Abstract
In recent years, various nanotechnology platforms in the area of medical biology, including both diagnostics and therapy, have gained remarkable attention. Moreover, research and development of engineered multifunctional nanoparticles as pharmaceutical drug carriers have spurred exponential growth in applications to medicine in the last decade. Design principles of these nanoparticles, including nano-emulsions, dendrimers, nano-gold, liposomes, drug-carrier conjugates, antibody-drug complexes, and magnetic nanoparticles, are primarily based on unique assemblies of synthetic, natural, or biological components, including but not limited to synthetic polymers, metal ions, oils, and lipids as their building blocks. However, the potential success of these particles in the clinic relies on consideration of important parameters such as nanoparticle fabrication strategies, their physical properties, drug loading efficiencies, drug release potential, and, most importantly, minimum toxicity of the carrier itself. Among these, lipid-based nanoparticles bear the advantage of being the least toxic for in vivo applications, and significant progress has been made in the area of DNA/RNA and drug delivery using lipid-based nanoassemblies. In this review, we will primarily focus on the recent advances and updates on lipid-based nanoparticles for their projected applications in drug delivery. We begin with a review of current activities in the field of liposomes (the so-called honorary nanoparticles), and challenging issues of targeting and triggering will be discussed in detail. We will further describe nanoparticles derived from a novel class of amphipathic lipids called bolaamphiphiles with unique lipid assembly features that have been recently examined as drug/DNA delivery vehicles. Finally, an overview of an emerging novel class of particles (based on lipid components other than phospholipids), solid lipid nanoparticles and nanostructured lipid carriers will be presented. We conclude with a few examples of clinically successful formulations of currently available lipid-based nanoparticles.
Keywords: lipid-based nanoparticles, drug delivery, solid lipid nanoparticles, liposomes, nanostructured lipid carriers, bolaamphiphiles, cancer therapy
I. INTRODUCTION
The formulation of bioactive molecules into relatively inert and non-toxic carriers coupled with site-specific targeting ligands for in vivo delivery constitutes a promising approach to improving their therapeutic index while reducing their side effects.1,2 In past years, extraordinary efforts have been made toward improving efficacy and bioavailability of drugs and pharmaceuticals by developing nanotechnology platforms.3–10 These efforts are further aided by the creation of outstanding excellence centers and other initiatives by the National Institutes of Health (NIH), including a national network of eight nanomedicine development centers, which serve as the intellectual and technological centerpiece of the NIH Nanomedicine Roadmap Initiative. In addition, the National Cancer Institute (NCI) Alliance for Nanotechnology in Cancer has established eight Centers of Cancer Nanotechnology Excellence (CCNEs), which are multi-institutional hubs that focus on integrating nanotechnology into basic and applied cancer research and providing new solutions for the diagnosis and treatment of cancer. Other initiatives of the NCI alliance include the establishment of 12 Cancer Nanotechnology Platform Partnerships that are designed to develop technologies for new products in molecular imaging and early detection, in vivo imaging, reporters of efficacy, multifunctional therapeutics, prevention and control, and research enablers; multidisciplinary research training and development teams; and the Nanotechnology Characterization Laboratory (NCL), which performs and standardizes the pre-clinical characterization of nanomaterials intended for cancer therapeutics and diagnostics developed by researchers from academia, government, and industry and funding opportunities worldwide.11
A diagram presented in Figure 1 shows the design principle of an ideal, multifunctional nanoparticle for targeted delivery of therapeutics that can also be envisioned as a nanobiologist’s “Holy Grail.” This nano-assembly includes imaging molecules, a payload of drugs, ligands for site-specific targeting, and a destabilizing lipid that allows for on-demand drug release at the desired site, as well as sensors that probe the efficacy of the drug in real time. Some widely examined nano-carriers aimed at delivering DNA, pharmaceuticals, and/or imaging agents include dendrimers,12,13 nano-gold shells,14,15 nano-emulsions,16 drug-polymer conjugates,17,18 drug-antibody conjugates,19 and quantum dots.20–22 Each of these is based on unique properties of the structural components used in fabricating the delivery vehicle and relies on self-assembly of the structural motifs, while accommodating the pharmaceutical agent and the targeting ligand. In spite of these efforts, only a limited number of the drug-loaded nanoparticles are successful for their clinical applications,23,24 suggesting that these nanotechnology platforms deserve a closer look to overcome several technical roadblocks to become ready for clinical applications.25 An important parameter of the delivery vehicle pertains to low or no toxicity of the carrier itself either in vivo or in the environment as a by-product. Therefore, nanoparticles fabricated using an assembly of natural biomolecules such as lipids, proteins, and carbohydrates are expected to be an appropriate choice for clinical applications.24
FIGURE 1.

Design principle of an ideal multifunctional lipid-based nanoparticle for targeted and triggered drug delivery. Liposomes consist of a matrix phospholipid (cyan), a destabilizing (pore forming) phospholipid (yellow), conjugation lipid (green), ligand attached via the conjugation lipid (brown), and a cell death marker such as an apoptotic detector (pink). The nanoparticle is loaded with a chemotherapeutic agent (red) and an imaging agent (blue) in the aqueous milieu.
Among various lipid-based formulations, classical examples are “liposomes,” which primarily consist of phospholipids (major components of biological membranes) and have been extensively studied.26,27 Lipoplexes (lipid-based assemblies of non-covalently associated DNA by charge-charge interactions) are used in gene-targeting studies.28,29 Recently, studies by Torchilin et al. reported novel micelle-like nanoparticles loaded with plasmid DNA and based on a covalent conjugate of DNA and polyethylenimine, which is coated with a lipid monolayer and polyethylene glycol (PEG) molecules. Their study suggests that micelle-like nanoparticles have architecture and properties suitable for in vivo application.30 Another well-studied example is tumor-targeted, liposome-based systemic gene delivery.31–35 Interestingly, bioactive lipids such as ceramide that are involved in cell signaling pathways have also been examined via nanoliposome-delivery systems.36,37 The above-mentioned nano-systems have been described elsewhere in detail.
In this review, we will limit ourselves to the history, current status, and future of lipid-based nanoparticles as carriers of drug delivery. The first section will deal with the drug-delivery status of liposomes, with a special focus on the principles of lipid packing, strategies for optimal formulations, phospholipid structural modifications, in vitro and in vivo triggering, and tumor-targeting modalities. In the second section, we will provide details about a novel class of amphipathic lipids, “bolaamphiphiles,” which bear distinct non-bilayer-forming properties and may become suitable drug-delivery platforms. The last section will briefly summarize current research activities in lipid-based nanoassemblies based on molecules other than phospholipids. These include solid lipid nanoparticles (SLN) and nano-structure lipid carriers (NLC); both systems are relatively new and present opportunities for further investigations for their application in drug delivery.
II. LIPOSOMES AS DRUG CARRIERS
The preparation of liposomes with entrapped solutes was first demonstrated in a published paper38 by Prof. A.D. Bangham of the United Kingdom. Since their inception, liposomes have been explored as carriers for delivering drugs and pharmaceuticals.4,39–41 They present a well-studied class of drug carriers generally characterized by the presence of a lipid bilayer that is primarily composed of amphipathic phospholipids (chemical structures shown in Fig. 2A) enclosing an interior aqueous space. Currently, a number of liposome formulations are in clinical use to combat cancer and infectious diseases, while others await clinical trial outcomes (for updated information, please visit the website www.clinicaltrials.org). Table 1 summarizes a partial list of liposomes approved for clinical applications, and this list is growing at a steady pace; Table 2 summarizes a partial list of liposomes that are currently undergoing clinical trials. It is notable to mention that Doxil®/caylex (a liposome-based formulation of the anticancer drug doxorubicin, Ortho-Biotech) was the first formulation approved for application in the clinic and therefore may be considered an honorary nanoparticle for patient care.23,24 Historically, the important milestones that led to the research and development of clinically suitable liposome formulations can be summed up in two major technological achievements: i) inclusion of pegylated lipids in the liposomes to bypass reticulo-endothelial system, resulting in significant accumulation in the tumors42,43; and ii) the strategic development of a remote drug-loading process based on the ammonium sulfate gradient method to achieve significantly high quantities of doxorubicin in the interior of the liposomes.44,45 These issues have been widely discussed elsewhere46 and therefore will only be briefly covered here.
FIGURE 2.
A, Chemical structures of commonly used phospholipids to prepare liposomes: Matrix lipid such as DPPC or DSPC (a); PEGylated lipid (usually DSPE-PEG2000) for longer circulation in vivo (b); lipid bilayer destabilizing lipid, such as lyso-lecithin or pore forming photoactivable lipid (c); and a functionalized lipid such as maleimide-DSPE-PEG2000 for conjugating ligands such as antibodies and/or peptides for site-specific targeting (d). R1 and R2 represent fatty acyl chains at sn-1 and sn-2 positions, respectively, and usually vary from C16 to C18. The selection of R1 and R2 will depend on the type of liposomes needed. B, Principle of assembly of various lipid molecules. The lipids assume various physical structures depending on their chemical structures. Top panel shows polymeric phases (PC, bilayer, lyso-PC, micellar, PE, hexagonal HI); bottom panel shows the corresponding self-assembly of these lipids as indicated.
TABLE 1.
Federal Drug Administration (FDA)-Approved Liposomes
| Drug name and company | Indications/Target | Reference |
|---|---|---|
| Marqib-Vincristine sulfate liposomes injection: Vincristine-encapsulated liposomes in a lipid bilayer of sphingomyelin (Hana Biosciences) | Treatment of adult patients with acute lymphoblastic leukemia | 245 |
| DaunoXome: Daunorubicin citrate-liposome injection (NeXstar Pharmaceuticals) | Advanced HIV-associated Kaposi’s sarcoma for first-line use | 246 |
| AmBisome®: Lipid-based formulations, including liposomal amphotericin B (Fujisawa Healthcare) | Treatment of fungal infection and visceral leishmaniasis | 247,248 |
| Doxil®, caylex: PEGylated liposomal loaded with doxorubicin (PLD) (Ben Venue Laboratories for Johnson & Johnson) | Treatment of metastatic breast cancer, advanced ovarian cancer, multiple myeloma, and AIDS-related Kaposi’s sarcoma | 249–252 |
| Amphocil: Lipid form of amphotericin B stabilized with cholesteryl sulfate (Samaritan Pharmaceuticals) | Amphocil binds to lipoproteins and ergosterol in cell membranes of infecting fungi; also indicated for the treatment of invasive aspergillosis and leishmaniasis | 253,254 |
| ABELCET®: Amphotericin B lipid complexed with two phospholipids (DMPC:DMPG, 7:3 molar ratio) in a 1:1 drug-to-lipid molar ratio (The Liposome Company) | ABELCET® consists of amphotericin B, a polyene, antifungal antibiotic for invasive fungal treatment | 255 |
| Depocyt™: Liposomal cytarabine or liposomal Ara-C are antimetabolite cytarabine, encapsulated into multivesicular lipid-based particles (Enzon Pharmaceuticals) | DepoCyt is a sustained release formulation of the chemotherapeutic agent cytarabine, used for the treatment of patients with lymphomatous meningitis | 256 |
TABLE 2.
Drug-Loaded Liposome Formulations Currently in Clinical Trials
| Drug name and company | Treatment | Status | Reference |
|---|---|---|---|
| Liposomal-annamycin semi-synthetic doxorubicin analog: Annamycin intercalates into DNA and inhibits topoisomerase II (Aronex Pharmaceuticals) | Acute myeloid and lymphoid leukemia (drug-resistant tumors) | Phase I/II | 257,258 |
| Lipoplatin: A new cisplatin-encapsulated liposome composed of DPPG, soy phosphatidyl choline (SPC-3), cholesterol, and mPEG2000-DSPE, designed to reduce cisplatin toxicities without reducing efficacy (HBio) | Carcinoma of the head and neck, pancreatic cancer, and continuing in advanced breast cancer and gastrointestinal cancers | Phase II clinical trial and one phase III non-inferiority clinical study | 259,260 |
| NX 211 (liposomal lurtotecan): Liposomal formulation of the lurtotecan a topoisomerase I inhibitor (Gilead and Glaxo) | Advanced or recurrent ovarian epithelial cancer | Phase II | 261,262 |
| Liposomal vincristine (ONCO-TCS™): Vincristine encapsulated in liposomes composed of sphingomyelin and cholesterol (INEX Pharmaceuticals) | Treatment of relapsed aggressive non-Hodgkin’s lymphoma and other cancers | Pivotal phase II/III | 245,263–265 |
| Doxorubicin HCL liposome for injection: Non-PEGylated liposomal doxorubicin Myocet made by Enzon Pharmaceticals for Cephelon in Europe and for Sopherion Therapeutics in the United States and Canada | Treatment for patients with stage II or III invasive breast cancer and with tests showing an overexpressing of human epidermal growth factor receptor 2; Myocet® is approved in Europe and Canada for treatment of metastatic breast cancer | Pivotal phase III global registrational trial for treatment of HER2-positive metastatic breast cancer | 266,267 |
| VentusTM: Prostaglandin E1 liposomes (The Liposome Company) | Pretreatment of acute respiratory distress syndrome | Phase III clinical trial | 268 |
| ThermoDoxTM: Lysolipid thermally sensitive liposomes are heat-activated liposomal encapsulation of doxorubicin (Celsion Corporation) | ThermoDoxTM can be used as treatment for hepatocellular carcinoma (primary liver cancer) and recurrent chest wall breast cancer | Phase III | 269 |
| Protein-stabilized liposome encapsulation of active drug Docetaxel (ATI-1123): Liposome product made in a single step and encapsulates Docetaxel a clinically well-established anti-mitotic chemotherapy medication (Azaya) | Docetaxel (trade name Taxotere) is used mainly for the treatment of breast, ovarian, and non-small cell lung cancer | Pre-clinical studies to FDA phase I clinical trial | 270 |
II.A. Design Principle of Liposomes and Assembly of Lipid Molecules
Phospholipids (phosphatidylcholines, usually called “lecithins”) are the main constituents of liposomes (Fig. 3). Due to their amphipathic properties, they readily form concentric bilayers (also initially called “bangosomes” by A.D. Bangham). There are several protocols and techniques available to convert these multilamellar lipid dispersions into single bilayer structures (called unilamellar liposomes or vesicles). The most commonly used laboratory methods include sonication, extrusion, reverse-phase evaporation, and solvent injection. The formulations that meet the regulatory standards by the Food and Drug Administration (FDA) require consideration of important parameters including their size, stability in circulation, batch-to-batch variations, efficiency of drug loading, etc.47 In addition to these features, the ability of liposomes to destabilize their membranes for localized drug delivery (triggerable liposomes) is another highly crucial aspect for improving the efficacy of liposome-entrapped drugs and pharmaceuticals. “Triggerable liposomes” will be discussed later in this section.
FIGURE 3.
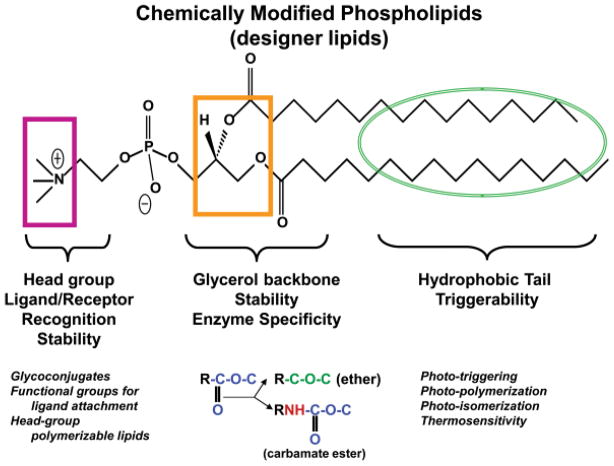
Sites of chemical modifications in the phospholipid molecules for tunable liposomes. The chemical structure of a typical PC is shown and the sites of various modifications in three major portions of this molecule are as follows: pink, head group modification; orange, glycerol backbone modifications; and green, fatty acyl modifications. The specific examples of modifications are given at the bottom.
Liposomes have also long served as excellent tools for model membranes and therefore lipid molecules other than phosphatidylcholine (PC) that may direct changes in the bilayer structure have been well studied.48 The polymeric structures of some of the phospholipids used in liposome formulations for biomedical applications are shown in Figure 2B. Among these, PC, which has zero spontaneous curvature, favors assembly in a lipid bilayer, whereas lyso-PC (usually called “lyso-lecithin”), which has positive spontaneous curvature, forms micelles when dispersed in an aqueous solution. Phosphatidylethanolamine (PE), which has negative spontaneous curvature, assumes a non-bilayer (inverted micellar or the hexagonal, H-II phase) structure at a given temperature and degree of hydration. If the desired outcome of a liposome formulation is fusion of its membrane with that of a cell, incorporation of PE into that formulation would favor that outcome because negative curvature lowers barriers to fusion.49 When lyso-PC is incorporated into a bilayer, its positive spontaneous curvature will lead to the formation of lipidic pores. Therefore, lyso-PC has been incorporated into thermosensitive liposomes, which have been designed to leak their contents when the tumor site is heated a few degrees above the physiological temperature. Thermosensitive liposomes are currently undergoing clinical trials,50,51 as discussed in detail below.
An alternate strategy to developing “designer liposomes” bearing desired properties includes discrete chemical modifications in the phospholipid structure, primarily PC. The PC molecule can be divided into three major parts, the head group, the glycerol backbone, and fatty acyl chains (Fig. 3). Each of these regions has been modified either by the introduction of additional groups or modification of existing chemical bonds. The head-group modifications include introduction of ligands,52–55 functional groups (such as malemide) for chemical conjugation of ligands (such as antibodies),56 and/or polymerizable moieties57 to produce stable liposomes. The carbonyl ester bonds of the glycerol backbone of PC (at both the sn-1 and sn-2 position) have been the choice for modifications with either ether58,59 or carbamyl esters,60–62 resulting in modulation of stability and in vivo circulation of these liposomes. The fatty acyl chain length and degree of unsaturation are important factors that govern bilayer packing properties of liposomes and contribute to the observed phase-transition (Tm) effect.
Thermosensitive liposomes were initially developed in early 1980s based on the Tm release properties of phospholipids,51 and since then have been further developed for their applications in the clinic (discussed in detail later in the review). Fatty acyl chains of the phospholipids have been further explored to introduce chemical modifications, and the resulting modified phospholipids have primarily yielded photo-activable liposomes.63 Design principles and current work on photo-triggerable liposomes will also be discussed later in this review.
II.B. Liposome-Triggering Modalities
Although the liposome drug-delivery field has made strides in overcoming crucial pitfalls (such as clearance rates, in vivo stability, etc.), the overall deliverable drug by these nanocarriers is expected to significantly increase by designing modalities to release drugs within a defined space and time in a localized area (such as the site of a tumor). It can be envisioned that strategic development of drug-loaded nanocarriers tuned to trigger drug release would significantly improve the efficacy of drugs and pharmaceuticals, potentially obliterating drug-resistance problems. Various triggering modalities for site-specific release of drugs from liposomes have been developed.64–69 The principles underlying these approaches revolve around one common feature, creating defects in the liposome membrane (Fig. 4A), and can be broadly classified into two main approaches: external and internal triggers (Fig. 4B).
FIGURE 4.
A, Principle of creating defects in the lipid bilayer. Top panel shows fatty acyl chain organization at the Tm temperature. The diagram was adapted from the website of Venable & Pastor (FDA/CBER). Lower panel shows release of drugs through localized defects in the membrane. B, Various strategies used for localized drug delivery.
Examples of external triggers include the utilization of two forces of nature, heat and light. Among these, liposomes sensitive to mild hyperthermia (currently under clinical trials) are the best-studied examples for triggered drug delivery, whereas light-sensitive liposomes (explored since early 1980s) have lately regained attention. Another relatively new modality at a very preliminary stage of development involves the concept of alternate magnetic field (Fig. 5A); however, the potential success of the approach is subject to future investigations. In addition to utilization of external triggering of liposomes, alternate triggering strategies are primarily based on exploiting the abnormalities in the biology of diseased cells, tissue, and/or organs. One well-studied example in this class includes utilization of enzymes that are up-regulated in certain tumors to cleave liposomal lipids and create defects in the membranes. Several excellent reports have recently dealt with enzyme-triggered release of liposomal contents.70–73 Among these, secretory phospholipase A2 appears to be a promising target.68,71,74 Specific examples in this class include generation of a pore-forming lipid (e.g., lyso PC, Fig. 2A) mentioned above. Significant efforts have also been made to develop liposomes that will undergo membrane reorganization in a low-pH environment (predominantly from a bilayer to a hexagonal HII lipid phase, Fig. 2). The lipid formulations for “pH-sensitive” liposomes typically include PE and cholesterol hemi-succinate for membrane destabilization in the endosome, resulting in localized drug release.3 A number of reviews on pH-sensitive liposomes and other nanoparticles have been published previously.75–78 In this review, we will only elaborate on the recent updates on thermo- and light-sensitive liposomes.
FIGURE 5.
Examples of external triggering modalities. A, Drug-loaded superparamagnetic iron oxide nanoparticles (SPIONs) encapsulated in liposome membrane (top panel). Exposure of these liposomes to alternative magnetic field results in local disruption of liposomes releasing SPIONS (step 1), and the drug is then released from the SPIONS (step 2). B, Timeline for development of thermosensitive liposomes. C and D, Chemical structures of designer lipids used for development of photo-activable liposomes: (a) Bis AzoPC, (b) Sorbyl PC, (c) o-nitrobenzyl conjugated lipid, (d) head-group polymerizable lipid, (e) DC8,9PC (1,2 bis(tricosa-10,12-diynoyl)-sn-glycero-3-phosphocholine), (f) principle of photopolymerization of DC8,9PC.
Thermosensitive liposomes are based on lipid-destabilizing mechanism(s), and thermal melting temperatures (Tm); this concept was first described by Yatvin et al.51 Since then, progress in the development of these liposomes has been substantial. Figure 5B shows a timeline of step-wise progress in the field of thermosensitive liposomes. It is now well-documented that local hyperthermia can be used to selectively enhance both the delivery and the rate of drug release from thermosensitive liposomes to targeted tissues.50,65 Recently, the Center for Interventional Oncology was established to develop and translate image-guided technologies for localized cancer treatments. For example, using thin needles, sound waves can be used to ablate tumors and enhance drug delivery from thermo-sensitive liposomes. Energy sources include high-intensity focused ultrasound, freezing, microwaves, laser, and radiofrequency. The advances in focused high-frequency ultrasound technologies has been used for noninvasively enhancing drug delivery and the clinical applications of liposomes.79 Thermosensitive liposomes are currently in clinical trials to treat hepatocellular carcinoma liver neoplasms and breast cancer (www.clinicaltrials.gov, identifier #NCT00441376 and #NCT00346229, respectively). The latest developments in this field include vascular targeting of thermosensitive liposomes.
Electromagnetic radiation-triggered release of liposomal drugs presents a promising approach that involves strategically designed phospholipid molecules to respond to a light trigger. These liposomes63 are based on the principle of photo-polymerization of lipids,80 photo-sensitization by membrane-anchored hydrophobic probes,72,73,81–83 or photo-isomerization of photo-reactive lipids.84 The design principles and mechanisms of light-triggered chemical changes in photo-reactive segments of these lipids have been recently reviewed in detail. Figure 5C and 5D show some of the designer lipids used for the development of light-activated liposomes. However, none of the formulations developed so far has been successful for in vivo applications, presumably due to the lack of adequate photon energy produced by the radiation source(s) or the inability of radiation to penetrate into biological tissues. We believe that the development of novel and innovative strategies to combine the unique chemistry of photoactivable lipids with “helper” components (such as metal ions) is needed to acquire high energy radiations. Therefore, we have recently pursued an alternative approach to create phase boundary defects in lipid model membranes using mixtures of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and a photo-polymerizable phospholipid, 1,2 bis(tricosa-10,12-diynoyl)-sn-glycero-3-phosphocholine (DC8,9PC) (Fig. 5D). This lipid bears highly reactive diacetylenic groups that can be polymerized by UV irradiation to form chains of covalently linked lipid molecules in the bilayer.80,85 We hypothesize that DC8,9PC is likely to form aggregates in the bilayer of phospholipids containing saturated acyl chains. We have recently reported that light-triggered calcein release occurs from liposomes containing a mixture of saturated phospholipids and DC8,9PC. The packing properties of DC8,9PC in triggerable formulations were in agreement with the modulation of the melting phase transitions (Tm) in these liposomes as determined by differential scanning calorimetry. Our initial in vivo experiments indicated that liposomes containing DC8,9PC are not toxic to mice, and these formulations have similar biodistribution to that of DPPC liposomes alone (unpublished observations). We surmise that the ability of diacetylenic groups to undergo chemical modifications in the presence of metal ions may result in the development of the next generation of radiation-sensitive liposomes for drug-delivery applications in the clinic.86
II.C. Liposome Targeting
As discussed in the previous sections, triggerable liposomes may serve as improved drug-delivery systems. It can be envisioned that grafting specific ligands on the liposome surface will further improve the delivery of drugs to targeted cells and/or tissues. To this end, antibody-coated liposomes (immunoliposomes) have been explored for decades. However, success of immunoliposomes in the clinic still remains to be seen. In addition to using antibodies as ligands,4,40,52,87 a number of other small molecules such as vitamins,17,18,88 peptides,89–92 aptamers,93 and Affibodies94–96 have also been examined to improve targeting of liposomes. However, the notion that “targeted liposomes” bear an advantage over non-targeted liposomes has been challenged and is subject to intense debate. In this section, we will provide an overview of various strategies used for liposome targeting (including specific examples of ligands, conjugation strategies, and biological systems used), and discuss the pros and cons of targeted versus non-targeted liposomes and their ability to be targeted to specific molecular signatures expressed on cell surfaces.
1. Targeted Liposomes: General Considerations
The advantage of using nanocarriers, including liposomes (size range 100–200 nm), to deliver anticancer agents to tumor tissue has been extensively discussed.2 This approach for anticancer therapy involves “passive targeting” of the drug-loaded nanocarriers, because these particles are known to accumulate in the tumor area due to the leaky vasculature-enhanced permeability and retention (EPR) effect (non-targeted liposomes). This effect represents the anatomic differences between normal and cancerous tissue because capillaries in the tumor area possess increased permeability. This passive targeting effect is highly dependent on a number of characteristics, including the degree of tumor vascularization and angiogenesis and the porosity and pore size of tumor vessels, which vary with the type and status of the tumors.97 These factors contribute to the pharmacokinetics and biodistribution of the liposomes. Additional issues related to “passive targeting,” such as the kinetics of drug release, efflux of released drug into the tumor cells, and tumor retention, are important factors that will dictate the outcome of effective treatment (Fig. 6A). For example, liposomes in the range of 100 to 150 nm have been shown to preferentially accumulate in tumors due to the EPR effect.98,99
FIGURE 6.
A, Targeting pathways of lipid-based nanoparticles to tumors. Schematic presentation of nanoparticles that passively or actively target tumors. These particles accumulate in the tumor area due to the leaky vasculature surrounding the tumors. However, targeted nanoparticles bind to the target tumor cells via ligand-receptor interactions and are internalized, whereas non-targeted nanoparticles are less efficient in interacting with tumor cells. B, Antibodies and antibody fragments used for targeting of lipid-based nanoparticles.
The design and development of ligand-bearing liposomes for “active targeting” involves specific interactions of liposomes via the receptors, followed by uptake of liposomal drugs by receptor-mediated endocytosis (targeted liposomes) (Fig. 6A). The development of targeted liposomes has been ongoing since the 1970s/1980s,40,87 while taking into consideration that liposome targeting does not compromise pharmacokinetics and the efficacy of drugs.
Ligands such as antibodies, peptides, and vitamins (e.g., folic acid), which can bind to up-regulated/overexpressed receptors on tumor tissue, have been investigated as biomarkers for targeted drug delivery. It is important to mention here that the concept of improving drug delivery by means of “targeted liposomes” has met with skepticism and has been challenged100,101; ligand-bearing liposomes showed improved efficacy of encapsulated drugs in some systems, whereas such an improvement was not observed in other experimental systems. We believe that a detailed analysis of the kinetics and extent of: i) liposome accumulation, ii) liposome internalization, iii) intracellular liposome degradation, and iv) intracellular fate of the drug are the factors that will play an important role in establishing the validity of targeted liposomes.102 The surface density of receptors, affinity with ligands (most importantly, the effect of multimerization of ligand binding), receptor recycling, and state of tumor development also contribute in determining the fate of targeted liposomes. Current studies using targeted liposomes are summarized in Table 3. For example, earlier work by Gabizon et al. (ref. 124) showed that attaching folate to liposomes did enhance uptake of liposomes in folate-expressing tumors in mouse biodistribution studies, but HER2-targeted immunoliposomes did not show any difference in biodistribution and tumor accumulation compared with non-targeted liposomes.103 However, targeted liposomes in the latter case had sixfold increased intracellular localization, suggesting that they are likely to bear an advantage for drug targeting. Recent studies by Laginha et al. support this notion, because their experiments, which were based on the examination of the HER2-targeted liposomes, showed improvement in doxorubicin-mediated cell killing and tumor regression in mice.104 Another example in support of targeted liposomes includes a number of recent studies by Torchilin’s group demonstrating improved targeting of doxorubicin using the anti-nucleosome antibody.105 Therefore, it can be postulated that the targeting molecules contribute (via the ligand-receptor interactions) subsequent to the nanocarrier accumulation in the tumor tissues.106,107
TABLE 3.
Ligand-Targeted Lipid-Based Particles for Anticancer Drug-Delivery Antibodies
| Targeted Agent/Antigen | Encapsulated Marker/Drug | Model | Reference |
|---|---|---|---|
| Anti CD-19 (Ab) | Doxorubicin | Human multiple lymphoma, ARH77 cells | 271, 272, 273 |
| Anti CD-19 (scFv) | Doxorubicin | Raji human lymphoma | 274 |
| Anti HER2 | Paclitaxel | HER2+ human breast cancer cells | 275 |
| Anti HER2 Fab′ or scFv | Human breast BT-474 adenocarcinoma cancer cells | 103,276 | |
| Recombinant human, anti-HER2-Fab′ or scFv C65 | Doxorubicin | HER2+ human breast cancer cells | 277 |
| Anti-nucleosome 2C5 mAb | Doxorubicin | Murine LLC, 4T1, C26; human BT-20, MCF-7, PC3 | 278,279 |
| scFv A5 with encapsulated Endothelin | Doxorubicin | Endothelial cells HU-VEC, HDMEC | 280 |
| Anti-GD2 and anti-GD2-Fab′ | Doxorubicin | Human neuroblastoma | 281 |
| Anti-idiotype mAb, S5A8 targeted to 38C13 | Doxorubicin | Murine D-cell lymphoma | 282 |
| Anti-51-kDa Fab′ | Doxorubicin | Mouse model of visceral leishmaniasis | 283 |
| Anti-MT1 (Fab′) (Metalloproteinase)-MMP- | Doxorubicin | Human HT1080 fibro-sarcoma | 284 |
| Anti-CD74 LL1 | Doxorubicin | Raji human B lymphoma | 285 |
| C225 mAb or Fab′ against EGF receptor | Doxorubicin | Human MDA-MB-468 | 286 |
| Anti-VCAM-1 targeted to Vascular cell adhesion molecule-1 | -- | Human endothelial cells | 287 |
| Anti epidermal growth factor receptor (EGFR) | Doxorubicin, epirubicin, or vinorelbine | EGFR-overexpressing MDA-MB-468 tumor cells | 287 |
|
Peptides | |||
| Antagonist G targeted to vasopressin | doxorubicin (DXR) | Human small cell lung cancer H69 | 288,289 |
| Vasoactive intestinal peptide targeted to VIP receptors | (fluorescent cholesterol) | Rat breast cancer | 290 |
| P0-protein targeted to intracellular adhesion molecule-1 | (Radioactive lipids) | Human M21 and A-375 melanoma | 291 |
| Arg-Gly-Asp peptide | Doxorubicin | Murine B16 melanoma | 292 |
|
Small Molecules | |||
| Folate vitamin targeted to folate recptor | Doxorubicin, anti-sense oligodeoxy-ronucleotides against growth factor receptor | M-109-R mouse carcinoma tumor, Chinese hamster ovary and KG-1 human acute myelogenouse leukemia cells | 121,122,124 |
| Folate vitamin targeted to folate receptor β | Doxorubicin | Murine acute myelogenouse leukemia cells | 293 |
|
Affibody | |||
| HER2-specific affibody-conjugated thermosensitive liposomes (affisomes) | Calcein/rhodamine | Breast cancer (formulation development) | 96 |
| Epidermal growth factor receptor-affibody liposomes | Mitoxanthrone | Breast cancer (cellular toxicity) | 95 |
| HER2-specific nanoparticles | Paclitaxel | Breast cancer (cellular toxicity) | 117 |
2. Targeting Ligands
Various candidate ligands have been examined to target liposomes to tumors that are aimed at exploiting overexpressed receptors (Table 3); these include antibodies, affibodies, and small ligands such as folate, aptamers, peptides, and lectins. Properties of a viable ligand that will bear the potential to succeed in targeting liposomes for site-specific drug delivery can be broadly classified based on the following factors: i) methodology of ligand production in large scale, ii) ease of purification and stability, and iii) ligand-liposome conjugation strategies without compromising the properties of either the ligand and/or liposomes. Among the various ligands examined thus far, antibodies have been extensively studied and will be discussed in detail. We will also provide an overview of affibodies and folate targeting in this section.
a. Immunoliposomes
Immunotherapy has been explored since detailed structural analysis of antibodies, hybridoma technology (monoclonal antibodies, mAb), and phage display technology (single-chain antibodies (scFv) became available (for details on therapeutic antibodies, the reader is referred to Therapeutic Antibodies: Methods and Protocols by A.S. Dimitrov, Humana Press, NY). Currently available therapeutic antibodies include mAbs (such as Herceptin for breast cancer and Epratuzumab for B-cell lymphoma), and therefore have been the preferred choice of molecules for generating immunoliposomes. One of the advantages of using mAbs is their stability and higher binding avidity that comes from the presence of two binding sites on the molecule. The Fc-receptor binding of mAb can lead to complement-dependent cytotoxicity and antibody-dependent cellular cytotoxicity, which may enhance tumor-cell killing. However, the Fc receptor-mediated responses may lead to high liver and spleen uptake of the immunoliposomes and might increase the immunogenicity of the molecule. The modifications in the whole antibody molecule include F(ab′)2, Fab′, and scFv fragments (Fig. 6B) that lack the Fc domain and the complement-activating region, which might reduce their immunogenicity.108 F(ab′)2 fragments retain two binding regions that are joined by disulfide bonds and can be quite stable during storage. Under reducing conditions, the disulfide bonds are cleaved to yield two Fab′ fragments, which is very useful for coupling the fragments to lipid based nano-particles. Fab′ fragments and scFv fragments have only one binding domain, which reduces their binding avidity; however, by attaching several fragments at the surface of immunoliposomes or by engineering bivalent or multivalent fragments, the multivalency and hence the avidity can be restored.109–111 The use of scFv fragments is attractive because of their ease of identification (from phage display) and production and because they decrease immunogenicity. However, these small fragments might be less stable during storage than Fab′ fragments or whole mAbs.109,110
Tremendous efforts are being made to apply antibody-directed nanotechnology platforms for diagnostics, imaging, and therapy. Among these, antibody-coated liposomes (immunoliposomes) are one of the long-studied nanoparticles and, as discussed previously, the practical applications of these particles are still subject to intense debates. Historically, initial attempts to link the whole-antibody molecules to the liposome surface involved chemical modifications of amine using bifunctional reagents. This method suffered drawbacks due to compromised active domains of the antibodies and lack of specificity.112 Since the availability of scFv (with engineered cysteines at the C-termini), antibody conjugation methods are now based on the maleimide-cysteine reaction, resulting in the formation of thio-ether bonds between proteins and liposomes, and are presently the preferred method of choice.56
Liposomes can be converted to targeted liposomes using two strategies. The first method (Fig. 7A) uses a versatile “post-insertion technique” in which ligands are coupled to end-functionalized groups in PEG lipid micelles. The ligand-PEG lipid conjugates are then transferred in a simple incubation step from the micelles into the outer monolayer of pre-formed, drug-loaded liposomes. This method allows for a combinatorial approach to the design of targeted liposomes that minimizes manufacturing complexities, allowing ligands to be inserted into pre-formed liposomes containing a variety of drugs; this process has been reviewed in detail previously.6,113 To date, HER2 scFv114,115 conjugated liposomes for drug delivery and anti-TfR scFv-lipoplexes for gene delivery have been successfully developed.31,32 In addition, an alternate protocol is based on preparing liposomes with grafted malemide-containing PEG lipid in the lipid bilayer. Antibodies bearing a cysteine (such as scFv) at the C-terminus are first reduced and then conjugated to the outer surface of liposomes using the same chemistry as above (Fig. 7B). The first method typically relies on using Doxil®, a formulation already being used clinically.104,114 However, insertion techniques are rather uncontrolled, and separation of micellar scFv-PEG lipid conjugate from the liposomes may become a technical challenge. On the other hand, direct conjugation of scFv to the exposed malemide groups on the liposome surface is relatively controlled and allows for only antibody conjugation on the outer surface.
FIGURE 7.
Scheme presenting steps in the conjugation of antibodies/targeting molecules to the liposomes. A, Post-insertion protocol. B, Antibody conjugation on the surface of liposomes.
To date, the issue of optimal ligand density per liposome remains to be resolved, mainly because of technical challenges in directly quantitating ligand density on liposomes. This is an underdeveloped research area and requires focused efforts. It is logical to predict that immuno-liposomes with high antibody densities may be desirable for antibody fragments, because this will lead to better binding avidity of the immuno-liposomes for the target antigen. In addition, high antibody densities on the surface of liposomes will provide multimeric binding sites to the cell surface receptors, potentially leading to the initiation of signal transduction mechanisms. Although high binding affinities are desirable, low-affinity ligands may be preferred in certain scenarios because they may allow liposomes to penetrate further into the tumor interior, decreasing the “binding site barrier.”116
b. Affisomes
Recently, a novel class of small molecules called “affibodies,” which can be considered antibody mimics, have been examined for liposome targeting. Affibody molecules are relatively small proteins (6–8 kDa) that offer the advantage of being extremely stable, highly soluble, and readily expressed in bacterial systems or produced by peptide synthesis. The binding affinities of affibody molecules are considerably higher compared with the corresponding antibodies. (Detailed information about the production and characterization of affibody molecules can be found at www.affibody.com).
Recently, we conjugated an 8.3-kDa HER2-specific affibody molecule (Z(HER2:342)-Cys) to the surface of thermosensitive liposomes (called “affisomes”) aimed at improving the targeting efficacy of these liposomes for breast cancer treatment.96 Another study by Beuttler et al.95 used a bivalent, high-affinity epidermal growth factor receptor (EGFR)-specific affibody molecule (14-kDa) for targeting PEGylated liposomes to EGFR-expressing tumor cell lines. Enhanced cytotoxicity toward EGFR-expressing cells was detected with mitoxantrone-loaded affibody targeted liposomes compared with untargeted liposomes in these studies.95 In another study, HER2-specific affibody molecules were used to fabricate nanoparticle-affibody conjugates composed of poly(D,L-lactic acid) and pegylated lipids. The resulting nanoparticles showed specific binding and uptake by HER2+ cells.117 Since the receptor-binding domains of affibodies may differ from that of antibodies, affisome uptake mechanisms may result in altered outcomes. Therefore, further studies in vitro and in animals are needed to establish the projected advantage of affibodies as targeting ligands for liposomes.
c. Folate as a Targeting Ligand
Folate receptor (FR) expression is known to be up-regulated in many human cancers, such as ovarian, lung, breast, kidney, brain, and colon cancers, and receptor density appears to increase as the disease progresses.117,118 FR has two glycosyl phosphatidylinositol-anchored isoforms: alpha and beta. FR-alpha expression is frequently amplified in epithelial cancers, whereas FR-beta expression is found in myeloid leukemia and activated macrophages associated with chronic inflammatory diseases. The vitamin folic acid binds to FR with high affinity and results in efficient internalization into cells. Therefore, FR is an attractive target for tumor-specific drug delivery.119 Interestingly, another feature of FR is its location on the apical membrane of epithelial cells, where it is not accessible from blood in normal cells. Studies by Low et al. show that folic-acid-drug conjugates are attractive molecules for folate targeting in vivo.118,120,121
In the field of lipid-based nanoparticles, folate lipid-bearing, drug-loaded liposomes have been investigated.122–127 Initial studies using the doxorubicin-loaded liposomes (folate lipid+) showed promising data; these liposomes displayed a 45-fold higher uptake than their non-targeted controls, and cytotoxicity of targeted liposomes was found to be 85 times greater than similarly loaded controls.122 These observations stimulated tremendous interest in the liposome field as well as other nano-carriers.88 Although initial animal studies showed an early increase in folate-targeted liposomes, overall uptake and tumor regressions were not significantly improved in vivo, and were tumor-type dependent.127 However, the same group has recently reported that folate-targeted liposomal doxorubicin formulations bear significant advantage, especially for intracavitary therapy, further suggesting tumor biology will be a major determinant in the treatment outcome.126 Therefore, the future of folate-targeted liposomes in the clinical setting remains uncertain at present.
III. BOLAAMPHIPHILES AND NANO-STRUCTURES FOR DRUG DELIVERY
III.A. Monolayer Membrane from Bolaamphiphiles
Bolaamphiphiles (also called bolalipids) are a unique class of lipids that bear two hydrophilic head groups situated at both ends of a hydrophobic domain (Figs. 8A and B). In contrast to single-hydrophilic head amphiphiles such as phospholipids (Fig. 2A), which form bilayer membranes, bolaamphiphiles may form monolayer membranes128 (Fig. 8A). Studies demonstrate that membranes made from bolaamphiphiles are less permeable and more durable than membranes composed of monopolar lipids.129,130 This unique combination of properties has ignited interest in the potential use of bolaamphiphiles as membrane-stabilizing agents in applications such as drug delivery and membrane-protein-based bio-sensors.131–133
FIGURE 8.
Bolaamphiphiles. A, Self-assembly of bolalipids into monolayers and comparison with bilayer forming lipids such as phosphatidylcholine. B, Chemical structures of various types of bolalipids: (a) bolaamphiphilic phosphocholine, (b) symmetrical bipolar phospholipid, and (c) unsymmetrical bipolar phospholipids. C, Possible assembly and aggregate structures of bolaamphiphiles.
Unsymmetrical bolaamphiphiles bearing two different hydrophilic head groups are attracting considerable attention in the field of nano- and biotechnologies because they can self-assemble in water to form unsymmetrical monolayer lipid membranes with parallel molecular packing,134–139 which results in nanostructures that possess different inner and outer surfaces covered with each head group. These nanostructures are applicable to construct delivery and medical diagnosis systems based on selective and effective encapsulation of functional nanomaterials and biomolecules into their inner space.137,140
In nature, bolaamphiphiles are found in archaebacteria, organisms that survive in volcanic environments under extreme conditions.141,142 The monolayer architecture of the archeabacteria enables them to survive in high-temperature and low-pH conditions,143 meaning that their bolaamphiphiles are excellent candidates as building blocks of nanoparticles for drug delivery.144,145 However, the chemical synthesis of bolaamphiphiles such as those found in archaebacteria is faced with technical challenges146–147; extraction techniques from the organism itself result in limited recovery of these lipids and therefore they are not yet commercially viable. Therefore efforts are focused on synthetic novel bolaamphiphiles and their ability to aggregate into nano-sized particles.148
III.B. Synthetic Bolaamphiphiles and Their Aggregation into Nano-structures
Synthetic analogs of the membrane-spanning lipids of archaebacteria have been prepared by Fuhrhop et al.149 In addition to Fuhrhop’s extensive work, efforts have been made by other groups to design and synthesize bolaamphiphiles with different structures (Fig. 8B) and to characterize their aggregation behavior in aqueous solutions.150–155 Depending on their molecular structure, bolaamphiphiles can form micelles, vesicles, multilayered sheet rings, and a variety of micro- and nano-structures with cylindrical geometry such as rods, tubules, ribbons, and helices (Fig. 8C).128 Polidori et al.154 reported the synthesis of symmetric bolaamphiphiles with systematic changes in their molecular structure, such as length and rigidity of the aliphatic chain, polar head volumes, and the presence of hydrogen-bonding groups. Studies with these synthetic bolaamphiphiles showed that the primary structures into which they aggregate are micelles. However, bolaamphiphiles with two hydrocarbon chains, as well as some single-chain bolaamphiphiles, formed spherical vesicles in aqueous solution.154 Studies with asymmetrical bolaamphiphiles showed that 1-galactosamide bolaamphiphile self-assembles to form a multilayer structure comprising unsymmetrical monolayer lipid membranes linked via a sugar–carboxylic acid H-bonding interface.156 The molecular parameters that seem to determine the natural shape and stability of the particles depend on the number and the length of the aliphatic chains, saturation versus unsaturation of the C-C bonds within the aliphatic chain, sizes of the head groups relative to the hydrophobic domain (see below), and the presence of hydrogen bond forming groups.
Typically, short-chain bolaamphiphiles form micelles, whereas long-chain bolaamphiphiles form vesicles.128 However, an incorporation of a diamide midsection into the hydrophobic chain of bolaamphiphiles157 caused short-chain bolaamphiphiles to form vesicles and long-chain bolaamphiphiles to form fibers. By comparison, when bolaamphiphiles with diester midsections157 were examined for their ability to form nanostructures, both short- and long-chain bolaamphiphiles formed vesicles, which were more stable than vesicles made from bolaamphiphiles with diamide midsection and therefore are more adequate for drug delivery.
III.C. Formation of Vesicles from Bolaamphiphiles for Drug Delivery
Liposomes made from phospholipid amphiphiles that aggregate into bilayer membranes have been extensively investigated as drug-delivery systems.158–162 However, in many cases, liposomes injected into the bloodstream are rapidly cleared from the system and only a fraction reach the target site, even when PEG-coated liposomes are used.163 Promising alternatives include vesicles composed of monolayer membranes made from bolaamphiphiles, which are potentially more stable than the classical bilayer liposomes and less likely to fuse with each other or with cell membranes due to reduced lipid exchange.150,164 This feature of reduced lipid exchange enables vesicles to cross biological membranes while maintaining their structure (see below). However, not all bolaamphiphiles aggregate into vesicles and some may form other nanostructures. Amphiphiles that form vesicles are mainly those having a ratio of the cross-sectional area of the apolar to polar regions of 0.74 to 1.0.165–167 When immersed in an aqueous solution at a concentration higher than the critical aggregation concentration (CAC) and at a temperature above the solid-ordered to liquid-disordered phase transition, the lipids aggregate spontaneously and form vesicles.167,168 Bolaamphiphiles generally have higher water solubility and a high CAC in the range of 10−4 to 10−6 M154,169 due to the presence of two head groups, resulting in vesicle membranes that are less stable upon dilution. In comparison, phospholipids have lower CAC values within the range of about 10–8 M,159 and from that point of view, they may form more stable vesicles than bolaamphiphiles. This point has to be taken into account when designing bolaamphiphiles to produce stable vesicles for drug-delivery applications—their CAC should be sufficiently low in order to maintain the vesicle structure independent of vesicle concentration.170 The choice of the fatty acyl chain length and the head group properties are important determinants to ensure stability of vesicles prepared from bolaamphiphiles. Indeed, it has been demonstrated that vesicles from bolaamphiphiles with short aliphatic chains were less stable than vesicles made from similar bolaamphiphiles with longer aliphatic chains.171
Other structural features of the bolaamphiphiles that are known to influence vesicle formation are the number of the aliphatic chains within the amphiphiles and the presence of symmetrical versus non-symmetrical head groups. Some single-chain bolaamphiphiles with symmetrical head groups were shown to form spherical vesicles, but only when they were formulated with single head amphiphiles and constituted no more than 20% of the formulation.172 Other single-chain symmetric bolaamphiphiles form tube-like vesicles136 or fibrous173 or helical175 structures. Investigators 154 have succeeded in preparing vesicles with monolayer membranes from a symmetrical single-chain bipolar ammonium salt. Monolayer membrane vesicles were shown to be formed from symmetrical bolaamphiphiles with relatively short (C16 and C20) membrane-spanning alkyl chains, but these vesicles were stable only when cholesterol was used in the formulation, presumably by adopting an asymmetric distribution of this component at opposing membrane interfaces, thus allowing the formation of highly curved surfaces.175 Alternatively, asymmetric bolaamphiphiles can be used to improve the curvature of the bolaamphiphiles within the membrane instead of using additives such as cholesterol. In this context, a highly stable monolayer membrane was obtained from asymmetric bolaamphiphiles extracted from the thermophilic archaeobacterium Sulfolobus solfataricus.132,176 Kai et al.132 demonstrated the formation of vesicles with high thermal stability with synthetic asymmetric bolaamphiphiles with long hydrophobic chains. Recently, Grinberg et al.157 demonstrated the formation of stable cationic vesicles from synthetic short-chain asymmetric bolaamphiphiles. The improved stability of vesicles made from short-chain asymmetric bolaamphiphiles can be attributed to the ability of the different-sized head groups to accommodate differences in the radii of curvature of the inner and outer surfaces of the monolayer membrane. Symmetric bolaamphiphiles do not generally form stable spherical vesicles because the same head group cross-sectional areas and volumes cannot accommodate the difference in the radii of the inside and outside surfaces of the monolayer membrane.128 Therefore, it can be predicted that a molecular design that results in adequate packing of bolaamphiphiles to form stable monolayer membrane vesicles will be well-suited for targeted drug delivery. From the studies described above, it can be assumed that monolayer membrane vesicles may have the characteristics needed for targeted delivery of drugs, as well as for releasing the encapsulated drug in a controlled manner. This assumption is based on the following considerations.
Bolaamphiphiles are expected to form more stable vesicles than liposomes made of bilayer membranes due to the high activation barrier towards pulling the inner charged head group through a hydrophobic matrix of a monolayer membrane.128,150 By comparison, bilayer vesicles and liposomes can grow by fusion because of an easy amphiphile exchange with the exterior. Although PEGylation of liposomes is used to reduce fusion, it does not completely eliminate it,177,178 and aggregation and fusion may still be a problem with PEGylated liposomes. In fact, under certain conditions, PEGylation may even increase fusion.179 Therefore, from this point of view, vesicles made from bolaamphiphiles that do not easily fuse may be superior to PE-Gylated liposomes.
A reduced lipid exchange is also deemed especially important for the transport of the liposomes intact through biological barriers. Because vesicles made from bolaamphiphiles are characterized by reduced lipid exchange, they may be superior to bilayer membrane liposomes when passage via a biological barrier is required. PEGylation of bilayer membrane liposomes is not sufficient to solve the problem, because steric hindrance by the PEG-shielding layer may interfere with cellular uptake.180–182
Monolayer membranes made of bolaamphiphiles with long aliphatic chains and polar groups within the chains offer the possibility of improved flexibility and elasticity (because they will be more resistant to shear forces) over that of bilayer membrane liposomes. Lipid bilayer membranes do not exhibit surface shear rigidity above the order-disorder transition temperature.183,184 Because the lipids in biological membranes exist above the order-disorder transition, such membranes may not tolerate shear forces well, which hampers their elasticity. Monolayer membranes, on the other hand, have higher elasticity185 because they tolerate greater shear forces. This would allow for shape change while penetrating though biological barriers, enabling the vesicles to remain intact during the penetration process.
The ability to obtain a more effective disruption mechanism in monolayer membranes compared with bilayer membranes is another advantage of bolaamphiphiles. This is based on the observation that bolaamphiphiles are known to form self-aggregating structures that readily convert from vesicles to fibers or sheets upon small changes in their structure,128,151 especially with small vesicles due to curvature considerations.186 Thus, vesicles made from bolaamphiphiles will have superior stability as described above, and yet, upon removal of the head groups, the content of the vesicles will be readily released.
Based on a calculation of the inner vesicle volume, a higher encapsulation volume can be achieved in nano-sized vesicles made from bolaamphiphiles. This is because the monolayer membrane is thinner than the bilayer membrane, resulting in a larger inner volume in monolayer vesicles compared with bilayer vesicles of the same size.
To date, only a few attempts have been made to obtain monolayer vesicles from bolaamphiphiles for targeted drug delivery, because the structural requirements for the formation of stable vesicles from such bolaamphiphiles were not well-understood. When analyzing lipid layer stability for drug-delivery applications, two main factors should be addressed: permeability of the vesicle membrane and prevention of delamination.186 Permeability in lipid membranes is related to the packing of the hydrocarbon chains. In the case of bolaamphiphiles, it was found that the permeability of the bolalipid layer is reduced compared with that of a monopolar lipid bilayer.187 Bilayer membranes can easily undergo delamination, since they are weakly bonded at the bilayer midplan, resulting in low mechanical stability.188 Delamination can also be prevented by using bolaamphiphiles because they have the ability to adopt a transmembrane configuration that completely spans the hydrophobic region of the lipid layer by placing the polar head groups at opposite membrane-water interfaces,189,190 thus making such bolaamphiphiles appropriate for several biotechnological applications because they can stabilize the membrane. Recently, a series of novel symmetric bolaamphiphiles that form stable vesicles with potential applications in targeted drug delivery have been described.157 Based on systematic changes in the bolaamphiphile structure and the relationships to the performance of the vesicles, new bolaamphiphiles that may form improved vesicles for targeted drug delivery can now be synthesized.171,191 Vesicles made from similar amphiphiles have already been shown to be effective in gene transfer192,193 and in the delivery of peptides to the brain192, the latter probably due to the stability and the flexibility of the monolayer membrane vesicles that allowed them to penetrate intact via the blood-brain barrier.
In summary, bolaamphiphiles are potential candidates for the formation of nanoparticles for targeted drug delivery and therefore deserve a closer look for the development of novel lipid-based nanoparticles. The suitability of bolaamphiphiles as building blocks of vesicles for drug delivery was well demonstrated with archeosomes, which are made from bolalipids extracted from archaebacteria,194–197 and with synthetic bolaamphiphiles based on the bolalipids of archaebacteria.145,154 These studies show that the structures of bolaamphiphiles determine the nature of the nanoparticles they form and their advantage in targeted drug delivery. In order to design optimal nanoparticles for drug delivery, understanding the rules of molecular self-organization is important. To this end, the structural requirements needed for bolaamphiphiles to form vesicles for targeted drug delivery are under investigation, and new efforts are focusing on rational design of bolaamphiphiles for targeted drug delivery.
IV. SOLID LIPID NANOPARTICLES (SLN) AND NANOSTRUCTURED LIPID CARRIERS (NLC)
Although liposomes have been the hallmark of lipid-based nanoparticles for site-specific delivery of drugs and pharmaceuticals, there is a need to develop alternate approaches for nanoparticles based on lipid components other than phospholipids. It is hoped that these drug carriers may allow for higher control over drug release and delivery of therapeutics, which may not efficiently load in to liposomes. Compared with other drug-delivery systems, SLN and NLC have been developed very recently and are potentially attractive, marketable choices due to their natural components and are easily scaled-up synthesis processes. Both SLN and NLC are well positioned for large-scale manufacturing, as solvent use can be avoided using the high-pressure homogenization method with extant machinery.198 In addition, their hydrophobic core provides a suitable environment for entrapment of hydrophobic drugs. This is important, as approximately 40% of newly developed drugs are hydrophobic in nature.198,199
Numerous reports have described various SLN formulations since the early 1990s, and NLC formulations since the late 1990s, that may find applications in drug-delivery systems. The SLN structure is composed of a solid lipid core, which may contain triglycerides, glyceride mixtures, or waxes that are solid at both room temperature and human body temperature.200,201 The diagram in Figure 9 depicts the possible assembly of triglycerides to generate SLN and NLC. SLN are interesting lipid-based drug-delivery carriers for a number of reasons, including: i) particle size is on the nano- to sub-micron scale (50–1000 nm) after drug encapsulation; ii) they are composed of biocompatible and biodegradable components (i.e., physiological lipids or lipid molecules) and do not require the use of organic solvents for their assembly; and iii) the particle synthesis process (e.g., high-pressure homogenization) can be performed at a lower cost and are easily scaled up. Therefore, these nanoparticles bear the positive aspects of other nano-lipid carrier systems, and they also overcome several of their disadvantages. For example, SLN are similar in nature to nanoemulsions, but feature a solid lipid core as opposed to a liquid lipid version. As a result, drug mobility decreases in the solid lipid state compared with the oily phase, thereby enhancing the controlled release of loaded drugs.198 SLN stability can be further improved by the addition of a surfactant coating.198 An additional advantage involves the production of SLN in a powder form, which may be loaded into pellets, capsules, or tablets for further enhancement of drug delivery. It is important to mention that applications of SLN formulations may be limited due to the undesired particle growth by agglomeration or coagulation resulting in rapid “burst release” of the drug. SLN have perfect crystal lipid matrices that accommodate the loaded drug in its molecular form between fatty acid chains. The formation and enhancement of the crystal structure during both production and storage of SLN often results in expulsion of the loaded drug solution, a major disadvantage of the nanoparticle.198,201
FIGURE 9.
Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC).
NLC, often referred to as the second generation of SLN, were first developed by Müller et al. in the late 1990s. In contrast to the lipid crystal matrix of SLN, the lipid matrix of NLC has an imperfect crystal or amorphous structure, which allows for drug loading in both the molecular form and in clustered aggregates at lattice imperfections (Fig. 9). As a result, NLC show enhanced drug loading and less pronounced drug expulsion by avoidance of a crystal structure.198
NLC, similar to SLN, are colloidal particles that typically range in size from 100 to 500 nm, depending on production parameters.199,200,203 A blend of solid- and liquid-phase lipids, NLC are generally solid at temperatures above 40°C. They have been successfully multifunctionalized to capture a payload of drugs, to target specific cells, and to release entrapped drugs in a controlled manner.198,199 NLC have been mostly researched for oral or dermal drug delivery applications, with little focus on parental administration; however, recent literature has demonstrated their potential as attractive candidates for the delivery of anticancer agents, as well as therapeutic proteins and peptides.198,199,202
IV.A. Formation of SLN and NLC
1. SLN
Encapsulation of drug solutions into SLN can be performed using numerous methods, including high-pressure homogenization, microemulsion formation, emulsification-solvent evaporation (precipitation), solvent injection (or solvent displacement),203,204 phase inversion,205 the multiple emulsion technique,206,207 ultrasonication,209 and the membrane contractor technique.209–211 A typical SLN formulation includes 0.1% to 30% solid lipid content, including one or more of the base ingredients (trimyristin, tristearin, trilaurin, stearic acid, glyceryl caprate as Capmul®MCM C10, theobroma oil, triglyceride coconut oil, 1-octadecanol, glycerol behenate as Compritol® 888 ATO, glycerol palmitostearate as Precirol® ATO 5, and cetyl palmitate wax); 0.5% to 30% surfactant stabilizer (examples previously mentioned); and 5% of the incorporated drug. For longer circulation time in vivo, curdlan and PEG molecules have been used.
Encapsulation of drug molecules can occur at various locations within SLN depending on their chemical properties. Lipophilic drugs will disperse well due to their miscibility in the lipid matrix, whereas hydrophilic drugs are thermodynamically immiscible and will separate to the outside of the lipid matrix. Typically, the SLN assembly process involves dispersion of drugs into a melted-lipid phase (precursor emulsion) either by using the appropriate solvent(s) or application of mechanical forces. For successful drug loading into SLN, the drug needs to adequately partition into the lipid droplets. In the synthesis step of SLN, fast cooling creates an unstable and disordered α-crystalline structure, which allows the desired drug to be stored in to the nanoparticle’s amorphous areas. During the storage period, this α-crystalline state can be converted to a thermodynamically stable state, which is β-crystalline in nature. The exact partitioning of the drug in SLN depends on the recrystallization rate of the lipid matrix and the resulting crystalline structure. Because drug molecules incorporate between the fatty acid chains, lipid layers, and in areas of crystal imperfections, a highly ordered and organized crystalline structure is not desirable for higher drug-loading capacities. It is important to note that the structural transformation from α-crystalline to β-crystalline can result in a “burst release” upon administration into the body, a significant drawback for SLN in the clinical setting. Recently, reverse micelle formation by the amphiphilic lipid lecithin within the lipid matrices has been shown to increase SLN drug-loading capacity.204
2. NLC
Similar to SLN, a mixture of solid- and liquid-phase lipids are used to create NLC. Usually, about 5% of drug (by weight) is incorporated in the lipid mixture upon initial NLC production, and approximately 3% to 4% drug loading is achieved (with typical encapsulation efficiencies of approximately 70%).199,212 Examples of solid-phase lipids typically utilized include monostearin, stearic acid, glyceryl dilaurate, hydrine, glyceryl, monostearate, cetyl alcohol, and imwitor 900; typical liquid phase lipids used are oleic acid, capmul glyceryl mono-dicaprylate, and caprylic/capric triglycerides.
Appropriate lipid selection is crucial for creating a stable drug-loaded NLC. The chemical stability of the drug is dependent on the type of solid lipid incorporated into the NLC. Similarly, the incorporation of drug into lattice defects of the NLC may alter particle stability (most likely enhancing stability). In addition, possible lipid interactions with the drug during and after NLC production should be considered. For example, auto-oxidation of lipid may cause drug degradation.213
Similarly, the percentage of liquid-phase lipid incorporated can influence the size and surface morphology of particles. Hu et al. showed that as the concentration of the liquid-phase lipid oleic acid increased up to about 30%, particle size decreased, and particle morphology became more spherical, smooth, and regular. In addition, their studies suggest that oleic acid concentration controls the initial rate of drug release.214
NLC may be subdivided into three categories based on the structure of their lipid matrix: the imperfect type, multiple types, and amorphous or structureless type (Fig. 9). The imperfect type of NLC has the least amount of liquid-phase lipid (oil) and is composed of saturated and unsaturated lipids with varying fatty acid chain lengths, which lead to defects in the lipid matrix and compartments for drug storage.213 However, less pronounced than in SLN, the imperfect NLC is prone to an expulsion of drugs during the crystallization process of production. Toward the end of production, the temperature is lowered, lipids transition from their melted state to a solid phase, and particles crystallize. This causes drug solubility to decrease and the subsequent release of entrapped drug from the lipid matrix.215
The multiple type of NLC avoids this drug expulsion by incorporating a higher concentration of liquid-phase lipids in the lipid matrix. During the cooling process, oil reaches its solubility limit and precipitates into nanocompartments. Compared with other SLN and NLC formulations, these nanocompartments can accommodate a higher drug concentration.216 In addition, a higher oil concentration is associated with faster drug release.217 The amorphous type of NLC forms a solid lipid that lacks any crystalline structure. This is achieved through the use of lipids such as hydroxyoctacosanylhydroxystearate and isopropyl-myristate.215 As expected, the lack of a crystalline structure avoids undesired drug expulsion during the cooling process.
3. Stabilization
The in vitro and in vivo stabilization of SLN and NLC is commonly achieved through PEGylation or polymer coating (e.g. PEG2000, PVA, poloxamers),218–221 which has been already used for doxorubicin-formulated liposome stabilization (i.e., Doxil®). The addition of PEG molecules prevents immunoprotein adsorption and minimizes phagocytic uptake by macrophages, thus increasing blood plasma circulation time.222,223 PEG has been successfully incorporated into lipid matrices by its conjugation to monostearate (PEG-SA). However, the incorporation of PEG-SA into NLC reduced drug encapsulation efficiency and increased the rate of drug release.217
Typically, PEG lipids are mixed with other solids and melted together. Past studies have found a five-fold enhancement of doxorubicin plasma concentration by a SLN carrier, and a seven-fold enhancement for PEG-stabilized stearic acid SLN have been reported.224 PEGylation has also been shown to increase oral delivery of peptide drugs, including calcitonin.225 However, literature studies show that SLN themselves increased drug circulation time significantly, providing higher drug concentration in the bloodstream when lecithin-based (e.g., Epikuron 200) surfactants were used for stabilization. The “stealth effect” was not found to be significant in these SLN, and more intensive studies are required to understand this effect with SLN through the use of PEGylation.
IV.B. Therapeutic Drug Delivery
There are several advantages of therapeutic drug delivery by SLN and NLC: i) they can control and extend drug release, ii) they can encapsulate various drugs, and iii) they can extend blood circulation time and utilize the EPR effect for enhancing treatment. Hydrophobic drugs with short circulation half-lives are ideal candidates for delivery via SLN and NLC. Many pharmaceutically active peptides and proteins are being developed. However, they are often characterized by a short half-life in the body and a limited ability to cross cell membranes. NLC may be an ideal carrier for their delivery, because it can protect the protein or peptide from degradation and may transport the therapeutic into the cell interior. However, the encapsulation of peptides and proteins into these lipid carriers is not always realizable. High temperatures associated with HPG and solvents associated with other production methods may denature and degrade proteins.199 Hydrophilic drugs are also candidates for delivery by lipid nanoparticles by using lipid drug conjugates.226
Therapeutic compounds can oftentimes be chemically reactive. SLN and NLC have the ability to protect labile anticancer drugs known to be susceptible to hydrolysis and, as a result, the active drugs remain in the bloodstream for a longer period of time. One example is the SN-38 compound, a relatively hydrophilic pro-drug of irinotecan that also carries a labile lactone structure such as camptothecin. SLN loaded with SN-38 protected the hydrolysis of the drug and increased the treatment effect. In addition, an extended half-life of the active lactone drug form in the whole blood was observed in in vivo nude mice studies.
A wide variety of drugs, such as prednisolone, doxorubicin, and retinol, have been successfully incorporated into SLN.227–231 Similarly, the anticancer therapeutics paclitaxel and doxorubicin have been successfully loaded into NLC.199 Compounds with the deaza skeleton of the antitumor drug temozolomide have also shown promising anticancer results when loaded in NLC.232 In addition, progesterone, valdecoxib, clobetasol propionate, closporine, retinol, Celecoxib, and etomidate have all been successfully loaded into NLC (some for transdermal drug delivery).212,214,217 The anticancer therapeutics paclitaxel and doxorubicin loaded into NLC have been found to overcome cell-multi drug resistance.199 In their study on the effects of a SLN formulation on the human colorectal cancer cell line HT-29, Serpe et al.233 demonstrated that SLN containing cholesteryl butyrate and doxorubicin showed significantly higher cytotoxic effect than the equivalent amount of free drug. In cancer chemotherapy, cancer cells continuously exposed to sub-optimal levels of cytotoxic agents may induce the expression of membrane-associated drug transporters (e.g., P-glycoprotein), thus rendering the cells more drug resistant. The enhanced chemotherapeutic effect by the lipid nanocarriers could be the result of efficient endocytosis into the cells, thereby bypassing the P-glycoprotein drug efflux mechanism. This effect has also been reported in doxorubicin-loaded SLN on murine and human breast cancer cell lines.
Drug administration can be facilitated by the submicron size and controlled drug release of SLN. Various administration routes (parenteral, pulmonary, mucosal, and topical) have been studied. Due to their nano-scale size, SLN can be delivered parenterally and can increase circulation times of therapeutic agents. Parenteral administration is suitable for drug targeting by SLN, and peptide and protein drugs are commonly supplied by this administration method due to the avoidance of enzymatic degradation in the GI tract, which is possibly in oral dosage. Initial work on SLN on the oral delivery of lipid nanopellets was reported, and a cyclosporine SLN formulation has been introduced to the market for oral administration. Interest in ocular administration of SLN has peaked due to their biocompatibility and mucoadhesive properties. Tobramycin delivery in rabbit eyes suggested that the drug bioavailability was significantly enhanced by SLN. Additionally, SLN formulations in a nebulized form have been used to carry anti-tubercular drugs for treatment of pulmonary tuberculosis, and showed an improved dug treatment effect. Finally, SLN have also been extensively applied to dermal applications in prolonging the shelf lives of sensitive compounds such as retinol and vitamin E.234
Entrapped therapeutics are released from NLC through natural diffusion and lipid degradation. Most NLC exhibit a biphasic drug release pattern consisting of an initial release burst followed by prolonged release afterward. The initial burst release rate can be controlled by the concentration of liquid lipid.199 An energetic impulse given to an NLC is expected to convert the disordered lipid matrix into a more crystal structure. In turn, this may drive out entrapped therapeutics. This phenomenon is evident in the transdermal delivery of cyclosporine in lipid particles. Burst release of the drug is initiated upon application to the skin, where it is exposed to body temperature and nearby evaporation of water, which convert the lipid matrix to a higher crystalline order, expelling the drug.198
IV.C. Targeting Ligands: SLN
Targeting strategies for tumor sites by SLN can also be combined to minimize side effects and enhance specificity to sites of interests. Liposomal-targeting approaches have been adapted directly to SLN formulations (i.e., ligand binding to the surface of nanoparticles). In one approach, researchers have prepared docetaxel-loaded SLN with a galactosylated conjugated DOPE lipid to specifically target the asialo-glycoprotein receptor on hepatocellular carcinoma cells.235 Additional work has been performed on a folate-targeted SLN system, which was developed for the delivery of the drug paclitaxel.121 The targeted formulation resulted in greater drug uptake and cytotoxicity in folate receptor cell lines than non-targeted SLN, and significantly improved in vivo tumor growth inhibition and tumor-bearing animal survival. For liver targeting in particular, SLN containing galactosylated or mannosylated lipids have been employed. Although SLN with and without galactosylation showed higher liver targeting compared with free drug solutions, galactosylated SLN displayed further enhancement when comparing in vitro activity and in vivo biodistribution of all formulations.236
Finally, these nanoparticles have also been utilized for drug delivery to the brain. Developments of nanocarriers to deliver drugs to the central nervous system have been limited due to their inability to cross the restrictive blood-brain barrier.237 On the other hand, the highly vascularized nature of brain tissue makes it an attractive choice for intravenous delivery of therapeutic drugs. SLN and NLC take advantage of the high capillary density of the brain, and at the same time possess the ability to overcome blood-brain barrier limitations.238 These formulations have been utilized to enhance delivery of antiretrovirals to the brain, such as the HIV protease inhibitor Atazanavir239 and drugs to treat cardio-cerebrovascular diseases, including daidzein.240 Treatment of Parkinson’s disease has also involved the use of SLN to deliver dopamine agonists to the brain. In comparing bromocriptine (BK)-loaded SLN to free BK in both in vitro and in vivo studies, Esposito et al. reported a faster preliminary release and subsequent slower gradual release of the drug associated with the SLN formulation.241 In 2007, Gupta et al. reported findings on the treatment of cerebral malaria with transferrin conjugated-SLN encapsulated with quinine hydrochloride.242 Drug release was found to be greater in the unconjugated SLN, whereas transferrin-SLN showed greater accumulation in brain tissue. Researchers have also demonstrated that thiamine-coated SLN successfully bind to the blood-brain barrier thiamine transporter, and result in a gradual buildup of SLN that is responsible for increased brain uptake.243 SLN and NLC are seen as promising drug-delivery systems, but in the case of the brain, they are even more important in terms of successfully overcoming the restrictions imposed by the blood-brain barrier.244
IV.D. Future Applications
The versatility of SLN and NLC as drug-delivery systems makes them an attractive choice as carriers of diverse anticancer cytotoxic agents and peptide drugs. The use of these lipid nanoparticles in drug vectorization is now being tested in both in vitro and in vivo studies for commercial applications. Surface modifications may make the SLN delivery system even more attractive, for example, through increased bioavailability with PEG coating.221 However, some disadvantages such as polymorphism and crystalline rearrangements in SLN and some formulations of NLC must be overcome to achieve controllable and stable drug delivery. Moreover, the release of the active molecules incorporated into these solid nanoparticles should be further investigated. For commercial applications, the quality control of the natural components in the SLN production has to be considered more seriously. NLC appear to be attractive vehicles for intravenous drug delivery; however, their application in this field is in the early exploratory stages of research. Future studies may enhance the controlled release of therapeutics from these carriers and improve the production process for more homogeneous samples pure of toxic compounds. Furthermore, their applications in cancer drug delivery remain to be seen.
V. FUTURE PERSPECTIVES OF LIPID-BASED NANOPARTICLES IN DRUG DELIVERY
Various nanotechnology platforms are currently being developed with the aim of improving drug delivery, especially to combat cancer. Among these, lipid-based nanoparticles present one of the promising drug-delivery candidates and have been the longest-studied nanocarriers. The various systems discussed here are summarized in Table 4. Despite tremendous efforts, only a few formulations are approved for clinical use thus far. In addition, the clinical applications of targeted nanoparticles remain to be seen. Therefore, it is imperative to re-visit considerations of current approaches and strategies employed in the design and development of these anticancer nanocarriers. In our opinion, efforts could be focused primarily on two areas: i) technical aspects such as fabrication strategies, the development of techniques for reproducible nanocarriers, large-scale production, and the conjugation of targeting molecules such as scFvs and peptides to the nanoparticle surface; and ii) novel concepts and approaches to accomplish on-demand release of drugs from the nanoparticles (based on the unique properties of the assembly components of lipid-based nanocarriers). We surmise that the future for on-demand drug-phospholipid assemblies (liposomes) is promising as strides are being made for on-demand drug release from targeted nanoparticles. Bolalipids bear several unique properties compared with glycerol-based phospholipids (used to fabricate liposomes), and their applications as nanocarriers are currently in the infant phase. Thus, the future of bolalipid drug-delivery assemblies remains to be seen. Similarly, SLN and NLC are very attractive drug-delivery candidates, primarily due to their relatively stable constituents and probable ease of drug encapsulations. However, their future in the clinical setting is also subject to extensive research.
TABLE 4.
Overview of Currently Available Lipid-Based Nanoparticles
| Lipid carrier | Base composition | Assembly | Clinical applications | References |
|---|---|---|---|---|
| Liposomes (elastic, transfer-somes, magnetic), 25–200 nm, 1965 to date | Phospholipids, sphingolipids, cholesterol, PEGylated lipids | Phospholipid bilayer surrounding an aqueous core (well-characterized) |
|
52,294–297 |
| SLN, 50–1000 nm, 1990s to date | Pure triglycerides, glyceride mixtures, waxes (primarily saturated fatty acid chains) | Solid lipid core, hydrophobic in nature, with a surfactant coating |
|
201,221,229,298 |
| NLC, 100–500 nm, 2000s to date | Pure triglycerides, glyceride mixtures, with saturated and unsaturated fatty acid chains, waxes | Second-generation SLN; blend of solid and liquid lipid phases |
|
212,299 |
| Monolayer membrane structures: archaeosomes, vesicles from synthetic bolalipids and micelles | Natural bolaamphiphiles from archaebacteria, synthetic bolaamphiphiles with and without cholesterol, cholesteryl hemisuccinate, PEGylated lipids, and chitosan-lipid conjugates | Monolayer membrane surrounding an aqueous core, micelles, and a variety of cylindrical and fiber structures |
|
157,171,300 |
Additionally, it is our viewpoint that two important aspects for clinically viable nanocarriers will facilitate their usefulness in the clinic: the development of triggering modalities that are amenable to human applications and the development of alternate strategies for in vivo stabilization of drug-delivery vehicles. Although the concept of PEGylation to increase half-life of nanoparticles revolutionized the nanoparticle-mediated drug-delivery field, significant improvements are warranted in this area.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research. We thank Dr. Kshitij Gupta for critical reading of the manuscript.
References
- 1.Wang AZ, Gu F, Zhang L, Chan JM, Radovic-Moreno A, Shaikh MR, Farokhzad OC. Biofunctionalized targeted nanoparticles for therapeutic applications. Expert Opin Biol Ther. 2008;8(8):1063–70. doi: 10.1517/14712598.8.8.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine. therapeutic applications and developments. Clin Pharmacol Ther. 2008;83(5):761–9. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 3.Alaouie AM, Sofou S. Liposomes with triggered content release for cancer therapy. J Biomed Nanotech. 2008;4(3):234–4. [Google Scholar]
- 4.Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303(5665):1818–22. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 5.Alonso MJ. Nanomedicines for overcoming biological barriers. Biomed Pharmacother. 2004;58(3):168–72. doi: 10.1016/j.biopha.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Sapra P, Allen TM. Ligand-targeted liposomal anticancer drugs. Prog Lipid Res. 2003;42(5):439–62. doi: 10.1016/s0163-7827(03)00032-8. [DOI] [PubMed] [Google Scholar]
- 7.Torchilin VP. Drug targeting. Eur J Pharm Sci. 2000;11(Suppl 2):S81–91. doi: 10.1016/s0928-0987(00)00166-4. [DOI] [PubMed] [Google Scholar]
- 8.Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm Res. 2007 Jan;24(1):1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Y. Lipid nanotubes: formation, templating nanostructures and drug nanocarriers. Critical Reviews in Solid State and Materials. Sciences. 2008;33(3–4):183–96. [Google Scholar]
- 10.Ganta S, Devalapally H, Shahiwala A, Amiji M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J Control Release. 2008;126(3):187–204. doi: 10.1016/j.jconrel.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 11.Kawasaki ES, Player A. Nanotechnology, nanomedicine, and the development of new, effective therapies for cancer. Nanomedicine. 2005;1(2):101–9. doi: 10.1016/j.nano.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Tomalia DA, Reyna LA, Svenson S. Dendrimers as multi-purpose nanodevices for oncology drug delivery and diagnostic imaging. Biochem Soc Trans. 2007;35(Pt. 1):61–7. doi: 10.1042/BST0350061. [DOI] [PubMed] [Google Scholar]
- 13.Majoros IJ, Williams CR, Baker JR., Jr Current dendrimer applications in cancer diagnosis and therapy. Curr Top Med Chem. 2008;8(14):1165–79. doi: 10.2174/156802608785849049. [DOI] [PubMed] [Google Scholar]
- 14.Liao JY. Construction of nanogold hollow balls with dendritic surface as immobilized affinity support for protein adsorption. Colloids Surf B Biointerfaces. 2007;57(1):75–80. doi: 10.1016/j.colsurfb.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Bakri SJ, Pulido JS, Mukherjee P, Marler RJ, Mukhopadhyay D. Absence of histologic retinal toxicity of intravitreal nanogold in a rabbit model. Retina. 2008;28(1):147–9. doi: 10.1097/IAE.0b013e3180dc9360. [DOI] [PubMed] [Google Scholar]
- 16.Tiwari SB, Amiji MM. Improved oral delivery of paclitaxel following administration in nanoemulsion formulations. J Nanosci Nanotechnol. 2006;6(9–10):3215–21. doi: 10.1166/jnn.2006.440. [DOI] [PubMed] [Google Scholar]
- 17.Leamon CP, Low PS. Folate-mediated targeting. from diagnostics to drug and gene delivery. Drug Discov Today. 2001;6(1):44–51. doi: 10.1016/s1359-6446(00)01594-4. [DOI] [PubMed] [Google Scholar]
- 18.Reddy JA, Low PS. Folate-mediated targeting of therapeutic and imaging agents to cancers. Crit Rev Ther Drug Carrier Syst. 1998;15(6):587–627. [PubMed] [Google Scholar]
- 19.Jaracz S, Chen J, Kuznetsova LV, Ojima I. Recent advances in tumor-targeting anticancer drug conjugates. Bioorg Med Chem. 2005;13(17):5043–54. doi: 10.1016/j.bmc.2005.04.084. [DOI] [PubMed] [Google Scholar]
- 20.Rzigalinski BA, Strobl JS. Cadmium-containing nanoparticles. perspectives on pharmacology and toxicology of quantum dots. Toxicol Appl Pharmacol. 2009;238(3):280–8. doi: 10.1016/j.taap.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park JH, Gu L, von MG, Ruoslahti E, Bhatia SN, Sailor MJ. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat Mater. 2009;8(4):331–6. doi: 10.1038/nmat2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cormode DP, Skajaa T, Fayad ZA, Mulder WJ. Nanotechnology in medical imaging. probe design and applications. Arterioscler Thromb Vasc Biol. 2009;29(7):992–1000. doi: 10.1161/ATVBAHA.108.165506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Batist G, Ramakrishnan G, Rao CS, Chandrasekharan A, Gutheil J, Guthrie T, Shah P, Khojasteh A, Nair MK, Hoelzer K, Tkaczuk K, Park YC, Lee LW. Reduced cardiotoxicity and preserved antitumor efficacy of liposome-encapsulated doxorubicin and cyclophosphamide compared with conventional doxorubicin and cyclophosphamide in a randomized, multicenter trial of metastatic breast cancer. J Clin Oncol. 2001;19(5):1444–54. doi: 10.1200/JCO.2001.19.5.1444. [DOI] [PubMed] [Google Scholar]
- 24.Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics. an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7(9):771–82. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 25.Peetla C, Stine A, Labhasetwar V. Biophysical interactions with model lipid membranes. applications in drug discovery and drug delivery. Mol Pharm. 2009;6(5):1264–76. doi: 10.1021/mp9000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fenske DB, Cullis PR. Liposomal nanomedicines. Expert Opin Drug Deliv. 2008;5(1):25–44. doi: 10.1517/17425247.5.1.25. [DOI] [PubMed] [Google Scholar]
- 27.Fenske DB, Chonn A, Cullis PR. Liposomal nanomedicines: an emerging field. Toxicol Pathol. 2008;36(1):21–9. doi: 10.1177/0192623307310960. [DOI] [PubMed] [Google Scholar]
- 28.Xu L, Frederik P, Pirollo KF, Tang WH, Rait A, Xiang LM, Huang W, Cruz I, Yin Y, Chang EH. Self-assembly of a virus-mimicking nanostructure system for efficient tumor-targeted gene delivery. Hum Gene Ther. 2002;13(3):469–81. doi: 10.1089/10430340252792594. [DOI] [PubMed] [Google Scholar]
- 29.Pirollo KF, Zon G, Rait A, Zhou Q, Yu W, Hogrefe R, Chang EH. Tumor-targeting nanoimmunoliposome complex for short interfering RNA delivery. Hum Gene Ther. 2006;17(1):117–24. doi: 10.1089/hum.2006.17.117. [DOI] [PubMed] [Google Scholar]
- 30.Ko YT, Kale A, Hartner WC, Papahadjopoulos-Sternberg B, Torchilin VP. Self-assembling micelle-like nanoparticles based on phospholipid-polyethyleneimine conjugates for systemic gene delivery. J Control Release. 2009;133(2):132–8. doi: 10.1016/j.jconrel.2008.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu L, Pirollo KF, Tang WH, Rait A, Chang EH. Transferrin-liposome-mediated systemic p53 gene therapy in combination with radiation results in regression of human head and neck cancer xenografts. Hum Gene Ther. 1999;10(18):2941–52. doi: 10.1089/10430349950016357. [DOI] [PubMed] [Google Scholar]
- 32.Xu L, Huang CC, Huang W, Tang WH, Rait A, Yin YZ, Cruz I, Xiang LM, Pirollo KF, Chang EH. Systemic tumor-targeted gene delivery by anti-transferrin receptor scFv-immunoliposomes. Mol Cancer Ther. 2002;1(5):337–46. [PubMed] [Google Scholar]
- 33.Rait AS, Pirollo KF, Ulick D, Cullen K, Chang EH. HER-2-targeted antisense oligonucleotide results in sensitization of head and neck cancer cells to chemotherapeutic agents. Ann N Y Acad Sci. 2003;1002:78–89. doi: 10.1196/annals.1281.018. [DOI] [PubMed] [Google Scholar]
- 34.Yu W, Pirollo KF, Yu B, Rait A, Xiang L, Huang W, Zhou Q, Ertem G, Chang EH. Enhanced transfection efficiency of a systemically delivered tumor-targeting immunolipoplex by inclusion of a pH-sensitive histidylated oligolysine peptide. Nucleic Acids Res. 2004;32(5):e48. doi: 10.1093/nar/gnh049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu W, Pirollo KF, Rait A, Yu B, Xiang LM, Huang WQ, Zhou Q, Ertem G, Chang EH. A sterically stabilized immunolipoplex for systemic administration of a therapeutic gene. Gene Ther. 2004;11(19):1434–40. doi: 10.1038/sj.gt.3302304. [DOI] [PubMed] [Google Scholar]
- 36.Stover TC, Sharma A, Robertson GP, Kester M. Systemic delivery of liposomal short-chain ceramide limits solid tumor growth in murine models of breast adenocarcinoma. Clin Cancer Res. 2005;11(9):3465–74. doi: 10.1158/1078-0432.CCR-04-1770. [DOI] [PubMed] [Google Scholar]
- 37.Stover TC, Kim YS, Lowe TL, Kester M. Thermoresponsive and biodegradable linear-dendritic nanoparticles for targeted and sustained release of a pro-apoptotic drug. Biomaterials. 2008;29(3):359–69. doi: 10.1016/j.biomaterials.2007.09.037. [DOI] [PubMed] [Google Scholar]
- 38.Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965;13(1):238–52. doi: 10.1016/s0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- 39.Allen TM. Liposomes. Opportunities in drug delivery. Drugs. 1997;54(Suppl 4):8–14. doi: 10.2165/00003495-199700544-00004. [DOI] [PubMed] [Google Scholar]
- 40.Gregoriadis G. Homing of Liposomes to Target-Cells. Biochem Soc Trans. 1975;3(5):613–8. doi: 10.1042/bst0030613. [DOI] [PubMed] [Google Scholar]
- 41.Torchilin VP. Liposomes as delivery agents for medical imaging. Mol Med Today. 1996;2(6):242–9. doi: 10.1016/1357-4310(96)88805-8. [DOI] [PubMed] [Google Scholar]
- 42.Papahadjopoulos D, Allen TM, Gabizon A, Mayhew E, Matthay K, Huang SK, Lee KD, Woodle MC, Lasic DD, Redemann C. Sterically stabilized liposomes. improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc Natl Acad Sci U S A. 1991;88(24):11460–4. doi: 10.1073/pnas.88.24.11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gabizon A, Martin F. Polyethylene glycol-coated (pegylated) liposomal doxorubicin. Rationale for use in solid tumours. Drugs. 1997;54(Suppl 4):15–21. doi: 10.2165/00003495-199700544-00005. [DOI] [PubMed] [Google Scholar]
- 44.Haran G, Cohen R, Bar LK, Barenholz Y. Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of amphipathic weak bases [erratum] Biochim Biophys Acta. 1994 Feb 23;1190(1):197. doi: 10.1016/0005-2736(93)90105-9. [DOI] [PubMed] [Google Scholar]
- 45.Haran G, Cohen R, Bar LK, Barenholz Y. Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of amphipathic weak bases. Biochim Biophys Acta. 1993 Sep 19;1151(2):201–15. doi: 10.1016/0005-2736(93)90105-9. [DOI] [PubMed] [Google Scholar]
- 46.Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of pegylated liposomal Doxorubicin: review of animal and human studies. Clin Pharmacokinet. 2003;42(5):419–36. doi: 10.2165/00003088-200342050-00002. [DOI] [PubMed] [Google Scholar]
- 47.Johnston MJ, Edwards K, Karlsson G, Cullis PR. Influence of drug-to-lipid ratio on drug release properties and liposome integrity in liposomal doxorubicin formulations. J Liposome Res. 2008;18(2):145–57. doi: 10.1080/08982100802129372. [DOI] [PubMed] [Google Scholar]
- 48.Hafez IM, Cullis PR. Roles of lipid polymorphism in intracellular delivery. Adv Drug Deliv Rev. 2001;47(2–3):139–48. doi: 10.1016/s0169-409x(01)00103-x. [DOI] [PubMed] [Google Scholar]
- 49.Blumenthal R, Clague MJ, Durell SR, Epand RM. Membrane fusion. Chem Rev. 2003 Jan;103(1):53–69. doi: 10.1021/cr000036+. [DOI] [PubMed] [Google Scholar]
- 50.Needham D, Anyarambhatla G, Kong G, Dewhirst MW. A new temperature-sensitive liposome for use with mild hyperthermia. characterization and testing in a human tumor xenograft model. Cancer Res. 2000;60(5):1197–201. [PubMed] [Google Scholar]
- 51.Yatvin MB, Weinstein JN, Dennis WH, Blumenthal R. Design of Liposomes for Enhanced Local Release of Drugs by Hyperthermia. Science. 1978;202(4374):1290–3. doi: 10.1126/science.364652. [DOI] [PubMed] [Google Scholar]
- 52.Torchilin V. Antibody-modified liposomes for cancer chemotherapy. Expert Opin Drug Deliv. 2008;5(9):1003–25. doi: 10.1517/17425247.5.9.1003. [DOI] [PubMed] [Google Scholar]
- 53.Gantert M, Lewrick F, Adrian JE, Rossler J, Steenpass T, Schubert R, Peschka-Suss R. Receptor-specific targeting with liposomes in vitro based on sterol-PEG(1300) anchors. Pharm Res. 2009;26(3):529–38. doi: 10.1007/s11095-008-9768-z. [DOI] [PubMed] [Google Scholar]
- 54.Dasgupta P, Bachhawat BK. Receptor-mediated uptake of asialoganglioside liposomes. sub-cellular distribution of the liposomal marker in isolated liver cell types. Biochem Int. 1985;10(3):327–36. [PubMed] [Google Scholar]
- 55.Terrell J, Yadava P, Castro C, Hughes J. Liposome fluidity alters interactions between the ganglioside GM1 and cholera toxin B subunit. J Liposome Res. 2008;18(1):21–9. doi: 10.1080/08982100801893929. [DOI] [PubMed] [Google Scholar]
- 56.Marty C, Schwendener RA. Cytotoxic tumor targeting with scFv antibody-modified liposomes. Methods Mol Med. 2005;109:389–402. doi: 10.1385/1-59259-862-5:389. [DOI] [PubMed] [Google Scholar]
- 57.Singh A, Marchywka S. Synthesis and characterization of head group modified 1,3 diacetylenic phospholipids. Abstracts of Papers of the American Chemical Society. 1989;198:147. [Google Scholar]
- 58.Agarwal K, Bali A, Gupta CM. Synthesis of carbamyl and ether analogs of phosphatidylcholines. Chem Phys Lipids. 1984;36(2):169–77. [Google Scholar]
- 59.Agarwal K, Bali A, Gupta CM. Influence of the phospholipid structure on the stability of liposomes in serum. Biochim Biophys Acta. 1986 Mar 27;856(1):36–40. doi: 10.1016/0005-2736(86)90006-4. [DOI] [PubMed] [Google Scholar]
- 60.Gupta CM, Bali A. Carbamyl analogs of phosphatidylcholines: synthesis, interaction with phospholipases and permeability behavior of their liposomes. Biochim Biophys Acta. 1981 Feb 23;663(2):506–15. doi: 10.1016/0005-2760(81)90178-8. [DOI] [PubMed] [Google Scholar]
- 61.Gupta CM, Bali A, Dhawan S. Modification of phospholipid structure results in greater stability if liposomes in serum. Biochim Biophys Acta. 1981 Nov 6;648(2):192–8. doi: 10.1016/0005-2736(81)90034-1. [DOI] [PubMed] [Google Scholar]
- 62.Bali A, Dhawan S, Gupta CM. Stability of liposomes in circulation is markedly enhanced by structural modification of their phospholipid component. FEBS Lett. 1983 Apr 18;154(2):373–7. doi: 10.1016/0014-5793(83)80185-9. [DOI] [PubMed] [Google Scholar]
- 63.Shum P, Kim JM, Thompson DH. Phototriggering of liposomal drug delivery systems. Adv Drug Deliv Rev. 2001;53(3):273–84. doi: 10.1016/s0169-409x(01)00232-0. [DOI] [PubMed] [Google Scholar]
- 64.Ponce AM, Vujaskovic Z, Yuan F, Needham D, Dewhirst MW. Hyperthermia mediated liposomal drug delivery. Int J Hyperthermia. 2006;22(3):205–13. doi: 10.1080/02656730600582956. [DOI] [PubMed] [Google Scholar]
- 65.Needham D, Dewhirst MW. The development and testing of a new temperature-sensitive drug delivery system for the treatment of solid tumors. Adv Drug Deliv Rev. 2001;53(3):285–305. doi: 10.1016/s0169-409x(01)00233-2. [DOI] [PubMed] [Google Scholar]
- 66.Khutoryanskiy VV, Tirelli N. Oxidation-responsiveness of nanomaterials for targeting inflammatory reactions. Pure and Applied Chemistry. 2008;80(8):1703–18. [Google Scholar]
- 67.Guo X, Szoka FC., Jr Chemical approaches to triggerable lipid vesicles for drug and gene delivery. Acc Chem Res. 2003;36(5):335–41. doi: 10.1021/ar9703241. [DOI] [PubMed] [Google Scholar]
- 68.Andresen TL, Davidsen J, Begtrup M, Mouritsen OG, Jorgensen K. Enzymatic release of antitumor ether lipids by specific phospholipase A2 activation of liposome-forming prodrugs. J Med Chem. 2004;47(7):1694–1703. doi: 10.1021/jm031029r. [DOI] [PubMed] [Google Scholar]
- 69.Andresen TL, Jensen SS, Jorgensen K. Advanced strategies in liposomal cancer therapy. problems and prospects of active and tumor specific drug release. Prog Lipid Res. 2005;44(1):68–97. doi: 10.1016/j.plipres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 70.Kaasgaard T, Andresen TL, Jensen SS, Holte RO, Jensen LT, Jorgensen K. Liposomes containing alkylated methotrexate analogues for phospholipase A(2) mediated tumor targeted drug delivery. Chem Phys Lipids. 2009;157(2):94–103. doi: 10.1016/j.chemphyslip.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 71.Andresen TL, Jensen SS, Kaasgaard T, Jorgensen K. Triggered activation and release of liposomal prodrugs and drugs in cancer tissue by secretory phospholipase A2. Curr Drug Deliv. 2005;2(4):353–62. doi: 10.2174/156720105774370203. [DOI] [PubMed] [Google Scholar]
- 72.Chandra B, Mallik S, Srivastava DK. Design of photocleavable lipids and their application in liposomal “uncorking”. Chem Commun (Camb ) 2005;(24):3021–3. doi: 10.1039/b503423j. [DOI] [PubMed] [Google Scholar]
- 73.Chandra B, Subramaniam R, Mallik S, Srivastava DK. Formulation of photo-cleavable liposomes and the mechanism of their content release. Org Biomol Chem. 2006;4(9):1730–40. doi: 10.1039/b518359f. [DOI] [PubMed] [Google Scholar]
- 74.Andresen TL, Jensen SS, Madsen R, Jorgensen K. Synthesis and biological activity of anticancer ether lipids that are specifically released by phospholipase A(2) in tumor tissue. J Med Chem. 2005;48(23):7305–14. doi: 10.1021/jm049006f. [DOI] [PubMed] [Google Scholar]
- 75.Midoux P, Pichon C, Yaouanc JJ, Jaffres PA. Chemical vectors for gene delivery. a current review on polymers, peptides and lipids containing histidine or imidazole as nucleic acids carriers. Brit J Pharmacol. 2009;157(2):166–78. doi: 10.1111/j.1476-5381.2009.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meng FH, Zhong ZY, Feijen J. Stimuli-responsive polymersomes for programmed drug delivery. Biomacromolecules. 2009;10(2):197–209. doi: 10.1021/bm801127d. [DOI] [PubMed] [Google Scholar]
- 77.Karve S, Alaouie A, Zhou YP, Rotolo J, Sofou S. The use of pH-triggered leaky heterogeneities on rigid lipid bilayers to improve intracellular trafficking and therapeutic potential of targeted liposomal immunochemotherapy. Biomaterials. 2009;30(30):6055–64. doi: 10.1016/j.biomaterials.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 78.Di Marzio L, Marianecci C, Cinque B, Nazzarri M, Cimini AM, Cristiano L, Cifone MG, Alhaique F, Carafa M. pH-sensitive non-phospholipid vesicle and macrophage-like cells. Binding, uptake and endocytotic pathway. Biochim Biophys Acta. 2008 Dec;1778(12):2749–56. doi: 10.1016/j.bbamem.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 79.Frenkel V. Ultrasound mediated delivery of drugs and genes to solid tumors. Adv Drug Deliv Rev. 2008;60(10):1193–1208. doi: 10.1016/j.addr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Regen SL, Singh A, Oehme G, Singh M. Polymerized phosphatidyl choline vesicles. Stabilized and controllable time-release carriers. Biochem Biophys Res Commun. 1981;101(1):131–6. doi: 10.1016/s0006-291x(81)80020-4. [DOI] [PubMed] [Google Scholar]
- 81.Lavi A, Weitman H, Holmes RT, Smith KM, Ehrenberg B. The depth of porphyrin in a membrane and the membrane’s physical properties affect the photosensitizing efficiency. Biophys J. 2002;82(4):2101–10. doi: 10.1016/S0006-3495(02)75557-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bisby RH, Mead C, Mitchell AC, Morgan CG. Fast laser-induced solute release from liposomes sensitized with photochromic lipid. effects of temperature, lipid host, and sensitizer concentration. Biochem Biophys Res Commun. 1999;262(2):406–10. doi: 10.1006/bbrc.1999.1206. [DOI] [PubMed] [Google Scholar]
- 83.Bisby RH, Mead C, Morgan CG. Wavelength-programmed solute release from photosensitive liposomes. Biochem Biophys Res Commun. 2000;276(1):169–73. doi: 10.1006/bbrc.2000.3456. [DOI] [PubMed] [Google Scholar]
- 84.Morgan CG, Bisby RH, Johnson SA, Mitchell AC. Fast solute release from photosensitive liposomes. an alternative to ‘caged’ reagents for use in biological systems. FEBS Lett. 1995;375(1–2):113–6. doi: 10.1016/0014-5793(95)01193-i. [DOI] [PubMed] [Google Scholar]
- 85.Rhodes DG, Blechner SL, Yager P, Schoen PE. Structure of polymerizable lipid bilayers. I--1,2-bis(10,12-tricosadiynoyl)-sn-glycero-3-phosphocholine, a tubule-forming phosphatidylcholine. Chem Phys Lipids. 1988;49(1–2):39–47. doi: 10.1016/0009-3084(88)90062-x. [DOI] [PubMed] [Google Scholar]
- 86.Yavlovich A, Singh A, Tarasov S, Capala J, Blumenthal R, Puri A. Design of liposomes containing photopolymerizable phospholipids for triggered release of contents. Journal of Thermal Analysis and Calorimetry. 2009;98(1):97–104. doi: 10.1007/s10973-009-0228-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leserman LD, Weinstein JN, Blumenthal R, Sharrow SO, Terry WD. Binding of antigen-bearing fluorescent liposomes to the murine myeloma tumor MOPC 315. 1979 Feb;122(2):585–91. [PubMed] [Google Scholar]
- 88.Low PS, Henne WA, Doorneweerd DD. Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Acc Chem Res. 2008;41(1):120–9. doi: 10.1021/ar7000815. [DOI] [PubMed] [Google Scholar]
- 89.Cressman S, Dobson I, Lee JB, Tam YY, Cullis PR. Synthesis of a labeled RGD-lipid, its incorporation into liposomal nanoparticles, and their trafficking in cultured endothelial cells. Bioconjug Chem. 2009;20(7):1404–11. doi: 10.1021/bc900041f. [DOI] [PubMed] [Google Scholar]
- 90.Garde SV, Forte AJ, Ge M, Lepekhin EA, Panchal CJ, Rabbani SA, Wu JJ. Binding and internalization of NGR-peptide-targeted liposomal doxorubicin (TVT-DOX) in CD13-expressing cells and its antitumor effects. Anticancer Drugs. 2007;18(10):1189–1200. doi: 10.1097/CAD.0b013e3282a213ce. [DOI] [PubMed] [Google Scholar]
- 91.Schiffelers RM, Fens MH, Janssen AP, Molema G, Storm G. Liposomal targeting of angiogenic vasculature. Curr Drug Deliv. 2005;2(4):363–8. doi: 10.2174/156720105774370186. [DOI] [PubMed] [Google Scholar]
- 92.Janssen AP, Schiffelers RM, ten Hagen TL, Koning GA, Schraa AJ, Kok RJ, Storm G, Molema G. Peptide-targeted PEG-liposomes in anti-angiogenic therapy. Int J Pharm. 2003;254(1):55–8. doi: 10.1016/s0378-5173(02)00682-8. [DOI] [PubMed] [Google Scholar]
- 93.Pangburn TO, Petersen MA, Waybrant B, Adil MM, Kokkoli E. Peptide- and aptamer-functionalized nanovectors for targeted delivery of therapeutics. J Biomech Eng. 2009;131(7):074005. doi: 10.1115/1.3160763. [DOI] [PubMed] [Google Scholar]
- 94.Alexis F, Rhee JW, Richie JP, Radovic-Moreno AF, Langer R, Farokhzad OC. New frontiers in nanotechnology for cancer treatment. Urol Oncol. 2008 Jan–Feb;26(1):74–85. doi: 10.1016/j.urolonc.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 95.Beuttler J, Rothdiener M, Muller D, Frejd FY, Kontermann RE. Targeting of epidermal growth factor receptor (EGFR)-expressing tumor cells with sterically stabilized affibody liposomes (SAL) Bioconjug Chem. 2009;20(6):1201–8. doi: 10.1021/bc900061v. [DOI] [PubMed] [Google Scholar]
- 96.Puri A, Kramer-Marek G, Campbell-Massa R, Yavlovich A, Tele SC, Lee SB, Clogston JD, Patri AK, Blumenthal R, Capala J. HER2-specific affibody-conjugated thermosensitive liposomes (Affisomes) for improved delivery of anticancer agents. J Liposome Res. 2008;18(4):293–307. doi: 10.1080/08982100802457377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Murphy EA, Majeti BK, Barnes LA, Makale M, Weis SM, Lutu-Fuga K, Wrasidlo W, Cheresh DA. Nanoparticle-mediated drug delivery to tumor vasculature suppresses metastasis. Proc Natl Acad Sci U S A. 2008;105(27):9343–8. doi: 10.1073/pnas.0803728105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Uchiyama K, Nagayasu A, Yamagiwa Y, Nishida T, Harashima H, Kiwada H. Effects of the size and fluidity of liposomes on their accumulation in tumors. A presumption of their interaction with tumors. Int J Pharm. 1995;121(2):195–203. [Google Scholar]
- 99.Ishida O, Maruyama K, Sasaki K, Iwatsuru M. Size-dependent extravasation and interstitial localization of polyethyleneglycol liposomes in solid tumor-bearing mice. Int J Pharm. 1999;190(1):49–56. doi: 10.1016/s0378-5173(99)00256-2. [DOI] [PubMed] [Google Scholar]
- 100.Ferrari M. Nanovector therapeutics. Curr Opin Chem Biol. 2005 Aug;9(4):343–6. doi: 10.1016/j.cbpa.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 101.Riehemann K, Schneider SW, Luger TA, Godin B, Ferrari M, Fuchs H. Nano-medicine--challenge and perspectives. Angew Chem Int Ed Engl. 2009;48(5):872–97. doi: 10.1002/anie.200802585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sidorov IA, Prabakaran P, Dimitrov DS. Non-covalent conjugation of nanoparticles to antibodies via electrostatic interactions--a computational model. Journal of Computational and Theoretical Nanoscience. 2007;4(6):1103–7. [Google Scholar]
- 103.Kirpotin D, Park JW, Hong K, Zalipsky S, Li WL, Carter P, Benz CC, Papahadjopoulos D. Sterically stabilized anti-HER2 immunoliposomes: design and targeting to human breast cancer cells in vitro. Biochemistry. 1997;36(1):66–75. doi: 10.1021/bi962148u. [DOI] [PubMed] [Google Scholar]
- 104.Laginha KM, Moase EH, Yu N, Huang A, Allen TM. Bioavailability and therapeutic efficacy of HER2 scFv-targeted liposomal doxorubicin in a murine model of HER2-overexpressing breast cancer. J Drug Target. 2008;16(7–8):605–10. doi: 10.1080/10611860802229978. [DOI] [PubMed] [Google Scholar]
- 105.ElBayoumi TA, Torchilin VP. Tumor-targeted nanomedicines: enhanced anti-tumor efficacy in vivo of doxorubicin-loaded, long-circulating liposomes modified with cancer-specific monoclonal antibody. Clin Cancer Res. 2009 Mar 15;15(6):1973–80. doi: 10.1158/1078-0432.CCR-08-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gullotti E, Yeo Y. Extracellularly activated nanocarriers: a new paradigm of tumor targeted drug delivery. Mol Pharm. 2009 Jul–Aug;6(4):1041–51. doi: 10.1021/mp900090z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Park JW, Benz CC, Martin FJ. Future directions of liposome- and immunoliposome-based cancer therapeutics. Semin Oncol. 2004;31(6 Suppl 13):196–205. doi: 10.1053/j.seminoncol.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 108.Pavlinkova G, Colcher D, Booth BJM, Goel A, Wittel UA, Batra SK. Effects of humanization and gene shuffling on immunogenicity and antigen binding of anti-TAG-72 single-chain Fvs. Int J Cancer. 2001;94(5):717–26. doi: 10.1002/ijc.1523. [DOI] [PubMed] [Google Scholar]
- 109.Allen TM, Sapra P, Moase E. Use of the post-insertion method for the formation of ligand-coupled liposomes. Cell Mol Biol Lett. 2002;7(3):889–94. [PubMed] [Google Scholar]
- 110.Allen TM. Ligand-targeted therapeutics in anticancer therapy. Nat Rev Cancer. 2002;2(10):750–63. doi: 10.1038/nrc903. [DOI] [PubMed] [Google Scholar]
- 111.Maynard J, Georgiou G. Antibody engineering. Annu Rev Biomed Eng. 2000;2:339–76. doi: 10.1146/annurev.bioeng.2.1.339. [DOI] [PubMed] [Google Scholar]
- 112.Torchilin VP, Klibanov AL, Ivanov NN, Gluckhova MA, Koteliansky VE, Kleinman HK, Martin GR. Binding of antibodies in liposomes to extracellular matrix antigens. J Cell Biochem. 1985;28(1):23–9. doi: 10.1002/jcb.240280105. [DOI] [PubMed] [Google Scholar]
- 113.Sapra P, Tyagi P, Allen TM. Ligand-targeted liposomes for cancer treatment. Curr Drug Deliv. 2005;2(4):369–81. doi: 10.2174/156720105774370159. [DOI] [PubMed] [Google Scholar]
- 114.Nellis DF, Giardina SL, Janini GM, Shenoy SR, Marks JD, Tsai R, Drummond DC, Hong K, Park JW, Ouellette TF, Perkins SC, Kirpotin DB. Preclinical manufacture of anti-HER2 liposome-inserting, scFv-PEG-lipid conjugate. 2. Conjugate micelle identity, purity, stability, and potency analysis. Biotechnol Prog. 2005;21(1):221–32. doi: 10.1021/bp049839z. [DOI] [PubMed] [Google Scholar]
- 115.Nellis DF, Ekstrom DL, Kirpotin DB, Zhu J, Andersson R, Broadt TL, Ouellette TF, Perkins SC, Roach JM, Drummond DC, Hong K, Marks JD, Park JW, Giardina SL. Preclinical manufacture of an anti-HER2 scFv-PEG-DSPE, liposome-inserting conjugate. 1. Gram-scale production and purification. Biotechnol Prog. 2005;21(1):205–20. doi: 10.1021/bp049840y. [DOI] [PubMed] [Google Scholar]
- 116.Lopes de Menezes DE, Pilarski LM, Allen TM. In vitro and in vivo targeting of immunoliposomal doxorubicin to human B-cell lymphoma. Cancer Res. 1998 Aug 1;58(15):3320–30. [PubMed] [Google Scholar]
- 117.Alexis F, Basto P, Levy-Nissenbaum E, Radovic-Moreno AF, Zhang L, Pridgen E, Wang AZ, Marein SL, Westerhof K, Molnar LK, Farokhzad OC. HER-2-targeted nanoparticle-affibody bioconjugates for cancer therapy. Chemmedchem. 2008;3(12):1839–43. doi: 10.1002/cmdc.200800122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Low PS, Antony AC. Folate receptor-targeted drugs for cancer and inflammatory diseases. Adv Drug Deliv Rev. 2004;56(8):1055–8. doi: 10.1016/j.addr.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 119.Leamon CP, Pastan I, Low PS. Cytotoxicity of folate-Pseudomonas exotoxin conjugates toward tumor cells. Contribution of translocation domain. J Biol Chem. 1993;268(33):24847–54. [PubMed] [Google Scholar]
- 120.Low PS, Kularatne SA. Folate-targeted therapeutic and imaging agents for cancer. Curr Opin Chem Biol. 2009;13(3):256–62. doi: 10.1016/j.cbpa.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 121.Stevens PJ, Sekido M, Lee RJ. A folate receptor-targeted lipid nanoparticle formulation for a lipophilic paclitaxel prodrug. Pharm Res. 2004;21(12):2153–7. doi: 10.1007/s11095-004-7667-5. [DOI] [PubMed] [Google Scholar]
- 122.Lee RJ, Low PS. Folate-mediated tumor cell targeting of liposome-entrapped doxorubicin in vitro. Biochim Biophys Acta. 1995;1233(2):134–44. doi: 10.1016/0005-2736(94)00235-h. [DOI] [PubMed] [Google Scholar]
- 123.Gabizon A, Shmeeda H, Horowitz AT, Zalipsky S. Tumor cell targeting of liposome-entrapped drugs with phospholipid-anchored folic acid-PEG conjugates. Adv Drug Deliv Rev. 2004;56(8):1177–92. doi: 10.1016/j.addr.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 124.Gabizon A, Horowitz AT, Goren D, Tzemach D, Shmeeda H, Zalipsky S. In vivo fate of folate-targeted polyethylene-glycol liposomes in tumor-bearing mice. Clin Cancer Res. 2003;9(17):6551–9. [PubMed] [Google Scholar]
- 125.Gabizon A, Horowitz AT, Goren D, Tzemach D, Mandelbaum-Shavit F, Qazen MM, Zalipsky S. Targeting folate receptor with folate linked to extremities of poly(ethylene glycol)-grafted liposomes. In vitro studies. Bioconjug Chem. 1999;10(2):289–98. doi: 10.1021/bc9801124. [DOI] [PubMed] [Google Scholar]
- 126.Gabizon A, Tzemach D, Gorin J, Mak L, Amitay Y, Shmeeda H, Zalipsky S. Improved therapeutic activity of folate-targeted liposomal doxorubicin in folate receptor-expressing tumor models. Cancer Chemother Pharmacol. 2009 Sep 25; doi: 10.1007/s00280-009-1132-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 127.Shmeeda H, Mak L, Tzemach D, Astrahan P, Tarshish M, Gabizon A. Intracellular uptake and intracavitary targeting of folate-conjugated liposomes in a mouse lymphoma model with up-regulated folate receptors. Mol Cancer Ther. 2006;5(4):818–24. doi: 10.1158/1535-7163.MCT-05-0543. [DOI] [PubMed] [Google Scholar]
- 128.Fuhrhop AH, Wang TY. Bolaamphiphiles. Chem Rev. 2004;104(6):2901–37. doi: 10.1021/cr030602b. [DOI] [PubMed] [Google Scholar]
- 129.Arakawa K, Eguchi T, Kakinuma K. Highly thermostable liposome from 72-membered macrocyclic tetraether lipid. Importance of 72-membered lipid for archaea to thrive under hyperthermal environments. Chem Lett. 2001;(5):440–1. [Google Scholar]
- 130.Arakawa K, Eguchi T, Kakinuma K. 36-membered macrocyclic diether lipid is advantageous for archaea to thrive under the extreme thermal environments. Bulletin of the Chemical Society of Japan. 2001;74(2):347–56. [Google Scholar]
- 131.Cornell BA, BraachMaksvytis VLB, King LG, Osman PDJ, Raguse B, Wieczorek L, Pace RJ. A biosensor that uses ion-channel switches. Nature. 1997;387(6633):580–3. doi: 10.1038/42432. [DOI] [PubMed] [Google Scholar]
- 132.Kai T, Sun XL, Faucher KM, Apkarian RP, Chaikof EL. Design and synthesis of asymmetric acyclic phospholipid bolaamphiphiles. J Org Chem. 2005;70(7):2606–15. doi: 10.1021/jo048167o. [DOI] [PubMed] [Google Scholar]
- 133.WeissWichert C, Smetazko M, ValinaSaba M, Schalkhammer T. A new analytical device based on gated ion channels: a peptide-channel biosensor. J Biomol Screen. 1997;2(1):11–8. [Google Scholar]
- 134.Claussen RC, Rabatic BM, Stupp SI. Aqueous self-assembly of unsymmetric peptide bolaamphiphiles into nanofibers with hydrophilic cores and surfaces. J Am Chem Soc. 2003;125(42):12680–1. doi: 10.1021/ja035882r. [DOI] [PubMed] [Google Scholar]
- 135.Fuhrhop JH, Tank H. Bolaamphiphiles with mannose and tetraalkylammonium head groups as coatings for nucleic-acids and possible reagents for transfections. Chem Phys Lipids. 1987;43(3):193–213. [Google Scholar]
- 136.Fuhrhop JH, Spiroski D, Boettcher C. Molecular monolayer rods and tubules made of alpha-(L-lysine),omega-(amino) bolaamphiphiles. J Am Chem Soc. 1993;115(4):1600–1. [Google Scholar]
- 137.Kameta N, Masuda M, Minamikawa H, Goutev NV, Rim JA, Jung JH, Shimizu T. Selective construction of supramolecular nanotube hosts with cationic inner surfaces. Advanced Materials. 2005;17(22):2732–6. [Google Scholar]
- 138.Kameta N, Masuda M, Minamikawa H, Shimizu T. Self-assembly and thermal phase transition behavior of unsymmetrical bolaamphiphiles having glucose- and amino-hydrophilic headgroups. Langmuir. 2007;23(8):4634–41. doi: 10.1021/la063542o. [DOI] [PubMed] [Google Scholar]
- 139.Masuda M, Shimizu T. Lipid nanotubes and microtubes. Experimental evidence for unsymmetrical monolayer membrane formation from unsymmetrical bolaamphiphiles. Langmuir. 2004;20(14):5969–77. doi: 10.1021/la049085y. [DOI] [PubMed] [Google Scholar]
- 140.Kameta N, Minamikawa H, Masuda M, Mizuno G, Shimizu T. Controllable biomolecule release from self-assembled organic nanotubes with asymmetric surfaces. pH and temperature dependence. Soft Matter. 2008;4(8):1681–7. doi: 10.1039/b803742f. [DOI] [PubMed] [Google Scholar]
- 141.Chang EL. Unusual thermal-stability of liposomes made from bipolar tetra-ether lipids. Biochem Biophys Res Commun. 1994 Jul 29;202(2):673–9. doi: 10.1006/bbrc.1994.1983. [DOI] [PubMed] [Google Scholar]
- 142.Chang EL. Can ether liposomes be used for oral vaccines? Biophys J. 1994;66(2):A383. [Google Scholar]
- 143.Li SY, Zheng FX, Zhang XR, Wang WC. stability and rupture of archaebacterial cell membrane: a model study. J Phys Chem B. 2009 Jan 29;113(4):1143–52. doi: 10.1021/jp808079h. [DOI] [PubMed] [Google Scholar]
- 144.Patel GB, Sprott GD. Archaeobacterial ether lipid liposomes (archaeosomes) as novel vaccine and drug delivery systems. Crit Rev Biotechnol. 1999;19(4):317–57. doi: 10.1080/0738-859991229170. [DOI] [PubMed] [Google Scholar]
- 145.Beduneau A, Pellequer Y, Lamprecht A. Supramolecular assemblies for the active drug targeting to the brain. Journal of Drug Delivery Science and Technology. 2009;19(3):155–63. [Google Scholar]
- 146.De RM, Gambacorta A. The lipids of archaebacteria. Prog Lipid Res. 1988;27(3):153–75. doi: 10.1016/0163-7827(88)90011-2. [DOI] [PubMed] [Google Scholar]
- 147.Eguchi T, Arakawa K, Terachi T, Kakinuma K. Total synthesis of archaeal 36-membered macrocyclic diether lipid. J Org Chem. 1997;62(7):1924–33. doi: 10.1021/jo962327h. [DOI] [PubMed] [Google Scholar]
- 148.Roussel M, Lognone V, Plusquellec D, Benvegnu T. Monolayer lipid membrane-forming dissymmetrical bolaamphiphiles derived from alginate oligosaccharides. Chemical Communications. 2006;(34):3622–4. doi: 10.1039/b607368a. [DOI] [PubMed] [Google Scholar]
- 149.Fuhrhop JH, David HH, Mathieu J, Liman U, Winter HJ, Boekema E. Bolaamphiphiles and monolayer lipid-membranes made from. 1,6,19,24-tetraoxa-3,21-cyclohexatriacontadiene-2,5,20,23-tetrone. J Am Chem Soc. 1986;108(8):1785–91. [Google Scholar]
- 150.Fuhrhop JH, Mathieu J. Routes to functional vesicle membranes without proteins. Angewandte Chemie-International Edition in English. 1984;23(2):100–13. [Google Scholar]
- 151.Lu T, Han F, Li ZC, Huang JB, Fu HL. Transitions of organized assemblies in mixed systems of cationic bolaamphiphile and anionic conventional surfactants. Langmuir. 2006;22(5):2045–9. doi: 10.1021/la0528100. [DOI] [PubMed] [Google Scholar]
- 152.Nieh MP, Harroun TA, Raghunathan VA, Glinka CJ, Katsaras J. Spontaneously formed monodisperse biomimetic unilamellar vesicles. The effect of charge, dilution, and time. Biophys J. 2004;86(4):2615–29. doi: 10.1016/S0006-3495(04)74316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Okahata Y, Kunitake T. Formation of stable monolayer membranes and related structures in dilute aqueous-solution from 2-headed ammonium amphiphiles. J Am Chem Soc. 1979;101(18):5231–4. [Google Scholar]
- 154.Polidori A, Wathier M, Fabiano AS, Olivier B, Pucci B. Synthesis and aggregation behaviour of symmetric glycosylated bolaamphiphiles in water. Arkivoc. 2006:73–89. [Google Scholar]
- 155.Wang XF, Shen YZ, Pan Y, Liang YQ. Bolaamphiphilic single-chain bis-Schiff base derivatives. Aggregation and thermal behavior in aqueous solution. Langmuir. 2001;17(11):3162–7. [Google Scholar]
- 156.Masuda M, Shimizu T. Multilayer structure of an unsymmetrical monolayer lipid membrane with a ‘head-to-tail’ interface. Chem Commun (Camb) 2001 Dec 7;(23):2442–3. doi: 10.1039/b106581p. [DOI] [PubMed] [Google Scholar]
- 157.Grinberg S, Kolot V, Linder C, Shaubi E, Kas’yanov V, Deckelbaum RJ, Heldman E. Synthesis of novel cationic bolaamphiphiles from vernonia oil and their aggregated structures. Chem Phys Lipids. 2008;153(2):85–97. doi: 10.1016/j.chemphyslip.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 158.Lasic D. Liposomes. American Scientist. 1992;80(1):20–31. [Google Scholar]
- 159.Lasic D. Liposomes: an industrial view. Chemistry & Industry. 1996;(6):210–4. [Google Scholar]
- 160.Lasic DD, Bolotin E, Brey RN. Polymerized liposomes. From biophysics to applications. Part I. Chimica Oggi-Chemistry Today. 2000;18(11–12):48–51. [Google Scholar]
- 161.Lasic DD, Bolotin E, Brey RN. Polymerized liposomes. From biophysics to applications. Part II. Chimica Oggi-Chemistry Today. 2001;19(1–2):45–48. [Google Scholar]
- 162.Schroeder A, Kost J, Barenholz Y. Ultrasound, liposomes, and drug delivery. principles for using ultrasound to control the release of drugs from liposomes. Chem Phys Lipids. 2009;162(1–2):1–16. doi: 10.1016/j.chemphyslip.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 163.Brochu H, Polidori A, Pucci B, Vermette P. Drug delivery systems using immobilized intact liposomes. a comparative and critical review. Curr Drug Deliv. 2004;1(3):299–312. doi: 10.2174/1567201043334678. [DOI] [PubMed] [Google Scholar]
- 164.Zana R, Yiv S, Kale KM. Chemical relaxation and equilibrium studies of association in aqueous-solutions of bolaform detergents. 3. Docosane-1,22- Bis(trimethylammonium bromide) Journal of Colloid and Interface Science. 1980;77(2):456–65. [Google Scholar]
- 165.Garbuzenko O, Barenholz Y, Priev A. Effect of grafted PEG on liposome size and on compressibility and packing of lipid bilayer. Chem Phys Lipids. 2005;135(2):117–29. doi: 10.1016/j.chemphyslip.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 166.Khazanov E, Priev A, Shillemans JP, Barenholz Y. Physicochemical and biological characterization of ceramide-containing liposomes: paving the way to ceramide therapeutic application. Langmuir. 2008;24(13):6965–80. doi: 10.1021/la800207z. [DOI] [PubMed] [Google Scholar]
- 167.Kumar VV. Complementary molecular shapes and additivity of the packing parameter of lipids. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):444–8. doi: 10.1073/pnas.88.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lichtenberg D, Barenholz Y. Liposomes: preparation, characterization, and preservation. Method Biochem Anal. 1988;33:337–462. doi: 10.1002/9780470110546.ch7. [DOI] [PubMed] [Google Scholar]
- 169.Kunitake T, Okahata Y, Shimomura M, Yasunami SI, Takarabe K. Formation of stable bilayer assemblies in water from single-chain amphiphiles--relationship between the amphiphile structure and the aggregate morphology. J Am Chem Soc. 1981;103(18):5401–13. [Google Scholar]
- 170.Kaler EW, Murthy AK, Rodriguez BE, Zasadzinski JAN. Spontaneous vesicle formation in aqueous mixtures of single-tailed surfactants. Science. 1989;245(4924):1371–4. doi: 10.1126/science.2781283. [DOI] [PubMed] [Google Scholar]
- 171.Popov M, Linder C, Deckelbaum RJ, Grinberg S, Hansen IH, Shaubi E, Waner T, Heldman E. Cationic vesicles from novel bolaamphiphilic compounds. J Liposome Res. 2009 Oct 22; doi: 10.3109/08982100903218900. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 172.Clary L, Gadras C, Greiner J, Rolland JP, Santaella C, Vierling P, Gulik A. Phase behavior of fluorocarbon and hydrocarbon double-chain hydroxylated and galactosylated amphiphiles and bolaamphiphiles. Long-term shelf-stability of their liposomes. Chem Phys Lipids. 1999;99(2):125–37. doi: 10.1016/s0009-3084(99)00032-8. [DOI] [PubMed] [Google Scholar]
- 173.Franceschi S, de Viguerie N, Riviere M, Lattes A. Synthesis and aggregation of two-headed surfactants bearing amino acid moieties. New Journal of Chemistry. 1999;23(4):447–52. [Google Scholar]
- 174.Nakashima N, Asakuma S, Kim JM, Kunitake T. Helical superstructures are formed from chiral ammonium bilayers. Chem Lett. 1984;(10):1709–12. [Google Scholar]
- 175.Di Meglio C, Rananavare SB, Svenson S, Thompson DH. Bolaamphiphilic phosphocholines. Structure and phase behavior in aqueous media. Langmuir. 2000;16(1):128–33. [Google Scholar]
- 176.Gliozzi A, Robello M, Relini A, Accardo G. Asymmetric black membranes formed by one monolayer of bipolar lipids at the air/water interface. Biochim Biophys Acta. 1994;1189(1):96–100. doi: 10.1016/0005-2736(94)90285-2. [DOI] [PubMed] [Google Scholar]
- 177.Cummings JE, Vanderlick TK. Aggregation and hemi-fusion of anionic vesicles induced by the antimicrobial peptide cryptdin-4. Biochim Biophys Acta. 2007;1768(7):1796–804. doi: 10.1016/j.bbamem.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 178.Holland JW, Hui C, Cullis PR, Madden TD. Poly(ethylene glycol)-lipid conjugates regulate the calcium-induced fusion of liposomes composed of phosphatidylethanolamine and phosphatidylserine. Biochemistry. 1996;35(8):2618–24. doi: 10.1021/bi952000v. [DOI] [PubMed] [Google Scholar]
- 179.Goryacheva YA, Vekshina OM, Yashin VA, Kim YA. Fusion and endocytosis of anionic liposomes with Ehrlich ascitic carcinoma cells. Bull Exp Biol Med. 2005;140(6):733–5. doi: 10.1007/s10517-006-0069-4. [DOI] [PubMed] [Google Scholar]
- 180.Gabizon A, Shmeeda H, Horowitz AT, Zalipsky S. Tumor cell targeting of liposome-entrapped drugs with phospholipid-anchored folic acid-PEG conjugates. Adv Drug Deliv Rev. 2004 Apr 29;56(8):1177–92. doi: 10.1016/j.addr.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 181.Pradhan P, Giri J, Rieken F, Koch C, Mykhaylyk O, Doblinger M, Banerjee R, Bahadur D, Plank C. Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. J Control Release. 2009 Oct 9; doi: 10.1016/j.jconrel.2009.10.002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 182.Yamada A, Taniguchi Y, Kawano K, Honda T, Hattori Y, Maitani Y. Design of folate-linked liposomal doxorubicin to its antitumor effect in mice. Clin Cancer Res. 2008;14(24):8161–8. doi: 10.1158/1078-0432.CCR-08-0159. [DOI] [PubMed] [Google Scholar]
- 183.Evans EA, Hochmuth RM. Membrane viscoplastic flow. Biophys J. 1976;16(1):13–26. doi: 10.1016/S0006-3495(76)85659-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Evans EA, Hochmuth RM. Membrane viscoelasticity. Biophys J. 1976;16(1):1–11. doi: 10.1016/S0006-3495(76)85658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lorite GS, Nobre TM, Zaniquelli ME, de PE, Cotta MA. Dibucaine effects on structural and elastic properties of lipid bilayers. Biophys Chem. 2009;139(2–3):75–83. doi: 10.1016/j.bpc.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 186.Longo GS, Thompson DH, Szleifer I. Stability and phase separation in mixed monopolar lipid/bolalipid layers. Biophys J. 2007;93(8):2609–21. doi: 10.1529/biophysj.106.102764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Thompson DH, Wong KF, Humphrybaker R, Wheeler JJ, Kim JM, Rananavare SB. Tetraether bolaform amphiphiles as models of archaebacterial membrane-lipids: raman-spectroscopy, P-31 NMR, X-ray-scattering, and electron-microscopy. J Am Chem Soc. 1992;114(23):9035–42. [Google Scholar]
- 188.Albertorio F, Diaz AJ, Yang TL, Chapa VA, Kataoka S, Castellana ET, Cremer PS. Fluid and air-stable lipopolymer membranes for biosensor applications. Langmuir. 2005;21(16):7476–82. doi: 10.1021/la050871s. [DOI] [PubMed] [Google Scholar]
- 189.Halter M, Nogata Y, Dannenberger O, Sasaki T, Vogel V. Engineered lipids that cross-link the inner and outer leaflets of lipid bilayers. Langmuir. 2004;20(6):2416–23. doi: 10.1021/la035817v. [DOI] [PubMed] [Google Scholar]
- 190.Kim JM, Patwardhan A, Bott A, Thompson DH. Preparation and electrochemical behavior of gramicidin-bipolar lipid monolayer membranes supported on gold electrodes. Biochim Biophys Acta. 2003;1617(1–2):10–21. doi: 10.1016/j.bbamem.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 191.Grinberg S. Exploiting vegetable oils for the delivery of hydrophilic drugs. In: Kipnisa N, Linderb C, Kolota V, Heldman E, editors. European Journal of Lipid Science and Technology. 1. 112 . 2010. pp. 137–51. [Google Scholar]
- 192.Grinberg S, Linder C, Kolot V, Waner T, Wiesman Z, Shaubi E, Heldman E. Novel cationic amphiphilic derivatives from vernonia oil. Synthesis and self-aggregation into bilayer vesicles, nanoparticles, and DNA complexants. Langmuir. 2005;21(17):7638–45. doi: 10.1021/la050091j. [DOI] [PubMed] [Google Scholar]
- 193.Wiesman Z, Ben Dom N, Sharvit E, Grinberg S, Linder C, Heldman E, Zaccai M. Novel cationic vesicle platform derived from vernonia oil for efficient delivery of DNA through plant cuticle membranes. J Biotechnol. 2007;130(1):85–94. doi: 10.1016/j.jbiotec.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 194.Elferink MG, de Wit JG, Driessen AJ, Konings WN. Stability and proton-permeability of liposomes composed of archaeal tetraether lipids. Biochim Biophys Acta. 1994;1193(2):247–54. doi: 10.1016/0005-2736(94)90160-0. [DOI] [PubMed] [Google Scholar]
- 195.Krishnan L, Dicaire CJ, Patel GB, Sprott GD. Archaeosome vaccine adjuvants induce strong humoral, cell-mediated, and memory responses: comparison to conventional liposomes and alum. Infect Immun. 2000;68(1):54–63. doi: 10.1128/iai.68.1.54-63.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Patel GB, Agnew BJ, Jarrell HC, Sprott GD. Stability of liposomes prepared from the total polar lipids of Methanosarcina mazei is affected by the specific salt form of the lipids. Journal of Liposome Research. 1999;9(2):229–45. [Google Scholar]
- 197.Patel GB, Chen W. Nanocarrier Technologies: Frontiers of Nanotherapy. Amsterdam: Springer; 2006. Archaeosomes as drug and vaccine nanodelivery systems. [Google Scholar]
- 198.Martins S, Sarmento B, Ferreira DC, Souto EB. Lipid-based colloidal carriers for peptide and protein delivery--liposomes versus lipid nanoparticles. Int J Nanomedicine. 2007;2(4):595–607. [PMC free article] [PubMed] [Google Scholar]
- 199.Zhang XG, Miao J, Dai YQ, Du YZ, Yuan H, Hu FQ. Reversal activity of nanostructured lipid carriers loading cytotoxic drug in multi-drug resistant cancer cells. Int J Pharm. 2008;361(1–2):239–44. doi: 10.1016/j.ijpharm.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 200.Wissing SA, Muller RH. The influence of solid lipid nanoparticles on skin hydration and viscoelasticity - in vivo study. Eur J Pharm Biopharm. 2003;56(1):67–72. doi: 10.1016/s0939-6411(03)00040-7. [DOI] [PubMed] [Google Scholar]
- 201.Wissing SA, Kayser O, Muller RH. Solid lipid nanoparticles for parenteral drug delivery. Adv Drug Deliv Rev. 2004;56(9):1257–72. doi: 10.1016/j.addr.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 202.Bondi ML, Fontana G, Carlisi B, Giammona G. Preparation and characterization of solid lipid nanoparticles containing cloricromene. Drug Delivery. 2003;10(4):245–50. doi: 10.1080/drd_10_4_245. [DOI] [PubMed] [Google Scholar]
- 203.Schubert MA, Muller-Goymann CC. Characterisation of surface-modified solid lipid nanoparticles (SLN). Influence of lecithin and nonionic emulsifier. Eur J Pharm Biopharm. 2005;61(1–2):77–86. doi: 10.1016/j.ejpb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 204.Schubert MA, Harms M, Muller-Goymann CC. Structural investigations on lipid nanoparticles containing high amounts of lecithin. Eur J Pharm Sci. 2006;27(2–3):226–36. doi: 10.1016/j.ejps.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 205.Heurtault B, Saulnier P, Pech B, Proust JE, Benoit JP. A novel phase inversion-based process for the preparation of lipid nanocarriers. Pharm Res. 2002;19(6):875–80. doi: 10.1023/a:1016121319668. [DOI] [PubMed] [Google Scholar]
- 206.Garcia-Fuentes M, Torres D, Martin-Pastor M, Alonso MJ. Application of NMR spectroscopy to the characterization of PEG-stabilized lipid nanoparticles. Langmuir. 2004;20(20):8839–45. doi: 10.1021/la049505j. [DOI] [PubMed] [Google Scholar]
- 207.Garcia-Fuentes M, Alonso MJ, Torres D. Design and characterization of a new drug nanocarrier made from solid-liquid lipid mixtures. J Colloid Interface Sci. 2005;285(2):590–8. doi: 10.1016/j.jcis.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 208.Puglia C, Bonina F, Castelli F, Micieli D, Sarpietro MG. Evaluation of percutaneous absorption of the repellent diethyltoluamide and the sunscreen ethyl-hexyl p-methoxycinnamate-loaded solid lipid nanoparticles. an in-vitro study. J Pharm Pharmacol. 2009;61(8):1013–9. doi: 10.1211/jpp/61.08.0004. [DOI] [PubMed] [Google Scholar]
- 209.Charcosset C, El-Harati A, Fessi H. Preparation of solid lipid nanoparticles using a membrane contactor. J Control Release. 2005;108(1):112–20. doi: 10.1016/j.jconrel.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 210.Charcosset C, El-Harati AA, Fessi H. A membrane contactor for the preparation of solid lipid nanoparticles. Desalination. 2006;200(1–3):570–1. doi: 10.1016/j.jconrel.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 211.El-Harati AA, Charcosset C, Fessi H. Influence of the formulation for solid lipid nanoparticles prepared with a membrane contactor. Pharm Dev Technol. 2006;11(2):153–7. doi: 10.1080/10837450600561182. [DOI] [PubMed] [Google Scholar]
- 212.Muller RH, Radtke M, Wissing SA. Nanostructured lipid matrices for improved microencapsulation of drugs. Int J Pharm. 2002;242(1–2):121–8. doi: 10.1016/s0378-5173(02)00180-1. [DOI] [PubMed] [Google Scholar]
- 213.Teeranachaideekul V, Muller RH, Junyaprasert VB. Encapsulation of ascorbyl palmitate in nanostructured lipid carriers (NLC) - Effects of formulation parameters on physicochemical stability. Int J Pharm. 2007;340(1–2):198–206. doi: 10.1016/j.ijpharm.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 214.Hu FQ, Jiang SP, Du YZ, Yuan H, Ye YQ, Zeng S. Preparation and characteristics of monostearin nanostructured lipid carriers. Int J Pharm. 2006;314(1):83–9. doi: 10.1016/j.ijpharm.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 215.Radtke M. Nanostructured lipid carriers. a novel generation of solid lipid drug carriers. Pharmaceutical Technology Europe. 2005;17(4):45–50. [Google Scholar]
- 216.Muller RH, Petersen RD, Hornmoss A, Pardeike J. Nanostructured lipid carriers (NLC) in cosmetic dermal products. Adv Drug Deliv Rev. 2007;59(6):522–30. doi: 10.1016/j.addr.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 217.Yuan H, Wang LL, Du YZ, You J, Hu FQ, Zeng S. Preparation and characteristics of nanostructured lipid carriers for control-releasing progesterone by melt-emulsification. Colloids Surf B Biointerfaces. 2007;60(2):174–9. doi: 10.1016/j.colsurfb.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 218.Goppert TM, Muller RH. Protein adsorption patterns on poloxamer- and poloxamine-stabilized solid lipid nanoparticles (SLN) Eur J Pharm Biopharm. 2005;60(3):361–72. doi: 10.1016/j.ejpb.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 219.Goppert TM, Muller RH. Polysorbate-stabilized solid lipid nanoparticles as colloidal carriers for intravenous targeting of drugs to the brain: comparison of plasma protein adsorption patterns. J Drug Target. 2005;13(3):179–87. doi: 10.1080/10611860500071292. [DOI] [PubMed] [Google Scholar]
- 220.Pandey R, Khuller GK. Solid lipid particle-based inhalable sustained drug delivery system against experimental tuberculosis. Tuberculosis. 2005;85(4):227–34. doi: 10.1016/j.tube.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 221.Uner M, Yener G. Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspectives. Int J Nanomedicine. 2007;2(3):289–300. [PMC free article] [PubMed] [Google Scholar]
- 222.Muller RH, Ruhl D, Runge S, SchulzeForster K, Mehnert W. Cytotoxicity of solid lipid nanoparticles as a function of the lipid matrix and the surfactant. Pharm Res. 1997;14(4):458–62. doi: 10.1023/a:1012043315093. [DOI] [PubMed] [Google Scholar]
- 223.Muller RH, Dingler A, Weyhers H, zurMuhlen A, Mehnert W. Solid lipid nano-particles--a novel carrier system for cosmetics and pharmaceutics. 3. Long-term stability, lyophilisation, spray drying toxicity, use in cosmetics and pharmaceutics. Pharmazeutische Industrie. 1997;59(7):614–9. [Google Scholar]
- 224.Fundaro A, Cavalli R, Bargoni A, Vighetto D, Zara GP, Gasco MR. Non-stealth and stealth solid lipid nanoparticles (SLN) carrying doxorubicin. Pharmacokinetics and tissue distribution after i.v. administration to rats. Pharmacol Res. 2000;42(4):337–43. doi: 10.1006/phrs.2000.0695. [DOI] [PubMed] [Google Scholar]
- 225.Almelda AJ, Souto E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv Drug Deliv Rev. 2007;59(6):478–90. doi: 10.1016/j.addr.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 226.Joshi MD, Muller RH. Lipid nanoparticles for parenteral delivery of actives. Eur J Pharm Biopharm. 2009;71(2):161–72. doi: 10.1016/j.ejpb.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 227.Wong HL, Rauth AM, Bendayan R, Wu XY. In vivo evaluation of a new polymer-lipid hybrid nanoparticle (PLN) formulation of doxorubicin in a murine solid tumor model. Eur J Pharm Biopharm. 2007;65(3):300–8. doi: 10.1016/j.ejpb.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 228.Wong HL, Bendayan R, Rauth AM, Li YQ, Wu XY. Chemotherapy with anti-cancer drugs encapsulated in solid lipid nanoparticles. Adv Drug Deliv Rev. 2007;59(6):491–504. doi: 10.1016/j.addr.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 229.Mehnert W, Mader K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2001;47(2–3):165–196. doi: 10.1016/s0169-409x(01)00105-3. [DOI] [PubMed] [Google Scholar]
- 230.Jenning V, Mader K, Gohla SH. Solid lipid nanoparticles (SLN) based on binary mixtures of liquid and solid lipids: a (1)H-NMR study. Int J Pharm. 2000;205(1–2):15–21. doi: 10.1016/s0378-5173(00)00462-2. [DOI] [PubMed] [Google Scholar]
- 231.Jenning V, Thunemann AF, Gohla SH. Characterisation of a novel solid lipid nanoparticle carrier system based on binary mixtures of liquid and solid lipids. Int J Pharm. 2000;199(2):167–77. doi: 10.1016/s0378-5173(00)00378-1. [DOI] [PubMed] [Google Scholar]
- 232.Bondi ML, Craparo EF, Giammona G, Cervello M, Azzolina A, Diana P, Martorana A, Cirrincione G. Nanostructured lipid carriers-containing anticancer compounds: preparation, characterization, and cytotoxicity studies. Drug Delivery. 2007;14(2):61–7. doi: 10.1080/10717540600739914. [DOI] [PubMed] [Google Scholar]
- 233.Serpe L, Catalano MG, Cavalli R, Ugazio E, Bosco O, Canaparo R, Muntoni E, Frairia R, Gasco MR, Eandi M, Zara GP. Cytotoxicity of anticancer drugs incorporated nanoparticles on HT-29 colorectal cancer in solid lipid cell line. Eur J Pharm Biopharm. 2004;58(3):673–80. doi: 10.1016/j.ejpb.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 234.Saha A, Ramakrishnan S. Unimolecular micelles and reverse micelles based on hyperbranched polyethers: comparative study of AB(2) + A-R and A(2) + B-3 + A-R type strategies. Journal of Polymer Science Part A-Polymer Chemistry. 2009;47(1):80–91. [Google Scholar]
- 235.Xu Z, Chen L, Gu W, Gao Y, Lin L, Zhang Z, Xi Y, Li Y. The performance of docetaxel-loaded solid lipid nanoparticles targeted to hepatocellular carcinoma. Biomaterials. 2009;30(2):226–32. doi: 10.1016/j.biomaterials.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 236.Lu W, He LC, Wang CH, Li YH, Zhang SQ. The use of solid lipid nanoparticles to target a lipophilic molecule to the liver after intravenous administration to mice. Int J Biol Macromol. 2008 Oct 1;43(3):320–4. doi: 10.1016/j.ijbiomac.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 237.Muthu MS, Singh S. Targeted nanomedicines. effective treatment modalities for cancer, AIDS and brain disorders. Nanomedicine. 2009;4(1):105–18. doi: 10.2217/17435889.4.1.105. [DOI] [PubMed] [Google Scholar]
- 238.Barbu E, Molnar E, Tsibouklis J, Gorecki DC. The potential for nanoparticle-based drug delivery to the brain. overcoming the blood-brain barrier. Expert Opinion on Drug Delivery. 2009;6(6):553–65. doi: 10.1517/17425240902939143. [DOI] [PubMed] [Google Scholar]
- 239.Chattopadhyay N, Zastre J, Wong HL, Wu XY, Bendayan R. Solid lipid nano-particles enhance the delivery of the HIV protease inhibitor, atazanavir, by a human brain endothelial cell line. Pharm Res. 2008;25(10):2262–71. doi: 10.1007/s11095-008-9615-2. [DOI] [PubMed] [Google Scholar]
- 240.Gao Y, Gu WW, Chen LL, Xu ZH, Li YP. The role of daidzein-loaded sterically stabilized solid lipid nanoparticles in therapy for cardio-cerebrovascular diseases. Biomaterials. 2008;29(30):4129–36. doi: 10.1016/j.biomaterials.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 241.Esposito E, Fantin M, Marti M, Drechsler M, Paccamiccio L, Mariani P, Sivieri E, Lain F, Menegatti E, Morari M, Cortesi R. Solid lipid nanoparticles as delivery systems for bromocriptine. Pharm Res. 2008;25(7):1521–30. doi: 10.1007/s11095-007-9514-y. [DOI] [PubMed] [Google Scholar]
- 242.Gupta Y, Jain A, Jain SK. Transferrin-conjugated solid lipid nanoparticles for enhanced delivery of quinine dihydrochloride to the brain. J Pharm Pharmacol. 2007;59(7):935–40. doi: 10.1211/jpp.59.7.0004. [DOI] [PubMed] [Google Scholar]
- 243.Lockman PR, Oyewumi MO, Koziara JM, Roder KE, Mumper RJ, Allen DD. Brain uptake of thiamine-coated nanoparticles. J Control Release. 2003;93(3):271–82. doi: 10.1016/j.jconrel.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 244.Kaur IP, Bhandari R, Bhandari S, Kakkar V. Potential of solid lipid nanoparticles in brain targeting. J Control Release. 2008;127(2):97–109. doi: 10.1016/j.jconrel.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 245.Rodriguez MA, Pytlik R, Kozak T, Chhanabhai M, Gascoyne R, Lu B, Deitcher SR, Winter JN. Vincristine sulfate liposomes injection (Marqibo) in heavily pre-treated patients with refractory aggressive non-Hodgkin lymphoma. report of the pivotal phase. 2 study. Cancer. 2009;115(15):3475–82. doi: 10.1002/cncr.24359. [DOI] [PubMed] [Google Scholar]
- 246.Fassas A, Anagnostopoulos A. The use of liposomal daunorubicin (DaunoXome) in acute myeloid leukemia. Leuk Lymphoma. 2005;46(6):795–802. doi: 10.1080/10428190500052438. [DOI] [PubMed] [Google Scholar]
- 247.Meunier F, Prentice HG, Ringden O. Liposomal amphotericin B (AmBisome): safety data from a phase II/III clinical trial. J Antimicrob Chemother. 1991 Oct;28(Suppl B):83–91. doi: 10.1093/jac/28.suppl_b.83. [DOI] [PubMed] [Google Scholar]
- 248.Meyerhoff A. US Food and Drug Administration approval of AmBisome (liposomal amphotericin B) for treatment of visceral leishmaniasis. Clin Infect Dis. 1999;28(1):42–8. doi: 10.1086/515085. [DOI] [PubMed] [Google Scholar]
- 249.Smylie MG, Wong R, Mihalcioiu C, Lee C, Pouliot JF. A phase II, open label, monotherapy study of liposomal doxorubicin in patients with metastatic malignant melanoma. Investigational New Drugs. 2007;25(2):155–9. doi: 10.1007/s10637-006-9002-y. [DOI] [PubMed] [Google Scholar]
- 250.Martin FJ. DOXIL (pegylated liposomal doxorubicin): clinical update. Abstracts of Papers of the American Chemical Society. 1996;211:34. [Google Scholar]
- 251.Andreopoulou E, Gaiotti D, Kim E, Downey A, Mirchandani D, Hamilton A, Jacobs A, Curtin J, Muggia F. Pegylated liposomal doxorubicin HCL (PLD;Caelyx/Doxil (R)). Experience with long-term maintenance in responding patients with recurrent epithelial ovarian cancer. Ann Oncol. 2007;18(4):716–21. doi: 10.1093/annonc/mdl484. [DOI] [PubMed] [Google Scholar]
- 252.Anon Doxil receives FDA market clearance. Aids Patient Care and Stds. 1996;10(2):135. [PubMed] [Google Scholar]
- 253.Timmers GJ, Zweegman S, Simoons-Smit AM, van Loenen AC, Touw D, Huijgens PC. Amphotericin B colloidal dispersion (Amphocil) vs fluconazole for the prevention of fungal infections in neutropenic patients. data of a prematurely stopped clinical trial. Bone Marrow Transplant. 2000;25(8):879–84. doi: 10.1038/sj.bmt.1702243. [DOI] [PubMed] [Google Scholar]
- 254.Berman JD, Ksionski G, Chapman WL, Waits VB, Hanson WL. Activity of amphotericin B cholesterol dispersion (Amphocil) in experimental visceral leishmaniasis. Antimicrob Agents Chemother. 1992;36(9):1978–80. doi: 10.1128/aac.36.9.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Trouet A. The amphotericin B lipid complex or abelcet its Belgian connection, its mode of action and specificity: a review. Acta Clinica Belgica. 2002;57(2):53–7. doi: 10.1179/acb.2002.014. [DOI] [PubMed] [Google Scholar]
- 256.Phuphanich S, Maria B, Braeckman R, Chamberlain M. A pharmacokinetic study of intra-CSF administered encapsulated cytarabine (DepoCyt (R)) for the treatment of neoplastic meningitis in patients with leukemia, lymphoma, or solid tumors as part of a phase III study. J Neurooncol. 2007;81(2):201–8. doi: 10.1007/s11060-006-9218-x. [DOI] [PubMed] [Google Scholar]
- 257.News in Brief. Expert review of anticancer therapy. 2004;1(1):3–5. [Google Scholar]
- 258.Shepard RC, Talluto CC, Jacob G. Phase I study results of nanomolecular liposomal annamycin in refractory ALL. J Clin Oncol (Meeting Abstracts) 2009;27(15S):7066. [Google Scholar]
- 259.Mylonakis N, Athanasiou A, Ziras N, Angel J, Rapti A, Lampaki S, Politis N, Karanikas C, Kosmas C. Phase II study of liposomal cisplatin (Lipoplatin) plus gemcitabine versus cisplatin plus gemcitabine as first line treatment in inoperable (stage IIIB/IV) non-small cell lung cancer. Lung Cancer. 2009 Jul 21; doi: 10.1016/j.lungcan.2009.06.017. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 260.Boulikas T. Clinical overview on Lipoplatin. a successful liposomal formulation of cisplatin. Expert Opin Investig Drugs. 2009;18(8):1197–218. doi: 10.1517/13543780903114168. [DOI] [PubMed] [Google Scholar]
- 261.Gelmon K, Hirte H, Fisher B, Walsh W, Ptaszynski M, Hamilton M, Onetto N, Eisenhauer E. A phase. 1 study of OSI-211 given as an intravenous infusion days. 1,. 2, and 3 every three weeks in patients with solid cancers. Invest New Drugs. 2004;22(3):263–75. doi: 10.1023/B:DRUG.0000026252.86842.e2. [DOI] [PubMed] [Google Scholar]
- 262.MacKenzie MJ, Hirte HW, Siu LL, Gelmon K, Ptaszynski M, Fisher B, Eisenhauer E. A phase I study of OSI-211 and cisplatin as intravenous infusions given on days 1, 2 and 3 every 3 weeks in patients with solid cancers. Ann Oncol. 2004;15(4):665–70. doi: 10.1093/annonc/mdh133. [DOI] [PubMed] [Google Scholar]
- 263.Thomas DA, Sarris AH, Cortes J, Faderl S, O’Brien S, Giles FJ, Garcia-Manero G, Rodriguez MA, Cabanillas F, Kantarjian H. Phase II study of sphingosomal vincristine in patients with recurrent or refractory adult acute lymphocytic leukemia. Cancer. 2006;106(1):120–7. doi: 10.1002/cncr.21595. [DOI] [PubMed] [Google Scholar]
- 264.Mitchell PL, Marlton P, Grigg A, Seymour JF, Hertzberg M, Enno A, Herrmann R, Bond R, Arthur C. A phase II study of liposomal daunorubicin, in combination with cyclophosphamide, vincristine and prednisolone, in elderly patients with previously untreated aggressive non-Hodgkin lymphoma. Leuk Lymphoma. 2008;49(5):924–31. doi: 10.1080/10428190802007700. [DOI] [PubMed] [Google Scholar]
- 265.Levine AM, Tulpule A, Espina B, Sherrod A, Boswell WD, Lieberman RD, Nathwani BN, Welles L. Liposome-encapsulated doxorubicin in combination with standard agents (cyclophosphamide, vincristine, prednisone) in patients with newly diagnosed AIDS-related non-Hodgkin’s lymphoma. results of therapy and correlates of response. J Clin Oncol. 2004;22(13):2662–70. doi: 10.1200/JCO.2004.10.093. [DOI] [PubMed] [Google Scholar]
- 266.Batist G, Barton J, Chaikin P, Swenson C, Welles L. Myocet (liposome-encapsulated doxorubicin citrate). a new approach in breast cancer therapy. Expert Opin Pharmacother. 2002;3(12):1739–51. doi: 10.1517/14656566.3.12.1739. [DOI] [PubMed] [Google Scholar]
- 267.Swenson CE, Perkins WR, Roberts P, Janoff AS. Liposome technology and the development of Myocet(TM) (liposomal doxorubicin citrate) The Breast. 2001;10(Suppl 2):1–7. [Google Scholar]
- 268.Lian T, Ho RJ. Trends and developments in liposome drug delivery systems. J Pharm Sci. 2001;90(6):667–80. doi: 10.1002/jps.1023. [DOI] [PubMed] [Google Scholar]
- 269.Needham D, Dewhirst MW. The development and testing of a new temperature-sensitive drug delivery system for the treatment of solid tumors. Adv Drug Deliv Rev. 2001;53(3):285–305. doi: 10.1016/s0169-409x(01)00233-2. [DOI] [PubMed] [Google Scholar]
- 270.Immordino ML, Brusa P, Arpicco S, Stella B, Dosio F, Cattel L. Preparation, characterization, cytotoxicity and pharmacokinetics of liposomes containing docetaxel. J Control Release. 2003;91(3):417–29. doi: 10.1016/s0168-3659(03)00271-2. [DOI] [PubMed] [Google Scholar]
- 271.Allen TM, Mumbengegwi DR, Charrois GJ. Anti-CD19-targeted liposomal doxorubicin improves the therapeutic efficacy in murine B-cell lymphoma and ameliorates the toxicity of liposomes with varying drug release rates. Clin Cancer Res. 2005;11(9):3567–73. doi: 10.1158/1078-0432.CCR-04-2517. [DOI] [PubMed] [Google Scholar]
- 272.Sapra P, Moase EH, Ma J, Allen TM. Improved therapeutic responses in a xenograft model of human B lymphoma (Namalwa) for liposomal vincristine versus liposomal doxorubicin targeted via anti-CD19 IgG2a or Fab′ fragments. Clin Cancer Res. 2004;10(3):1100–11. doi: 10.1158/1078-0432.ccr-03-0041. [DOI] [PubMed] [Google Scholar]
- 273.Cheng WWK, Allen TM. Targeted delivery of anti-CD19 liposomal doxorubicin in B-cell lymphoma. A comparison of whole monoclonal antibody, Fab′ fragments and single chain Fv. J Control Release. 2008;126(1):50–8. doi: 10.1016/j.jconrel.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 274.Cheng WWK, Das D, Suresh M, Allen TM. Expression and purification of two anti-CD19 single chain Fv fragments for targeting of liposomes to CD19-expressing cells. Biochim Biophys Acta. 2007;1768(1):21–9. doi: 10.1016/j.bbamem.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 275.Yang T, Choi MK, Cui FD, Lee SJ, Chung SJ, Shim CK, Kim DD. Antitumor effect of paclitaxel-loaded PEGylated immunoliposomes against human breast cancer cells. Pharm Res. 2007;24(12):2402–11. doi: 10.1007/s11095-007-9425-y. [DOI] [PubMed] [Google Scholar]
- 276.Kirpotin DB, Drummond DC, Shao Y, Shalaby MR, Hong K, Nielsen UB, Marks JD, Benz CC, Park JW. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006;66(13):6732–40. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- 277.Park JW, Hong K, Kirpotin DB, Colbern G, Shalaby R, Baselga J, Shao Y, Nielsen UB, Marks JD, Moore D, Papahadjopoulos D, Benz CC. Anti-HER2 immunoliposomes: enhanced efficacy attributable to targeted delivery. Clin Cancer Res. 2002;8(4):1172–81. [PubMed] [Google Scholar]
- 278.Elbayoumi TA, Torchilin VP. Enhanced accumulation of long-circulating liposomes modified with the nucleosome-specific monoclonal antibody 2C5 in various tumours in mice: gamma-imaging studies. European Journal of Nuclear Medicine and Molecular Imaging. 2006;33(10):1196–205. doi: 10.1007/s00259-006-0139-x. [DOI] [PubMed] [Google Scholar]
- 279.Elbayoumi TA, Torchilin VP. Tumor-specific antibody-mediated targeted delivery of Doxil (R) reduces the manifestation of auricular erythema side effect in mice. Int J Pharm. 2008;357(1–2):272–9. doi: 10.1016/j.ijpharm.2008.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Volkel T, Muller R, Kontermann RE. Isolation of endothelial cell-specific human antibodies from a novel fully synthetic scFv library. Biochem Biophys Res Commun. 2004;317(2):515–21. doi: 10.1016/j.bbrc.2004.03.074. [DOI] [PubMed] [Google Scholar]
- 281.Pastorino F, Brignole C, Marimpietri D, Sapra P, Moase EH, Allen TM, Ponzoni M. Doxorubicin-loaded Fab′ fragments of anti-disialoganglioside immunoliposomes selectively inhibit the growth and dissemination of human neuroblastoma in nude mice. Cancer Res. 2003;63(1):86–92. [PubMed] [Google Scholar]
- 282.Tseng YL, Hong RL, Tao MH, Chang FH. Sterically stabilized anti-idiotype immunoliposomes improve the therapeutic efficacy of doxorubicin in a murine B-cell lymphoma model. Int J Cancer. 1999;80(5):723–30. doi: 10.1002/(sici)1097-0215(19990301)80:5<723::aid-ijc16>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 283.Mukherjee S, Das L, Kole L, Karmakar S, Datta N, Das PK. Targeting of parasite-specific immunoliposome-encapsulated doxorubicin in the treatment of experimental visceral leishmaniasis. J Infect Dis. 2004;189(6):1024–34. doi: 10.1086/382048. [DOI] [PubMed] [Google Scholar]
- 284.Atobe K, Ishida T, Ishida E, Hashimoto K, Kobayashi H, Yasuda J, Aoki T, Obata KI, Kikuchi H, Akita H, Asai T, Harashima H, Oku N, Kiwada H. In vitro efficacy of a sterically stabilized immunoliposomes targeted to membrane type 1 matrix metalloproteinase (MT1-MMP) Biol Pharm Bull. 2007;30(5):972–8. doi: 10.1248/bpb.30.972. [DOI] [PubMed] [Google Scholar]
- 285.Lundberg BB, Griffiths G, Hansen HJ. Cellular association and cytotoxicity of doxorubicin-loaded immunoliposomes targeted via Fab′ fragments of an anti-CD74 antibody. Drug Delivery. 2007;14(3):171–5. doi: 10.1080/10717540601036831. [DOI] [PubMed] [Google Scholar]
- 286.Mamot C, Drummond DC, Noble CO, Kallab V, Guo Z, Hong K, Kirpotin DB, Park JW. Epidermal growth factor receptor-targeted immunoliposomes significantly enhance the efficacy of multiple anticancer drugs in vivo. Cancer Res. 2005;65(24):11631–8. doi: 10.1158/0008-5472.CAN-05-1093. [DOI] [PubMed] [Google Scholar]
- 287.Voinea M, Manduteanu I, Dragomir E, Capraru M, Simionescu M. Immunoliposomes directed toward VCAM-1 interact specifically with activated endothelial cells - A potential tool for specific drug delivery. Pharm Res. 2005;22(11):1906–17. doi: 10.1007/s11095-005-7247-3. [DOI] [PubMed] [Google Scholar]
- 288.Moreira JN, Gaspar R, Allen TM. Targeting stealth liposomes in a murine model of human small cell lung cancer. Biochim Biophys Acta. 2001;1515(2):167–76. doi: 10.1016/s0005-2736(01)00411-4. [DOI] [PubMed] [Google Scholar]
- 289.Moreira JN, Hansen CB, Gaspar R, Allen TM. A growth factor antagonist as a targeting agent for sterically stabilized liposomes in human small cell lung cancer. Biochim Biophys Acta. 2001;1514(2):303–17. doi: 10.1016/s0005-2736(01)00386-8. [DOI] [PubMed] [Google Scholar]
- 290.Dagar S, Sekosan M, Lee BS, Rubinstein I, Onyuksel H. VIP receptors as molecular targets of breast cancer. implications for targeted imaging and drug delivery. J Control Release. 2001;74(1–3):129–34. doi: 10.1016/s0168-3659(01)00326-1. [DOI] [PubMed] [Google Scholar]
- 291.Jaafari MR, Foldvari M. P-o protein mediated targeting of liposomes to melanoma cells with high level of ICAM-1 expression. J Drug Target. 1999;7(2):101–12. doi: 10.3109/10611869909085495. [DOI] [PubMed] [Google Scholar]
- 292.Xiong XB, Huang Y, Lu WL, Zhang X, Zhang H, Nagai T, Zhang Q. Enhanced intracellular delivery and improved antitumor efficacy of doxorubicin by sterically stabilized liposomes modified with a synthetic RGD mimetic. J Control Release. 2005;107(2):262–75. doi: 10.1016/j.jconrel.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 293.Pan XQ, Zheng X, Shi GF, Wang HQ, Ratnam M, Lee RJ. Strategy for the treatment of acute myelogenous leukemia based on folate receptor beta-targeted liposomal doxorubicin combined with receptor induction using all-trans retinoic acid. Blood. 2002;100(2):594–602. doi: 10.1182/blood.v100.2.594. [DOI] [PubMed] [Google Scholar]
- 294.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–60. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 295.Benson HA. Elastic liposomes for topical and transdermal drug delivery. Curr Drug Deliv. 2009;6(3):217–26. doi: 10.2174/156720109788680813. [DOI] [PubMed] [Google Scholar]
- 296.Rai K, Gupta Y, Jain A, Jain SK. Transfersomes: self-optimizing carriers for bioactives. PDA J Pharm Sci Technol. 2008 Sep–Oct;62(5):362–79. [PubMed] [Google Scholar]
- 297.Soenen SJ, Hodenius M, De Cuyper M. Magnetoliposomes. versatile innovative nanocolloids for use in biotechnology and biomedicine. Nanomedicine. 2009;4(2):177–91. doi: 10.2217/17435889.4.2.177. [DOI] [PubMed] [Google Scholar]
- 298.Muller RH, Mader K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery - a review of the state of the art. Eur J Pharm Biopharm. 2000;50(1):161–77. doi: 10.1016/s0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 299.Sawant KK, Dodiya SS. Recent advances and patents on solid lipid nanoparticles. Recent Pat Drug Deliv Formul. 2008;2(2):120–35. doi: 10.2174/187221108784534081. [DOI] [PubMed] [Google Scholar]
- 300.Fuhrhop JH, Wang T. Bolaamphiphiles. Chem Rev. 2004;104(6):2901–37. doi: 10.1021/cr030602b. [DOI] [PubMed] [Google Scholar]