Abstract
Aging is associated with an altered ability to match oxygen delivery (Qo2) to consumption (V̇o2) in skeletal muscle and differences in the temporal profile of vasodilation may provide a mechanistic basis for the Qo2-to-V̇o2 mismatching during the rest-to-exercise transition. Therefore, we tested the hypothesis that the speed of vasodilation will be blunted in skeletal muscle first-order arterioles from old vs. young rats. Arterioles from the soleus and the red portion of the gastrocnemius (GastRed) muscles were isolated from young (Y, 6 mo; n = 9) and old (O, 24 mo; n = 9) Fischer 344 rats and studied in vitro. Vessels were exposed to acetylcholine (ACh; 10−6 M), sodium nitroprusside (SNP; 10−4 M), and increased intraluminal flow, and the subsequent vasodilation was recorded at 30 frames/s. The data were fit to a monoexponential model and the dynamics of vasodilation [i.e., time delay, time constant (tau), and rate of change (delta/tau)] were calculated. With old age, the rate of vasodilation was significantly blunted in resistance vessels from the soleus to ACh (Y, 27.9 ± 3.6; O, 8.8 ± 2.6 μm/s) and flow (Y, 12.8 ± 2.1; O, 3.1 ± 0.9 μm/s). In the GastRed the old age-associated impairment of endothelium-dependent vasodilator dynamics was even greater than that of the soleus. With SNP neither the magnitude nor time constant of vasodilation was affected by age in either muscle. The results indicate that aging impairs the dynamics of vasodilation in resistance vessels from the soleus and GastRed muscles mediated, in part, through the endothelium. Thus the old age-associated slower rate and magnitude of vasodilation could inhibit the delivery of O2 during the critical transition from rest to exercise in moderate to highly oxidative skeletal muscle.
Keywords: isolated arteriole, endothelium-dependent vasodilation
aging is associated with a decline in maximal aerobic capacity (V̇o2 max) (41–43, 56) due, in part, to the reduced ability of the cardiovascular system to provide adequate blood flow to the active muscles. Specifically, with advancing age there is a lower bulk blood flow (21, 29, 47, 61) and an altered distribution of perfusion within muscle (39) during physical activity. Recently, Behnke et al. (6) have further demonstrated a temporal mismatching of oxygen delivery (Qo2) to oxygen consumption (V̇o2) across the rest-to-contractions transition in the spinotrapezius muscle of old rats, resulting in a transiently lowered microvascular Po2. These altered microvascular Po2 dynamics will, according to Fick's law, impair blood-myocyte O2 transfer in the elderly. Using the modeling techniques of Barstow and Móle (3), the old age-related microvascular Po2 response described by Behnke et al. (6) can arise only if blood flow dynamics are compromised (increased time constant) vs. the normal hyperemic time course observed in skeletal muscle from young subjects (31). There are several mechanisms responsible for the old age-associated perturbations of muscle blood flow, including a reduction in the number of feed arteries (5) and an impairment of endothelium-dependent vasodilation (20, 37, 55, 62) mediated by a reduced bioavailability of nitric oxide (NO) (18). These changes in resistance artery structure and function with advancing age culminate in significant impairments in capillary hemodynamics at rest (46) and during exercise (14). Despite the resolution of several mechanisms of the old age-related vasomotor dysfunction, the effects of aging on the dynamic responses of resistance arterioles (i.e., the speed of vasodilation) remain unknown.
Given the quintessential role of targeted arterial vasodilation in the matching of Qo2 to V̇o2 during the exercise on-transient, the purpose of this investigation was to quantify the effects of aging on skeletal muscle resistance artery vasomotor dynamics. We hypothesized that the speed of vasodilation will be blunted in arterioles from oxidative skeletal muscles from old vs. young animals. To test this hypothesis, resistance arterioles from the soleus and the red portion of the gastrocnemius (GastRed), two highly oxidative muscles (19), were studied since this muscle type demonstrates large old age-associated deficits in blood flow during exercise (39). In addition, with old age, blood flow is elevated to several low oxidative muscles of primarily fast glycolytic fiber type composition (39). Therefore, to further determine how aging affects vasomotor function, a second set of experiments was performed to quantify the speed of endothelium-dependent vasodilation in the low oxidative white portion of the gastrocnemius (GastWhite) muscle. Given the key role of NO in regulating resting vasomotor tone (28, 48, 49) and our interest in the dynamic transition from a resting state to a steady-state vasodilation, these studies focus primarily on NO-mediated vasodilation.
METHODS
All procedures performed in this study were approved by the University of Florida Institutional Animal Use and Care Committee. Twelve young (6 mo old; 382 ± 15 g) and 12 old (24 mo old; 421 ± 14 g) male Fischer 344 rats were obtained from the National Institute on Aging colony. The rats were housed in a temperature-controlled (23 ± 2°C) room with a 12:12-h light-dark cycle. Water and rat chow were provided ad libitum.
Arteriolar preparation.
Animals were anesthetized with pentobarbital sodium (85 mg/kg ip) and killed by exsanguination. The gastrocnemius-plantaris-soleus muscle complex was carefully excised from each leg and placed in cold (4°C) physiological saline solution (PSS). From the soleus and Gastred muscles, 1A arterioles (i.e., resistance arterioles, intraluminal diameter 85–205 μm, length 0.5–1.0 mm), defined as the first branch off the feed artery that perforates the muscle, were isolated. The arterioles were cleared of surrounding muscle fibers, removed from the muscle, and cannulated on both ends to glass micropipettes. After cannulation, the arterioles were transilluminated by an inverted microscope (Olympus IX71) equipped with a video camera (Orca-HR, Hamamatsu) attached to a digital video recorder (Sony DSR-30 DVR). Resistance arterioles were initially pressurized to 60 cmH2O with two independent hydrostatic pressure reservoirs. Leaks were detected by pressurizing the vessel and determining whether vessel diameter was maintained. Arterioles that exhibited leaks were discarded. Arterioles were warmed to 37°C and allowed to develop spontaneous tone during a 30- to 60-min equilibration period.
Assessment of vasomotor time courses followed similar methods as previously described (13, 63). Briefly, on displaying a steady level of spontaneous tone, vessels were exposed to either acetylcholine (ACh: 5 × 10−8 and 1 × 10−6 M), increased intraluminal flow, or sodium nitroprusside (SNP: 1 × 10−4 M), and images were time referenced by frame and stored on digital media (Maxell Mini DV, DVM60SE) for off-line analysis via digital video recorder (Sony DSR-30 DVR). Both ACh and SNP were administered via a microinjector placed near the vessel wall. The increase in intraluminal flow was accomplished by altering the heights of the independent fluid reservoirs in equal and opposite directions so that a pressure difference was created across the vessel without altering mean intraluminal pressure. In a preliminary set of experiments vasodilation dynamics to two rates of flow were investigated. Specifically, diameter measurements were determined in response to pressure differences from the static state to 10 (n = 5) or 60 (n = 5) cmH2O to represent low and high intraluminal flow rates, respectively. No difference in the temporal profile of vasodilation was observed between the two flow rates. Therefore, flow data presented herein are from a pressure difference of 10 cmH2O, which corresponds to an intraluminal flow rate of 13.4 ± 0.5 nl/s (37). On completion of the dynamics study, the vessel was incubated in calcium-free PSS solution for 60 min to determine maximal passive diameter (37).
Based on the results from the high oxidative skeletal muscle and the findings of Musch et. al. (39) of an old age-associated increased blood flow to low oxidative glycolytic muscles during exercise, an additional protocol was added to quantify the effects of aging on the dynamics of vasodilation in the low oxidative GastWhite muscle. Arterioles (young, n = 7; old, n = 6) were isolated from the superficial portion of the GastWhite and, on displaying a steady level of spontaneous tone, exposed to ACh (1 × 10−6 M); images were recorded as described above.
Off-line analysis.
To determine the rate of dispersion of a given compound in the buffer solution surrounding the vessel, India ink was initially infused through the microinjector during high-speed video recording. It was found that dispersion of the compound was complete within three frames or 1/10th of a second. Changes in arteriolar luminal diameter with exposure to ACh, increased intraluminal flow, and SNP were determined during frame-by-frame playback (30 frames/s) using a video caliper (Colorado Video, 307A, Boulder, CO). Subsequently, the time-diameter values were curve-fit to a monoexponential plus delay model (8) using an iterative least-squares technique by means of a commercial graphing/analysis package (KaleidaGraph 3.5).
Vasodilation was expressed as a percentage of maximal dilation using the following equation:
where Dt is the diameter recorded at a given time point, Di is the diameter recorded immediately before addition of the vasoactive agent (i.e., initial diameter), and Dm is the maximal diameter recorded.
Statistics.
For the KaleidaGraph analysis program a user-defined function to the data was fit using the following equation:
where Diametert is the change in diameter at time t, Diameterb is baseline diameter, ΔDiameterss is the change in diameter from baseline (preintervention) to the steady-state value, TD is the time delay, and τ is the time constant of the response, which estimates the time taken to reach 63% of the final exponential response. The same model was applied to diameter responses of arterioles from the soleus, GastRed, and GastWhite of young and old animals. From the mathematical modeling results the rate of vasodilation [change in diameter (Δ)/τ] and the time to reach a steady-state diameter (4τ) were calculated.
A two-way ANOVA was used to determined differences in vessel characteristics and vessel dynamics data between the young and old groups and between the soleus and gastrocnemius muscles. When a significant F value was demonstrated a Student-Newman-Keuls (SNK) post hoc test was performed to determine differences among mean values. Significance was accepted at P ≤ 0.05. Values are presented as means ± SE.
RESULTS
Vessel characteristics.
Vessel characteristics are shown in Table 1. There was no difference in maximal diameter between age groups for the soleus and a tendency (P = 0.09) for a greater maximal diameter in the GastRed from the old age group. The level of spontaneous tone before each intervention is shown in Table 1. Consistent with previous findings in soleus muscle arterioles (37), there was no significant difference in the level of spontaneous tone between the young and old groups. In the GastRed the initial diameter after the equilibration period was significantly smaller in young; however, spontaneous tone was not different between the two groups (Table 1).
Table 1.
Characteristics of resistance arterioles from the soleus and red portion of the gastrocnemius muscles of young and old animals
| Soleus Muscle |
GastRed Muscle |
|||
|---|---|---|---|---|
| Young (n = 9) | Old (n = 9) | Young (n = 9) | Old (n = 9) | |
| Maximal diameter, μm | 135±9 | 139±7 | 158±14 | 176±18* |
| Spontaneous tone, % | ||||
| Flow | 40±4 | 39±3 | 36±5 | 37±4 |
| ACh | 41±5 | 38±6 | 38±3 | 34±4 |
| Nitroprusside | 43±4 | 41±5 | 39±5 | 39±6 |
Values are means ± SE. GastRed, red portion of the gastrocnemius; maximal diameter, diameter measured in Ca2+-free solution.
P < 0.1 vs. corresponding young diameter.
Vasodilator dynamics to increased intraluminal flow.
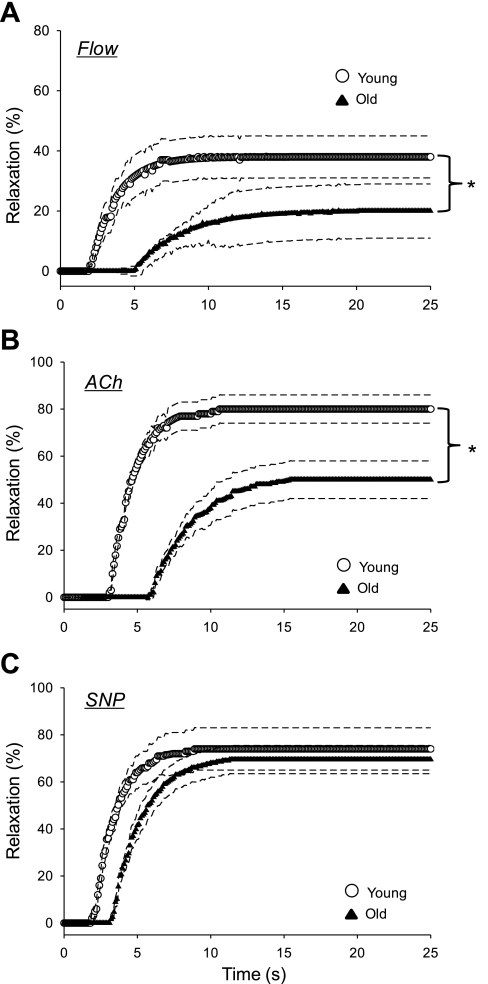
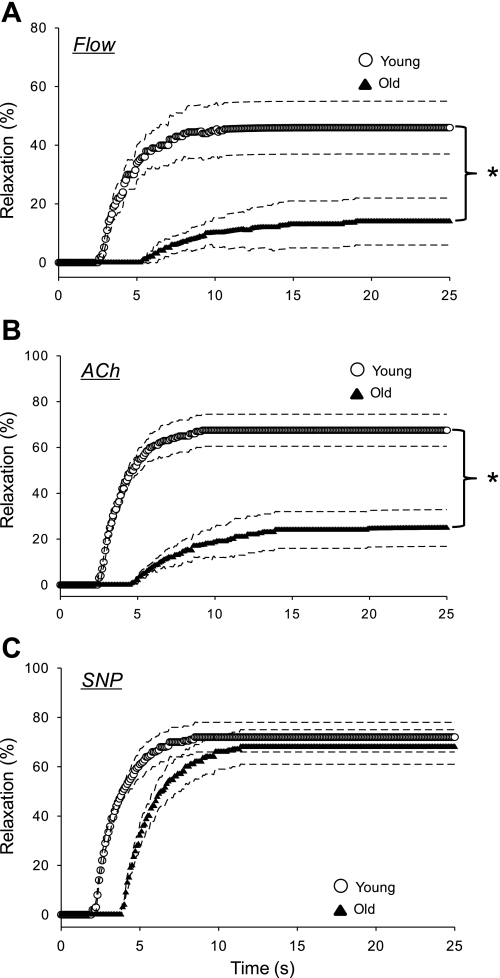
The magnitude of vasodilation to an increase in intraluminal flow of 13.4 ± 0.5 nl/s was reduced in resistance arterioles of both the soleus (Fig. 1A) and GastRed (Fig. 2A) muscles from old vs. young animals. After the increase in intraluminal flow, the time to reach a steady-state diameter was over twice as long in arterioles from the soleus muscle, and almost three times as long in those from the GastRed muscle from old animals (Table 2). In resistance arterioles from both muscles of the old animals, the time delay before a statistically significant change in intraluminal diameter occurred was increased ∼170% (Table 2). The time constant of vasodilation to flow was significantly longer in the GastRed vs. the soleus in the old group (Table 2). The slower dynamics and smaller magnitude of vasodilation culminated in a reduced rate of vasodilation (i.e., delta/time constant) with old age in both the soleus (old 3.1 ± 0.9 vs. young 12.8 ± 2.1 μm/s) and GastRed (old 1.9 ± 0.7 vs. young 15.7 ± 1.9 μm/s) muscles.
Fig. 1.
Vasodilator dynamics of arterioles from the soleus muscle of young and old rats to flow (A), ACh (B), and sodium nitroprusside (SNP; C). Exposure to a given condition began at time 0. Dashed lines represent the 95% confidence intervals. *P < 0.05.
Fig. 2.
Vasodilator dynamics of arterioles from the red portion of the gastrocnemius muscle (GastRed) of young and old rats to flow (A), ACh (B), and SNP (C). Exposure to a given condition began at time 0. Dashed lines represent the 95% confidence intervals. *P < 0.05.
Table 2.
Temporal characteristics of resistance arterioles from the soleus and red portion of the gastrocnemius (GastRed) muscles from young and old animals
| Soleus Muscle |
GastRed Muscle |
|||
|---|---|---|---|---|
| Young | Old | Young | Old | |
| Flow | n = 9 | n = 8 | n = 8 | n = 8 |
| Vasodilation, % | 42±9 | 18±8*‡ | 48±7 | 12±7*‡ |
| Time to S-S, s | 8.6±1.6 | 17.5±2.7*‡ | 8.8±1.5 | 22.1±3.4*‡ |
| Time delay, s | 1.9±0.4 | 5.1±1.1* | 2.0±0.4 | 5.5±0.9*‡ |
| Tau, s | 1.7±0.3 | 3.1±0.8*‡ | 1.7±0.4 | 4.2±1.1*‡ |
| Delta, μm | 22±6‡ | 9±4*‡ | 27±6‡ | 8±3*‡ |
| ACh | n = 9 | n = 9 | n = 9 | n = 9 |
| Vasodilation, % | 81±6 | 54±8*‡ | 67±7 | 25±8*‡ |
| Time to S-S, s | 9.5±1.2 | 17.9±2.6*‡ | 8.4±0.5 | 20.0±3.0*‡ |
| Time delay, s | 3.1±0.4 | 4.4±1.6 | 2.5±0.2 | 4.5±1.6* |
| Tau, s | 1.6±0.4 | 3.4±0.7*‡ | 1.5±0.3 | 3.9±0.6*‡ |
| Delta, μm | 45±6 | 30±7*‡ | 40±6 | 15±5*‡ |
| Nitroprusside | n = 9 | n = 9 | n = 9 | n = 9 |
| Vasodilation, % | 74±9 | 70±6 | 72±6 | 68±7 |
| Time to S-S, s | 7.8±1.1 | 11.2±2.0* | 8.2±1.3 | 11.3±2.4* |
| Time delay, s | 2.0±0.6 | 3.2±1.1† | 2.0±0.5 | 3.8±1.1* |
| Tau, s | 1.5±0.5 | 2.0±1.1 | 1.6±0.2 | 1.9±0.7 |
| Delta, μm | 43±5 | 40±4 | 44±6 | 47±8 |
Values are means ± SE. Vasodilation, percent difference between the steady-state (postintervention) and initial diameter; time to S-S, time taken to reach a stable (i.e., steady-state) diameter after intervention; time delay, initial delay before a significant change in diameter; tau, time taken to reach 63% of the final steady-state diameter
P < 0.05 vs. corresponding young value.
P < 0.1 vs. corresponding young value.
P < 0.05 vs. value from sodium nitroprusside.
Vasodilator dynamics to ACh.
In response to 5 × 10−8 M ACh there was no significant change in intraluminal diameter vs. initial diameter in either group (data not shown). Therefore, dynamics data are presented only for the 1 × 10−6 M dose of ACh, which elicited a significant vasodilation in arterioles from both muscles in young and old animals (Figs. 1B and 2B). The magnitude of vasodilation to ACh was reduced in resistance arterioles from both the soleus (Fig. 1B) and GastRed (Fig. 2B) muscles of old vs. young animals. The temporal responses of vasodilation to ACh are demonstrated in Figs. 1B and 2B and reported in Table 2. In the soleus muscle, the time delay before the onset of vasodilation to ACh was not significant between young (3.1 ± 0.4 s) and old (4.4 ± 1.6 s; P = 0.16), whereas the time delay was less in the GastRed of young (2.5 ± 0.2 s) vs. old (4.5 ± 1.6 s; P < 0.05) animals. The time constant of vasodilation from both muscles was over twice as long in the old vs. young group (Table 2). The blunted dynamics in the resistance arterioles of the old group resulted in a longer time to attain a steady-state luminal diameter in both the soleus (old 17.1 ± 2.8 vs. young 9.4 ± 1.1 s; P < 0.05) and GastRed (old 20.1 ± 3.2 vs. young 7.8 ± 0.5 s; P < 0.05) muscles. The rate of vasodilation as a function of the exponential response was reduced in old compared with young in both the soleus (old 8.8 ± 2.6 vs. young 27.9 ± 3.6; μm/s; P < 0.05) and GastRed (old 3.8 ± 1.3 vs. young 26.9 ± 4.3; μm/s; P < 0.05) muscles.
Vasodilator dynamics to SNP.
In response to the NO donor SNP (1 × 10−4 M), the magnitude of vasodilation was not different between young and old in either muscle (Figs. 1C and 2C; Table 2). In the soleus muscle, there was a trend (P < 0.1) for an increased time delay before the onset of vasodilation to SNP in the old vs. young group, whereas the time delay was significantly longer in the GastRed with old age (Table 2). There was no age-associated difference in the time constant of vasodilation in either muscle. The rate of change was not different depending on age in either the soleus (old 22.3 ± 4.6 vs. young 27.5 ± 4.5 μm/s) or the GastRed (old 25.1 ± 4.7 vs. young 27.4 ± 5.1 μm/s) muscles.
Vasodilation dynamics in the GastWhite.
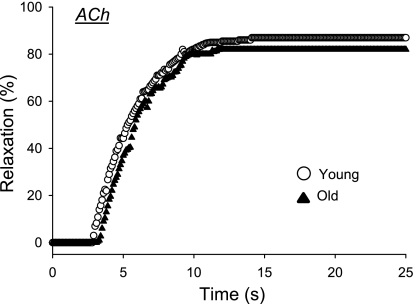
From the second series of investigations the maximal diameter of arterioles from the GastWhite was significantly greater in old (192 ± 18 μm) vs. young (158 ± 12 μm), but consistent with previous findings (37) the percent relaxation to ACh was not different between age groups (Fig. 3). With exposure to ACh, there were no age-associated differences in the time delay (old 3.2 ± 0.7; young 3.0 ± 0.6 s), time constant (old 2.4 ± 0.7; young 2.2 ± 0.6 s), or rate of vasodilation (old 28.6 ± 9.3; young 27.1 ± 8.2 μm/s).
Fig. 3.
Vasodilator dynamics of arterioles from the low oxidative white portion of the gastrocnemius muscle (GastWhite) of young and old rats to the endothelium-dependent vasodilator ACh. No age-related differences in the dynamics of vasodilation or the percent relaxation were observed.
DISCUSSION
Previous work has demonstrated that aging results in a temporal mismatching of Qo2 to V̇o2 in skeletal muscle during the rest-to-exercise transition that results in a low microvascular Po2 (6). Modeling analysis indicates that this old age-induced transient reduction in microvascular Po2 can only arise through a delay in the muscle hyperemia at the onset of contractions. However, to date there have been no studies describing whether aging alters vasodilation dynamics in isolated resistance arterioles. Therefore, the primary purpose of this study was to determine whether there is an old age-related blunting of the dynamics of vasodilation in resistance arterioles from highly oxidative skeletal muscle. The results demonstrate that with advancing age there is a prolonged time taken to reach a steady-state diameter and a reduced magnitude of endothelium-dependent vasodilation in resistance arterioles from both the soleus and GastRed muscles. The key finding of this study is a decrease of >70% and >85% in the rate of endothelium-dependent vasodilation in resistance arterioles from the soleus and GastRed muscles of old animals, respectively. Conversely, there were no age-related differences in the rate or magnitude of vasodilation from either muscle to the endothelium-independent NO donor SNP. The latter suggests that neither vascular structural nor mechanical properties can account for the slower rate of endothelium-dependent vasodilation in arterioles from old rats. The finding of an old age-associated blunting of endothelium-dependent vasodilator dynamics provides a plausible mechanism for the rapid decline in microvascular Po2 across the exercise on-transient (6) and the greater depletion of high-energy phosphates (57) in older individuals. An additional study utilizing the low oxidative GastWhite muscle was performed to investigate whether aging blunts the dynamics of vasodilation uniformly across muscle fiber types. Neither the speed nor magnitude of endothelium-dependent vasodilation was affected by age in the GastWhite (Fig. 3). Compared with the GastWhite, the speed of vasodilation in arterioles from the soleus and GastRed muscles was faster in the young and slower in the old groups, suggesting that old age-related alterations in the speed of vasodilation are fiber type specific.
Time course of vasodilation.
The time course of exercise hyperemia has been of interest for well over a century since Gaskell (23) postulated a metabolite-associated vasodilator mechanism. However, the mechanistic bases for the rapid increase in blood flow with contractions remains unknown and is a matter of some debate, although it is likely a combination of the mechanical augmentation of blood flow (i.e., muscle pump) in skeletal muscle and vasodilation (for review, see Refs. 11, 17, 58). However, to our knowledge the literature contains no previous reports of the dynamics of isolated resistance arteriolar vasodilation from aged skeletal muscle.
At the onset of muscular contractions there is an immediate increase in red blood cell (RBC) velocity through the capillary bed (31) and feed arteries (60), whereas RBC flux does not increase quite as rapidly (31). There is disagreement regarding the latency before the onset of arteriolar vasodilation, with values ranging from 1–2 s (4), 3–6 s (36), and 2–18 s (35), up to >20 s (25). In isolated arterioles we have consistently observed a time delay of ∼3–4 s before the onset of vasodilation (present study and 63). In contrast, in isolated soleus muscle feed arteries from young animals, Clifford et al. (12) have demonstrated that mechanical deformation of the vessel by external compression elicits an immediate vasodilation that is complete within 3–4 s. The differences between these studies are likely due, in part, to dissimilar vasodilator stimuli (i.e., mechanical compression vs. pharmacological agents) and the spatially dependent physiochemical milieu of feed arteries (extramuscular) vs. resistance arterioles (i.e., intramuscular). For example, in smaller arterioles (i.e., 20- to 30-μm luminal diameter) located within the gluteus maximus muscle (and therefore subject to large changes in intramuscular pressure), after the onset of muscular contractions there is a clear delay before the onset of vasodilation (4).
With advancing age much less is known regarding the time course of exercise hyperemia. There is an old age-related deficit in steady-state blood flow (44) and vascular conductance (33) during dynamic leg exercise and electrically induced muscular contractions (27). Recently, Copp et al. (14) have investigated the effects of aging on the capillary hemodynamic response to muscular contractions. With advancing age these researchers found virtually no increase in RBC flux (index of O2 delivery) over time with muscular contractions, indicating impaired peripheral circulatory control. In highly oxidative muscle such as the soleus and GastRed NO plays a significant role in the regulation of vascular tone in vivo (28). Therefore, the slowed endothelium-dependent vasodilator dynamics with advancing age (Figs. 1, 2, and 4; Table 2) and the results of previous work demonstrating reduced bioavailability of NO in arterioles during endothelium-dependent vasodilation (18, 51) provide a plausible mechanism for the blunted capillary hemodynamics (14) and the lower microvascular Po2 (6) following the onset of muscle contractions with aging. Indeed, Ferreira et al. (22) have demonstrated that NO bioavailability is crucial in ensuring adequate Qo2-to-V̇o2 matching in skeletal muscle across the rest-to-exercise transition. In addition, the slower dynamics and reduced magnitude of endothelium-dependent vasodilation in the soleus and GastRed from old animals are consistent with the diminished blood flow to these muscles during exercise in conscious animals (39). However, the lack of change in the dynamics of vasodilation in the GastWhite (Fig. 3) is unlikely responsible for the enhanced exercise hyperemic response in glycolytic muscle with aging (39). The old age-associated elevation of blood flow during exercise in low oxidative muscle may be the result of 1) an increased recruitment of white glycolytic fibers due to an inadequate perfusion of high oxidative motor units and/or 2) an impaired myogenic autoregulation of vasomotor tone observed in resistance vessels from this muscle fiber type (38). Prospective studies are required to address this issue mechanistically.
Fig. 4.
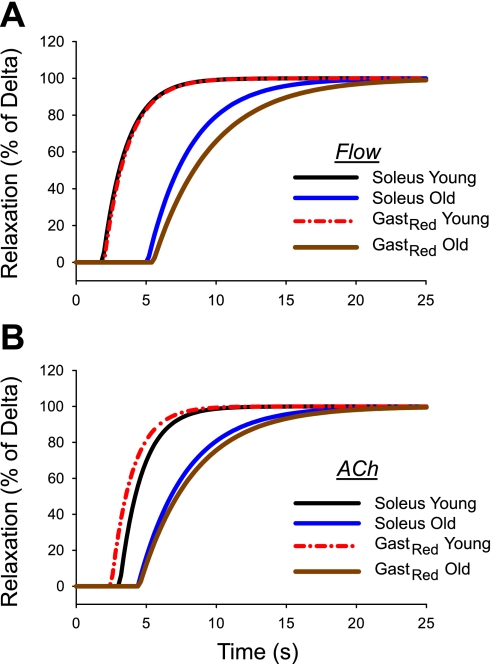
Time course of vasodilation to endothelium-dependent stimuli increased intraluminal flow (A) and ACh (B) in arterioles from the soleus and GastRed of young and old rats. Data are plotted as a function of the delta (i.e., change in diameter between pre- and postintervention) to normalize the percent vasodilation between the means of the two groups.
Mechanistic bases for aging-induced alterations in the speed of vasodilation.
Many of the investigations focusing on vasomotor dysfunction with advancing age have demonstrated impaired endothelium-dependent vasodilation (20, 24, 37, 55, 62). Muller-Delp and colleagues (37, 52) have further determined that a large component of the old age-associated endothelial dysfunction originates from the NO signaling pathway. In the present study, a degree of old age-associated endothelial dysfunction on the dynamics of vasodilation is also evident (Fig. 4, A and B). In the soleus muscle, for example, the normalized vasodilator responses through endothelium-dependent mechanisms (i.e., flow and ACh) are significantly blunted with old age (Fig. 4, A and B). Interestingly, advancing age impaired the speed of vasodilation to a greater extent in arterioles from the GastRed vs. that from the soleus (Fig. 4A) muscle, although the mechanistic basis for this difference is not entirely clear. Given the GastRed is a locomotory muscle and the soleus is primarily a postural muscle (19, 32), it is likely that the reduced spontaneous physical activity with advancing age (5) may affect vessels from the GastRed to a greater extent than those from the soleus muscle.
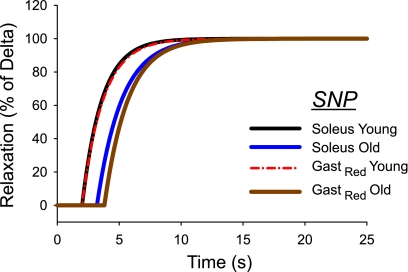
Although there was no change in the rate of vasodilation to SNP, there was a tendency in the soleus muscle (Figs. 1C and 5) and a statistically longer delay in arterioles from the GastRed muscle (Fig. 2C and 5) before the onset of vasodilation to SNP with aging. The mechanism(s) responsible for this increased delay is currently unclear; however, an impaired diffusion of NO into the vascular smooth muscle or an inertia within the cGMP signaling pathway may be contributing factors. However, NO freely diffuses across cell membranes due to its small size and lack of charge making an impaired diffusion of NO into the vessel unlikely. The intransigent rate of vasodilation to SNP between age groups suggests the prolonged time delay of vasodilation with old age is manifest in the initial steps of the NO/cGMP pathway. To our knowledge, there are no studies reporting old age-associated changes in enzyme kinetics that would explain a slower onset of vasodilation. However, advancing age affects several key enzymes in the NO/cGMP signaling pathway, including 1) loss of expression of the β-subunit of soluble guanylyl cyclase (sGC) (10) and 2) decreased activation of cGMP-dependent protein kinase by cGMP (34), both of which have the potential to affect smooth muscle relaxation. In addition, with increasing age there is an elevated superoxide (O2−) production (15) due, in part, to a loss of extracellular superoxide dismutase (SOD) (54) located within the vascular intima. Superoxide can exhibit a modulatory effect of NO concentration by scavenging NO to form the oxidant peroxynitrite (26), which likely contributes to the reduced bioavailability of the former with aging (30, 51, 59). Therefore, with exogenous NO administration to a milieu of increased O2− production, the time taken to reach the critical concentration of NO sufficient to activate cGMP may be prolonged, resulting in a delay in the onset of vasodilation (Figs. 1C, 2C, and 5).
Fig. 5.
Time course of vasodilation to endothelium-independent vasodilator SNP in arterioles from the soleus and GastRed of young and old rats. Data are plotted as a function of the delta (i.e., change in diameter between pre- and postintervention) to normalize the percent vasodilation between the means of the two groups.
Ramifications of slowed vasodilatory dynamics.
Skeletal muscle possesses a tremendous ability to augment blood flow from rest to exercise (1, 2, 9, 31, 40, 45, 50). Therefore, alterations in the fidelity in which the resistance vasculature can modulate vascular conductance can have severe ramifications on exercise tolerance. Across the rest-to-exercise transition, the time course of the whole muscle hyperemic response reflects the summation of the individual resistance arterioles vasodilator dynamic profiles. Blunted arteriolar vasodilator (and therefore Qo2) dynamics (Figs. 1, A and B, and 2, A and B) would have a downstream affect on the individual microvascular units they perfuse. Specifically, a slower rate of Qo2 would force a compensatory increase in fractional O2 extraction at the capillary level to maintain O2 flux and cellular energetics. Indeed, in both aged humans (16) and animals (6) the muscle oxygenation kinetic profile across the exercise on-transient supports a slower adaptation of muscle blood flow vs. that of V̇o2. The blunted vasodilator dynamics with advancing age will likely potentiate the vascular dysfunction elicited by pathological conditions such as chronic heart failure (CHF) and diabetes. Indeed, an even slower skeletal muscle hyperemic response with contractions is predicted in aged subjects with CHF based on microvascular O2 exchange profiles (7).
Conclusion.
In summary, data from the present study demonstrate that the time course of endothelium-dependent vasodilation in isolated arterioles is significantly slower in skeletal muscle from old animals. These blunted dynamics concurrent with a reduced magnitude of vasodilation would have severe ramifications on the ability to rapidly augment blood flow to exercising muscle in the elderly. Therefore, the ability to match Qo2 to V̇o2, which is crucial to maintain cellular energetics and exercise tolerance, is compromised in the aged muscle. It is known that chronic exercise training can mitigate the old-age associated vascular endothelial dysfunction (52, 53); however, whether exercise training can speed the dynamics of vasodilation in skeletal muscle from aged individuals remains to be determined.
GRANTS
This study was supported by National Institutes of Health Grant KO1-AG-031327-01 (B. J. Behnke).
DISCLOSURES
No conflicts of interest are declared by the authors.
Supplementary Material
REFERENCES
- 1. Adams DB, Baccelli G, Mancia G, Zanchetti A. Relation of cardiovascular changes in fighting to emotion and exercise. J Physiol 212: 321–335, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Armstrong RB, Laughlin MH. Metabolic indicators of fibre recruitment in mammalian muscles during locomotion. J Exp Biol 115: 201–213, 1985 [DOI] [PubMed] [Google Scholar]
- 3. Barstow TJ, Mole PA. Simulation of pulmonary O2 uptake during exercise transients in humans. J Appl Physiol 63: 2253–2261, 1987 [DOI] [PubMed] [Google Scholar]
- 4. Bearden SE. Advancing age produces sex differences in vasomotor kinetics during and after skeletal muscle contraction. Am J Physiol Regul Integr Comp Physiol 293: R1274–R1279, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Behnke B, Prisby RD, Lesniewski LA, Donato AJ, Olin H, Delp MD. Influence of aging and physical activity on vascular morphology in rat skeletal muscle. J Physiol 575: 617–626, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Behnke BJ, Delp MD, Dougherty PJ, Musch TI, Poole DC. Effects of aging on microvascular oxygen pressures in rat skeletal muscle. Respir Physiol Neurobiol 146: 259–268, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Behnke BJ, Delp MD, Poole DC, Musch TI. Aging potentiates the effect of congestive heart failure on muscle microvascular oxygenation. J Appl Physiol 103: 1757–1763, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Behnke BJ, Kindig CA, Musch TI, Koga S, Poole DC. Dynamics of microvascular oxygen pressure across the rest-exercise transition in rat skeletal muscle. Respir Physiol 126: 53–63, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Buckwalter JB, Ruble SB, Mueller PJ, Clifford PS. Skeletal muscle vasodilation at the onset of exercise. J Appl Physiol 85: 1649–1654, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Chen L, Daum G, Fischer JW, Hawkins S, Bochaton-Piallat ML, Gabbiani G, Clowes AW. Loss of expression of the beta subunit of soluble guanylyl cyclase prevents nitric oxide-mediated inhibition of DNA synthesis in smooth muscle cells of old rats. Circ Res 86: 520–525, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Clifford PS. Skeletal muscle vasodilatation at the onset of exercise. J Physiol 583: 825–833, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clifford PS, Kluess HA, Hamann JJ, Buckwalter JB, Jasperse JL. Mechanical compression elicits vasodilatation in rat skeletal muscle feed arteries. J Physiol 572: 561–567, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Colleran PN, Behnke BJ, Wilkerson MK, Donato AJ, Delp MD. Simulated microgravity alters rat mesenteric artery vasoconstrictor dynamics through an intracellular Ca2+ release mechanism. Am J Physiol Regul Integr Comp Physiol 294: R1577–R1585, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Copp SW, Ferreira LF, Herspring KF, Musch TI, Poole DC. The effects of aging on capillary hemodynamics in contracting rat spinotrapezius muscle. Microvasc Res 77: 113–119, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res 90: 1159–1166, 2002 [DOI] [PubMed] [Google Scholar]
- 16. DeLorey DS, Kowalchuk JM, Paterson DH. Adaptation of pulmonary O2 uptake kinetics and muscle deoxygenation at the onset of heavy-intensity exercise in young and older adults. J Appl Physiol 98: 1697–1704, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Delp MD. Control of skeletal muscle perfusion at the onset of dynamic exercise. Med Sci Sports Exerc 31: 1011–1018, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Delp MD, Behnke BJ, Spier SA, Wu G, Muller-Delp JM. Ageing diminishes endothelium-dependent vasodilatation and tetrahydrobiopterin content in rat skeletal muscle arterioles. J Physiol 586: 1161–1168, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol 80: 261–270, 1996 [DOI] [PubMed] [Google Scholar]
- 20. DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS. Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol 290: H272–H278, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Ferreira LF, Padilla DJ, Williams J, Hageman KS, Musch TI, Poole DC. Effects of altered nitric oxide availability on rat muscle microvascular oxygenation during contractions. Acta Physiol (Oxf) 186: 223–232, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Gaskell WH. On the tonicity of the heart and blood vessels. J Physiol 3: 48–75, 1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gerhard M, Roddy MA, Creager SJ, Creager MA. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension 27: 849–853, 1996 [DOI] [PubMed] [Google Scholar]
- 25. Gorczynski RJ, Klitzman B, Duling BR. Interrelations between contracting striated muscle and precapillary microvessels. Am J Physiol Heart Circ Physiol 235: H494–H504, 1978 [DOI] [PubMed] [Google Scholar]
- 26. Gryglewski RJ, Palmer RM, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature 320: 454–456, 1986 [DOI] [PubMed] [Google Scholar]
- 27. Hammer LW, Boegehold MA. Functional hyperemia is reduced in skeletal muscle of aged rats. Microcirculation 12: 517–526, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Hirai T, Visneski MD, Kearns KJ, Zelis R, Musch TI. Effects of NO synthase inhibition on the muscular blood flow response to treadmill exercise in rats. J Appl Physiol 77: 1288–1293, 1994 [DOI] [PubMed] [Google Scholar]
- 29. Irion GL, Vasthare US, Tuma RF. Age-related change in skeletal muscle blood flow in the rat. J Gerontol 42: 660–665, 1987 [DOI] [PubMed] [Google Scholar]
- 30. Kang LS, Reyes RA, Muller-Delp JM. Aging impairs flow-induced dilation in coronary arterioles: role of NO and H2O2. Am J Physiol Heart Circ Physiol 297: H1087–H1095, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kindig CA, Richardson TE, Poole DC. Skeletal muscle capillary hemodynamics from rest to contractions: implications for oxygen transfer. J Appl Physiol 92: 2513–2520, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Laughlin MH, Armstrong RB. Muscular blood flow distribution patterns as a function of running speed in rats. Am J Physiol Heart Circ Physiol 243: H296–H306, 1982 [DOI] [PubMed] [Google Scholar]
- 33. Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol 285: H1023–H1031, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Lin CS, Liu X, Tu R, Chow S, Lue TF. Age-related decrease of protein kinase G activation in vascular smooth muscle cells. Biochem Biophys Res Commun 287: 244–248, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Marshall JM, Tandon HC. Direct observations of muscle arterioles and venules following contraction of skeletal muscle fibres in the rat. J Physiol 350: 447–459, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mihok ML, Murrant CL. Rapid biphasic arteriolar dilations induced by skeletal muscle contraction are dependent on stimulation characteristics. Can J Physiol Pharmacol 82: 282–287, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 283: H1662–H1672, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Muller-Delp JM, Spier SA, Ramsey MW, Lesniewski LA, Papadopoulos A, Humphrey JD, Delp MD. Effects of aging on vasoconstrictor and mechanical properties of rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 282: H1843–H1854, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Musch TI, Eklund KE, Hageman KS, Poole DC. Altered regional blood flow responses to submaximal exercise in older rats. J Appl Physiol 96: 81–88, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Naik JS, Valic Z, Buckwalter JB, Clifford PS. Rapid vasodilation in response to a brief tetanic muscle contraction. J Appl Physiol 87: 1741–1746, 1999 [DOI] [PubMed] [Google Scholar]
- 41. Ogawa T, Spina RJ, Martin WH, 3rd, Kohrt WM, Schechtman KB, Holloszy JO, Ehsani AA. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation 86: 494–503, 1992 [DOI] [PubMed] [Google Scholar]
- 42. Paterson DH, Cunningham DA, Koval JJ, St Croix CM. Aerobic fitness in a population of independently living men and women aged 55–86 years. Med Sci Sports Exerc 31: 1813–1820, 1999 [DOI] [PubMed] [Google Scholar]
- 43. Proctor DN, Joyner MJ. Skeletal muscle mass and the reduction of V̇o2max in trained older subjects. J Appl Physiol 82: 1411–1415, 1997 [DOI] [PubMed] [Google Scholar]
- 44. Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL, Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol 85: 68–75, 1998 [DOI] [PubMed] [Google Scholar]
- 45. Radegran G, Saltin B. Muscle blood flow at onset of dynamic exercise in humans. Am J Physiol Heart Circ Physiol 274: H314–H322, 1998 [DOI] [PubMed] [Google Scholar]
- 46. Russell JA, Kindig CA, Behnke BJ, Poole DC, Musch TI. Effects of aging on capillary geometry and hemodynamics in rat spinotrapezius muscle. Am J Physiol Heart Circ Physiol 285: H251–H258, 2003 [DOI] [PubMed] [Google Scholar]
- 47. Saltin B. Blood flow and substrate exchange in skeletal muscle of man: techniques relevant for use in the study of the ageing process of muscle. Muscle Nerve Suppl 5: S107–S109, 1997 [PubMed] [Google Scholar]
- 48. Schrage WG, Eisenach JH, Joyner MJ. Ageing reduces nitric-oxide- and prostaglandin-mediated vasodilatation in exercising humans. J Physiol 579: 227–236, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schrage WG, Joyner MJ, Dinenno FA. Local inhibition of nitric oxide and prostaglandins independently reduces forearm exercise hyperaemia in humans. J Physiol 557: 599–611, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shoemaker JK, Halliwill JR, Hughson RL, Joyner MJ. Contributions of acetylcholine and nitric oxide to forearm blood flow at exercise onset and recovery. Am J Physiol Heart Circ Physiol 273: H2388–H2395, 1997 [DOI] [PubMed] [Google Scholar]
- 51. Sindler AL, Delp MD, Reyes R, Wu G, Muller-Delp JM. Effects of ageing and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. J Physiol 587: 3885–3897, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW, Muller-Delp J.M. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol 556: 947–958, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Spier SA, Delp MD, Stallone JN, Dominguez JM, Muller-Delp JM. Exercise training enhances flow-induced vasodilation in skeletal muscle resistance arteries of aged rats: role of PGI2 and NO. Am J Physiol Heart Circ Physiol 292: H3119–H3127, 2007 [DOI] [PubMed] [Google Scholar]
- 54. Sun D, Huang A, Yan EH, Wu Z, Yan C, Kaminski PM, Oury TD, Wolin MS, Kaley G. Reduced release of nitric oxide to shear stress in mesenteric arteries of aged rats. Am J Physiol Heart Circ Physiol 286: H2249–H2256, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation 91: 1981–1987, 1995 [DOI] [PubMed] [Google Scholar]
- 56. Tanaka H, Desouza CA, Jones PP, Stevenson ET, Davy KP, Seals DR. Greater rate of decline in maximal aerobic capacity with age in physically active vs. sedentary healthy women. J Appl Physiol 83: 1947–1953, 1997 [DOI] [PubMed] [Google Scholar]
- 57. Taylor DJ, Kemp GJ, Thompson CH, Radda GK. Ageing: effects on oxidative function of skeletal muscle in vivo. Mol Cell Biochem 174: 321–324, 1997 [PubMed] [Google Scholar]
- 58. Tschakovsky ME, Sheriff DD. Immediate exercise hyperemia: contributions of the muscle pump vs. rapid vasodilation. J Appl Physiol 97: 739–747, 2004 [DOI] [PubMed] [Google Scholar]
- 59. van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Luscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med 192: 1731–1744, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. VanTeeffelen JW, Segal SS. Rapid dilation of arterioles with single contraction of hamster skeletal muscle. Am J Physiol Heart Circ Physiol 290: H119–H127, 2006 [DOI] [PubMed] [Google Scholar]
- 61. Wahren J, Saltin B, Jorfeldt L, Pernow B. Influence of age on the local circulatory adaptation to leg exercise. Scand J Clin Lab Invest 33: 79–86, 1974 [DOI] [PubMed] [Google Scholar]
- 62. Woodman CR, Price EM, Laughlin MH. Aging induces muscle-specific impairment of endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol 93: 1685–1690, 2002 [DOI] [PubMed] [Google Scholar]
- 63. Wunsch SA, Muller-Delp J, Delp MD. Time course of vasodilatory responses in skeletal muscle arterioles: role in hyperemia at onset of exercise. Am J Physiol Heart Circ Physiol 279: H1715–H1723, 2000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.