Abstract
This study investigated the effect of sildenafil on uterine volumetric flow (UVF) and vascular impedance in nonpregnant, nulliparous women. Fifteen women were randomized in a double-blind fashion to receive either placebo, or sildenafil (25 or 100 mg) during the luteal phase of the menstrual cycle. Color Doppler ultrasound of both uterine arteries was performed at baseline and 1 and 3- hours post-dosing to calculate resistance index (RI) and UVF. Those who received sildenafil significantly increased UVF and decreased RI over the three hour monitoring period. When UVF responses to sildenafil were examined as a function of baseline UVF, a significant increase in UVF was observed in only those subjects with higher baseline UVF. Overall, women in the luteal phase demonstrated a significant increase in UVF in response to sildenafil. However, this increase appears to be directly associated with basal UVF.
Keywords: uterine volumetric blood flow, sildenafil, flow-mediated vasodilation
Introduction
Abnormal uterine hemodynamics in early pregnancy have been linked to increased risk of intrauterine growth restriction (IUGR), reduced birth weight and preeclampsia 1–3. Animal models designed to evaluate the effect of uterine blood flow on fetal growth have shown a strong association with attenuation of uteroplacental blood flow and incidence of IUGR 4–6. Prenatal preventative treatments and therapies for IUGR, aimed at improving uteroplacental perfusion, have included pharmacologic agents such as low dose aspirin, low molecular weight heparin, betamimetics, calcium channel blockers and nitric oxide (NO) donors 7, 8. However, these pharmacologic agents are not always effective as preventative treatments or as therapies. For example, studies have failed to show a beneficial effect of betamimetics and calcium channel blockers for the treatment of IUGR 8. NO donors have also been suggested as therapies for IUGR, as NO is a key component of the vasodilatory mechanism in arteries and facilitates increased uteroplacental blood flow 9–11. Contrary to the studies using betamimetics and calcium channel blockers, research conducted in pregnant women evaluated the effect of L-arginine, an NO precursor, as a treatment for IUGR has demonstrated a significant increase in the weight of growth-restricted newborns 11.
NO is a key signaling molecule involved in the vasodilatory response of smooth muscle cells. NO activates the cyclic guanosine monophosphate (cGMP)/protein kinase G (PKG) pathway within smooth muscle cells to promote smooth muscle cell relaxation. Animal studies were among the first to identify NO as a vasodilatory agent that increased uterine blood flow 12, 13. Sildenafil citrate inhibits phosphodiesterase 5 (PDE5) maintaining activation of cGMP and PKG and maximizing the effect of existing NO thus facilitating smooth muscle cell relaxation. The potent vasodilatory action of sildenafil has led researchers to evaluate sildenafil as a treatment in assisted reproduction where low uterine blood flow is perceived to be a contributor to implantation failure 14, 15. Interestingly, uterine pulsatility indices in women who received sildenafil while concurrently undergoing in vitro fertilization were decreased suggesting reduced uterine vascular impedance and increased uterine blood flow. These women also had a thicker endometrium 15. In women with inadequate endometrial development, sildenafil was noted to increase endometrial development and improve in vitro fertilization outcome 14. The profound vasodilatory effect of NO on vascular tissue coupled with the effect of sildenafil on the endometrium in nonpregnant women suggests the NO pathway as a potential candidate for manipulation in the search for a successful treatment of pregnancy complications associated with reduced uterine perfusion. The current study was designed to investigate the hemodynamic effect of sildenafil on the uterine artery in nonpregnant women in a placebo controlled double blind fashion.
Methods
This study was designed as a pilot investigation with IRB approval up to 30 subjects with a planned analysis after 15 subjects were randomized and studied to determine if study endpoints had been met, or if not, if additional recruitment of 15 more subjects would be warranted. At the time of initial analysis it was clear that the primary endpoint had been met and the effects of different dosages of sildenafil on uterine blood flow would not be clarified by increasing the subject recruitment to 30.
Fifteen young, healthy, primarily Caucasian (93%, 14/15), nulligravid women were recruited during the luteal phase of their menstrual cycle. Women were recruited over a two year period starting in May 2006 and ending in June 2008. Study subjects were between the ages of 18 and 40, nonsmokers, and free from major medical illness, including cardiovascular disease or diabetes mellitus. Each subject was asked to abstain from alcohol and caffeine for at least 24 hours prior to their study visit and to avoid the use of decongestants and nonsteroidal medications for at least 48 hours before their visit. Patients were randomized to receive orally delivered placebo, sildenafil 25 mg, or sildenafil 100 mg (1:1:1) in a double blind manner.
Each assessment was conducted between 8 AM and 10 AM. Subjects were admitted to the University of Vermont General Clinical Research Center on the day of the study after an overnight fast. Studies were performed during the luteal phase of the menstrual cycle (cycle day 22 ± 4.9). A first-void urine was obtained to confirm the nonpregnant state. Following height and weight determination, subjects rested in the supine position for the remainder of the study and for a minimum of 30 minutes prior to hemodynamic assessment. The research protocol was approved by the University of Vermont Human Investigational Committees. All women provided written informed consent.
UVF, RI, CO and UI
Using color Doppler ultrasound, uterine artery vascular impedance (resistance index, RI), uterine artery blood flow, and cardiac output (CO) were measured at baseline (0 hours) and 1 and 3 hours post-dosing. The 1 and 3 hour time points were chosen because they are closely associated with peak serum concentrations following oral ingestion and a first half-life of 4 hours. All ultrasound measurements were made by the same individual. Uterine blood flow was assessed using color Doppler ultrasound with an 8.0 MHz transvaginal transducer employing a Vivid 7 General Electric ultrasound unit, (Milwaukee WI). Uterine artery measurements were obtained lateral to the cervix at the level of the internal os prior to uterine artery branching. Vessel diameter was measured during power color Doppler imaging. Five estimates of vessel diameter were obtained for the right uterine artery. For determination of average mean velocity angle correction was employed and all angles of sono-incidence were less than or equal to 60 degrees. Five velocity measurements were taken of the vessel and average mean velocity was calculated over an observation window of 1 to 3 minutes. Mean vessel diameter was used to calculate uterine volumetric blood flow (UVF) after integration with average mean velocity to express flow in mL/min: [(time averaged mean velocity) × cross sectional area (calculated as π r2) × 60].
CO was determined by Doppler echocardiographic examination using a 4.0 MHz matrix array transducer. Doppler-derived forward stroke volume (FSV) across the aortic valve was calculated as the product of the left ventricular outflow tract area and the outflow tract velocity time integral as assessed by pulsed Doppler using previously described methods 16. Five complete spectral envelopes with the largest Doppler shift were recorded and averaged for each patient. The Doppler stroke volume was calculated as the product of the outflow tract area and the velocity time integral. CO is expressed as mL/minute after integration of stroke volume with pulse rate.
Uterine index (UI) was calculated as UVF divided by CO. This measurement was used to differentiate from a global generalized change in blood flow versus a redistribution of systemic blood flow favoring the uterine vascular bed during pregnancy.
Flow-mediated vasodilation
Flow-mediated vasodilation is known to be an NO mediated effect and indicate endothelial cell function 17, 18. Therefore, we sought to examine flow-mediated vasodilation of the brachial and popliteal arteries to determine whether baseline endothelial cell function could assist in predicting the individualized response to sildenafil.
Prior to randomization to either placebo or sildenafil, baseline flow-mediated vasodilation studies were performed in both the brachial and popliteal arteries using color Doppler ultrasound. For brachial artery volumetric flow, a blood pressure cuff was placed over the distal forearm, approximately 4 cm below the antecubital fossae, and inflated to 50 mmHg above the research subject’s systolic blood pressure. Five minutes after inflation, the blood pressure cuff was deflated. Vessel diameter was calculated at 50, 60 and 70 seconds after deflation. Popliteal artery blood flow was calculated using the same procedure as for brachial artery blood flow with the blood pressure cuff placed approximately 4 cm below the popliteal fossae.
Mean arterial pressure and pulse
Blood pressure and pulse were also obtained using an automated blood pressure cuff and recorded at baseline, 60, 90, 120, 150 and 180 minutes post-dosing.
Statistical Analysis
Two-way repeated measures analyses of variance were used to compare subjects randomized to placebo and those receiving sildenafil with respect to changes in mean arterial pressure (MAP), pulse rate, UBF and RI. The two factors represented group (an across-subject function) and time (a within-subject function). Initial analyses examined differences between the three randomized groups (placebo, 25 mg sildenafil and 100 mg sildenafil). Because the trend in the two sildenafil groups was similar with no evidence of a dose effect, these two groups were combined for subsequent analyses to increase power for comparisons relative to placebo. Subsequent to the overall ANOVA, F-tests corresponding to simple effects were used to examine temporal trends within sildenafil and placebo groups. Similar analyses were conducted within the sildenafil group to investigate whether baseline values were predictive of who would respond to active treatment. For this latter analysis, two groups of subjects were defined based on performing a median split depending on baseline UVF. Pearson’s correlation coefficient (r) was used to examine the correlation between flow-mediated vasodilation responses in both the popliteal and brachial arteries and the change in UI at the one hour and three hour time points. Analyses were performed using SAS Statistical Software Version 9 (SAS Institute, Cary NC). All data are expressed as mean ± S.E.M.
Results
Baseline characteristics
There were no significant differences in any measured physiologic characteristics between subjects randomized to placebo and sildenafil treatment groups (Table 1).
Table 1.
Baseline Demographics and Physiological Variables (Mean ± SE)
| Characteristic | Placebo (n = 5) | Sildenafil Treated (n = 10) | p-value§ |
|---|---|---|---|
| Weight | 61.2 ± 5.4 | 64.4 ± 2.9 | 0.59 |
| Age | 26.6 ± 3.7 | 26.6 ± 1.9 | 1.00 |
| Cycle day | 21.2 ± 2.9 | 22.5 ± 1.3 | 0.64 |
| Body mass index | 22.9 ± 1.6 | 23.2 ± 0.9 | 0.90 |
| Hematocrit | 12.5 ± 0.2 | 12.2 ± 0.3 | 0.43 |
| Mean arterial pressure | 84.3 ± 2.2 | 75.9 ± 2.8 | 0.08 |
| Pulse | 68.2 ± 4.8 | 63.7 ± 2.9 | 0.40 |
Significance of difference in baseline characteristics of placebo versus sildenafil treated group determined by t-test.
MAP and pulse
MAP significantly decreased over time across groups in association with supine rest (p = .007). However, there was no evidence this decrease was treatment dependent (p = .14) and there were no significant differences in MAP between placebo and sildenafil treated groups at any of the post-dose assessments (baseline: 84 ± 2 vs. 76 ± 3, 60 min: 81 ± 2 vs. 74 ± 2, 180 min: 76 ± 2 vs. 72 ± 2 for placebo and sildenafil groups, respectively). Pulse rate did not significantly change over time in either group (p = .53) and there were no significant difference in the temporal patterns in pulse between placebo and sildenafil treated groups (p = .76).
UVF, RI, UI and CO
UVF was significantly increased in the sildenafil treated group at the one hour and three hour post-dosing time points as compared to baseline and the placebo treated group (p < .05, Figure 1). Overall, there was a trend towards UVF increasing over time (p = .09) in a treatment specific manner (p = .10) (Table 2). Within group analyses showed that this increase was due to significant changes over time in the sildenafil treated group (p = .003), but not in the placebo group (p = .99) (Table 2). With respect to RI, there was a significant time effect (p = .03). Within group analyses indicated that this temporal effect was driven by a significant decrease within the sildenafil treated group (p = .03, Table 2). No significant change over time was observed in the placebo group (p = .28). Analyses of UI resulted in no significant change over time being observed across groups (p = .11, Table 2). However, a significant increase over the three hour time period was observed within subjects randomized to sildenafil treatment (p = .006), while no significant change was observed over time in the placebo treated group (p = .97) (Table 2).
Figure 1.
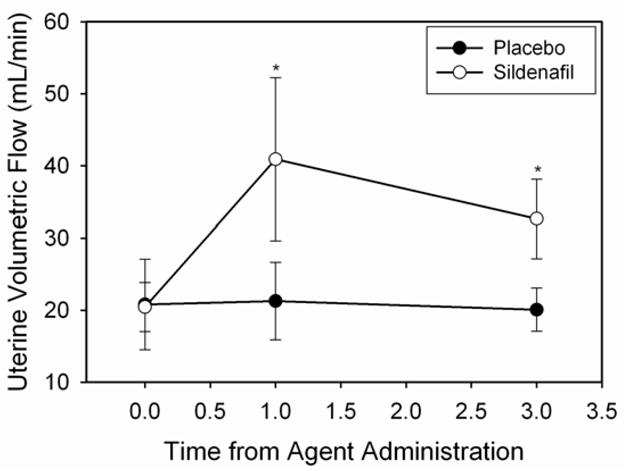
Sildenafil significantly increases UVF one and three hours post-dosing (Fisher’s LSD, p < 0.05).
Table 2.
Mean uterine volumetric blood flow (UVF), resistance index (RI), uterine index (UI), cardiac output (CO), mean arterial pressure (MAP) and heart rate (HR) at baseline and over the three hour treatment period in placebo (n = 5) and sildenafil (n = 10) treated subjects.
| Hemodynamic Measure | Group | Baseline | 1 hour | 3 hour | p-value# |
|---|---|---|---|---|---|
| UVF (mL/min) | Placebo | 20.8 ± 6.3 | 21.3 ± 5.4 | 20.1 ± 3.0 | .99 |
| Sildenafil | 20.4 ± 3.4a | 40.9 ± 11.3b | 32.6 ± 5.5b | .003 | |
| RI | Placebo | 0.84 ± 0.03 | 0.84 ± 0.04 | 0.79 ± 0.03 | .28 |
| Sildenafil | 0.90 ± 0.02a | 0.86 ± 0.04ab | 0.83 ± 0.02b | .03 | |
| UI (%) | Placebo | 0.45 ± 0.15 | 0.47 ± 0.13 | 0.44 ± 0.09 | .97 |
| Sildenafil | 0.45 ± 0.07a | 0.85 ± 0.23b | 0.70 ± 0.11b | .006 | |
| CO (mL/min) | Placebo | 4902 ± 330 | 4698 ± 457 | 4754 ± 336 | .80 |
| Sildenafil | 4534 ± 245 | 4733 ± 335 | 4608 ± 234 | .66 | |
Significance of time effect within each group based on repeated measures two-way ANOVA. Within-group means across time sharing a common letter are not significantly different (Fisher’s LSD, p < .05).
There was no evidence of changes in CO over time in subjects randomized to either the sildenafil or placebo group (p = .66 and p = .80, respectively, Table 2).
As a post hoc exploratory analysis, we investigated whether subjects’ baseline UVF was predictive of who might respond to sildenafil treatment. We partitioned the sildenafil treated subjects into two groups based on a median split: those whose baseline UVF was below the median (15.2 mL/min) and those with baseline UVF was above the median, and compared their response at the one and three hour post-dosing time points. When the low UVF group was compared to the high UVF group, there was a significant difference between the low-flow group and the high-flow group in UVF across time (p = .003). A significant increase in UVF was observed in the high-flow group over time (p = .001, Table 3) while there was no significant increase in the five subjects with lower baseline UVF. Within group analyses showed a significant post-dose increase in sildenafil treated subjects who had higher baseline UVF (p < .001), but no evidence of a change in those with lower UVF at baseline (p = .62) (Table 3). Results for UI paralleled the patterns observed for UVF. With respect to RI, there was a borderline significant time effect in the low UVF group (p = .05) but not the high UVF group (p = .31) (Table 3). For subjects with lower UVF, mean RI values were decreased at 3 hours compared to pre-dosing. No significant change was observed in mean CO in either the low or high UVF group (Table 3).
Table 3.
Mean uterine volumetric blood flow (UVF), resistance index (RI), uterine index (UI) and cardiac output (CO) in response to sildenafil. Subjects (n = 10) were separated into low (n = 5) and high (n = 5) baseline UVF groups based on a median split.
| Hemodynamic Measure | UVF Group | Baseline | 1 hour | 3 hour | p-value# |
|---|---|---|---|---|---|
| UVF (mL/min) | Low | 11.7 ± 1.3 | 13.9 ± 2.3 | 17.9 ± 1.4 | .62 |
| High | 29.2 ± 3.7a | 67.9 ± 14.3c | 47.3 ± 5.3b | <.001 | |
| RI | Low | 0.96 ± 0.02a | 0.93 ± 0.03ab | 0.86 ± 0.04b | .05 |
| High | 0.85 ± 0.01 | 0.79 ± 0.06 | 0.80 ± 0.02 | .31 | |
| UI (%) | Low | 0.29 ± 0.04 | 0.33 ± 0.06 | 0.42 ± 0.03 | .65 |
| High | 0.61 ± 0.10a | 1.36 ± 0.32c | 0.97 ± 0.13b | <.001 | |
| CO (mL/min) | Low | 4109 ± 317 | 4319 ± 509 | 4274 ± 337 | .74 |
| High | 4958 ± 282 | 5147 ± 400 | 4942 ± 277 | .72 | |
Significance of time effect within each group based on repeated measures two-way ANOVA. Within-group means across time sharing a common letter are not significantly different (Fisher’s LSD, p < .05).
Flow- mediated vasodilation and correlative statistical analysis
We evaluated the correlation between the flow-mediated vasodilation responses in both the popliteal and brachial arteries and the change in UI at the one hour and three hour time points. We found no significant association between the flow-mediated dilation in either vascular bed and the change in UI. At one and three hour time points, the popliteal artery demonstrated the following correlations with UI (one hour: r = 0.41, p = 0.27; three hour: r = 0.45, p = 0.22) compared to the brachial artery association with UI (one hour: r = 0.11, p = 0.76; three hour: r = −0.01, p = 0.97).
Discussion
Uterine perfusion is a key component in facilitating fetal growth (reviewed by 19). Abrogated uterine blood flow is often correlated with fetal growth restriction, lower birth weights and increased fetal mortality and neonatal morbidity and mortality (reviewed by 19). Increasing numbers of studies are investigating therapies targeted towards augmenting uterine perfusion in hopes of successfully increasing fetal growth. A dysfunctional NO signaling pathway has been implicated in contributing to vascular incompetence leading to inadequate uterine perfusion seen in preeclamptic and IUGR- complicated pregnancies 20–22. Research in both humans and animals has focused on therapies that manipulate the NO pathway including administration of NO donors and sildenafil 9, 11, 23–25. While NO donors provide a source of NO, phosphodiesterase-5 inhibitors such as sildenafil, act downstream of NO production and augments the vasodilatory effect of existing NO by inhibiting the hydrolysis of cyclic guanine monophosphate (cGMP) thus sustaining NO signaling.
Animal models have been used to study the effect of sildenafil on uterine blood flow with varying results 26 One study found that only when pregnant rats were exposed to hypoxia did sildenafil increase fetal weights 24. However, in another study, treatment of pregnant rats with sildenafil alone increased fetal weights 26. Though uterine blood flow in these experiments was not evaluated, a subsequent study performed in ovariectomized nonpregnant sheep showed increased uterine blood flow with sildenafil treatment 26. We observed a similar result in our study indicating that in nonpregnant, nulliparous women, sildenafil induced a short-term increase in UVF. However, upon examination of individual responses to sildenafil, there was a clear delineation between subjects with higher baseline flow versus those with low baseline flow. We found that the high UVF subjects had an increased UVF response to sildenafil while the opposing group was not significantly affected by sildenafil treatment. Surprisingly, RI decreased in those subjects with low UVF, yet UVF was not significantly affected. This dichotomy suggests that sildenafil affects UVF and RI by separate and exclusive means, or that the relative effect of sildenafil on uterine vascular impedance, compared with the peripheral vasculature, is not sufficient to create significant volumetric flow redistribution.
Our observation that there may be sildenafil responders and non-responders was unexpected and disappointing since patients needing to augment UVF during pregnancy are most likely those with low physiologic UVF. Interestingly, NO donors such as isosorbide dinitrate and L-arginine, have been shown to decrease uterine vascular impedance and ameliorate IUGR 9, 11. Given the NO donor literature and our own data we suggest that there may be a preexisting dysfunction in the vascular physiology of some women that either inhibits the production, activity, release and/or availability of NO or, impairs the response of downstream signaling such as cGMP. Without adequate availability of NO, therapies targeting the cGMP portion of the NO pathway or upstream NOS enzymes may not be effective. Interestingly, others have shown that estrogen signaling through the NOS pathways increases uterine blood flow and the type of estrogen exposure (acute vs. long term) dictates the type of NOS activated 27, 28. Further studies investigating NO/cGMP pathways within the nonpregnant ovine uterine circulation suggest that activation of both calcium-dependant potassium channels (BKCa) and NOS is necessary for acute increases in estrogen-mediated uterine blood flow 29. If both BKCa channels and a functional NO pathway are needed to maximize estrogen-mediated uterine blood flow, compromised activation of BKCa channels or an intrinsic deficiency in estrogen would attenuate NO/estrogen-mediated vasodilation and uterine blood flow. Though we did not evaluate estrogen levels in our subjects, it is possible that those with low UVF may have lower estrogen levels, altered estrogen/progesterone ratios or impaired signaling through BKCa channels and therefore lower NOS signaling. In this case, targeting signaling molecules downstream of these signaling pathways might not work.
Increased vascular impedance within the maternal uterine artery often accompanies IUGR and preeclampsia and can contribute to growth restriction 30–34. Our observations suggest that sildenafil treatment could decrease vascular impedance over time in nonpregnant nulliparous women. This is consistent with the literature indicating that NO donors decrease vascular impedance when given during late pregnancy to preeclamptic women and women at risk for preterm labor 9, 23, 35.
Flow-mediated vasodilation studies on the brachial artery have traditionally been used to evaluate endothelial cell function in pathologies that are characterized by abnormal endothelial cell function such as coronary artery disease 17, 36, 37. These flow-mediated vasodilation studies indicate an association between abnormal vasodilatory responses and coronary artery disease 38–40. Studies have emerged describing the value of using the popliteal artery to gauge endothelial cell function in comparison to the brachial artery 41, 42. Since endothelial cell dysfunction has been suggested as a contributor to improper vascular adaptation to pregnancy resulting in pregnancy disease, we were interested in determining if flow-mediated vasodilation in either the brachial or the popliteal artery could be used to predict responders versus non-responders to sildenafil 43, 44. Therefore, we evaluated the correlation between the popliteal and brachial arteries and the uterine vascular bed upon sildenafil treatment. Although the correlations were not significant, probably due to small sample size, the correlation coefficients for popliteal flow with UI were notably higher than those for brachial flow. These data suggest that the physiology of the popliteal vascular bed may more accurately reflect the physiology of the uterine vascular bed, though more studies are clearly needed to confirm this speculation.
Sildenafil has been suggested as a therapy for IUGR by targeting the uterine vascular bed to decrease vascular impedance and increase uterine blood flow. We found that in nonpregnant, nulliparous women UVF increased over time as a result of sildenafil treatment while vascular impedance decreased. However, due to the segregation of sildenafil responders and nonresponders, we propose that some women may have preexisting endothelial cell dysfunction leading to changes in NO signaling pathways that contributes to low UVF and likewise, might prevent these women from responding to treatments targeted downstream of NO synthesis such as therapies aimed at the cGMP portion of the NO pathway. This challenges the potential role of sildenafil as an effective treatment for increasing uterine blood flow in those at highest risk for sub-optimal uterine perfusion.
Acknowledgments
Grant support: NIH HL 71944 (IMB), M01 RR109 (UVM GCRC), NIH HL 073895 (GO)
Footnotes
Research was performed at the University of Vermont, Burlington, VT
Contributor Information
Sarah A. Hale, Email: sarah.hale@uvm.edu.
Cresta W. Jones, Email: cresta.jones@wfhc.org.
George Osol, Email: george.osol@uvm.edu.
Adrienne Schonberg, Email: Adrienne.schonberg@uvm.edu.
Gary J. Badger, Email: gary.badger@uvm.edu.
References
- 1.Hollis B, Prefumo F, Bhide A, Rao S, Thilaganathan B. First-trimester uterine artery blood flow and birth weight. Ultrasound Obstet Gynecol. 2003;22(4):373–376. doi: 10.1002/uog.231. [DOI] [PubMed] [Google Scholar]
- 2.Melchiorre K, Wormald B, Leslie K, Bhide A, Thilaganathan B. First-trimester uterine artery Doppler indices in term and preterm pre-eclampsia. Ultrasound Obstet Gynecol. 2008;32(2):133–137. doi: 10.1002/uog.5400. [DOI] [PubMed] [Google Scholar]
- 3.Rang S, van Montfrans GA, Wolf H. Serial hemodynamic measurement in normal pregnancy, preeclampsia, and intrauterine growth restriction. Am J Obstet Gynecol. 2008;198(5):519, e511–519. doi: 10.1016/j.ajog.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Lang U, Baker RS, Braems G, Zygmunt M, Künzel W, Clark KE. Uterine blood flow--a determinant of fetal growth. Eur J Obstet Gynecol Reprod Biol. 2003;110(Supplement 1):S55–S61. doi: 10.1016/s0301-2115(03)00173-8. [DOI] [PubMed] [Google Scholar]
- 5.Lang U, Baker RS, Khoury J, Clark KE. Effects of chronic reduction in uterine blood flow on fetal and placental growth in the sheep. Am J Physiol Regul Integr Comp Physiol. 2000 July 1;279(1):R53–59. doi: 10.1152/ajpregu.2000.279.1.R53. [DOI] [PubMed] [Google Scholar]
- 6.Boyle DW, Lecklitner S, Liechty EA. Effect of prolonged uterine blood flow reduction on fetal growth in sheep. Am J Physiol Regul Integr Comp Physiol. 1996 January 1;270(1):R246–253. doi: 10.1152/ajpregu.1996.270.1.R246. [DOI] [PubMed] [Google Scholar]
- 7.Hossain N, Paidas MJ. Adverse pregnancy outcome, the uteroplacental interface, and preventive strategies. Semin Perinatol. 2007;31(4):208–212. doi: 10.1053/j.semperi.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Figueroa R, Maulik D. Prenatal therapy for fetal growth restriction. Clin Obstet Gynecol. 2006;49(2):308–319. doi: 10.1097/00003081-200606000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Nakatsuka M, Takata M, Tada K, et al. A Long-term Transdermal Nitric Oxide Donor Improves Uteroplacental Circulation in Women With Preeclampsia. J Ultrasound Med. 2002;21(8):831–836. doi: 10.7863/jum.2002.21.8.831. [DOI] [PubMed] [Google Scholar]
- 10.Nakatsuka M, Tada K, Kimura Y, et al. Clinical experience of long-term transdermal treatment with nitric oxide donor for women with preeclampsia. Gynecol Obstet Invest. 1999;47(1):13–19. doi: 10.1159/000010055. [DOI] [PubMed] [Google Scholar]
- 11.Sieroszewski P, Suzin J, Karowicz B, Ska A. Ultrasound evaluation of intrauterine growth restriction therapy by a nitric oxide donor (L-arginine) Journal of Maternal-Fetal & Neonatal Medicine. 2004;15(6):363–366. doi: 10.1080/14767050410001725280. [DOI] [PubMed] [Google Scholar]
- 12.Miller SL, Jenkin G, Walker DW. Effect of nitric oxide synthase inhibition on the uterine vasculature of the late-pregnant ewe. Am J Obstet Gynecol. 1999 May;180(5):1138–1145. doi: 10.1016/s0002-9378(99)70607-1. [DOI] [PubMed] [Google Scholar]
- 13.Van Buren GA, Yang DS, Clark KE. Estrogen-induced uterine vasodilatation is antagonized by L-nitroarginine methyl ester, an inhibitor of nitric oxide synthesis. Am J Obstet Gynecol. 1992 Sep;167(3):828–833. doi: 10.1016/s0002-9378(11)91597-x. [DOI] [PubMed] [Google Scholar]
- 14.Sher G, Fisch JD. Effect of vaginal sildenafil on the outcome of in vitro fertilization (IVF) after multiple IVF failures attributed to poor endometrial development. Fertil Steril. 2002;78(5):1073–1076. doi: 10.1016/s0015-0282(02)03375-7. [DOI] [PubMed] [Google Scholar]
- 15.Sher G, Fisch JD. Vaginal sildenafil (Viagra): a preliminary report of a novel method to improve uterine artery blood flow and endometrial development in patients undergoing IVF. Hum Reprod. 2000;15(4):806–809. doi: 10.1093/humrep/15.4.806. [DOI] [PubMed] [Google Scholar]
- 16.Lewis JF, Kuo LC, Nelson JG, Limacher MC, Quinones MA. Pulsed Doppler echocardiographic determination of stroke volume and cardiac output: clinical validation of two new methods using the apical window. Circulation. 1984;70(3):425–431. doi: 10.1161/01.cir.70.3.425. [DOI] [PubMed] [Google Scholar]
- 17.Neunteufl T, Katzenschlager R, Hassan A, et al. Systemic endothelial dysfunction is related to the extent and severity of coronary artery disease. Atherosclerosis. 1997;129(1):111–118. doi: 10.1016/s0021-9150(96)06018-2. [DOI] [PubMed] [Google Scholar]
- 18.Uehata A, Lieberman EH, Gerhard MD, et al. Noninvasive assessment of endothelium-dependent flow-mediated dilation of the brachial artery. Vasc Med. 1997;2(2):87–92. doi: 10.1177/1358863X9700200203. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds LP, Caton JS, Redmer DA, et al. Evidence for altered placental blood flow and vascularity in compromised pregnancies. J Physiol. 2006;572(Pt 1):51–58. doi: 10.1113/jphysiol.2005.104430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris NH, Sooranna SR, Learmont JG, et al. Nitric oxide synthase activities in placental tissue from normotensive, pre-eclamptic and growth retarded pregnancies. Br J Obstet Gynaecol. 1995;102(9):711–714. doi: 10.1111/j.1471-0528.1995.tb11428.x. [DOI] [PubMed] [Google Scholar]
- 21.Seligman SP, Buyon JP, Clancy RM, Young BK, Abramson SB. The role of nitric oxide in the pathogenesis of preeclampsia. Am J Obstet Gynecol. 1994;171(4):944–948. doi: 10.1016/s0002-9378(94)70064-8. [DOI] [PubMed] [Google Scholar]
- 22.Smarason AK, Allman KG, Young D, Redman CW. Elevated levels of serum nitrate, a stable end product of nitric oxide, in women with pre-eclampsia. Br J Obstet Gynaecol. 1997;104(5):538–543. doi: 10.1111/j.1471-0528.1997.tb11528.x. [DOI] [PubMed] [Google Scholar]
- 23.Luzi G, Caserta G, Iammarino G, Clerici G, Di Renzo GC. Nitric oxide donors in pregnancy: fetomaternal hemodynamic effects induced in mild pre-eclampsia and threatened preterm labor. Ultrasound Obstet Gynecol. 1999;14(2):101–109. doi: 10.1046/j.1469-0705.1999.14020101.x. [DOI] [PubMed] [Google Scholar]
- 24.Refuerzo JS, Sokol RJ, Aranda JV, et al. Sildenafil citrate and fetal outcome in pregnant rats. Fetal Diagn Ther. 2006;21(3):259–263. doi: 10.1159/000091352. [DOI] [PubMed] [Google Scholar]
- 25.Wareing M, Myers JE, O’Hara M, Baker PN. Sildenafil citrate (Viagra) enhances vasodilatation in fetal growth restriction. J Clin Endocrinol Metab. 2005;90(5):2550–2555. doi: 10.1210/jc.2004-1831. [DOI] [PubMed] [Google Scholar]
- 26.Villanueva-Garcia D, Mota-Rojas D, Hernandez-Gonzalez R, et al. A systematic review of experimental and clinical studies of sildenafil citrate for intrauterine growth restriction and pre-term labour. J Obstet Gynaecol. 2007;27(3):255–259. doi: 10.1080/01443610701194978. [DOI] [PubMed] [Google Scholar]
- 27.Salhab WA, Shaul PW, Cox BE, Rosenfeld CR. Regulation of types I and III NOS in ovine uterine arteries by daily and acute estrogen exposure. Am J Physiol Heart Circ Physiol. 2000 Jun;278(6):H2134–2142. doi: 10.1152/ajpheart.2000.278.6.H2134. [DOI] [PubMed] [Google Scholar]
- 28.Rosenfeld CR, Cox BE, Roy T, Magness RR. Nitric oxide contributes to estrogen-induced vasodilation of the ovine uterine circulation. The Journal of Clinical Investigation. 1996 Nov 01;98(9):2158–2166. doi: 10.1172/JCI119022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenfeld CR, White RE, Roy T, Cox BE. Calcium-activated potassium channels and nitric oxide coregulate estrogen-induced vasodilation. Am J Physiol Heart Circ Physiol. 2000 July 1;279(1):H319–328. doi: 10.1152/ajpheart.2000.279.1.H319. [DOI] [PubMed] [Google Scholar]
- 30.Coleman MA, McCowan LM, North RA. Mid-trimester uterine artery Doppler screening as a predictor of adverse pregnancy outcome in high-risk women. Ultrasound Obstet Gynecol. 2000;15(1):7–12. doi: 10.1046/j.1469-0705.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 31.Dugoff L, Lynch AM, Cioffi-Ragan D, et al. First trimester uterine artery Doppler abnormalities predict subsequent intrauterine growth restriction. Am J Obstet Gynecol. 2005;193(3, Supplement 1):1208–1212. doi: 10.1016/j.ajog.2005.06.054. [DOI] [PubMed] [Google Scholar]
- 32.Fratelli N, Rampello S, Guala M, Platto C, Frusca T. Transabdominal uterine artery Doppler between 11 and 14 weeks of gestation for the prediction of outcome in high-risk pregnancies. J Matern Fetal Neonatal Med. 2008;21(6):403–406. doi: 10.1080/14767050802053073. [DOI] [PubMed] [Google Scholar]
- 33.Özkaya U, Özkan S, Özeren S, Çorakç A. Doppler examination of uteroplacental circulation in early pregnancy: Can it predict adverse outcome? J Clin Ultrasound. 2007;35(7):382–386. doi: 10.1002/jcu.20370. [DOI] [PubMed] [Google Scholar]
- 34.Li H, Gudnason H, Olofsson P, Dubiel M, Gudmundsson S. Increased uterine artery vascular impedance is related to adverse outcome of pregnancy but is present in only one-third of late third-trimester pre-eclamptic women. Ultrasound in Obstetrics and Gynecology. 2005;25(5):459–463. doi: 10.1002/uog.1895. [DOI] [PubMed] [Google Scholar]
- 35.Kahler C, Schleussner E, Moller A, Seewald HJ. Nitric oxide donors: effects on fetoplacental blood flow. Eur J Obstet Gynecol Reprod Biol. 2004;115(1):10–14. doi: 10.1016/S0301-2115(02)00429-3. [DOI] [PubMed] [Google Scholar]
- 36.Werns SW, Walton JA, Hsia HH, Nabel EG, Sanz ML, Pitt B. Evidence of endothelial dysfunction in angiographically normal coronary arteries of patients with coronary artery disease. Circulation. 1989;79(2):287–291. doi: 10.1161/01.cir.79.2.287. [DOI] [PubMed] [Google Scholar]
- 37.Inoue T, Matsuoka H, Higashi Y, et al. Flow-mediated vasodilation as a diagnostic modality for vascular failure. Hypertens Res. 2008;31(12):2105–2113. doi: 10.1291/hypres.31.2105. [DOI] [PubMed] [Google Scholar]
- 38.Kuvin JT, Patel AR, Sliney KA, et al. Peripheral vascular endothelial function testing as a noninvasive indicator of coronary artery disease. Journal of the American College of Cardiology. 2001;38(7):1843–1849. doi: 10.1016/s0735-1097(01)01657-6. [DOI] [PubMed] [Google Scholar]
- 39.Matsushima Y, Takase B, Uehata A, et al. Comparative predictive and diagnostic value of flow-mediated vasodilation in the brachial artery and intima media thickness of the carotid artery for assessment of coronary artery disease severity. International Journal of Cardiology. 2007;117(2):165–172. doi: 10.1016/j.ijcard.2006.04.063. [DOI] [PubMed] [Google Scholar]
- 40.Perrone-Filardi P, Cuocolo A, Brevetti G, et al. Relation of Brachial Artery Flow-Mediated Vasodilation to Significant Coronary Artery Disease in Patients With Peripheral Arterial Disease. The American Journal of Cardiology. 2005;96(9):1337–1341. doi: 10.1016/j.amjcard.2005.06.084. [DOI] [PubMed] [Google Scholar]
- 41.Angerer P, Negut C, Stork S, von Schacky C. Endothelial function of the popliteal artery in patients with coronary artery disease. Atherosclerosis. 2001;155(1):187–193. doi: 10.1016/s0021-9150(00)00536-0. [DOI] [PubMed] [Google Scholar]
- 42.Spacil J, Ceska R, Haas T. Flow-Dependent Vasomotor Dysfunction of the Popliteal Artery Related to Common Carotid Artery Intima-Media Thickness. Angiology. 2001;52(10):689–695. doi: 10.1177/000331970105201005. [DOI] [PubMed] [Google Scholar]
- 43.Roberts JM, Redman CWG. Pre-eclampsia: more than pregnancy-induced hypertension. The Lancet. 1993;341(8858):1447–1451. doi: 10.1016/0140-6736(93)90889-o. [DOI] [PubMed] [Google Scholar]
- 44.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161(5):1200–1204. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]


