Abstract
In children, exercise modulates systemic anabolism, muscle growth, and overall physiological development through the growth hormone (GH)-insulin-like growth factor I (IGF-I) axis. GH secretion, at rest and during exercise, changes with age and maturational status and can be blunted by hyperlipidemia and obesity, with possible negative effects on physiological growth. However, little is known about the effect of progressively more severe pediatric obesity on the GH response to exercise and its relationship to pubertal status. We therefore studied 48 early- or late-pubertal obese children [body mass index (BMI) >95th percentile, separated in tertiles with progressively greater BMI] and 42 matched controls (BMI <85th percentile), who performed ten 2-min cycling bouts at ∼80% of maximal O2 consumption, separated by 1-min rest intervals. Plasma GH and IGF-I were measured at baseline and end exercise. GH responses were systematically blunted in obese children, with more pronounced blunting paralleling increasing BMI. Although overall the GH response to exercise was greater in late-pubertal than in younger children, this blunting pattern was observed in early- and late-pubertal children. Our results reveal insight into the interaction between pediatric obesity and key modulators of physiological growth and development and underscore the necessity of optimizing physical activity strategies for specific pediatric dysmetabolic conditions.
Keywords: adolescence, growth factors, adiposity, sexual maturation
the growth hormone (GH)-insulin-like growth factor I (IGF-I) axis is a primary regulator of systemic anabolism and muscle growth (5). Although GH continues to play a major homeostatic role in the metabolism of energy substrates later in life (3, 22), it is especially crucial for physiological growth and development during childhood and adolescence. Genetic GH insensitivity, in fact, leads to a number of pathological consequences, including abnormal shortness of the trunk and limbs (31, 42). Conditions that can interfere with GH action in pediatric populations are therefore likely to alter physiological growth and development, with possible negative repercussions on future health and well-being. Obesity has been clearly demonstrated to affect, through multiple biochemical pathways, GH metabolism and function (13, 28); the correlations between severity of obesity and regulation of GH secretion, however, remain relatively understudied, especially during the metabolically critical years of sexual maturation. These issues are gaining particular relevance in light of the rapid development of pediatric obesity into an unprecedented health, social, and financial challenge (35).
Baseline systemic GH concentration is determined by pulsatile releases of the hormone from the anterior pituitary periodically throughout the day, with the maximal secretion occurring during deep sleep (20, 47). A number of physiological stimuli can also acutely increase GH secretion. Among these stimuli, the quantitatively most important is arguably physical activity, which in childhood and adolescence occurs spontaneously after a pattern of repeated, relatively brief, often intense bursts of exercise (2), each resulting in GH elevations of up to 10- to 20-fold over resting levels (14, 18). The robustness and consistency of this response suggest that its acute or chronic alteration may affect the physiological effects of the GH-IGF-I axis.
A number of factors, such as drugs [arginine and ghrelin acutely increase GH secretion (1, 9)], metabolic changes (acute hypoglycemia causes an abrupt rise in plasma GH, but repeated hypoglycemia reduces the GH response to subsequent stimuli), and the presence of certain chronic endocrine pathologies, are known to potentially alter physiological GH levels. Particularly relevant to the GH response to exercise are the observations that ingestion of a single high-fat meal (7, 16), adult and pediatric obesity (14, 25), and the performance of a series of prior exercise bouts of variable intensity and duration (17, 44, 45) result in significant blunting of GH secretion during a subsequent exercise challenge. An additional important effect on GH secretion pattern and systemic concentrations is exerted by age and maturational status, with baseline levels and responsiveness to stimuli increasing in adolescents compared with early-pubertal children and then decreasing again in older individuals (6, 19, 49).
The above-mentioned considerations suggest that alterations in the GH response to exercise may become progressively more pronounced in children with obesity of greater severity and that this dose-response pattern may persist through the general increase in GH responses with sexual maturation. However, no direct documentation concerning the interaction of pubertal status and regulation of GH response to exercise in progressively increasing stages of juvenile obesity has been published. We therefore designed the present study in which GH levels were measured in early- and late-pubertal children with normal weight or obesity of increasing severity after a standardized intermittent exercise challenge simulating real-life spontaneous physical activity.
MATERIALS AND METHODS
All aspects of the studies were conducted at the University of California, Irvine (UCI), Institute for Clinical and Translational Science (ICTS) by specialized personnel. The UCI Institutional Review Board approved all protocols.
A total of 113 children of both sexes, aged 9–17 yr, underwent preliminary screening. To clearly separate groups by obesity and maturational status, we dismissed 23 children between 12 and 14 yr of age or with body mass index (BMI) between the 85th and 95th percentiles from the study. The final study subject pool therefore consisted of 90 children, of which 44 were late pubertal (15.8 ± 0.2 yr old, 23 girls, Tanner stage 4.1 ± 0.1) and 46 were early pubertal (10.4 ± 0.2 yr old, 24 girls, Tanner stage 2.0 ± 0.1; Table 1).
Table 1.
Demographics
| Early Pubertal |
Late Pubertal |
|||
|---|---|---|---|---|
| Obese | Healthy | Obese | Healthy | |
| Age, yr | 10.5 ± 0.2 | 10.2 ± 0.3 | 15.4 ± 0.3 | 16.2 ± 0.3 |
| Sex, M/F | 14/12 | 8/12 | 11/11 | 10/12 |
| Tanner stage | 2.2 ± 0.2 | 1.8 ± 0.2 | 4.0 ± 0.2 | 4.3 ± 0.1 |
| Height, cm | 146 ± 2 | 143 ± 3 | 169 ± 2 | 168 ± 1 |
| Body wt, kg | 59.1 ± 2.7 | 37.1 ± 2.2* | 90.8 ± 3.6 | 59.9 ± 1.5* |
| BMI | ||||
| kg/m2 | 27.5 ± 0.8 | 17.7 ± 0.5* | 31.9 ± 1.2 | 21.1 ± 0.4* |
| Percentile | 97.8 ± 0.3 | 57.2 ± 5.4* | 97.5 ± 0.3 | 53.2 ± 4.6* |
| Whole body fat, % | 41.2 ± 1.4 | 23.6 ± 1.5* | 36.1 ± 1.9 | 21.7 ± 2.5* |
Values are means ± SE. BMI, body mass index.
P < 0.05 vs. corresponding obese group.
Preliminary visit.
All subjects and respective parents/guardians reported to the UCI ICTS and were fully informed of all procedures and possible risks and signed consent/assent forms.
A medical history and physical examination ensured that all participants were in good health, as documented by no history of recent or chronic pathology (including severe childhood illnesses, diabetes, asthma, recent common colds, or minor injuries). Participants were also not taking prescription or over-the-counter medications and displayed vital signs within normal limits. To confirm a balanced composition of study groups with respect to maturational status, we determined Tanner stage via a validated standard questionnaire (38). Individual BMI percentile was then determined utilizing the most recent Centers for Disease Control criteria (26). Forty-two children comprised the normal weight (NW) group (24 girls, BMI <85th percentile, mean 55.1 ± 3.5 percentile). Forty-eight children comprised the obese (OW) group (23 girls, BMI >95th percentile, mean 97.7 ± 0.2 percentile) and were further subdivided into three tertiles on the basis of increasing BMI: 16 (5 girls) with BMI between the 95th and 97th percentile; 15 (7 girls) with BMI between the 97th and 98.5th percentile, and 17 (11 girls) with BMI >98.5th percentile. Body fat was measured by dual-energy X-ray absorptiometry (QDR 4500 densitometer, Hologic, Bedford, MA).
All participants completed a preliminary cycling test (Ergoline 800S, SensorMedics, Yorba Linda, CA) conducted by an exercise physiologist from the UCI ICTS. After 2 min of unloaded pedaling, the resistance on the ergometer was increased by 10–20 W/min (∼10% of individually predicted maximal workload/min) until subjects were unable to sustain pedaling (12). Breath-by-breath gas exchange was measured by a standard metabolic cart (SensorMedics), and the anaerobic threshold (AT, a level beyond which cellular aerobic energy production must be accompanied by anaerobic processes) and maximal aerobic capacity [maximal O2 consumption (V̇o2 max) , the gold-standard indicator of overall fitness for adults and children] were determined.
Main study visit.
At ≥48 h after the preliminary visit, subjects were admitted to the UCI ICTS at ∼8 AM after an overnight fast (during which they were permitted to consume water ad libitum). Upon admission, vital signs were obtained to confirm healthy status, and an intravenous line was inserted on the left forearm at the median cubital vein for easy access for phlebotomy. To limit the effect of stress associated with catheter insertion, participants rested for 45 min before the exercise protocol was started.
Thirty-minute exercise protocol.
Subjects performed ten 2-min cycling bouts separated by 1-min rest intervals. During each bout, the work rate was midway between individual predetermined AT and V̇o2 max, which corresponded on average to ∼80% V̇o2 max. This work intensity was chosen because it corresponds to a work rate approximately midway between AT and V̇o2 max that significantly activates growth factor and other physiological neuroendocrine and metabolic adaptive responses to exercise (10, 19). The intermittent pattern renders the challenge more acceptable to children and more closely mimics spontaneous physical activity (2); it has therefore become a successful standard research protocol for thousands of studies involving children at the UCI ICTS. Blood samples were drawn at baseline and immediately after the end of exercise in sterile EDTA Vacutainer tubes (BD Diagnostics, Franklin Lakes, NJ) and centrifuged at 300 rpm for 15 min. Plasma glucose concentrations were determined immediately in triplicate using a glucose analyzer (Glucose II Analyzer, Beckman Coulter, Fullerton, CA); aliquots of the remaining plasma were frozen in smaller tubes at −80°C until assay.
Laboratory procedures.
Samples were thawed and processed at the UCI ICTS Bioassay Laboratory as follows. GH was measured by human GH ELISA DSL-10-1900 (Diagnostic Systems Laboratories, Webster, TX). GH standards were prepared by the manufacturer according to World Health Organization specifications (directive 88/505) in human serum with a non-mercury-containing preservative. Intra-assay coefficient of variation (CV) was 3.3–4.3%, interassay CV was 6.3–6.6%, and assay sensitivity was 0.03 ng/ml. IGF-I was measured by IGF-I ELISA DSL-10-5600 (Diagnostic Systems Laboratories). Intra-assay CV was 4.5–7.1%, interassay CV was 4.8–8.8%, and assay sensitivity was 0.03 ng/ml. Insulin levels in plasma were determined by a human insulin ELISA kit (EZHI-14K, Linco Research, St. Charles, MO) after extraction by the acid-ethanol method. Intra-assay CV was 4.6–7.0%, interassay CV was 9.1–11.4%, and assay sensitivity was 2 μU/ml.
Statistics.
Subjects' demographic characteristics and circulating variables are presented as group means ± SE. Comparisons of subgroups with different maturational status and BMI percentile categories were performed by Student's t-tests and considered statistically significant when P < 0.05 or appropriate Bonferroni correction for multiple comparisons was detected.
RESULTS
Effect of increasing BMI on the GH exercise response.
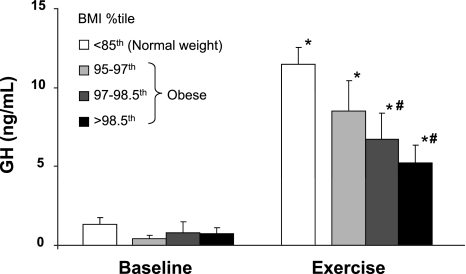
GH concentrations were similar in all groups of children at rest (1.4 ± 0.4 ng/ml in NW and 0.5 ± 0.2, 0.9 ± 0.7, and 0.5 ± 0.2 ng/ml in 95th-97th, 97th-98.5th, and >98.5th BMI percentiles, respectively), and all significantly increased over baseline at the end of the exercise challenge. The response was greater, however, in normal-weight than in obese children (11.5 ± 1.0 vs. 6.5 ± 0.9 ng/ml, P < 0.01). Within the obese group, the degree of blunting followed a dose-dependent pattern with increasing BMI: 8.5 ± 2.0 ng/ml in the tertile with the lowest BMI, 6.5 ± 1.4 ng/ml in the middle tertile (P < 0.01 vs. NW), and 5.3 ± 1.3 ng/ml in the tertile with the highest BMI (P < 0.001 vs. NW; Fig. 1).
Fig. 1.
Baseline and end-exercise growth hormone (GH) concentrations in 1 group of normal-weight children and 3 groups of obese children with progressively greater body mass index (BMI) percentile. *P < 0.01 vs. corresponding baseline value. #P < 0.01–0.001 vs. normal-weight group.
Effect of maturational status on the GH exercise response.
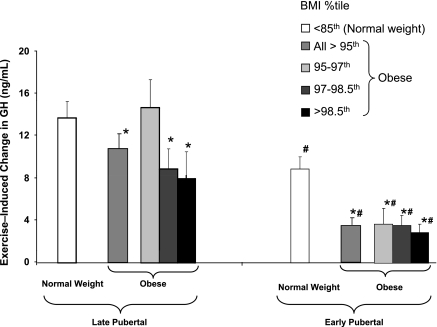
Within the normal-weight group, the GH exercise response was significantly smaller in early- than late-pubertal children (8.9 ± 1.2 vs. 13.8 ± 1.5 ng/ml, P < 0.02). This pattern was maintained in obese children, among whom the magnitude of the blunting of the responses was even more pronounced in early- than late-pubertal children (3.4 ± 0.7 vs. 10.3 ± 1.4 ng/ml, P < 0.001; Fig. 2).Furthermore, the obese late- and early-pubertal groups displayed a significantly reduced response compared with their corresponding normal-weight group. It should be noted that although baseline blood lactate was slightly elevated in obese compared with normal-weight children (2.3 vs. 1.6 mmol/l, P < 0.001), the blood lactate exercise response between all BMI groups in children with the same maturational status was comparable (P > 0.05; Table 2).
Fig. 2.
Exercise-induced GH responses in 1 group of normal-weight children and 3 groups of obese children with progressively greater BMI percentile, subdivided by maturational status into late- and early-pubertal subgroups (mean Tanner stages ∼4 and ∼2, respectively). *P < 0.05 vs. corresponding normal-weight group. #P < 0.05 vs. corresponding late-pubertal group.
Table 2.
Plasma glucose, insulin, IGF-I, and lactate
| Resting Glucose, mg/dl |
Resting Insulin, μU/ml |
Resting IGF-I, pg/ml |
Postexercise Lactate, mmol/l |
|||||
|---|---|---|---|---|---|---|---|---|
| BMI Percentile | Late pubertal | Early pubertal | Late pubertal | Early pubertal | Late pubertal | Early pubertal | Late pubertal | Early pubertal |
| <85 | 88 ± 2 | 89 ± 1 | 6.4 ± 0.7 | 4.9 ± 0.6 | 570 ± 40 | 379 ± 45† | 6.2 ± 0.5 | 2.7 ± 0.4† |
| 95–97 | 96 ± 3* | 91 ± 2 | 9.2 ± 1.4 | 13.9 ± 2.7* | 584 ± 51 | 366 ± 47† | 5.7 ± 1.0 | 2.1 ± 0.5† |
| 97–98.5 | 93 ± 4 | 92 ± 2 | 16.1 ± 1.7* | 8.3 ± 1.0*† | 559 ± 64 | 370 ± 107 | 6.2 ± 0.7 | 3.1 ± 1.3† |
| >98.5 | 110 ± 9* | 93 ± 2† | 15.3 ± 2.5* | 12.5 ± 1.4* | 481 ± 96 | 377 ± 28 | 5.9 ± 0.3 | 2.8 ± 0.3† |
Values are means ± SE. IGF-I, insulin-like growth factor I.
P < 0.05 vs. corresponding normal-weight (BMI <85th percentile) children.
P < 0.05 vs. corresponding late-pubertal children.
Because of the marked differences between early- and late-pubertal children, we proceeded to analyze the effect of increasing BMI on the exercise GH response within each maturational group. In general, the pattern of a dose-response relationship between increasing BMI and blunting of GH, which was observed in the groups as a whole, persisted after the children were divided by pubertal status. Among late-pubertal children, the exercise GH response for the normal-weight group was 13.8 ± 1.5 ng/ml. The GH response was similar (14.7 ± 2.7 ng/ml) in the tertile of obese children with the lowest BMI, 8.9 ± 1.9 ng/ml in the middle tertile (P < 0.05 vs. NW), and 8.0 ± 2.5 ng/ml in the highest tertile (P < 0.05 vs. NW; Fig. 2). In this group, therefore, blunting of the GH response did not begin until the middle tertile, approximately when BMI exceeded the 97th percentile. Among early-pubertal children, on the other hand, the exercise GH response was markedly blunted in all obese tertiles compared with the normal-weight response of 8.9 ± 1.2 ng/ml: 3.7 ± 1.5 ng/ml in the tertile of obese children with the lowest BMI (P < 0.01 vs. NW), 3.5 ± 0.9 ng/ml in the middle tertile (P < 0.01 vs. NW), and 2.9 ± 0.9 ng/ml in the highest tertile (P < 0.005 vs. NW). In this group, despite marked suppression of the GH response, a dose-response pattern was somewhat maintained, albeit with relatively small differences among subgroups.
Plasma glucose, insulin, and IGF-I.
In resting conditions, there was no difference in IGF-I that could be ascribed to increased BMI; however, IGF-I concentrations were greater in late-pubertal than in corresponding early-pubertal children [albeit only reaching statistical significance (P < 0.01) in NW and the lowest obese tertile]. All subjects displayed small, nonsignificant increases in systemic IGF-I concentrations after exercise; no significant differences across subgroups were detected.
As expected, plasma glucose and insulin concentrations were significantly greater in overweight children than in normal-weight controls in early- and late-pubertal groups. Within each maturational group, glycemia appeared to display a dose-dependent pattern relative to increasing BMI; this effect was less clear for insulin, especially in the early-pubertal group (Table 2).
DISCUSSION
The main finding of our study is that, in a cohort of obese children (BMI >95th percentile), who as a whole displayed significantly blunted GH responses to a standard exercise challenge compared with age-matched controls, the degree of blunting was proportional to the severity of obesity. This pattern was maintained in early- and late-pubertal children, while, in general, late-pubertal children displayed greater GH responses, independent of their BMI status.
Physical activity of even very moderate intensity results in manifold increases in GH (14, 18); as children spontaneously engage in repeated, often intense exercise patterns (2), their body is naturally exposed to a series of GH peaks that substantially add to the circadian, baseline GH production. Growing evidence indicates that these repeated GH peaks are physiologically meaningful, modulating key aspects of systemic growth (11, 33). In animal models, the whole body rate of growth was observed to be enhanced by physical activity and paralleled by increased frequency and amplitude of the GH pulse (24). In humans, insufficient exercise during childhood has been associated with later osteoporosis and sarcopenia through reduced bone mineralization (32) and muscle mass development (43). The role of the GH-IGF-I axis in this context is documented by the close correlation among systemic concentrations of these hormones, fitness, and muscle mass in children, adolescents, and adults (15, 39, 48). These multiple lines of evidence suggest that exercise-induced GH responses are likely to play a relevant role in physiological growth and development; conversely, conditions, such as pediatric obesity, that can alter acutely or chronically the pattern of GH response to physical activity during childhood may disrupt this physiological pattern of effects mediated by the GH-IGF-I axis. Although documentation of these long-term effects was obviously beyond the scope of our work, our findings are clearly consistent with these concepts.
Interestingly, despite the documented presence of marked sex effects in multiple aspects of physiological adaptation to stress in general and exercise in particular, no sex differences were observed in the present study. Inasmuch as these experiments were not powered to detect such differences, we cannot exclude the possibility that they could be revealed by a larger subject pool; however, the marked homogeneity across sexes of the blunting of GH responses seems to indicate that obesity-related, downstream effects of this altered GH-IGF-I modulation may indeed be sex independent.
Age also appears to have a strong modulatory effect on the magnitude of the GH response to exercise, initially during the transition from childhood to adolescence and later abating through adulthood toward old age. In this context, our data are in agreement with prior reports comparing GH responses in early- and late-pubertal healthy children challenged with various exercise formats [e.g., 15 min of continuous exercise at 70% V̇o2 max (51) and 30 min at ∼95% of individual ventilatory threshold (40)]; similar to our observations, the GH response to exercise was consistently greater in older children. However, our results also show, for the first time, that, within this general late-pubertal enhancement, obesity systematically attenuates exercise-induced GH secretion in a dose-dependent fashion. Later in life, GH responses to exercise are known to become gradually reduced, as clearly documented by a series of studies reporting that peak GH secretion after similar exercise is higher in children than in adults (6) and in young adults (early 20s) than in 40- to 50-yr-old (19) and 60- to 70-yr-old (49) men. To our knowledge, studies in which the effects of obesity on exercise GH responses are compared between pediatric and adult populations have not been published.
The three subgroups into which our obese children were divided may appear deceivingly small, each encompassing 1.5–2 BMI percentile units. In reality, because of the recent exponential increase in pediatric obesity, the number of children with BMI >95th percentile is very large, estimated to range from 16% to >30% of all US children and adolescents (21, 27, 34). Therefore, each of the three subgroups with BMI >95th percentile is likely to include 5–10% of the US pediatric population. Also, inasmuch as BMI percentile values are based on a Gaussian distribution of absolute BMI values, the differences in absolute values are much greater at the end of the curve than in the middle. For instance, in an 8-yr-old boy who is 4 ft 6 in. tall, a shift of BMI from the 50th to the 51st percentile would imply a weight gain of only 0.2 pounds (from 66.3 to 66.5 pounds), or 0.1 BMI unit (from 16.0 to 16.1 BMI units). In a boy of the same age and height, a shift from the 96th to the 97th BMI percentile would require a weight gain of 9 pounds (from 87 to 96 pounds), or 2.2 BMI units (from 21.0 to 23.2 BMI units). Even larger weight gains would be needed to reach the 98th or 99th percentile, underscoring the possible clinical relevance of the apparently small BMI percentile changes at the very upper end of the range. It should also be remembered that, although BMI is extremely useful and widely used, it is still an indirect and imperfect index of adiposity: high BMI values could theoretically be recorded in overly muscular, lean individuals. In our study, however, dual-energy X-ray absorptiometry data confirmed that true excessive adiposity indeed paralleled measured BMI values, as our data on subject percent body fat clearly indicate (Table 1). In fact, body fat percentage displayed the strongest individual correlation with GH responses among the variables tested in this study, further supporting the robustness of our observations.
Definition of the pathogenetic mechanisms underlying our observations was beyond the scope of our experimental design. Our results, however, are compatible with at least three modulating mechanisms for GH secretion: two are associated with increased concentrations of free fatty acids (FFAs), which have been reported to suppress GH release, either through a direct effect on pituitary somatotrophs (likely through FFA interference with integral proteins in the cellular lipid bilayer) (8, 36, 37) or indirectly through a hypothalamic-mediated pathway, involving the release of somatostatin (4, 23, 29, 30); a third mechanism involves GH-insulin interaction. Although obesity is not necessarily always associated with hyperlipidemia, this is commonly the case (50), and, indeed, in our study, average FFA concentrations were 372 ± 35 μM in normal-weight children and 547 ± 49 μM in obese children. The contributory role of FFAs is supported by the observation that when lipolysis is pharmacologically prevented during an antecedent exercise bout, subsequent GH secretion is not suppressed; in fact, it is increased, even if FFA concentrations drop below preexercise levels (46). Furthermore, even in normal-weight subjects, acute increases in FFA through ingestion of a high-fat meal 30–45 min before exercise can reduce the GH response by 40–50%. This was first demonstrated in adults performing a 10-min exhaustive exercise bout (7) and more recently confirmed in our laboratory in children performing an intermittent exercise protocol identical to that used in the present study (16). These observations suggest that not only chronic dyslipidemia associated with pediatric obesity, but also excessive fat consumption before physical activity in children with healthy body weight, may alter the physiological release of growth factors during physical activity. The latter scenario may be especially pertinent to the Western society lifestyle, in which feeding children a high-fat fast-food meal purchased on the way to sports practice is a very common event. Insulin is also known to potentially modulate GH function through signaling cross talk, inasmuch as several intracellular signaling pathways (e.g., phosphatidylinositol 3-kinase and ERK1/2) are activated by the GH and insulin receptors (53). Prolonged hyperinsulinemia, in particular, has been shown to inhibit GH-induced signaling at the receptor and postreceptor level (52). In our study, in fact, plasma insulin was, in general, markedly elevated in obese compared with normal-weight children. The level of hyperinsulinemia, however, did not follow a clear dose-response pattern with increasing BMI, especially in prepubertal children, suggesting that it may have contributed only in part to the reported effects on GH secretion.
The exercise protocol used in this study was carefully developed by our research group to reproduce the type of exertion and adaptive responses that may be expected during a soccer/basketball practice or recreational-level match. Therefore, the repeat pattern of individual bouts and their relative intensity render any findings derived from its application likely applicable to everyday life. In the specific context of GH responses, however, children may be exposed to a different range of exercise formats, especially if they are participating in elite sport divisions; although we have no reason to expect that the effect of obesity or dyslipidemia on the GH response to these more intense and prolonged exercise challenges would be different, this will have to be demonstrated by specifically designed, future studies.
The choice of studying subgroups of children clearly separated by maturational status and presence of obesity (i.e., not including children 12–14 yr old or those with BMI between 85th and 95th percentiles) was made with the intent of maximally reducing possible confounding factors in data interpretation. It may be argued, however, that not having a “continuous” data set may have reduced the extent of potential information obtainable through the reported experiments. For instance, additional information could have been gained through regression analysis, such as the relative, progressive contribution of multiple covariates (age, Tanner stage, BMI, and fitness) in explaining the variation of the GH response to exercise over the whole age range of our subject pool. We believe the straightforwardness of our somewhat simpler statistical approach, however, helped keep our main message clear and to the point. Another design issue requiring attention is that our sampling schedule was limited to pre- and end-exercise measurements. Technically, our observation of lower end-exercise GH peaks in obese children could also be explained with a different GH time course: GH in obese children could have peaked earlier and started to return to preexercise levels by end exercise. This trend could only have been detected by multiple blood draws a few minutes apart, during and after exercise, which logistical and ethical considerations (limited blood draw volume allowed in children) unfortunately did not allow us to implement. However, several earlier studies support the concept that exercise-induced GH peaks occur consistently at the end of exercise. This has been demonstrated during 30-min exercise protocols (same duration as in our study) over a range of exercise intensities below and above the AT (41). Furthermore, in adults with both upper and lower body obesity, GH responses to 30 min of exercise at 70% V̇o2 max were significantly blunted compared with healthy controls, again with GH peaks occurring in all subjects immediately at end exercise (25). We therefore believe that although the possibility that our obese subjects had an altered GH time course cannot be completely excluded, this is unlikely, and our data reflect a genuine GH suppression in obese children.
In conclusion, in a cohort of peripubertal obese children, the GH response to exercise was markedly reduced compared with age-matched controls, with the degree of blunting increasing with greater severity of obesity. Although overall the GH response to exercise was greater in late-pubertal than in younger children, this blunting pattern was observed in early- and late-pubertal children. Our observations provide a strong rationale for future studies aimed at defining the impact of altered GH homeostasis in pediatric obesity and underscore the necessity of optimizing physical activity strategies in specific subject subgroups, such that the expected health benefits of exercise can be maximized.
GRANTS
This project was funded by National Institutes of Health Grants M01-RR-00827-28 and P01 HD-048721-01A1.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
These findings would not have been achievable without the extraordinary work by all the UCI ICTS nursing staff (Barbara Bodenhoefer, A. Diane Capobianco, Connie Parido, and Minh Phan), laboratory personnel and specialists (Georgia Bachman, Antonio Zambrano, Bridgette Duarte, Milagros Ibardolaza, and Frank Zaldivar), and administrators.
REFERENCES
- 1. Arvat E, Maccario M, Di Vito L, Broglio F, Benso A, Gottero C, Papotti M, Muccioli G, Dieguez C, Casanueva FF, Deghenghi R, Camanni F, Ghigo E. Endocrine activities of ghrelin, a natural growth hormone secretagogue (GHS), in humans: comparison and interactions with hexarelin, a nonnatural peptidyl GHS, and GH-releasing hormone. J Clin Endocrinol Metab 86: 1169–1174, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Berman N, Bailey R, Barstow TJ, Cooper DM. Spectral and bout detection analysis of physical activity patterns in healthy, prepubertal boys and girls. Am J Hum Biol 10: 289–297, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Brandou F, Aloulou I, Razimbaud A, Fedou C, Mercier J, Brun JF. Lower ability to oxidize lipids in adult patients with growth hormone (GH) deficiency: reversal under GH treatment. Clin Endocrinol (Oxf) 65: 423–428, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Brazeau P, Vale W, Burgus R, Ling N, Butcher M, Rivier J, Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science 179: 77–79, 1973 [DOI] [PubMed] [Google Scholar]
- 5. Butler AA, Roith DL. Control of growth by the somatropic axis: growth hormone and the insulin-like growth factors have related and independent roles. Annu Rev Physiol 63: 141–164, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Cappa M, Bizzarri C, Martinez C, Porzio O, Giannone G, Turchetta A, Calzolari A. Neuroregulation of growth hormone during exercise in children. Int J Sports Med 21 Suppl 2: S125–S128, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Cappon JP, Ipp E, Brasel JA, Cooper DM. Acute effects of high fat and high glucose meals on the growth hormone response to exercise. J Clin Endocrinol Metab 76: 1418–1422, 1993 [DOI] [PubMed] [Google Scholar]
- 8. Casanueva FF, Villanueva L, Dieguez C, Diaz Y, Cabranes JA, Szoke B, Scanlon MF, Schally AV, Fernandez-Cruz A. Free fatty acids block growth hormone (GH) releasing hormone-stimulated GH secretion in man directly at the pituitary. J Clin Endocrinol Metab 65: 634–642, 1987 [DOI] [PubMed] [Google Scholar]
- 9. Collier SR, Casey DP, Kanaley JA. Growth hormone responses to varying doses of oral arginine. Growth Horm IGF Res 15: 136–139, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Cooper DM, Barstow TJ, Bergner A, Lee WN. Blood glucose turnover during high- and low-intensity exercise. Am J Physiol Endocrinol Metab 257: E405–E412, 1989 [DOI] [PubMed] [Google Scholar]
- 11. Cooper DM, Nemet D, Galassetti P. Exercise, stress, and inflammation in the growing child: from the bench to the playground. Curr Opin Pediatr 16: 286–292, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Cooper DM, Weiler-Ravell D, Whipp BJ, Wasserman K. Aerobic parameters of exercise as a function of body size during growth in children. J Appl Physiol 56: 628–634, 1984 [DOI] [PubMed] [Google Scholar]
- 13. Dieguez C, Casanueva FF. Influence of metabolic substrates and obesity on growth hormone secretion. Trends Endocrinol Metab 6: 55–59, 1995 [DOI] [PubMed] [Google Scholar]
- 14. Eliakim A, Nemet D, Zaldivar F, McMurray RG, Culler FL, Galassetti P, Cooper DM. Reduced exercise-associated response of the GH-IGF-I axis and catecholamines in obese children and adolescents. J Appl Physiol 100: 1630–1637, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Eliakim A, Scheett TP, Newcomb R, Mohan S, Cooper DM. Fitness, training, and the growth hormone→insulin-like growth factor I axis in prepubertal girls. J Clin Endocrinol Metab 86: 2797–2802, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Galassetti P, Larson J, Iwanaga K, Salsberg SL, Eliakim A, Pontello A. Effect of a high-fat meal on the growth hormone response to exercise in children. J Pediatr Endocrinol Metab 19: 777–786, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Galassetti P, Mann S, Tate D, Neill RA, Costa F, Wasserman DH, Davis SN. Effects of antecedent prolonged exercise on subsequent counterregulatory responses to hypoglycemia. Am J Physiol Endocrinol Metab 280: E908–E917, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Galassetti PR, Iwanaga K, Crisostomo M, Zaldivar FP, Larson J, Pescatello A. Inflammatory cytokine, growth factor and counterregulatory responses to exercise in children with type 1 diabetes and healthy controls. Pediatr Diabetes 7: 16–24, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Gilbert KL, Stokes KA, Hall GM, Thompson D. Growth hormone responses to 3 different exercise bouts in 18- to 25- and 40- to 50-year-old men. Appl Physiol Nutr Metab 33: 706–712, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Hartman ML, Faria AC, Vance ML, Johnson ML, Thorner MO, Veldhuis JD. Temporal structure of in vivo growth hormone secretory events in humans. Am J Physiol Endocrinol Metab 260: E101–E110, 1991 [DOI] [PubMed] [Google Scholar]
- 21. Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA 291: 2847–2850, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Hussain MA, Schmitz O, Mengel A, Glatz Y, Christiansen JS, Zapf J, Froesch ER. Comparison of the effects of growth hormone and insulin-like growth factor I on substrate oxidation and on insulin sensitivity in growth hormone-deficient humans. J Clin Invest 94: 1126–1133, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Imaki T, Shibasaki T, Masuda A, Hotta M, Yamauchi N, Demura H, Shizume K, Wakabayashi I, Ling N. The effect of glucose and free fatty acids on growth hormone (GH)-releasing factor-mediated GH secretion in rats. Endocrinology 118: 2390–2394, 1986 [DOI] [PubMed] [Google Scholar]
- 24. Kaji H, Asanuma Y, Ide H, Saito N, Hisamura M, Murao M, Yoshida T, Takahashi K. The auto-brewery syndrome—the repeated attacks of alcoholic intoxication due to the overgrowth of Candida (albicans) in the gastrointestinal tract. Mater Med Pol 8: 429–435, 1976 [PubMed] [Google Scholar]
- 25. Kanaley JA, Weatherup-Dentes MM, Jaynes EB, Hartman ML. Obesity attenuates the growth hormone response to exercise. J Clin Endocrinol Metab 84: 3156–3161, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Adv Data 314: 1–27, 2000 [PubMed] [Google Scholar]
- 27. Levi J, Trust for America's Health F as in Fat: How Obesity Policies Are Failing in America 2009. Washington, DC: Trust for America's Health, 2009 [Google Scholar]
- 28. Maccario M, Grottoli S, Procopio M, Oleandri SE, Rossetto R, Gauna C, Arvat E, Ghigo E. The GH/IGF-I axis in obesity: influence of neuro-endocrine and metabolic factors. Int J Obes Relat Metab Disord 24 Suppl 2: S96–S99, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Maccario M, Procopio M, Grottoli S, Oleandri SE, Boffano GM, Taliano M, Camanni F, Ghigo E. Effects of acipimox, an antilipolytic drug, on the growth hormone (GH) response to GH-releasing hormone alone or combined with arginine in obesity. Metab Clin Exp 45: 342–346, 1996 [DOI] [PubMed] [Google Scholar]
- 30. Maccario M, Procopio M, Loche S, Cappa M, Martina V, Camanni F, Ghigo E. Interaction of free fatty acids and arginine on growth hormone secretion in man. Metab Clin Exp 43: 223–226, 1994 [DOI] [PubMed] [Google Scholar]
- 31. Maheshwari HG, Bouillon R, Nijs J, Oganov VS, Bakulin AV, Baumann G. The impact of congenital, severe, untreated growth hormone (GH) deficiency on bone size and density in young adults: insights from genetic GH-releasing hormone receptor deficiency. J Clin Endocrinol Metab 88: 2614–2618, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Mora S, Gilsanz V. Establishment of peak bone mass. Endocrinol Metab Clin North Am 32: 39–63, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Nemet D, Cooper DM. Exercise, diet, and childhood obesity: the GH-IGF-I connection. J Pediatr Endocrinol Metab 15 Suppl 2: 751–757, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003–2006. JAMA 299: 2401–2405, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA 288: 1728–1732, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Peino R, Cordido F, Penalva A, Alvarez CV, Dieguez C, Casanueva FF. Acipimox-mediated plasma free fatty acid depression per se stimulates growth hormone (GH) secretion in normal subjects and potentiates the response to other GH-releasing stimuli. J Clin Endocrinol Metab 81: 909–913, 1996 [DOI] [PubMed] [Google Scholar]
- 37. Perez FR, Casabiell X, Camina JP, Zugaza JL, Casanueva FF. cis-Unsaturated free fatty acids block growth hormone and prolactin secretion in thyrotropin-releasing hormone-stimulated GH3 cells by perturbing the function of plasma membrane integral proteins. Endocrinology 138: 264–272, 1997 [DOI] [PubMed] [Google Scholar]
- 38. Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolesc 17: 117–133, 1988 [DOI] [PubMed] [Google Scholar]
- 39. Poehlman ET, Copeland KC. Influence of physical activity on insulin-like growth factor-I in healthy younger and older men. J Clin Endocrinol Metab 71: 1468–1473, 1990 [DOI] [PubMed] [Google Scholar]
- 40. Pomerants T, Tillmann V, Karelson K, Jurimae J, Jurimae T. Impact of acute exercise on bone turnover and growth hormone/insulin-like growth factor axis in boys. J Sports Med Phys Fitness 48: 266–271, 2008 [PubMed] [Google Scholar]
- 41. Pritzlaff CJ, Wideman L, Weltman JY, Abbott RD, Gutgesell ME, Hartman ML, Veldhuis JD, Weltman A. Impact of acute exercise intensity on pulsatile growth hormone release in men. J Appl Physiol 87: 498–504, 1999 [DOI] [PubMed] [Google Scholar]
- 42. Rosenfeld RG, Rosenbloom AL, Guevara-Aguirre J. Growth hormone (GH) insensitivity due to primary GH receptor deficiency. Endocr Rev 15: 369–390, 1994 [DOI] [PubMed] [Google Scholar]
- 43. Roubenoff R. Sarcopenia: effects on body composition and function. J Gerontol A Biol Sci Med Sci 58: 1012–1017, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Stokes K, Nevill M, Frystyk J, Lakomy H, Hall G. Human growth hormone responses to repeated bouts of sprint exercise with different recovery periods between bouts. J Appl Physiol 99: 1254–1261, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Stokes KA, Nevill ME, Hall GM, Lakomy HK. Growth hormone responses to repeated maximal cycle ergometer exercise at different pedaling rates. J Appl Physiol 92: 602–608, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Stokes KA, Tyler C, Gilbert KL. The growth hormone response to repeated bouts of sprint exercise with and without suppression of lipolysis in men. J Appl Physiol 104: 724–728, 2008 [DOI] [PubMed] [Google Scholar]
- 47. Takahashi Y, Kipnis DM, Daughaday WH. Growth hormone secretion during sleep. J Clin Invest 47: 2079–2090, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tirakitsoontorn P, Nussbaum E, Moser C, Hill M, Cooper DM. Fitness, acute exercise, and anabolic and catabolic mediators in cystic fibrosis. Am J Respir Crit Care Med 164: 1432–1437, 2001 [DOI] [PubMed] [Google Scholar]
- 49. Weltman A, Weltman JY, Roy CP, Wideman L, Patrie J, Evans WS, Veldhuis JD. Growth hormone response to graded exercise intensities is attenuated and the gender difference abolished in older adults. J Appl Physiol 100: 1623–1629, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, Sowers MR. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Arch Intern Med 168: 1617–1624, 2008 [DOI] [PubMed] [Google Scholar]
- 51. Wirth A, Trager E, Scheele K, Mayer D, Diehm K, Reischle K, Weicker H. Cardiopulmonary adjustment and metabolic response to maximal and submaximal physical exercise of boys and girls at different stages of maturity. Eur J Appl Physiol Occup Physiol 39: 229–240, 1978 [DOI] [PubMed] [Google Scholar]
- 52. Xu J, Ji S, Venable DY, Franklin JL, Messina JL. Prolonged insulin treatment inhibits GH signaling via STAT3 and STAT1. J Endocrinol 184: 481–492, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Xu J, Keeton AB, Franklin JL, Li X, Venable DY, Frank SJ, Messina JL. Insulin enhances growth hormone induction of the MEK/ERK signaling pathway. J Biol Chem 281: 982–992, 2006 [DOI] [PubMed] [Google Scholar]