Abstract
Evolution depends on the acquisition of genomic mutations that increase cellular fitness. Here, we evolved Escherichia coli MG1655 cells to grow at extreme temperatures. We obtained a maximum growth temperature of 48.5 °C, which was not increased further upon continuous cultivation at this temperature for >600 generations. Despite a permanently induced heat shock response in thermoresistant cells, only exquisitely high GroEL/GroES levels are essential for growth at 48.5 °C. They depend on the presence of lysyl-tRNA-synthetase, LysU, because deletion of lysU rendered thermoresistant cells thermosensitive. Our data suggest that GroEL/GroES are especially required for the folding of mutated proteins generated during evolution. GroEL/GroES therefore appear as mediators of evolution of extremely heat-resistant E. coli cells.
Keywords: Bacteria, Chaperone Chaperonin, Evolution, Heat Shock Protein, Protein Stability, Proteomics
Introduction
Extended stress favors mutations that fuel evolution of resistance mechanisms (1–3). Bacteria have evolved to grow under a habitat-defined temperature regime. Yet, specific stress responses, genome instability, and cellular adaptability to environmental challenges may expand their biotope. For instance, a sudden increase in cultivation temperature induces the evolutionary highly conserved heat shock response upon which heat shock proteins (Hsps)2 are increasingly synthesized (for review, see Ref. 4). Hsps include chaperones, which assist the folding of newly synthesized proteins, prevent stress-induced unfolding and irreversible aggregation of proteins, as well as proteases, which degrade cellular proteins that are damaged beyond repair (5–8). Both mechanisms, degradation and prevention of aggregation, ensure protein homeostasis that is essential for the viability of cells. The importance of chaperones is most intriguingly demonstrated for GroE, which is the only indispensable chaperone system in Escherichia coli because it assists the folding of several essential E. coli proteins (9).
Growth of laboratory E. coli strains is, depending on the respective strain and cultivation medium, inhibited above 44 °C–46 °C (10–12). This is due to limitations of membrane permeability (13) and protein stability (12, 14). The synthesis of Hsps following exposure to elevated temperatures confers thermotolerance beyond the duration of stress. However, simple overexpression of heat shock genes is not sufficient (15), suggesting that additional factors are required for thermotolerance. Here, we cultivated E. coli MG1655 cells for several hundreds of generations and evolved them for growth at 48.5 °C. This temperature represents the absolute maximum at which growth of this E. coli strain is possible and exceeds the maximum growth temperature of MG1655 in LB medium by 3 °C. We show that adaptation to high temperatures is accompanied by an increase in steady-state levels of Hsps and lysyl-tRNA-synthetase, LysU. However, only high GroEL/GroES levels, which exceed the nonstress level in wild-type cells by 16-fold, in combination with the presence of LysU are required for thermoresistance.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Plasmids
E. coli MG1655 zba::kan (10) was used for evolution. BM3 lysU::cam and BM28 lysU::cam were generated by P1 transduction with MC4100 lysU::cam as donor. BM3 cells were transformed with pBB541 (16); GroESL levels similar to BM28 were obtained upon induction with 25 μm isopropyl 1-thio-β-d-galactopyranoside contained in the overnight culture.
Generation of Heat-adapted Strains
Three lines of E. coli were founded from one common ancestor (MG1655 zba::kan). All cultivations during adaptation were performed in 10-ml test tubes filled with 7 ml of LB medium. Ten serial transfers into fresh LB medium were done at 37 °C to allow regeneration from freezing. The ancestor was continued to propagate for a total of 2,937 generations at 37 °C for comparative experiments (BM3). The three replicate lines were adapted to high temperature in a stepwise manner. First, cultures were gradually shifted to 45 °C; cells were propagated for 104 generations at 42 °C and 104 generations at 45 °C. After all periods of adaptation, samples of each culture were stored as glycerol stocks at −80 °C thereby conserving different stages of adaptation. Cells were then directly shifted to 47 °C. After 24 generations the three lines stopped growing and had to be propagated for 15 generations again at lower temperature (46 °C). Then, cells were transferred for 45 generations at 47 °C, 126 generations at 47.5 °C, and 84 generations at 48 °C. It took a total of 620 generations and about 2 years to reach the actual highest growth temperature of 48.5 °C. In total, cells were propagated for 1,256 generations at high temperature. Some cultures stopped growing and were lost at high temperatures and had to be restored from existing lines or glycerol stocks. During the process of adaptation to high temperature the cells were always incubated for 48 h to reach late stationary phase. This is a growth phase with a high deleterious genomic mutation rate (17) promoting evolution and adaptation to new growth conditions. At temperatures above 45 °C, cells cultivated in test tubes were not able to reach high densities (maximum OD600 2.0) or to grow after high dilutions (optimal dilution factor 1:8). The strains BM3 (2,937 generations at 37 °C), BM15 (evolved at 42 °C), BM16 (45 °C), BM25 (48 °C), and BM28 (48.5 °C) were used for further characterization.
Culture Conditions and Heat Stress Treatment
Strains were cultivated in 10–20 ml of LB medium containing kanamycin (50 μg/ml) at the indicated temperature in 125-ml flasks with baffles until stationary phase (total of 16 h). For growth curves, cultures were diluted to OD600 0.2 and further incubated at their specific growth temperature or at 37 °C. Relative fitness was calculated from the doubling times of heat-evolved strains at the given temperature compared with that of the control strain BM3 at the same temperature. For the analysis of viability at 49 °C, 300 μl of overnight cultures were transferred into 1.5-ml tubes and incubated for 3 h at 1,000 rpm. Samples were removed, serially diluted, and spotted onto LB plates, and colony-forming units were counted after 24 h at 37 °C.
Analysis of Proteins on Two-dimensional Gels
Strains were cultivated in LB medium at the indicated temperature in flasks with baffles until stationary phase (16 h). Cells were harvested (5 min, 5,000 rpm, 8 °C) and washed once with ice-cold water. Cell pellets corresponding to OD600 2 were resuspended in 1 ml of two-dimensional sample buffer (7 m urea, 2 m thiourea, 1% (w/v) Serdolit MB-1, 1% (w/v) dithiothreitol, 4% (w/v) Chaps, and 0.5% (v/v) Pharmalyte 3–10), incubated at 20 °C, 1,000 rpm for 1 h and then lysed by repeated freeze-thawing (four times). Samples were centrifuged (13,300 rpm, 45 min, 4 °C), the supernatant was transferred to a new tube and the centrifugation repeated. For the first dimension (i.e. isoelectric focusing), identical protein amounts (450-μl samples corresponding to 0.9 × OD) were applied onto 24-cm IPG strips (pH 4–7; GE Healthcare). Two-dimensional gel electrophoresis was performed exactly as described previously (18, 19). Two-dimensional gels were stained with Coomassie Blue and destained with 10% acetic acid and subsequently scanned. The spot intensity of elongation factor TU (TufA) was very similar in all cell samples and was used as an internal loading control. Protein spots of interest were excised from the two-dimensional gels, subjected to trypsin digestion (according to Ref. 20), and afterward analyzed using an Ultraflex I ToF/ToF mass spectrometer (Bruker Daltonik). Data analysis was performed using the BioTools (Bruker Daltonik) and Mascot (Matrix Science) software packages.
Sequencing of Heat Shock Genes
Chromosomal DNA was extracted from BM3, BM15, BM16, BM25, and BM28 cells (GenEluteTM Bacterial Genomic DNA kit, Sigma-Aldrich), and the heat shock genes were amplified using primers annealing 200 bp downstream and upstream of the respective gene. DNA sequencing was performed using the purified PCR products as template and the mentioned primers.
Quantitative Western Blot Analysis
The amount of GroEL and GroES in heat-adapted cells (BM28) and control cells (BM3) was analyzed using cell extracts of overnight cultures grown for 16 h at 37 °C in LB medium. Six different concentrations of cell extracts (BM3: OD 0.001–0.004/lane (for GroEL) and OD 0.0015–0.025/lane (for GroES); BM28: OD 0.00005–0.00025/lane (for GroEL) and OD 0.0001–0.0025/lane (for GroES)) along with five different concentrations of GroEL (1.3–5 ng/lane) and GroES (2.5–12.5 ng/lane) were separated on Neutral gradient gels (Serva). Proteins were transferred onto nitrocellulose membrane, and GroEL/GroES was detected using antibodies raised against GroES and GroEL, respectively. The amount of GroEL/GroES in BM3 and BM28 cell extracts was quantified by comparison with the signals in the linear range obtained for the reference proteins. Average values were determined based on five independently repeated analyses.
Analysis of the Maximum Growth Temperature
BM3, BM3 pBB541, and BM28 cells grown on LB plates containing kanamycin (50 μg/ml) at 37 °C were used to inoculate aerobic or anaerobic LB medium or M9 minimal medium. Anerobic media were obtained by adding Oxyrase for broth (Oxyrase). Freshly inoculated media were either transferred into 125-ml flasks with baffles (5–10-ml volume, aerobic growth) or into 1.5-ml reaction tubes (filled with 1.5 ml, anaerobic growth) and incubated at 44 °C–49 °C with (aerobic) or without (anaerobic) shaking. After 24 h, growth was analyzed by measuring OD600. The temperature at which growth was barely detectable is considered the maximum growth temperature. It should be mentioned that anaerobic media incubated at high temperatures form a slight precipitate, which results in an OD600 of 0.06 (44 °C) to 0.12 (47 °C) and should be regarded.
Analysis of the Heat Shock Response
Overnight cultures grown in LB medium at 37 °C for 16 h were diluted 1:40 into fresh LB medium and cultivated further until an OD600 of 1.5 was reached. Then, 700 μl of cells was transferred to 1.5-ml tubes and cultivated at 37 °C or 45 °C (1,000 rpm). After 20 min the complete cultures were harvested (5 min, 13,300 rpm, 4 °C), resuspended in two-dimensional sample buffer, and analyzed on two-dimensional gels as described above.
RESULTS AND DISCUSSION
Adaptation of E. coli MG1655 Cells to Heat
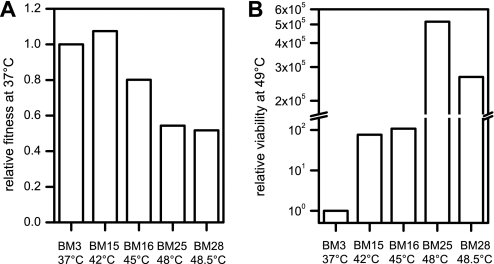
Nonphysiological conditions induce survival mechanisms, e.g. altered transcription or expression patterns or mutations that can result in an increased ability to cope with this stress (2). To study the process and mechanism of evolutionary adaptation of bacteria to heat, we used the E. coli strain MG1655. E. coli cells were cultivated in LB medium in which genes involved in amino acid biosynthesis, such as of the met operon required for methionine biosynthesis, are neither induced nor required and should therefore not limit growth (11). We determined the maximum growth temperature of MG1655 cells in liquid LB medium to 45.5 °C, which is about 1 °C less than on agar plates (10). MG1655 cells were either maintained at 37 °C (BM3) or successively shifted to higher temperatures, i.e. 42 °C (BM15), 45 °C (BM16), 48 °C (BM25), and 48.5 °C (BM28). The maximum growth temperature of 48.5 °C (BM28) was reached after a stepwise increase in temperature for 620 generations. Continuous cultivation at 48.5 °C for about 600 generations did not result in a further increase in thermoresistance. For comparison, the maximum growth temperature of BM3 cells, which were cultivated at 37 °C for almost 3,000 generations, was identical to that of the ancestral strain (45.5 °C). The relative fitness at 37 °C (i.e. the growth rate of the evolved strain at 37 °C compared with that of the ancestor at 37 °C) increased for cells adapted to 42 °C (BM15) but decreased for cells continuously cultivated at 45 °C-48.5 °C (BM16, BM25, BM28) (Fig. 1A). This indicates stress-induced mutations and suggests that adaptation to higher temperatures occurs on the expense of generation times. Cultivation of cells at 49 °C resulted in a decreased viability, with a decline specific to each culture (Fig. 1B, 3 h). BM3 cells completely lost viability whereas that of BM15 and BM16 cells was reduced to about 0.0006%. BM25 and BM28 cells, in contrast, showed only a small decrease in the number of surviving cells, which corresponded to an at least 2 × 105-fold increased viability at 49 °C compared with the control cells.
FIGURE 1.
Fitness of heat-evolved E. coli strains. A, overnight cultures of BM3, BM15, BM16, BM25, and BM28 grown at 37 °C, 42 °C, 45 °C, 48 °C, and 48.5 °C, respectively, were diluted and growth further followed at 37 °C. B, 300-μl cultures from A were shifted to 49 °C and the number of viable cells determined after 3 h. Relative fitness/viability was calculated by determining the doubling times/viability of the evolved strain relative to that of BM3, which was set to 1.
All cells grown at their specific growth temperature showed a similar morphology. Cells were analyzed by scanning electron microscopy and showed the typical rod-like shape with a length of ∼1.3–1.5 μm and a width of ∼0.5–0.7 μm (compare with Ref. 21). Wild-type-like dimensions suggest that heat adaptation is independent of elevated σS levels, which would render cells significantly shorter (22). To test whether heat adaptation confers cross-protection against other stresses, we tested the viability of BM3 and BM28 cells upon exposure to hydrogen peroxide (0–8 mm, 37 °C, 3 h). Both strains showed a similar viability during the oxidative stress treatment, suggesting that BM28 cells are only adapted to extreme growth temperatures. This is in line with earlier observations by Cullum and co-workers (23) who showed that adaptation of E. coli cells to 42 °C does not confer any cross-protection or preadaptation to other, not yet encountered, stress types.
Heat-adapted Cells Show Increased Hsp Levels
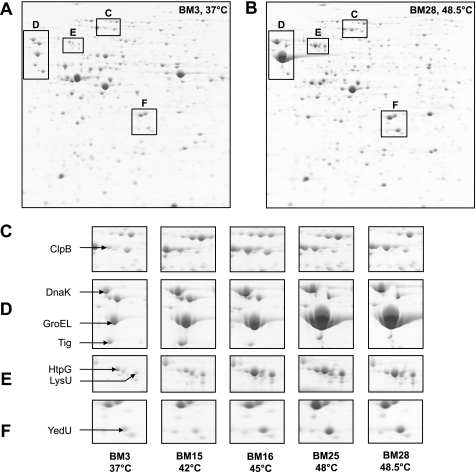
The decreased fitness of BM28 cells at 37 °C suggests that they acquired mutations during adaptation to high temperatures. It has been demonstrated earlier that the level of the Hsp GroEL is increased in mutated bacterial lineages (24, 25). To analyze potential changes in the proteome, we analyzed the control cells (BM3) and heat-adapted cells (BM15, BM16, BM25, BM28) on two-dimensional gels. The most significant differences between BM3 and heat-adapted cells were an increase in steady-state levels of Hsps (i.e. ClpB, DnaK, GroEL, GroES, HtpG, YedU) and LysU (Fig. 2). In contrast, the level of the chaperone trigger factor, Tig (Fig. 2A) was reduced. Tig assists the folding of newly synthesized proteins and has been shown to increase the viability of E. coli cells at low temperatures (26) but is apparently not required for survival at high temperatures. Also, other proteins with functions unrelated to protein folding show significantly altered steady-state levels. They include the metabolic enzymes tryptophanase, thymidine phosphorylase, deoxyribose-phosphate aldolase, and purine nucleoside phosphorylase with decreased levels and asparaginase II and cysteine synthase A with increased levels (not indicated in the two-dimensional gels). Because Hsps are vital for survival at high temperatures and are therefore likely to support heat adaptation, we focused only on the cellular levels of the chaperones.
FIGURE 2.
High Hsp levels in heat-evolved E. coli cells. Overnight cultures of BM3, BM15, BM16, BM25, and BM28 grown at their specific growth temperature were analyzed on two-dimensional gels. A and B, two-dimensional gels of BM3 and BM28 with the regions comprising Hsps marked with boxes, respectively. Separated proteins show an isoelectric point of 3–10 (left to right) and a molecular mass from 150 to 10 kDa (upper to lower part). C–F, selected regions of two-dimensional gels showing changes in the spot intensities of Hsps (labeled by an arrow).
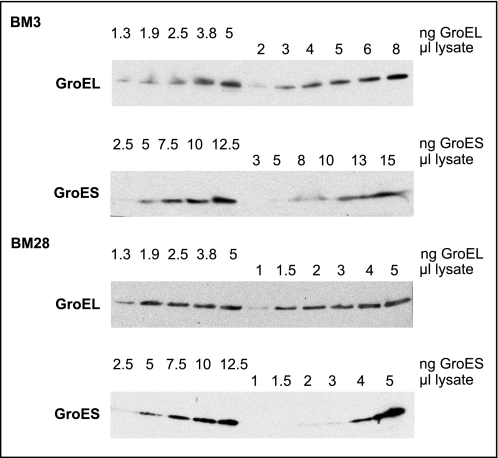
Of note, GroEL/GroES levels in BM15 and BM16 are elevated, and BM25 and BM28 cells show GroEL/GroES levels that largely exceeded the respective amount produced upon heat shock in wild-type cells (27) (compare also Figs. 2D and 5A) and observed in mutated lineages (24, 25). In fact, according to quantitative Western blot analysis, GroEL and GroES levels in BM28 cells are increased 16 times compared with BM3 cells (average of five independent analyses; representative data shown in Fig. 3). Although BM3 cells contained about 1.2 μg of GroEL and GroES/OD unit, BM28 cells contained about 20 μg of GroEL and 19 μg of GroES/OD unit, which corresponds roughly to 20% of the total cellular protein. High GroE (i.e. GroEL/GroES) levels during heat adaptation suggest a requirement for a significantly increased protein folding capacity for thermolabile proteins. GroE is a promiscuous, ATP-dependent chaperone system in the E. coli cytosol, which entraps proteins during the functional cycle and promotes their folding to the native state (6, 28–31). Upon heat shock conditions, GroE is required to shift the equilibrium of folding intermediates toward a productive folding pathway, whereby it facilitates the native state of thermolabile proteins under usually nonpermissive folding conditions (32).
FIGURE 5.
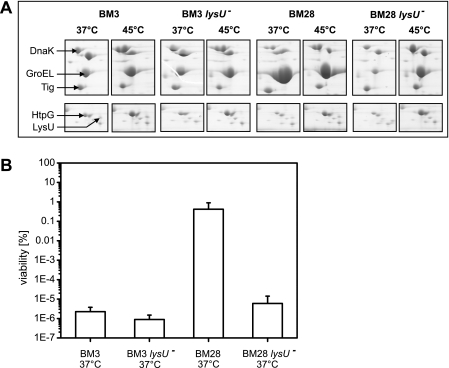
LysU is required for thermoresistance and high GroEL/GroES levels but not the heat shock response. A, exponentially growing BM3, BM3 ΔlysU, BM28, and BM28 ΔlysU cells were either maintained at 37 °C or shifted to 45 °C for 20 min and analyzed on two-dimensional gels. Selected regions of two-dimensional gels are presented to show changes in the Hsps upon heat shock. B, 300 μl of cells as in A were grown to stationary phase and shifted to 49 °C, and viability was determined after 3 h. The viability of nonstressed cells was set to 100%. Given are the averages ± S.D. (error bars) of three independent experiments.
FIGURE 3.
Quantitative Western blot analysis of GroE levels in BM3 and BM28 cells. Lysates of overnight cultures were analyzed by immunoblotting using GroEL- and GroES-specific antibodies. Purified GroEL (1.3- 5 ng) or GroES (2.5–12.5 ng) was loaded along with lysates of BM3 (OD 0.0005/μl) and BM28 (0.00005/μl), respectively. GroEL and GroES signals in lysates were compared with those of the reference proteins and quantified to 1.2 μg/OD (BM3, GroEL, and GroES), 20 μg/OD (BM28, GroEL), and 19 μg/OD (BM28, GroES).
High Levels of GroEL/GroES Are Maintained at 37 °C
Elevated levels of Hsps usually result from increased gene expression in response to stress, mediated by the heat shock transcription factor, σ32 (RpoH). To analyze whether mutations caused the high steady-state levels of Hsps in heat-evolved cells, we sequenced the rpoH, dnaK, groEL, groES, htpG, and yedU genes, including their promoter regions. Neither in cells that were maintained at 37 °C nor in any heat-adapted cells did we identify mutations in these genes/promoters. This excludes the possibility of a direct mutation-derived cause for altered transcription/expression of heat shock genes or increased stability/activity of the respective Hsp that could be derived by amino acid substitution. However, we cannot exclude that an unknown factor is responsible for altered transcription, translation, or stability of Hsps.
We reasoned that the high Hsp levels are only induced upon high temperatures and should resume wild-type levels upon return to non–heat shock temperatures. However, GroE levels in BM28 cells remained uniquely high upon cultivation at 37 °C whereas the levels of all other Hsps, Tig, and the metabolic enzymes that showed drastically altered levels returned to wild-type levels (see Fig. 5A and data not shown). Surprisingly, the viability of BM28 cells that were either shifted from 37 °C to 49 °C or from 48.5 °C to 49 °C was very similar (0.5% versus 2.2%), indicating that high GroE levels are sufficient for thermoresistance. Therefore, we analyzed the maximum growth temperature and the viability at 49 °C of BM3 cells that expressed groELS from a plasmid (BM3 pBB541) to the levels observed in BM28 cells (Table 1 and Fig. 4). High plasmid-derived GroE levels (see Fig. 4, inset) increased the maximum growth temperature of BM3 cells from 46 °C to 47.5 °C (Table 1), and their viability at 49 °C compared with BM3 cells by at least 1,000-fold (Fig. 4). Of note, BM3 cells that overexpressed groELS to even higher levels showed a viability at 49 °C similar to the above mentioned groELS overexpressor (Fig. 4). We conclude that high GroE levels contribute to heat resistance but that additional factors are required for the extreme heat resistance of BM28 cells during aerobic growth in LB medium.
TABLE 1.
Maximum growth temperatures of E. coli strains during aerobic and anaerobic growth in LB medium and M9 medium
| Strain | Maximum growth temperature |
|||
|---|---|---|---|---|
| Aerobic growth |
Anaerobic growth |
|||
| LB medium | M9 medium | LB medium | M9 medium | |
| °C | °C | °C | °C | |
| BM3 | 46 | 45.5 | 46 | 46 |
| BM3 pBB541 | 47.5 | 46 | 47.5 | 47.5 |
| BM28 | 48.5 | 47.5 | 46 | 46 |
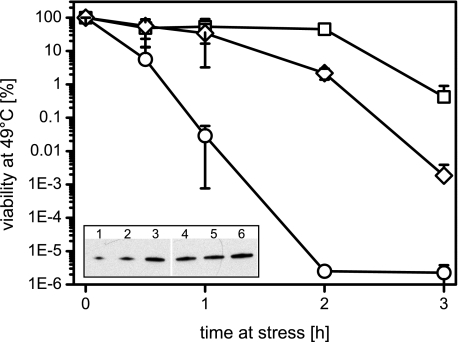
FIGURE 4.
High GroE levels confer heat resistance. 300-μl overnight cultures of BM3 (circles), BM3 pBB541 (diamonds), and BM28 (squares) were shifted to 49 °C, and viability was determined at the indicated time points. The viability of nonstressed cells was set to 100%. Given are the averages ± S.D. (error bars) of three to four independent experiments. Inset, Western blot analysis of GroEL levels in BM3, BM3 pBB541, and BM28 cells. 10, 20, and 40 ng of GroEL (lanes 1-3) were loaded along with 5 μl of lysates of BM3 (lane 4, OD 0.0005/μl), BM3 pBB541 (lane 5, OD 0.00005/μl), and BM28 (lane 6, OD 0.00005/μl). GroEL signals in lysates were quantified to 1.2 μg/OD (BM3), 15 μg/OD (BM3 pBB541), and 20 μg/OD (BM28).
To test whether high GroE levels confer general thermoresistance, we tested the maximum growth temperature of the BM3, BM3 pBB541, and BM28 strains also in M9 minimal medium as well as under anaerobic conditions (Table 1). The maximum growth temperature of the BM3 and BM3 pBB541 strains was 46 °C and 47.5 °C, respectively, with the exception of aerobic growth in M9 medium, which was only possible until 45.5 °C (BM3) and 46 °C (BM3 pBB541). Surprisingly, BM28 cells showed a high maximum growth temperature of 48.5 °C (LB) and 47.5 °C (M9) during aerobic growth, but their growth during anaerobic conditions was very similar to that of the BM3 strain (46 °C). These data indicate that BM28 cells are only evolved for high temperature tolerance during aerobic growth. Supposedly, certain mutations arose in BM28 cells during the time course of heat adaptation that limit anaerobic growth to 46 °C despite very high GroE levels. Given that high GroE levels as observed in BM3 pBB541 enable growth at 47.5 °C during aerobic and anaerobic growth in LB medium, we conclude that high cellular GroE concentrations confer significant thermotolerance to E. coli cells.
Role of LysU in the Heat Resistance of Evolved Cells
We analyzed the relationship between LysU and GroE to understand the reason for high GroE levels in heat-adapted cells. Neither GroEL nor GroES carries any post-translational modifications that could account for a decreased turnover (data not shown). We can also exclude the specific induction of groESL because the entire heat shock genes are induced in response to heat shock or accumulating unfolded proteins (33). A gene duplication event as described by Riehle and co-workers in E. coli cells evolved at 41.5 °C for 2,000 generations (34) seems an unlikely cause for high GroE levels in our heat-adapted cells as it could not account for the massively increased GroE levels. High GroE levels at high temperatures may result indirectly from increased LysU levels (Fig. 2B). LysU is heat-inducible; it synthesizes a number of adenyl dinucleotides, such as AppppA (Ap4A), which accumulate upon heat shock (35) and were shown to bind to GroEL and other Hsps (36, 37). Ap4A may be involved in the expression of groELS; it could affect the transcriptional or translational efficiency of groELS; however, nothing is known about the effect of Ap4A on tRNAs associated with GroE translation. LysU/Ap4A could also be involved in controlling the expression of other thermotolerance genes that in turn affect the expression of groELS. Although this may apply to 48.5 °C, the situation is different at 37 °C. GroE levels apparently did not directly correlate with LysU levels because GroE levels were identical when BM28 cells were cultivated at 48.5 °C or 37 °C (see above), and LysU levels were elevated at 48.5 °C but wild-type like at 37 °C. We tested whether GroE was LysU-dependently stabilized in cell extracts. For this we tested the trypsin sensitivity of GroES and GroEL in heat-resistant BM28 cells that were either cultivated at 48.5 °C or 37 °C and BM28 ΔlysU cells expressing plasmid-encoded groELS. However, GroEL/GroES were similarly degraded by trypsin in both strains at both temperatures (data not shown), indicating that LysU/Ap4A did not or not significantly affect GroE stability.
Dinucleotides, including Ap4A, have been proposed to act as modulators of the heat shock response (38); yet, their direct involvement has not been tested so far. Therefore, we analyzed the heat shock response in exponentially growing BM3 lysU+/− and BM28 lysU+/− cells. All strains produced similarly increased amounts of Hsps upon heat shock in a LysU-independent manner (Fig. 5A). The only exception was GroE, whose high levels in BM28 cells were not further increased during the heat shock treatment. Therefore, LysU is neither required for nor does it apparently modulate the heat shock response in E. coli. In this line, if LysU is required for the production of other thermotolerance proteins, their influence on the heat shock response and heat shock survival is only marginal. Strikingly, BM28 lysU− cells lack the permanently increased GroE levels (Fig. 5A). We tested whether LysU is also responsible for thermoresistance by analyzing the viability of BM3 lysU+/− and BM28 lysU+/− cells at 49 °C. Deletion of lysU only slightly reduced the viability of BM3 cells at 49 °C (Fig. 5B). In contrast, deletion of lysU in BM28 cells decreased the viability at 49 °C by more than 100,000-fold to a BM3-like viability (Fig. 5B). Therefore, LysU is indispensable for the thermoresistance of BM28 cells. This links the thermoresistance of BM28 cells to both the presence of LysU and permanently increased GroE levels, but, interestingly, not to the permanent heat shock response in general.
What could be the benefit of high GroE levels in heat-evolved cells? Heat-evolved cells (BM16, BM25, BM28 but not BM15) showed a fitness decline at 37 °C (see Fig. 1A), which either could be indicative for the acquisition of mutations or for the prolonged interaction of GroE with cellular proteins. We can exclude the latter possibility because the GroE levels in BM15 and BM16 cells are similarly high, but their relative fitness at 37 °C is largely different. We thus speculate that high GroE levels in heat-evolved cells are primarily required to buffer mutations and stabilize (mutated) cellular proteins at high temperatures. This is in line with previous work by Todd et al. who suggested GroE could ameliorate the effects of mutations (39). Besides GroE, the chaperone Hsp90 was also shown to promote evolutionary change. Hsp90 keeps genetic determinants silent in eukaryotes; however, when Hsp90 is mutant or impaired, then genetic variation occurs (40). Tanner et al. (37) showed that Ap4A binds to GroEL, thereby enhancing its chaperone activity at high temperatures, resulting in a higher folding capacity during stress. This likely applies also to our heat-evolved E. coli cells, at least when they were cultivated at 48.5 °C at which a large number of thermolabile cellular proteins require assistance to maintain their active structure. The fact that from all Hsps only GroE is required for heat resistance indicates that some, supposedly mutated, proteins are especially aggregation-prone and now depend on GroE for folding. This is supported by the observation that GroE-dependent substrates (9) show a high propensity to aggregate (41). Further support for the hypothesis that high GroE levels are especially required for the folding of mutated proteins is derived from previous reports showing that GroEL is up-regulated in bacteria encountering a high mutation rate and mitigates the consequences of deleterious mutations (24, 25). Vice versa, elevated GroE levels allow for an increased number of accumulating mutations in proteins and assist their folding even if they carry mutations in the core or mutations that lead to a large destabilization (42, 43). Therefore, the primary role of GroE in heat-evolved cells is to specifically keep thermolabile or mutated proteins active. Taken together, our results imply that the extreme up-regulation of GroEL/GroES is central to the evolution of thermoresistance in E. coli.
Acknowledgments
We thank Drs. J. Bardwell and B. Bukau for providing strains or plasmids; Dr. S. Gleiter and members of the Winter laboratory for discussion; B. Richter for performing two-dimensional gels and electron microscopy; B. Ludwig and T. Kriehuber for performing mass spectrometry analysis; and P. Baer, J. Kobuch, and K. Ganzinger for technical assistance.
This work was supported by the Elitenetzwerk Bayern (to K. M. G.), the Fonds der chemischen Industrie and Sonderforschungsbereich 594 (to J. B.), and the Emmy-Noether program of the Deutsche Forschungsgemeinschaft (to J. W.).
- Hsp
- heat shock protein
- LysU
- lysyl-tRNA-synthetase
- Chaps
- 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid.
REFERENCES
- 1.Galhardo R. S., Hastings P. J., Rosenberg S. M. (2007) Crit. Rev. Biochem. Mol. Biol. 42, 399–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matic I., Taddei F., Radman M. (2004) Res. Microbiol. 155, 337–341 [DOI] [PubMed] [Google Scholar]
- 3.McKenzie G. J., Rosenberg S. M. (2001) Curr. Opin. Microbiol. 4, 586–594 [DOI] [PubMed] [Google Scholar]
- 4.Guisbert E., Yura T., Rhodius V. A., Gross C. A. (2008) Microbiol. Mol. Biol. Rev. 72, 545–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bukau B., Weissman J., Horwich A. (2006) Cell 125, 443–451 [DOI] [PubMed] [Google Scholar]
- 6.Hartl F. U., Hayer-Hartl M. (2002) Science 295, 1852–1858 [DOI] [PubMed] [Google Scholar]
- 7.Hinault M. P., Ben-Zvi A., Goloubinoff P. (2006) J. Mol. Neurosci. 30, 249–265 [DOI] [PubMed] [Google Scholar]
- 8.Walter S., Buchner J. (2002) Angew. Chem. Int. Ed. Engl. 41, 1098–1113 [DOI] [PubMed] [Google Scholar]
- 9.Kerner M. J., Naylor D. J., Ishihama Y., Maier T., Chang H. C., Stines A. P., Georgopoulos C., Frishman D., Hayer-Hartl M., Mann M., Hartl F. U. (2005) Cell 122, 209–220 [DOI] [PubMed] [Google Scholar]
- 10.Bardwell J. C., Craig E. A. (1988) J. Bacteriol. 170, 2977–2983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mordukhova E. A., Lee H. S., Pan J. G. (2008) Appl. Environ. Microbiol. 74, 7660–7668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ron E. Z., Davis B. D. (1971) J. Bacteriol. 107, 391–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van de Vossenberg J. L., Ubbink-Kok T., Elferink M. G., Driessen A. J., Konings W. N. (1995) Mol. Microbiol. 18, 925–932 [DOI] [PubMed] [Google Scholar]
- 14.Gur E., Biran D., Gazit E., Ron E. Z. (2002) Mol. Microbiol. 46, 1391–1397 [DOI] [PubMed] [Google Scholar]
- 15.VanBogelen R. A., Acton M. A., Neidhardt F. C. (1987) Genes Dev. 1, 525–531 [DOI] [PubMed] [Google Scholar]
- 16.Tomoyasu T., Mogk A., Langen H., Goloubinoff P., Bukau B. (2001) Mol. Microbiol. 40, 397–413 [DOI] [PubMed] [Google Scholar]
- 17.Loewe L., Textor V., Scherer S. (2003) Science 302, 1558–1560 [DOI] [PubMed] [Google Scholar]
- 18.Hiniker A., Bardwell J. C. (2004) J. Biol. Chem. 279, 12967–12973 [DOI] [PubMed] [Google Scholar]
- 19.Winter J., Linke K., Jatzek A., Jakob U. (2005) Mol. Cell 17, 381–392 [DOI] [PubMed] [Google Scholar]
- 20.Schäfer H., Nau K., Sickmann A., Erdmann R., Meyer H. E. (2001) Electrophoresis 22, 2955–2968 [DOI] [PubMed] [Google Scholar]
- 21.Barth E., Gora K. V., Gebendorfer K. M., Settele F., Jakob U., Winter J. (2009) Microbiology 155, 1680–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lange R., Hengge-Aronis R. (1991) J. Bacteriol. 173, 4474–4481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cullum A. J., Bennett A. F., Lenski R. E. (2001) Evolution 55, 2194–2202 [DOI] [PubMed] [Google Scholar]
- 24.Fares M. A., Ruiz-González M. X., Moya A., Elena S. F., Barrio E. (2002) Nature 417, 398. [DOI] [PubMed] [Google Scholar]
- 25.Maisnier-Patin S., Roth J. R., Fredriksson A., Nyström T., Berg O. G., Andersson D. I. (2005) Nat. Genet. 37, 1376–1379 [DOI] [PubMed] [Google Scholar]
- 26.Kandror O., Goldberg A. L. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 4978–4981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mogk A., Tomoyasu T., Goloubinoff P., Rüdiger S., Röder D., Langen H., Bukau B. (1999) EMBO J. 18, 6934–6949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beissinger M., Rutkat K., Buchner J. (1999) J. Mol. Biol. 289, 1075–1092 [DOI] [PubMed] [Google Scholar]
- 29.Horwich A. L., Fenton W. A. (2009) Q. Rev. Biophys. 42, 83–116 [DOI] [PubMed] [Google Scholar]
- 30.Lilie H., Buchner J. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 8100–8104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thirumalai D., Lorimer G. H. (2001) Annu. Rev. Biophys. Biomol. Struct. 30, 245–269 [DOI] [PubMed] [Google Scholar]
- 32.Grallert H., Buchner J. (1999) J. Biol. Chem. 274, 20171–20177 [DOI] [PubMed] [Google Scholar]
- 33.Parsell D. A., Sauer R. T. (1989) Genes Dev. 3, 1226–1232 [DOI] [PubMed] [Google Scholar]
- 34.Riehle M. M., Bennett A. F., Long A. D. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 525–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brevet A., Chen J., Lévêque F., Plateau P., Blanquet S. (1989) Proc. Natl. Acad. Sci. U.S.A. 86, 8275–8279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnstone D. B., Farr S. B. (1991) EMBO J. 10, 3897–3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanner J. A., Wright M., Christie E. M., Preuss M. K., Miller A. D. (2006) Biochemistry 45, 3095–3106 [DOI] [PubMed] [Google Scholar]
- 38.Lee P. C., Bochner B. R., Ames B. N. (1983) Proc. Natl. Acad. Sci. U.S.A. 80, 7496–7500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Todd M. J., Lorimer G. H., Thirumalai D. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 4030–4035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rutherford S. L., Lindquist S. (1998) Nature 396, 336–342 [DOI] [PubMed] [Google Scholar]
- 41.Niwa T., Ying B. W., Saito K., Jin W., Takada S., Ueda T., Taguchi H. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 4201–4206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moran N. A. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 2873–2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tokuriki N., Tawfik D. S. (2009) Nature 459, 668–673 [DOI] [PubMed] [Google Scholar]