Abstract
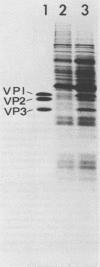
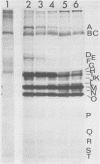
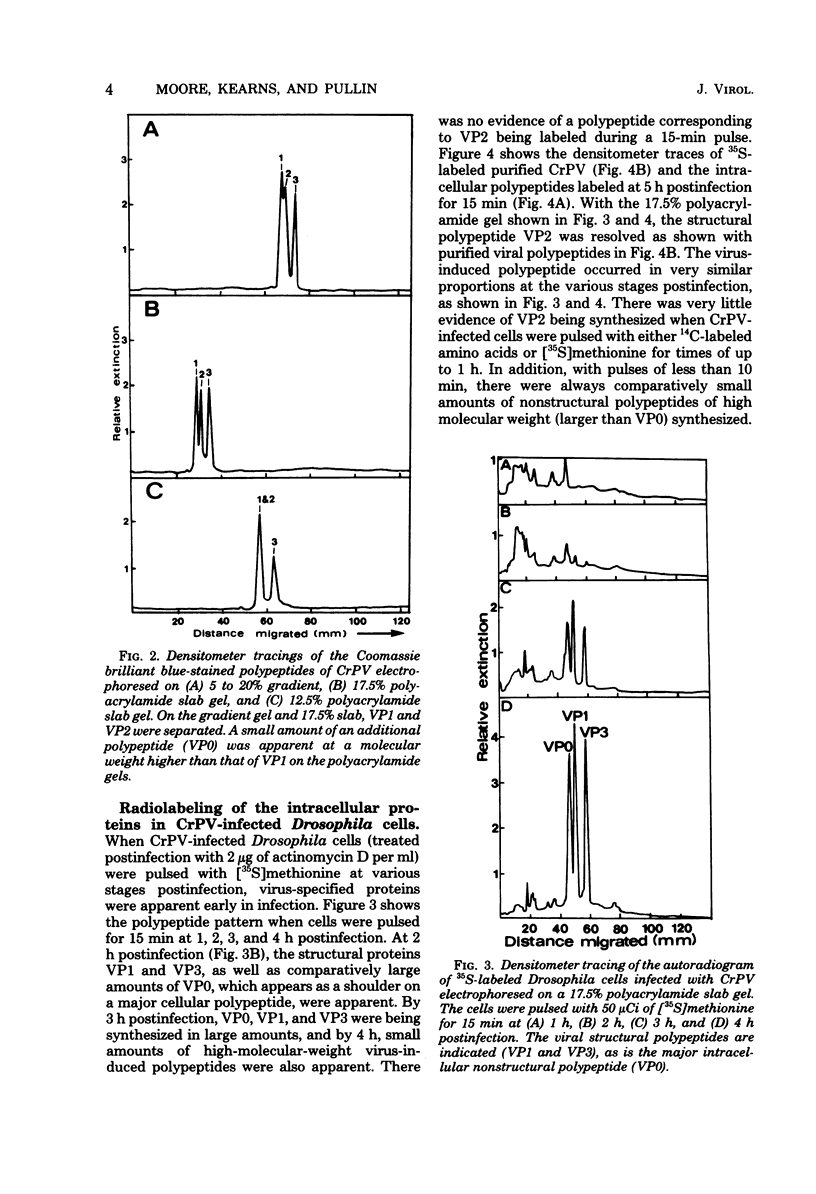
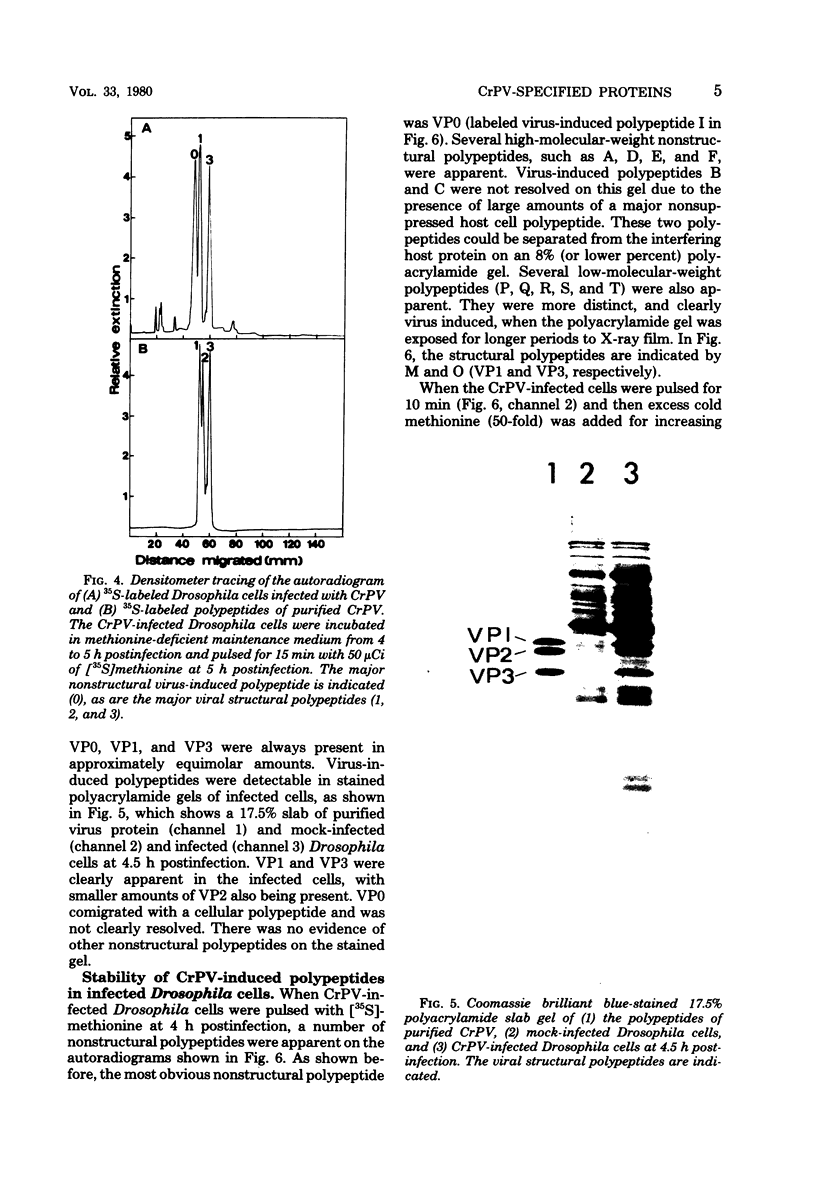
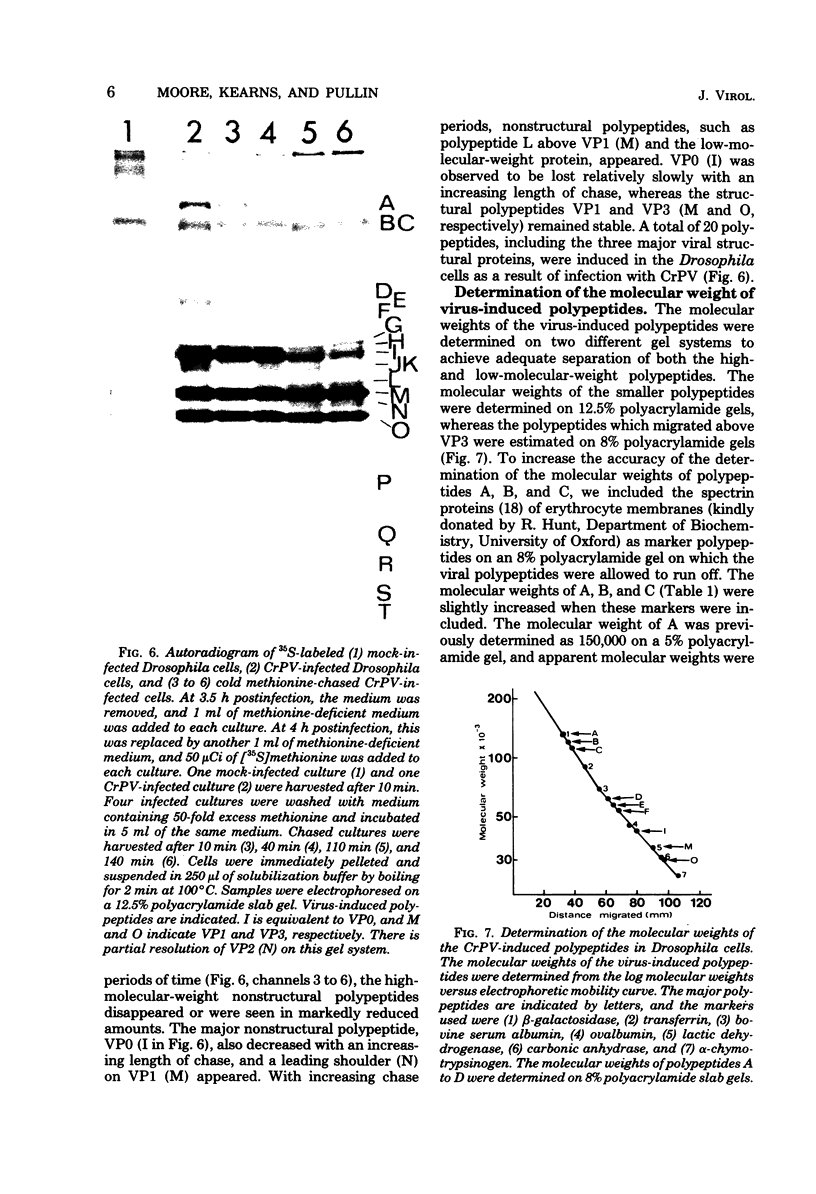
Cricket paralysis virus purified from Galleria mellonella larvae was shown to be similar to virus purified from Drosophila melanogaster cells. Cricket paralysis virus contained three major structural polypeptides of similar molecular weight (around 30,000), had a buoyant density of 1.344 g/ml, and had a capsid diameter of 27 nm. Twenty virus-induced polypeptides could be detected in CrPV-infected Drosophila cells. Two major polypeptides found in the infected cells corresponded to two structural viral polypeptides (VP1 and VP3), whereas the third major intracellular polypeptide was the apparent precursor of the third viral structural polypeptide (VP2). Three of the primary virus-induced polypeptides had molecular weights of 144,000, 124,000, and 115,000. These and other polypeptides were chased into lower-molecular-weight proteins when excess cold methionine was added after a short [35S]methionine pulse. Although cricket paralysis virus has a number of characteristics in common with the mammalian enteroviruses, the extremely fast processing of high-molecular-weight polypeptides into viral proteins seems atypical. Also, no VP4 (8,000 to 10,000 molecular weight) has been found in the virus particles.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAILEY L., GIBBS A. J., WOODS R. D. TWO VIRUSES FROM ADULT HONEY BEES (APIS MELLIFERA LINNAEUS). Virology. 1963 Nov;21:390–395. doi: 10.1016/0042-6822(63)90200-9. [DOI] [PubMed] [Google Scholar]
- Bailey L., Newman J. F., Porterfield J. S. The multiplication of Nodamura virus in insect and mammalian cell cultures. J Gen Virol. 1975 Jan;26(1):15–20. doi: 10.1099/0022-1317-26-1-15. [DOI] [PubMed] [Google Scholar]
- Bailey L., Woods R. D. Three previously undescribed viruses from the honey bee. J Gen Virol. 1974 Nov;25(2):175–186. doi: 10.1099/0022-1317-25-2-175. [DOI] [PubMed] [Google Scholar]
- Butterworth B. E., Korant B. D. Characterization of the large picornaviral polypeptides produced in the presence of zinc ion. J Virol. 1974 Aug;14(2):282–291. doi: 10.1128/jvi.14.2.282-291.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner F. Classification and nomenclature of viruses. Second report of the International Committee on Taxonomy of Viruses. Intervirology. 1976;7(1-2):1–115. doi: 10.1159/000149938. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Asso J., Baltimore D. Further evidence on the formation of poliovirus proteins. J Mol Biol. 1970 May 14;49(3):657–669. doi: 10.1016/0022-2836(70)90289-5. [DOI] [PubMed] [Google Scholar]
- Jousset F. X., Bergoin M., Revet B. Characterization of the Drosophila C virus. J Gen Virol. 1977 Feb;34(2):269–283. doi: 10.1099/0022-1317-34-2-269. [DOI] [PubMed] [Google Scholar]
- Jousset F. X. Etude expérimentale du spectre d'hôtes du virus C de Drosophila melanogaster chez quelques diptères et lépidoptères. Ann Microbiol (Paris) 1976 May-Jun;127(4):529–544. [PubMed] [Google Scholar]
- Jousset F. X., Plus N., Croizier G., Thomas M. Existence chez Drosophila de deux groupes de picornavirus de propriétés sérologiques biologiques différentes. C R Acad Sci Hebd Seances Acad Sci D. 1972 Dec 18;275(25):3043–3046. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Longworth J. F. Small isometric viruses of invertebrates. Adv Virus Res. 1978;23:103–157. doi: 10.1016/s0065-3527(08)60099-8. [DOI] [PubMed] [Google Scholar]
- Lucas-Lenard J. Cleavage of mengovirus polyproteins in vivo. J Virol. 1974 Aug;14(2):261–269. doi: 10.1128/jvi.14.2.261-269.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi V. T., Furthmayr H., Tomita M. The red cell membrane. Annu Rev Biochem. 1976;45:667–698. doi: 10.1146/annurev.bi.45.070176.003315. [DOI] [PubMed] [Google Scholar]
- Murphy F. A., Scherer W. F., Harrison A. K., Dunne H. W., Gary G. W., Jr Characterization of Nodamura virus, an arthropod transmissible picornavirus. Virology. 1970 Apr;40(4):1008–1021. doi: 10.1016/0042-6822(70)90147-9. [DOI] [PubMed] [Google Scholar]
- Newman J. F., Brown F. Absence of poly (A) from the infective RNA of Nodamura virus. J Gen Virol. 1976 Jan;30(1):137–140. doi: 10.1099/0022-1317-30-1-137. [DOI] [PubMed] [Google Scholar]
- Newman J. F., Brown F. Further physicochemical characterization of Nodamura virus. Evidence that the divided genome occurs in a single component. J Gen Virol. 1978 Jan;38(1):83–95. doi: 10.1099/0022-1317-38-1-83. [DOI] [PubMed] [Google Scholar]
- Paucha E., Seehafer J., Colter J. S. Synthesis of viral-specific polypeptides in Mengo virus-infected L cells: evidence for asymmetric translation of the viral genome. Virology. 1974 Oct;61(2):315–326. doi: 10.1016/0042-6822(74)90269-4. [DOI] [PubMed] [Google Scholar]
- Plus N., Croizier G., Duthoit J. L., David J., Anxolabéhère D., Périquet G. Découverte, chez la Drosophile, de virus appartenant à trois nouveaux groupes. C R Acad Sci Hebd Seances Acad Sci D. 1975 Mar 24;280(12):1501–1504. [PubMed] [Google Scholar]
- Plus N., Croizier G., Jousset F. X., David J. Picornaviruses of laboratory and wild Drosophila melanogaster: geographical distribution and serotypic composition. Ann Microbiol (Paris) 1975 Jan;126(1):107–117. [PubMed] [Google Scholar]
- Plus N., Croizier G., Reinganum C., Scott P. D. Cricket paralysis virus and drosophila C virus: serological analysis and comparison of capsid polypeptides and host range. J Invertebr Pathol. 1978 May;31(3):296–302. doi: 10.1016/0022-2011(78)90219-7. [DOI] [PubMed] [Google Scholar]
- Plus N., Croizier G., Veyrunes J. C., David J. A comparison of buoyant density and polypeptides of Drosophila P, C and A viruses. Intervirology. 1976;7(6):346–350. doi: 10.1159/000149975. [DOI] [PubMed] [Google Scholar]
- Plus N., Duthoit J. L. Un nouveau virus de Drosphila melanogaster, le virus P. C R Acad Sci Hebd Seances Acad Sci D. 1969 May 5;268(18):2313–2315. [PubMed] [Google Scholar]
- Reinganum C., Scotti P. D. Serological relations between twelve small RNA viruses of insects. J Gen Virol. 1976 Apr;31(1):131–134. doi: 10.1099/0022-1317-31-1-131. [DOI] [PubMed] [Google Scholar]
- Reinganum C. The isolation of cricket paralysis virus from the emperor gum moth, Antheraea eucalypti Scott, and its infectivity towards a range of insect species. Intervirology. 1975;5(1-2):97–102. doi: 10.1159/000149886. [DOI] [PubMed] [Google Scholar]
- Scherer W. F., Hurlbut H. S. Nodamura virus from Japan: a new and unusual arbovirus resistant to diethyl ether and chloroform. Am J Epidemiol. 1967 Sep;86(2):271–285. doi: 10.1093/oxfordjournals.aje.a120737. [DOI] [PubMed] [Google Scholar]
- Scherer W. F., Verna J. E., Richter W. Nodamura virus, an ether- and chloroform-resistant arbovirus from Japan: physical and biological properties, with ecologic observations. Am J Trop Med Hyg. 1968 Jan;17(1):120–128. doi: 10.4269/ajtmh.1968.17.120. [DOI] [PubMed] [Google Scholar]
- Schneider I. Cell lines derived from late embryonic stages of Drosophila melanogaster. J Embryol Exp Morphol. 1972 Apr;27(2):353–365. [PubMed] [Google Scholar]
- Scotti P. D. Cricket paralysis virus replicates in cultured Drosophila cells. Intervirology. 1975;6(6):333–342. doi: 10.1159/000149489. [DOI] [PubMed] [Google Scholar]
- Scotti P. D. End-point dilution and plaque assay methods for titration of cricket paralysis virus in cultured Drosophila cells. J Gen Virol. 1977 May;35(2):393–396. doi: 10.1099/0022-1317-35-2-393. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Teninges D., Plus N. P virus of Drosophila melanogaster, as a new picornavirus. J Gen Virol. 1972 Jul;16(1):103–109. doi: 10.1099/0022-1317-16-1-103. [DOI] [PubMed] [Google Scholar]