Pediatrics. 2006;117:763–770. doi: 10.1542/peds.2005-0451. [DOI] [PubMed] [Google Scholar]
- 3.Mellins CA, Brackis-Cott E, Leu CS, Elkington KS, Dolezal C, Wiznia A, McKay M, Bamji M, Abrams EJ. Rates and types of psychiatric disorders in perinatally human immunodeficiency virus-infected youth and seroreverters. J Child Psychol Psychiatry. 2009 Sep;50(9):1131–8. doi: 10.1111/j.1469-7610.2009.02069.x. Epub 2009 Feb 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gadow K, Chernoff M, Williams P, Brouwers P, Morse E, Heston J, Hodge J, DiPoalo V, Deygoo NS, Nachman S, the PACTG P1055 Study Team Cooccuring psychiatric symptoms in children perinatally-infected with HIV and peer comparison sample. J Dev Behav Pediatr. 2010;31(2):116–128. doi: 10.1097/DBP.0b013e3181cdaa20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donenberg GR, Pao M. Youths and HIV/AIDS: psychiatry's role in a changing epidemic. J Am Acad Child Adolesc Psychiatry. 2005 Aug;44(8):728–47. doi: 10.1097/01.chi.0000166381.68392.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007 Jan;8(1):33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- 7.Woods SP, Morgan EE, Marquie-Beck J, Carey CL, Grant I, Letendre S, the HIV Neurobehavioral Research Center (HNRC) Group Markers of macrophage activation and axonal injury are associated with prospective memory in HIV-1 disease. Cog Behav Neurol. 2006 Dec;4:217–221. doi: 10.1097/01.wnn.0000213916.10514.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li G, Kim YJ, Mantel C, Broxmeyer HE. P-selectin enhances generation of CD14+CD16+ dendritic-like cells and inhibits macrophage maturation from human peripheral blood monocytes. J Immunol. 2003 Jul 15;171(2):669–77. doi: 10.4049/jimmunol.171.2.669. [DOI] [PubMed] [Google Scholar]
- 9.Ludwig A, Weber C. Transmembrane chemokines: versatile ‘special agents’ in vascular inflammation. Thromb Haemost. 2007 May;97(5):694–703. [PubMed] [Google Scholar]
- 10.Galkina E, Ley K. Double jeopardy: how soluble P-selectin activates leukocytes in peripheral arterial occlusive disease. Circ Res. 2006 Jan 6;98(1):12–4. doi: 10.1161/01.RES.0000200409.65882.14. [DOI] [PubMed] [Google Scholar]
- 11.Woollard KJ, Kling D, Kulkarni S, Dart AM, Jackson S, Chin-Dusting J. Raised plasma soluble P-selectin in peripheral arterial occlusive disease enhances leukocyte adhesion. Circ Res. 2006 Jan 6;98(1):149–56. doi: 10.1161/01.RES.0000199295.14073.69. [DOI] [PubMed] [Google Scholar]
- 12.Kaul M, Lipton SA. Mechanisms of neuroimmunity and neurodegeneration associated with HIV-1 infection and AIDS. J Neuroimmune Pharmacol. 2006;1:138–151. doi: 10.1007/s11481-006-9011-9. [DOI] [PubMed] [Google Scholar]
- 13.Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath MS. Unique monocyte subset in patients with AIDS dementia. Lancet. 1997 Mar 8;349(9053):692–5. doi: 10.1016/S0140-6736(96)10178-1. [DOI] [PubMed] [Google Scholar]
- 14.Miller TL, Jacobson DL, Mendez A, Hazra R, Geffner M, Siberry G, Borkowsky W, Patel K, McFarland E, Van Dyke R, for the Pediatric HIV/AIDS Cohort Study (PHACS) CROI. Montreal: 2009. Biomarkers of Vascular Dysfunction in HIV-Infected Children With and Without Hyperlipidemia. [Google Scholar]
- 15.Ballantyne CM, Nambi V. Markers of inflammation and their clinical significance. Atheroscler Suppl. 2005 May;6(2):21–9. doi: 10.1016/j.atherosclerosissup.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Papanicolaou DA, Vgontzas AN. Interleukin-6: the endocrine cytokine. J Clin Endocrinol Metab. 2000 Mar;85(3):1331–3. doi: 10.1210/jcem.85.3.6582. [DOI] [PubMed] [Google Scholar]
- 17.Wang Q, Doerschuk CM. The signaling pathways induced by neutrophilendothelial cell adhesion. Antioxid Redox Signal. 2002 Feb;4(1):39–47. doi: 10.1089/152308602753625843. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg RB. Cytokine and cytokine-like inflammation markers, endothelial dysfunction, and imbalanced coagulation in development of diabetes and its complications. J Clin Endocrinol Metab. 2009 Sep;94(9):3171–82. doi: 10.1210/jc.2008-2534. [DOI] [PubMed] [Google Scholar]
- 19.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Hotta K, Nishida M, Takahashi M, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000 Sep 12;102(11):1296–301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 20.Wechsler D. Wechsler Intelligence Scale for Children-Fourth Edition. Harcourt Assessment, Inc.; San Antonio, Texas: 2003. [Google Scholar]
- 21.Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, Retzlaff BM, Knopp RH, Brunzell JD, Kahn SE. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003 Apr;46(4):459–69. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 22.Andersen KK, Frystyk J, Wolthers OD, Heuck C, Flyvbjerg A. Gender differences of oligomers and total adiponectin during puberty: a cross-sectional study of 859 Danish school children. J Clin Endocrinol Metab. 2007 May;92(5):1857–62. doi: 10.1210/jc.2006-2310. [DOI] [PubMed] [Google Scholar]
- 23.Böttner A, Kratzsch J, Müller G, Kapellen TM, Blüher S, Keller E, Blüher M, Kiess W. Gender differences of adiponectin levels develop during the progression of puberty and are related to serum androgen levels. J Clin Endocrinol Metab. 2004 Aug;89(8):4053–61. doi: 10.1210/jc.2004-0303. [DOI] [PubMed] [Google Scholar]
- 24.Jeremy RJ, Kim S, Nozyce M, Nachman S, McIntosh K, Pelton SI, Yogev R, Wiznia A, Johnson GM, Krogstad P, Stanley K, Pediatric AIDS Clinical Trials Group (PACTG) 338 & 377 Study Teams Neuropsychological functioning and viral load in stable antiretroviral therapy-experienced HIV-infected children. Pediatrics. 2005 Feb;115(2):380–7. doi: 10.1542/peds.2004-1108. [DOI] [PubMed] [Google Scholar]
- 25.Mialky E, Vagnoni J, Rutstein R. School-age children with perinatally acquired HIV infection: medical and psychosocial issues in a Philadelphia cohort. AIDS Patient Care STDS. 2001 Nov;15(11):575–9. doi: 10.1089/108729101753287667. [DOI] [PubMed] [Google Scholar]
- 26.Malee K, Williams PL, Montepiedra G, Nichols S, Sirois PA, Storm D, Farley J, Kammerer B, PACTG 219C Team The role of cognitive functioning in medication adherence of children and adolescents with HIV infection. J Pediatr Psychol. 2009 Mar;34(2):164–75. doi: 10.1093/jpepsy/jsn068. Epub 2008 Jul 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Connolly ES, Jr, Winfree CJ, Prestigiacomo CJ, Kim SC, Choudhri TF, Hoh BL, Naka Y, Solomon RA, Pinsky DJ. Exacerbation of cerebral injury in mice that express the P-selectin gene: identification of P-selectin blockade as a new target for the treatment of stroke. Circ Res. 1997 Sep;81(3):304–10. doi: 10.1161/01.res.81.3.304. [DOI] [PubMed] [Google Scholar]
- 28.Shiramizu B, Marshall A, Agsalda M, Troelstrup D, Shikuma C, Valcour V. CROI. San Francisco, CA: 2010. HAART-experienced Subjects with HIV-associated Neurocognitive Disorders Have Longitudinally Persistent Elevated HIV DNA Levels in CD14+, CD16+ Monocytes. [Google Scholar]
- 29.Yang H, Lang S, Zhai Z, Li L, Kahr WH, Chen P, Brkic J, Spring CM, Flick MJ, Degen JL, Freedman J, Ni H. Fibrinogen is required for maintenance of platelet intracellular and cell surface P-selectin expression. Blood. 2009 Mar 30; doi: 10.1182/blood-2008-03-145821. [DOI] [PubMed] [Google Scholar]
- 30.Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci. 2006 Jan 25;26(4):1098–106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalez E, Rovin BH, Sen L, Cooke G, Dhanda R, Mummidi S, Kulkarni H, Bamshad MJ, Telles V, Anderson SA, Walter EA, Stephan KT, Deucher M, Mangano A, Bologna R, Ahuja SS, Dolan MJ, Ahuja SK. HIV-1 infection and AIDS dementia are influenced by a mutant MCP-1 allele linked to increased monocyte infiltration of tissues and MCP-1 levels. Proc Natl Acad Sci U S A. 2002 Oct 15;99(21):13795–800. doi: 10.1073/pnas.202357499. Epub 2002 Oct 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Letendre S, Marquie-Beck J, Singh KK, de Almeida S, Zimmerman J, Spector SA, Grant I, Ellis R, HNRC Group The monocyte chemotactic protein-1 -2578G allele is associated with elevated MCP-1 concentrations in cerebrospinal fluid. J Neuroimmunol. 2004 doi: 10.1016/j.jneuroim.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 33.Gendelman HE, Ding S, Gong N, Liu J, Ramirez SH, Persidsky Y, Mosley RL, Wang T, Volsky DJ, Xiong H. Monocyte chemotactic protein-1 regulates voltage-gated K+ channels and macrophage transmigration. J Neuroimmune Pharmacol. 2009 Mar;4(1):47–59. doi: 10.1007/s11481-008-9135-1. Epub 2008 Nov 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weber C, Belge KU, von Hundelshausen P, Draude G, Steppich B, Mack M, Frankenberger M, Weber KS, Ziegler-Heitbrock HW. Differential chemokine receptor expression and function in human monocyte subpopulations. J Leukoc Biol. 2000 May;67(5):699–704. doi: 10.1002/jlb.67.5.699. [DOI] [PubMed] [Google Scholar]
- 35.Fischer-Smith T, Croul S, Sverstiuk AE, Capini C, L'Heureux D, Régulier EG, Richardson MW, Amini S, Morgello S, Khalili K, Rappaport J. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol. 2001 Dec;7(6):528–41. doi: 10.1080/135502801753248114. [DOI] [PubMed] [Google Scholar]
- 36.Sasseville VG, Lackner AA. Neuropathogenesis of simian immunodeficiency virus infection in macaque monkeys. J Neurovirol. 1997 Feb;3(1):1–9. doi: 10.3109/13550289709015787. [DOI] [PubMed] [Google Scholar]
- 37.Persidsky Y, Nottet HS, Sasseville VG, Epstein LG, Gendelman HE. The development of animal model systems for HIV-1 encephalitis and its associated dementia. J Neurovirol. 1995 Sep;1(3-4):229–43. doi: 10.3109/13550289509114019. [DOI] [PubMed] [Google Scholar]
- 38.Galea P, Vermot-Desroches C, Le Contel C, Wijdenes J, Chermann JC. Circulating cell adhesion molecules in HIV1-infected patients as indicator markers for AIDS progression. Res Immunol. 1997 Feb;148(2):109–17. doi: 10.1016/s0923-2494(97)82482-0. [DOI] [PubMed] [Google Scholar]
- 39.Hurwitz AA, Berman JW, Lyman WD. The role of the blood-brain barrier in HIV infection of the central nervous system. Adv Neuroimmunol. 1994;4(3):249–56. doi: 10.1016/s0960-5428(06)80263-9. [DOI] [PubMed] [Google Scholar]