Abstract
A miniature pinscher-cross was evaluated for chronic coughing. Computed tomography and bronchoscopy revealed severe, diffuse, cylindrical bronchiectasis secondary to eosinophilic bronchopneumopathy. Computed tomography is the gold standard for diagnosis of bronchiectasis in humans, and should be further investigated in dogs as a means of characterizing severity and pattern of disease.
Résumé
Bronchectasie cylindrique diffuse attribuable à la bronchopneumopathie éosinophile chez un chien. Un Pinscher-cross miniature a été évalué pour une toux chronique. Une tomodensitométrie et une bronchoscopie ont révélé une bronchectasie cylindrique diffuse secondaire à une bronchopneumopathie éosinophile. La tomodensitométrie est la méthodeétalon pour le diagnostic de la bronchectasie chez les humains et devrait faire l’objet de nouvelles recherches chez les chiens comme méthode de caractérisation de la gravité et de la tendance de la maladie.
(Traduit par Isabelle Vallières)
A 9-year-old male, intact miniature pinscher mixed breed dog was referred to the Purdue University School of Veterinary Medicine for evaluation of a chronic cough of approximately 1-year duration. The cough was productive, exacerbated by excitement, and had increased in severity over the past several months; occasional terminal gagging was also reported. Empiric treatment with several courses of antibiotics had not affected the frequency or severity of clinical signs. The dog had no previous significant medical history and had been receiving heartworm preventative therapy monthly. The dog was kept indoors except for supervised walks. The owners reported that they and the dog had moved into a new house at approximately the time coughing was first noted. Diffuse mold growth was found on several walls in the house at that time, and was treated by a professional cleaning company. The owner reported some respiratory symptoms as well.
Case description
On physical examination, the patient had increased expiratory effort and several severe coughing episodes. Cough was easily elicited by palpation of the trachea. Thoracic auscultation revealed severe crackles and expiratory wheezes over all lung fields. The remainder of the physical examination was unremarkable. Complete blood (cell) count, serum chemistry panel, and urinalysis were unremarkable. Fecal zinc sulfate flotation, Baerman sedimentation, and examination of a direct smear did not reveal any parasites or ova. Tests for heartworm (antigen and antibody) were negative. Primary differential diagnoses for the cough and other associated clinical signs at the time of diagnostic imaging studies included tracheal collapse, chronic bronchitis, eosinophilic bronchopneumopathy, heartworm disease, pulmonary parasite infection, bronchopneumonia, and pulmonary blastomycosis.
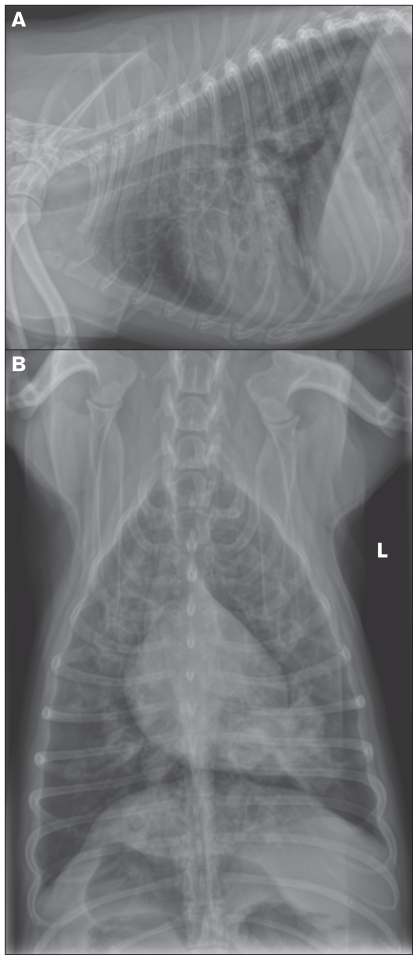
Thoracic radiographs revealed multiple rounded bullae-like structures, predominantly found in the ventral lung fields, consistent with diffuse, severe, cylindrical bronchiectasis (Figure 1). Rare tubular structures were also noted in the caudal and cranioventral lung fields and were suspected, based on location, to be soft tissue or fluid-filled bronchi. A discrete area of increased opacity with irregular margins in the left caudal lung lobe surrounding the associated bronchus was also noted. The diameter of the trachea at the level of the thoracic inlet was slightly reduced on the lateral view. Fluoroscopic examination of the neck and thorax confirmed Grade III-IV intra-thoracic tracheal collapse, primarily noted on expiration.
Figure 1.
Thoracic radiographs from a dog with chronic coughing demonstrate diffuse severe cylindrical bronchiectasis with occasional filling of bronchi with soft-tissue opacities. A — Left lateral view of the thorax. Bronchiectasis is most severe in the cranioventral lung lobes. B — Dorsoventral view. A soft-tissue opacity with irregular margins is present in the left caudal lung lobe.
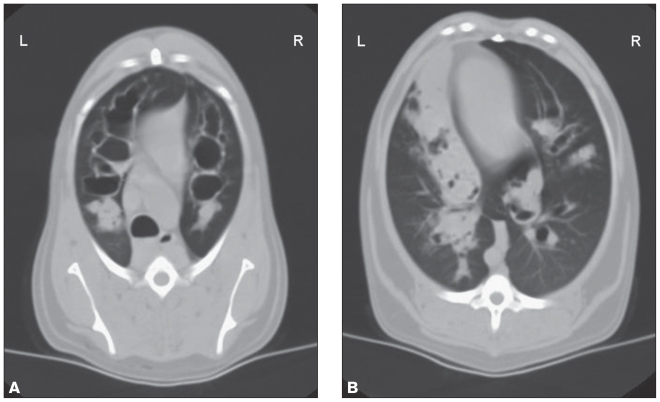
Computed tomographic (CT) examination of the thorax (3 mm collimation; HiSpeed Advantage-RP helical CT unit; GE Medical Systems, Waukesha, Wisconsin, USA) was performed to better characterize the left caudal opacity noted on thoracic radiographs, fully characterize the severity and extent of the bronchiectasis, and possibly identify concurrent pulmonary disease. Computed tomography revealed globally dilated air-filled bronchi, consistent with bronchiectasis, most severe in the cranial lung fields. Dilated bronchi were uniformly cylindrical in appearance with the most severely affected bronchi approximately 1.6 cm in diameter. Multiple dilated bronchi were filled with soft tissue-attenuating material, predominantly in the caudal lung fields (Figure 2). Bronchial:arterial ratios were calculated for 3 lobar bronchi (right cranial, cranial half of the left cranial, caudal half of the left cranial), and ranged from 3.5 to 5.0. The final radiologic diagnosis was diffuse, severe cylindrical bronchiectasis with multifocal complete to partially obstructive accumulations of fluid or tissue.
Figure 2.
Transverse CT images of the thorax. Severe, diffuse cylindrical bronchiectasis is present in all lung fields. Soft tissue-attenuating material is present in the dilated bronchi of the ventral lung fields. A — Transverse image of the cranial thorax. Severe bronchiectasis is evident in all lung lobes. B — Transverse image of the caudal lung fields. Multiple dilated bronchi are filled with soft tissue-attenuating material, most pronounced in the left hemithorax adjacent to the heart.
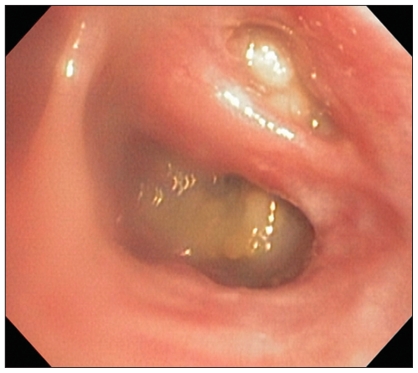
Bronchoscopy and bronchoalveolar lavage were performed [Q180 Video Bronchoscope (60 cm working length, 5.1 mm outer diameter); Olympus, Center Valley, Pennsylvania, USA] in an attempt to determine an underlying cause of the severe, diffuse bronchial disease. The tracheal lumen was of expected size and shape, but diffusely hyperemic and with markedly prominent vasculature. The carina and all inter-bronchial septae were moderately thickened and all the pulmonary mucosa was uniformly hyperemic. Secondary and tertiary bronchial lumen were approximately 50% collapsed and irregular in shape. Partially obstructive, pale green to yellow, mucoid to caseous mass-like lesions were seen in greater than 90% of lobar, secondary, and tertiary bronchi of all lung lobes (Figure 3); there was also increased mucus within secondary and tertiary bronchi that was clear to pale yellow with occasional globules of thicker yellow material. Bronchoalveolar lavage samples were collected from 3 different lung lobes and submitted for cytologic examination, and a sample from the right caudal lung lobe was submitted for aerobic, anaerobic, and fungal culture. Cytologic examination revealed eosinophilic inflammation (56% to 66% eosinophils, 22% to 44% neutrophils, 0% to 10% macrophages, 0% to 2% mast cells) in all lavage samples, with mild degeneration of most non-eosinophil inflammatory cells. Several clusters of cuboidal epithelial cells with intense basophilic cytoplasmic staining were noted, consistent with marked epithelial reactivity and hyperplasia. Rare Curschmann spirals were noted; no infectious agents were seen. Cultures did not result in any growth of organisms. A pinch biopsy sample of one of the mass-like lesions in the right caudal lung lobe was submitted for histopathologic examination, but no tissue was present following formalin fixation and tissue processing.
Figure 3.
Endoscopic image of the right caudal and accessory lung lobes. Partially to fully obstructive yellow-green mucopurulent material is evident in both lobar bronchi. Mild diffuse mucosal hyperemia is also evident.
The owner was re-questioned about possible allergen exposure (such as cigarette smoke, powdered or aerosolized carpet cleaners or air fresheners, cat litter) but no possible inciting cause of an allergic response was identified. The house was again inspected for mold but no traces were found. Repeat fecal examinations for parasites again failed to reveal any ova or organisms. The final diagnosis was eosinophilic bronchopneumopathy.
Initial treatment while culture results were pending consisted of amoxicillin-clavulanic acid, 12.5 mg/kg, PO, q12h, for 3 wk, prednisone at an anti-inflammatory dose, 0.5 mg/kg, PO, q12h, and fenbendazole, 50 mg/kg, PO, q24h, for 2 wk. The prednisone dose was then increased to 1 mg/kg, PO, q12h when results of the cultures and repeat fecal examinations became available. Two months after diagnosis the owner reported that the dog had an excellent response to therapy with a significant reduction of coughing episodes and an increased energy level. The owner had elected to taper and then discontinue the prednisone once clinical signs had significantly improved, but coughing recurred. Thoracic radiographs were repeated; although the severity of bronchiectasis was unchanged, fewer soft-tissue opacity-filled bronchi were noted. Prednisone was reinstated at an immunosuppressive dose, 1 mg/kg, PO, q12h, and the dog’s clinical signs again improved.
Discussion
Bronchiectasis is due to irreversible dilatation of bronchi following loss of bronchial wall structural integrity. This pathologic finding is a secondary, non-specific consequence of chronic, recurrent airway inflammation, secondary bacterial infections, and obstruction of bronchi (1). Bronchiectasis is rare in dogs, with a reported prevalence of 0.05% to 0.08% (1,2). Diseases which have been associated with bronchiectasis in dogs include eosinophilic bronchopneumopathy (EBP), pulmonary parasites, aspergillosis, bacterial pneumonia, neoplasia, allergic pneumonopathies, tracheal collapse, and heartworm disease (1–3).
Computed tomography is currently the gold standard for diagnosis of bronchiectasis in humans because of the relatively high sensitivity (84% to 90%), specificity, and ease with which pattern and extent of disease can be assessed (4,5). Several veterinary publications acknowledge that use of CT for evaluation of bronchiectasis would likely be superior to radiography in dogs and cats, and CT images of focal bronchiectasis in dogs have been published (1,6). Comparison of our radiographic and CT findings supports this claim, as the severity and distribution of bronchiectasis in this dog would have been underestimated without the latter imaging study.
Despite the preferred use of CT for diagnosis of bronchiectasis in human medicine, the sensitivity and specificity of chest radiography is similar to CT (87.8% and 74.4%, respectively); however, they may be insufficient to diagnose mild bronchiectasia (7). Nevertheless, unremarkable radiographs are sufficient to exclude clinically relevant bronchiectasis, and there is a linear relationship between the severity of bronchiectasis as determined by CT and the presence of radiographic abnormalities (7). Sensitivity and specificity of CT for diagnosis of bronchiectasis are also dependent on the type of scanner used. For example, multidetector CT using 1-mm slices is superior to 10-mm slices using high resolution computed tomography (HRCT) for determining presence and extent of bronchiectasis (8).
Four patterns of bronchiectasis have been described: cylindrical (the most common form in dogs, cats, and people), saccular, cystic, and varicose (1,2,9). The cylindrical pattern is due to uniform dilatation and loss of distal tapering of the largest, thick-walled bronchi, as was seen in the dog of this report. Saccular bronchiectasis has a “grape cluster” appearance resulting from circumscribed sacculations of bronchial walls at their terminal ends; intermediate-sized bronchi are primarily affected. Cystic bronchiectasis is thought to be an advanced stage of saccular bronchiectasis primarily involving the terminal bronchi (1,2). Varicose bronchiectasis, the only pattern not reported in dogs and cats, is due to dilatation of bronchi with circumferential constrictions resulting in a “beaded” appearance (1).
In people, diagnosis of bronchiectasis by CT is confirmed by comparison of bronchial diameter to that of the adjacent pulmonary artery; in healthy individuals the diameter of the bronchi and adjacent arteries should be approximately equal at any level of branching of the pulmonary tree (10). Whether or not the same relationship between these anatomic structures holds true in dogs has not been systematically studied. A maximal bronchial/arterial ratio of 2 has been suggested as a reasonable upper range in normal animals, and the measurements we obtained in the dog of this report were significantly larger than this value (6).
Eosinophilic bronchopneumopathy is one of the most common diseases associated with bronchiectasis in dogs, with 26% of patients with EBP demonstrating some degree of pathologic bronchial dilatation in 1 study (11). Diagnosis is by demonstration of an eosinophilic inflammatory cell infiltrate within respiratory tract cytological specimens (tracheal wash or bronchoalveolar lavage fluid). Our bronchoscopic findings of abundant yellow-green mucus, concreted mucopurulent material, and thickened bronchial mucosa are typical features of EBP (12). Although the etiology of primary EBP is unknown, a hypersensitivity to inhaled allergens is suspected (12). Suspected agents include fungi or mold, drugs, bacteria, and parasites. Persistent antigenic exposure is thought to result in chronic irritation of the tracheal and bronchial mucous membranes and inflammation, with eventual epithelial desquamation, hyperplasia of the mucous glands, and airway obstruction (12). These changes impair mucociliary clearance and predispose to secondary bacterial infections, ultimately resulting in bronchiectasis. The increase in intrapulmonary pressures created by coughing against the partially obstructed airways also contributes to destruction of the elastic and muscular layers of the bronchial wall.
The computed tomographic appearance of the various eosinophilic lung diseases which occur in people has been reviewed (13,14). Simple pulmonary eosinophilia, acute or chronic eosinophilic pneumonia, idiopathic hypereosinophilic syndrome, eosinophilic granulomata, and eosinophilic vasculitis, all have characteristic appearances on CT images (14). The clinical course and histopathologic changes seen in dogs with EBP share features with both chronic eosinophilic pneumonia and eosinophilic bronchitis (12). The most common CT lesion seen in humans with chronic eosinophilic pneumonia is peripheral air-space consolidation predominantly affecting the lung periphery; however, the inflammatory infiltrate, which occurs with eosinophilic bronchitis in humans, is confined to the upper respiratory tract and is not typically evaluated by CT. The authors are not aware of any study reporting the CT findings in dogs with eosinophilic bronchopneumopathy, and the dog in this report did not have lesions similar to those reported in humans. A systematic study of a larger number of patients is clearly needed before any conclusions can be drawn as to whether or not our CT findings represent a typical or atypical case of canine EBP. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office ( hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Marolf A, Blaik M. Bronchiectasis. Compend Contin Educ Small Anim Pract. 2006;28:766–775. [Google Scholar]
- 2.Hawkins EC, Basseches J, Berry CR, Stebbins ME, Ferris KK. Demographic, clinical, and radiographic features of bronchiectasis in dogs: 316 cases (1988–2000) J Am Vet Med Assoc. 2003;223:1628–1635. doi: 10.2460/javma.2003.223.1628. [DOI] [PubMed] [Google Scholar]
- 3.Marolf A, Blaik M, Specht A. A retrospective study of the relationship between tracheal collapse and bronchiectasis in dogs. Vet Radiol Ultrasound. 2007;48:199–203. doi: 10.1111/j.1740-8261.2007.00229.x. [DOI] [PubMed] [Google Scholar]
- 4.Kumar NA, Nguyen B, Maki D. Bronchiectasis: Current clinical and imaging concepts. Seminars in roentgenology. 2001;36:41–50. doi: 10.1053/sroe.2001.20462. [DOI] [PubMed] [Google Scholar]
- 5.King P, Holdsworth S, Freezer N, Holmes P. Bronchiectasis. Intern Med J. 2006;36:729–37. doi: 10.1111/j.1445-5994.2006.01219.x. [DOI] [PubMed] [Google Scholar]
- 6.Johnson EG, Wisner ER. Advances in respiratory imaging. Vet Clin North Am Small Anim Pract. 2007;37:879–900. vi. doi: 10.1016/j.cvsm.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 7.van der Bruggen-Bogaarts BA, van der Bruggen HM, van Waes PF, Lammers JW. Screening for bronchiectasis. A comparative study between chest radiography and high-resolution CT. Chest. 1996;109:608–611. doi: 10.1378/chest.109.3.608. [DOI] [PubMed] [Google Scholar]
- 8.Dodd JD, Souza CA, Muller NL. Conventional high-resolution CT versus helical high-resolution MDCT in the detection of bronchiectasis. Ajr. 2006;187:414–420. doi: 10.2214/AJR.05.0723. [DOI] [PubMed] [Google Scholar]
- 9.Norris CR, Samii VF. Clinical, radiographic, and pathologic features of bronchiectasis in cats: 12 cases (1987–1999) J Am Vet Med Assoc. 2000;216:530–534. doi: 10.2460/javma.2000.216.530. [DOI] [PubMed] [Google Scholar]
- 10.McGuinness G, Naidich DP. CT of airways disease and bronchiectasis. Radiologic clinics of North America. 2002;40:1–19. doi: 10.1016/s0033-8389(03)00105-2. [DOI] [PubMed] [Google Scholar]
- 11.Clercx C, Peeters D, Snaps F, et al. Eosinophilic bronchopneumopathy in dogs. J Vet Internal Med. 2000;14:282–291. doi: 10.1892/0891-6640(2000)014<0282:ebid>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Clercx C, Peeters D. Canine eosinophilic bronchopneumopathy. Vet Clin North Am Small Anim Pract. 2007;37:917–935. vi. doi: 10.1016/j.cvsm.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Mayo JR, Muller NL, Road J, Sisler J, Lillington G. Chronic eosinophilic pneumonia: CT findings in six cases. Ajr. 1989;153:727–730. doi: 10.2214/ajr.153.4.727. [DOI] [PubMed] [Google Scholar]
- 14.Ooi GC, Khong PL, Chan-Yeung M, et al. High-resolution CT quantification of bronchiectasis: Clinical and functional correlation. Radiology. 2002;225:663–672. doi: 10.1148/radiol.2253011575. [DOI] [PubMed] [Google Scholar]