Abstract
High resolution separation of α,β-tubulin by SDS-PAGE on minigels can be rapidly performed rapidly using simple modifications of the standard Laemmli procedure. Separation of the subunits can be observed even in high protein loads (up to 40 µg of protein).
Keywords: tubulin, microtubules, SDS-PAGE
Tubulin, the major structural component of microtubules, is a heterodimeric protein consisting of similar subunits designated as α- and β-, each having an approximate molecular weight of 50 kDa [1]. The tubulin dimers, present in equimolar amounts per sample and being of approximately equal molecular weight, are difficult to separate adequately on polyacrylamide minigels. Taking advantage of the disparate detergent binding of the α- and β-subunits under denaturing conditions, electrophoretic separation of the heterodimers has been achieved in slab gels [2]. It was demonstrated that the subunits would separate well when low-grade SDS was used [3], and subsequently, the two subunits were shown to differentially bind lower and higher homologues of SDS [4]. In previous slab gel experiments, the monomers were separated when the amount of tubulin loaded was low (≤ 5 µg) [4, 5, 6], though separation of alkylated, dansyl-tagged B-tubulin in one milligram quantities has been reported in cylindrical gels [7]. Slab gel separation was often accomplished with addition of urea and running times of several hours [2, 8, 9]. Though such conditions are modifications of the original Laemmli protocol optimized for larger slab gels (e.g., 14 × 10 cm), we found these procedures do not directly translate to 10 × 8 cm minigels, now standard. We therefore undertook a systematic study designed to optimize α- and β-tubulin separation on SDS-PAGE minigels, taking into consideration factors known to be important in resolving the subunits. This paper describes a simple and rapid procedure at which we arrived for separating large amounts of tubulin monomers in minigels.
Methods
Polyacrylamide gel electrophoresis was carried out in a Bio-Rad minigel system. Tris-OH, glycine, PIPES (piperazine-1,4-bis[2-ethanesulfonic acid]), EGTA (glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid), MgSO4 (magnesium sulfate), SDS (sodium dodecyl sulfate, 95% pure, catalog number L5750), acrylamide, and N,N′-methylenebisacrylamide were obtained from Sigma. TEMED (N,N,N,N-tetramethyl-ethane-1,2-diamine) and APS (ammonium persulfate) were obtained from Acros Organics. Sephadex G-50 resin for size exclusion chromatography column was purchased from GE Healthcare. Purified bovine brain tubulin, free of microtubule associated proteins, was prepared by the method of Williams and Lee [10]. The purified tubulin was drop frozen and stored under liquid nitrogen. Freshly thawed tubulin was used for each experiment after equilibrating it with PME buffer (0.1 M PIPES, 1 mM MgSO4, 2 mM EGTA, pH 6.90) by gel filtration. The concentration was measured with a UV-visible spectrophotometer (Hewlett Packard 8453A UV/Vis Spectrophotometer) using the molar extinction coefficient of 1.23 (mL/mg)−1 cm−1 at 278 nm [11]. Acrylamide minigels (10.1 cm W × 8.2 cm L × 1.5 mm thick) were freshly cast for all experiments. A stock solution of 30% acrylamide and 0.8% N,N′-methylenebisacrylamide (w/v) was prepared. The stacking layer (6% acrylamide final concentration) was prepared by mixing 2 mL acrylamide/bis stock solution, 5.4 mL ddH2O, 2.5 mL stacking buffer (0.5 M Tris-OH, pH 6.8) and 100 µL 10% SDS. Polymerization was initiated by adding 50 µL of freshly prepared 10% APS and 10 µL TEMED. Parameters varied in the resolving layer depending on the experiment. The optimum conditions were a resolving layer solution prepared using 5 mL acrylamide/bis stock solution (30% acrylamide, 0.8% bis-acrylamide) to produce a final acrylamide concentration of 7.5%, 8 mL ddH2O, 5 mL resolving buffer made with 1.5 M tris-OH (pH 9.8), and 200 µL 10% SDS. Polymerization was initiated with 250 µL 10% APS and 16 µL TEMED. The amount of acrylamide/bisacrylamide used in the resolving layer was varied from 7.5% to 12.5%. Samples of purified bovine brain tubulin were treated with 3x Laemmli sample buffer (prepared with 0.325 mL ddH2O, 1.875 mL 0.5 M tris-OH, pH 6.8, 3.75 mL glycerol, 3.0 mL 10% (w/v) SDS, 0.3 mL 0.5% (w/v) bromophenol blue, which was divided into aliquots of 925 µL. Just prior to sample preparation, 75 µL β-mercaptoethanol was added to each sample buffer. Samples of tubulin were diluted with 3x sample buffer to a final sample buffer concentration of 1x, boiled for 5 minutes, then centrifuged in a microfuge (Brinkmann Eppendorf Microcentrifuge 5414) for 1 minute, and loaded onto the gels. In some experiments, urea was added in each of stacking, resolving and sample buffers at various concentrations (6 M and 8 M) to detect any improvement in the monomer separation. The running buffer contained 0.1% SDS, 0.025 M Tris-OH, 0.192 M glycine (pH 8.8). The gels were run at a constant voltage ranging from 60 V to 120 V and for different time intervals (1 h, 1.5 h, and 3 h). Optimal separation was achieved at 120 V for 1.5 h. After running, gels were stained overnight with 0.25% Coomassie Brilliant Blue R-250 (w/v) in 45% methanol, 10% acetic acid, and destained in 40% methanol, 10% acetic acid. The distained gels were then photographed and stored in Ziploc bags containing 5 mL of weak destaining solution (7% acetic acid, 5% methanol).
Results
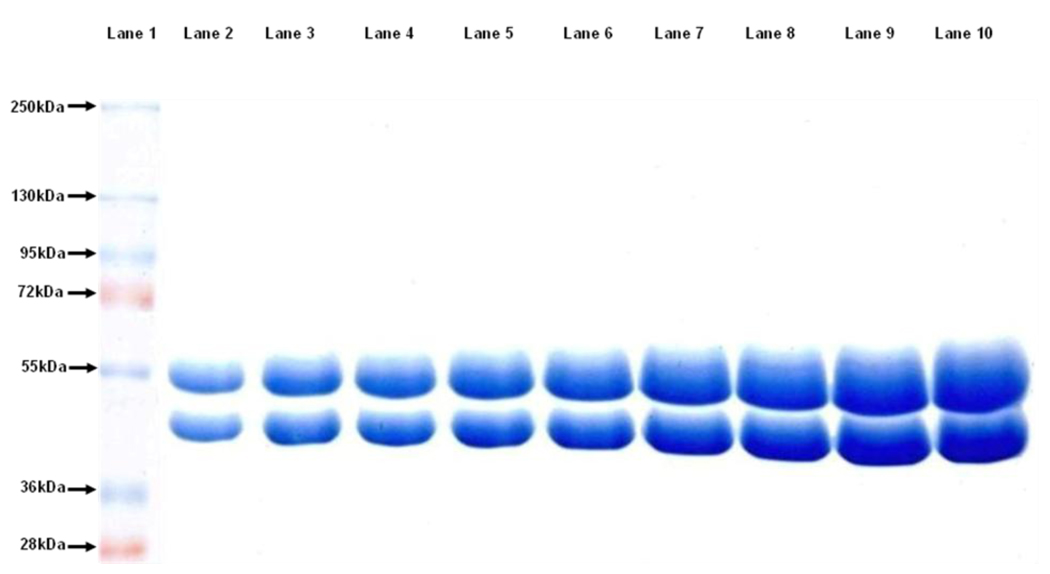
Fig. 1 shows the separation of bovine brain α- and β-tubulin using the optimal conditions. Note that separation of the subunits can be discerned even at high loading mass (40 µg).
Figure 1.
Increasing masses of pure bovine brain tubulin were loaded in a 7.5% resolving layer and 6% stacking layer. The pH of resolving and stacking buffers were maintained at 9.8 and 6.8, respectively. The gel was run at 120 V for 1.5 hrs in a running buffer containing 0.1% SDS. Molecular weight markers are shown in lane 1. The mass of tubulin in lane 2 to lane 10 was 8 µg, 12 µg, 16 µg, 20 µg, 24 µg, 28 µg, 32 µg, 36 µg, and 40 µg, respectively.
It has been reported that inclusion of urea in the stacking, resolving, running, and sample buffers can improve the separation of tubulin subunits [12, 13]. Inclusion of 6 M urea in the standard tubulin separation procedure (resolving buffer pH 8.8) provided visible separation of the subunits, but the resolution was less than that afforded by the conditions optimized in this work.
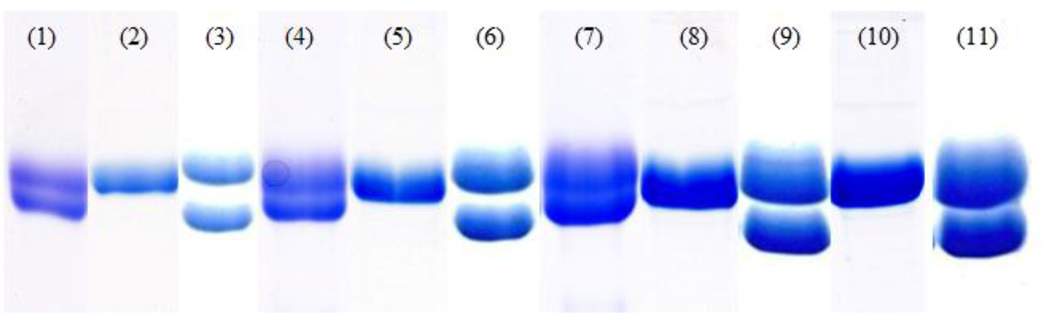
Another factor that affects the separation of α-,β-tubulin by SDS-PAGE is the pH of the resolving buffer. In the Laemmli protocol, the resolving buffer is pH 8.8. [14]. Stephens achieved a large separation of α-,β-tubulin by deliberately adding octadecyl sulfate to the running buffer [4]. Increasing the pH of the resolving buffer in slab gels to 9.1 also positively correlated with increased monomer separation [15]. The maximum protein load presented in the illustrations was 1.2 µg tubulin, so it was unclear if larger masses of the protein would be equally well resolved. We therefore explored the effect of the pH of the resolving buffer on the separation of α-,β-tubulin, varying from pH 8.8 to 10.5. Results from three different pH values are illustrated in Fig. 2. A pH of 9.8 proved to be most effective for high mass tubulin dimmer separation.
Figure 2.
Comparison of tubulin separations as a function of the pH of the resolving buffer on 7.5% polyacrylamide gels. Lanes (1), (4), (7): tubulin separation on pH 9.1 resolving buffer gel at 9 µg, 19 µg, and 30 µg protein loaded, respectively. Lanes (2), (5), (8), (10): tubulin separation on pH 10.5 resolving buffer gel at 9 µg, 19 µg, 33 µg, and 42 µg protein loaded, respectively. Lanes (3), (6), (9), (11): tubulin separation on pH 9.8 resolving buffer gel at 8 µg, 20 µg, 32 µg, and 40 µg protein loaded, respectively. Electrophoresis was performed at 120 V for 1.5 hours for all gels.
Another parameter considered was the concentration of acrylamide in the separating gel, which was varied from 7.5% to 12.5%. We observed that at pH 9.8, the best separation of tubulin monomers was obtained at 7.5% acrylamide concentration in the separating gel. With increased acrylamide concentrations, the separation between the tubulin monomers was observed to decrease. The tubulin monomers were indistinguishable when the acrylamide concentration was raised to 12.5% in the separation gel (data not shown).
Finally, we examined the effect of the voltage and running time on tubulin subunit separation. A 90 minute running time at 120 V yielded good resolution in the minigel without reported diffusing of bands [16]. Gels were also run at lower voltages (60 to 100 V) and for longer times, but resolution and separation of the tubulin monomers was not improved.
In summary, a resolving buffer of pH 9.8, a resolving layer containing 7.5% acrylamide/bis-acrylamide, use of 95% pure Sigma SDS, and a running time of 90 minutes at 120 V proved to be very effective in separating tubulin monomers, even when loaded in high mass (40 µg) in the minigels. For its simplicity, brief running duration, and ability to separate tubulin by maintaining a high resolution at high mass, we anticipate that this method will be useful for separation the α- and β-monomers of tubulin for preparative as well as analytical purposes.
Acknowledgments
We gratefully acknowledge David Tuttle for taking the photographs of the gels and assisting in preparing the final figures. The work was supported by NIH Grant CA-69571.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ludueña RF. Multiple forms of tubulin: different gene products and covalent modifications. Int. Rev. Cytol. 1998;178:207–275. doi: 10.1016/s0074-7696(08)62138-5. [DOI] [PubMed] [Google Scholar]
- 2.Ludueña RF, Woodward DO. α- and β-Tubulin: separation and partial sequence analysis. Ann. New York Acad. Sci. 1975;253:272–283. doi: 10.1111/j.1749-6632.1975.tb19206.x. [DOI] [PubMed] [Google Scholar]
- 3.Best D, Warr PJ, Gull K. Influence of the composition of commercial sodium dodecyl sulfate preparations on the separation of α- and β-tubulin during polyacrylamide gel electrophoresis. Anal. Biochem. 1981;114:281–284. doi: 10.1016/0003-2697(81)90481-4. [DOI] [PubMed] [Google Scholar]
- 4.Stephens RE. Electrophoretic resolution of tubulin and tektin subunits by differential interaction with long-chain alkyl sulfates. Anal. Biochem. 1998;265:356–360. doi: 10.1006/abio.1998.2909. [DOI] [PubMed] [Google Scholar]
- 5.Knipling L, Hwang J, Wolff J. Preparations and properties of pure tubulin S. Cell Motil. Cytoskeleton. 1999;43:63–71. doi: 10.1002/(SICI)1097-0169(1999)43:1<63::AID-CM7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 6.Bode CJ, Gupta ML, Suprenant KA, Himes RH. The two α-tubulin isotypes in budding yeast have opposing effects on microtubule dynamics in vitro. EMBO Rep. 2003;4:94–99. doi: 10.1038/sj.embor.embor716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stephens RE. High-resolution preparative SDS-polyacrylamide gel electrophoresis: fluorescent visualization and electrophoretic elution-concentration of proteins bands. Anal. Biochem. 1975;65:369–379. doi: 10.1016/0003-2697(75)90521-7. [DOI] [PubMed] [Google Scholar]
- 8.Marotta CA, Harris JL, Gilbert JM. Characterization of multiple forms of brain tubulin subunits. J. Neurosci. 1978;30:1431–1440. doi: 10.1111/j.1471-4159.1978.tb10475.x. [DOI] [PubMed] [Google Scholar]
- 9.Melki R, Kerjan P, Waller JP, Carlier MF, Pantaloni D. Interaction of microtuble-associated proteins with microtubules: yeast lysyl- and valyl-tRNA synthetases and τ 218–235 synthetic peptide as model systems. Biochemistry. 1991;30:11536–11545. doi: 10.1021/bi00113a008. [DOI] [PubMed] [Google Scholar]
- 10.Williams RC, Jr., Lee JC. Preparation of tubulin from brain. Meth. Enzymol. 1982;85:376–385. doi: 10.1016/0076-6879(82)85038-6. [DOI] [PubMed] [Google Scholar]
- 11.Detrich HW, Williams RC. Reversible dissociation of the αβ dimer of tubulin from bovine brain. Biochemistry. 1978;17:3900–3907. doi: 10.1021/bi00612a002. [DOI] [PubMed] [Google Scholar]
- 12.Rüdiger M, Plessman U, Klöppel K, Wehland J, Weber K. Class II tubulin, the major brain β tubulin isotype is polyglutamylated on glutamic acid residue 435. FEBS Lett. 1992;308:101–105. doi: 10.1016/0014-5793(92)81061-p. [DOI] [PubMed] [Google Scholar]
- 13.Cleveland DW, Lopata MA, Sherline P, Kirschner MW. Unpolymerized tubulin modulates the level of tubulin mRNAs. Cell. 1981;25:537–546. doi: 10.1016/0092-8674(81)90072-6. [DOI] [PubMed] [Google Scholar]
- 14.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Murphy DB, Wallis KT. Brain and erythrocyte microtubules from chicken contain different β-tubulin polypeptides. J. Biol. Chem. 1983;258:7870–7875. [PubMed] [Google Scholar]
- 16.Williams RC, Shah C, Sackett D. Separation of tubulin isoforms by isoelectric focusing in immobilized pH gradient gels. Anal. Biochem. 1999;275:265–267. doi: 10.1006/abio.1999.4326. [DOI] [PubMed] [Google Scholar]