Abstract
Dendritic cells (DCs) express cell surface lectins that are potentially involved in the recognition, uptake, and presentation of glycosylated foreign substances. A unique calcium-type (C-type) lectin, the macrophage galactose (Gal)-type C-type lectin (MGL/CD301) expressed on DCs, is thought to participate in the recognition of molecules from both altered self and pathogens due to its monosaccharide specificity for Gal and N-acetylgalactosamine (GalNAc). Although mice have two MGL genes, Mgl1 and Mgl2, their distinct roles have not been previously explored. The present report characterizes the properties of MGL2 by examining its distribution and its role in antigen presentation by DCs. We generated an MGL2-specific monoclonal antibody and examined MGL2 expression in tissues by immunohistochemistry and in isolated cells by flow cytometry. The cells reactive with this antibody were shown to be a portion of MGL1-expressing cells, mostly conventional DCs. Internalization of soluble polyacrylamide polymers (PAA) with α-GalNAc residues (GalNAc-PAA) by bone marrow-derived DCs (BM-DCs) was mediated by MGL2, as revealed by a comparison of Mgl1−/− and Mgl2−/− BM-DCs with wild-type BM-DCs. Biotinylated GalNAc-PAA conjugated to streptavidin (SAv) was more efficiently presented to SAv-primed T cells by BM-DCs than β-N-acetylglucosamine-PAA conjugated to SAv or SAv alone as shown by thymidine uptake and cytokine production. This is the first report that demonstrates the involvement of GalNAc residues in antigen uptake and presentation by DCs that lead to CD4+ T cell activation.
Keywords: Antigen Presentation, Dendritic Cell, Glycosylation, Immunology, Lectin, Mucin
Introduction
It is estimated that more than half of all proteins produced by eukaryotes are glycosylated (1). Most, although not all, cell surface and secreted proteins are thought to be glycosylated. Therefore, the immune responses to self and foreign proteins are likely to be modulated through the functions of carbohydrate-recognition molecules (i.e. lectins) expressed on antigen presenting cells. Dendritic cells (DCs)3 are antigen presenting cells that are capable of activating or tolerizing antigen-specific naive T cells (2). Surface lectins are potentially involved in this process as endocytic receptors, signaling receptors, or regulators of cellular localization and trafficking. C-type lectins expressed on DCs and Langerhans cells include type I multi-carbohydrate recognition domain (CRD) lectins, such as the mannose receptor (MR) and DEC-205, and type II single-CRD lectins, such as macrophage galactose (Gal)-type C-type lectin (MGL/CD301), DCIR, DC-SIGN, dectin-1, dectin-2, BDCA-2, and Langerin (3–5). These C-type lectins have previously been shown to be involved with the internalization of antigens and their subsequent presentation to antigen-specific T cells (4, 6–9). However, the involvement of glycoforms in antigen uptake for presentation is not well understood because these previous conclusions have been obtained with anti-lectin antibodies. Among the lectins studied, monosaccharide specificity of MR, DC-SIGN, Langerin, and dectin-2 is mannose, and dectin-1 is specific for β-glucan (5). The roles of C-type lectins on antigen presenting cells with monosaccharide specificity for Gal or GalNAc are not thoroughly investigated even though these residues are thought to serve as terminals of glycans involved in tumor immunity, infection, and recognition of altered self.
Examples of Gal and GalNAc epitopes important in tumor immunity are the Tn (GalNAcα-Ser/Thr) and Thomsen-Friedenreich (TF: Galβ1–3GalNAcα-Ser/Thr) antigens in carcinoma-associated mucins (10). Cancer cells express MUC1, which has shorter carbohydrate chains that have been proposed to be recognized by the immune system (11), and MUC1-specific cytotoxic T lymphocytes and anti-MUC1 humoral reactions have been detected in breast, pancreatic, and ovarian carcinoma patients. Furthermore, early breast cancer patients with naturally occurring MUC1-specific antibodies have a better prognosis (12). The MUC1-specific immune response might be modulated by its TF/Tn carbohydrate chains, as suggested by a previous report (13). Whether such recognition leads to effective tumor immunity or tumor-induced immune suppression is currently unknown.
MGL is known as a type II transmembrane glycoprotein that contains a single CRD specific for monosaccharides Gal/GalNAc. A single gene encodes human MGL/CD301 (14), whereas mice have two genes encoding MGL1/CD301a (15) and MGL2/CD301b (16). MGL was originally detected on tumoricidal macrophages (15) and was found to be expressed on histiocytic macrophages but not on Langerhans cells (17, 18). Recently, we identified MGL expression on immature DCs in humans and mice (19, 20). However, the precise cellular distribution of MGL1 and MGL2 has not been investigated because the MGL-specific monoclonal antibodies (mAbs) used previously (mAb LOM-14 and mAb ER-MP23) recognize a common epitope between MGL1 and MGL2, although mAb LOM-8.7, specific for MGL1, was also used in some studies (16, 21). In the present report, the protein expression of MGL1 and MGL2 is individually shown from investigation with the combined use of mAb LOM-8.7 and novel MGL2-specific mAb URA-1. We describe in detail for the first time the unique characteristics of mAb URA-1. Furthermore, generation of Mgl2 knock-out (Mgl2−/−) mice allows for investigating the immunological functions of MGL2 separately from that of MGL1 at the cellular level; once the backcross has been completed, further studies beyond the cellular level may proceed.
Based on available knowledge, MGL is the sole Gal-type lectin among the C-type lectins expressed on DCs. Furthermore, the MGL expressed on immature DCs has been shown to mediate the uptake of antigens containing GalNAc residues (19, 20). Mouse and human MGLs have a high affinity for clusters of O-linked GalNAc residues (16, 22, 23). MGL on immature human monocyte-derived DCs has been shown to recognize and internalize the three tandem repeat peptides of MUC1 carrying Tn (Tn-MUC1) (13). An important question still remaining to be answered is whether human or mouse DCs, after internalizing antigens that have GalNAc residues, are able to elicit antigen-specific T cell responses. Thus, the final part of the report is designed to approach this question on the regulation of immune response in mice. The results indicate that polypeptides associated with mucins and mucin-like molecules with GalNAc residues are taken up by bone marrow (BM)-DCs through MGL2 and presented to specific CD4+ T cells. This is the first report demonstrating that GalNAc residues on a complex antigen influence both its uptake by DCs and the subsequent antigen-specific immune response.
EXPERIMENTAL PROCEDURES
Animals
Specific pathogen-free female C57BL/6 mice were obtained from CLEA Japan, Inc. (Tokyo, Japan). We used Mgl1−/− mice backcrossed eight times to C57BL/6 background (24). Mgl2−/− mice were generated as described under supplemental Experimental Procedures. Mgl1 or Mgl2 heterozygotes were bred for homozygotes of the deficient Mgl1 or Mgl2 allele and their wild-type (WT) littermates. Specific pathogen-free F344/Du rats were obtained from Charles River Japan, Inc. (Yokohama, Japan). Animals were housed under specific pathogen-free conditions. All experiments were approved by the Animal Care and Use Committee of the Graduate School of Pharmaceutical Sciences of the University of Tokyo and performed according to the guidelines of the Bioscience Committee of the University of Tokyo.
Preparation of MGL2-specific mAbs
Recombinant proteins corresponding to the extracellular domain (ECD) of MGL1 and MGL2 were prepared as described previously (16). An F344/Du rat was subcutaneously immunized with 100 μg of MGL2-ECD in complete Freund's adjuvant (BD Biosciences). One month later, a second immunization with 100 μg of MGL2-ECD in incomplete Freund's adjuvant (BD Biosciences) was given to the same rat followed by an intraperitoneal booster injection of 100 μg of MGL2-ECD 4 days before fusion. Hybridoma cells were prepared as described previously (25). Cells in the wells that produced antibodies with binding capacity for MGL2-ECD, but not for MGL1-ECD, were subjected to limiting dilution for cloning. The subclass of mAbs was determined using a Rat MonoAB ID/SP kit (Zymed Laboratories Inc., San Francisco, CA) according to the manufacturer's instructions.
Enzyme-linked Immunosorbent Assay
Binding assays for antibodies were carried out as previously described (16).
Immunohistochemical Staining
The binding sites of mAb LOM-8.7 or mAb URA-1 were immunohistochemically detected as previously described with slight modifications (18). Avidin binding sites were blocked using an Avidin/Biotin Blocking kit (Vector Laboratories, Burlingame, CA).
Preparation of Thioglycollate-induced Peritoneal Macrophages and in Vitro Cytokine Stimulation
C57BL/6 mice were injected peritoneally with 4.05% thioglycollate broth (BD Biosciences). Three days later, peritoneal exudate cells (PEC) were harvested by a lavage with cold, serum-free RPMI 1640 media. Cells were stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD11b (eBioscience, San Diego, CA) and phycoerythrin (PE)-conjugated F4/80 (eBioscience), and the double-positive cells were purified as peritoneal macrophages (PEC-macrophages) using a FACSAria cell sorter (BD Biosciences) (>95% purity). The isolated cells were cultured in the presence of 10 ng/ml mouse recombinant IL-4 (Peprotech, Rocky Hill, NJ) or recombinant IFN-γ (Peprotech) in a Petri dish (AGC Techno Glass Co., LTD, Chiba, Japan) overnight. Cells were collected by pipetting with cold Dulbecco's modified phosphate-buffered saline without calcium and magnesium (DPBS−).
Cell Preparations from BM, Spleens, and Lungs
BM cells were isolated by flushing femurs and tibias with DPBS−. Spleens were digested for 20 min at 37 °C with 1 mg/ml collagenase from Clostridium histolyticum (Sigma) in calcium and magnesium-free Hanks' balanced salt solution. The digested tissue was resuspended by pipetting. Erythrocytes were lysed by ammonium chloride treatment. Splenocytes were treated with 10 mm EDTA to disrupt T cell-DC complexes.
Lung parenchymal cells were prepared by a modification of the method reported by Corti et al. (26). Lung tissue digested with dispase (Invitrogen) was gently teased from the bronchi and transferred to the tube and incubated with gentle stirring with 200 units/ml collagenase (Wako, Osaka, Japan) for 10 min at 37 °C. The cell suspension was filtered, centrifuged, and resuspended in DPBS−. Cells were layered onto a 20%/70% Percoll gradient (GE Healthcare) and centrifuged at 1800 rpm for 20 min at room temperature. Cells between the 20 and 70% layers were collected and washed with DPBS−.
Preparation of BM-macrophages
BM cells (2–4 × 106 cells/ml) were cultured in a 6-cm dish (BD Biosciences) in 5 ml of RPMI 1640 medium supplemented with 10% fetal calf serum, 10 mm HEPES, 50 mm β-mercaptoethanol, 100 units/ml penicillin, 100 μg/ml streptomycin, and 20% conditioned media of L929 cells (Cell Resource Center for Biomedical Research, Tohoku University) as a source of macrophage colony-stimulating factor. After culturing overnight, non-adherent cells were transferred to a new 10-cm dish (BD Biosciences) with the same media and 5 ml of fresh media and then cultured for 5 days. Adherent cells were collected after treatment with 2.5 mm EDTA on ice for 10 min. Induction of macrophages was verified by flow cytometric analysis of CD11b and F4/80 expression.
Preparation and Purification of BM-DCs
BM-DCs were generated using murine recombinant granulocyte-macrophage colony-stimulating factor (provided from the Kirin Brewery Co.) as previously described (19). After 5 days of culture, non-adherent cells were collected and used as immature BM-DCs. In some experiments, immature BM-DCs were incubated with anti-mouse CD11c microbeads (Miltenyi Biotec, Bergisch Glabach, Germany), and CD11c+ DCs were positively separated by LS columns or AutoMACS (Miltenyi Biotec) according to the manufacturer's instructions. More than 95% purity was confirmed by flow cytometric analysis using FITC-conjugated anti-CD11c.
Flow Cytometric Analysis
CHO-K1 cells stably transfected with pCMV-Tag4-Mgl1, pCMV-Tag4-Mgl2, or pCMV-Tag4 were incubated with rat IgG, mAb LOM-14 (rat IgG2b), mAb LOM-8.7, or mAb URA-1 (10 μg/ml) followed by FITC-conjugated goat anti-Rat IgG (1/200 dilution, Invitrogen).
Cells isolated from mouse tissues and primary cultured cells were incubated with anti-mouse CD16/CD32 (1/100 dilution of ammonium sulfate-precipitated hybridoma culture supernatant; the 2.4G2 hybridoma was purchased from ATCC) and 5% normal mouse serum to reduce nonspecific binding 5 min before the addition of the first antibodies. Cells were then incubated with biotin-, FITC-, PE-, PE-Cy5-, and/or allophycocyanin (APC)-conjugated antibodies for 30 min. Biotinylated antibodies were visualized with APC- or PE-Cy7-labeled streptavidin (SAv) (BioLegend, San Diego, CA). To exclude dead cells, all cells were resuspended in DPBS− containing 0.1% bovine serum albumin and 0.1% sodium azide (FCM buffer) containing 7-amino-actinomycin D (eBioscience). A biotin-conjugated anti-MGL1 mAb LOM-8.7, a biotin-conjugated anti-MGL2 mAb URA-1, and an APC-conjugated anti-MGL2 mAb URA-1 were prepared using purified antibodies from the hybridoma culture supernatant; the following additional antibodies were used: FITC-conjugated anti-CD11c (BioLegend), PE-conjugated anti-CD4, CD40, and Gr-1 (BioLegend), CD8 (Immunotech), CD11b, B220, CD86, major histocompatibility complex (MHC) class II, and F4/80 (eBioscience), mPDCA-1 and DEC-205 (Miltenyi Biotec), PE-Cy5-conjugated anti-CD3 and CD19 (eBioscience), isotype controls bio-rat IgG2a, PE-rat IgG2a, PE-rat IgG2b, APC-rat IgG2a (eBioscience), and FITC-hamster IgG (Santa Cruz Biotechnology, Santa Cruz, CA).
All procedures were performed on ice. Antibodies and reagents were diluted in FCM buffer. The cells were rinsed twice with FCM buffer at the end of each incubation period. Samples were analyzed on an EPICS XL flow cytometer (Beckman Coulter, Fullerton, CA) or a FACSAria cell sorter. Data were analyzed using FlowJo software (Tree Star, Ashland, OR).
Carbohydrate Binding and Internalization Assessed by Flow Cytometric Analysis
The binding and uptake assays were performed as previously described (19).
Antigen Presentation Assays
Mice were immunized subcutaneously in the hind limb footpad and the tail base with 10–50 μg of SAv (Sigma) emulsified in complete Freund's adjuvant. WT or Mgl1 or Mgl2 heterozygotes were used for the immunization when Mgl1−/− or Mgl2−/− BM-DCs were used as stimulators in the antigen presentation assays. Seven days after the immunization, inguinal, popliteal, and iliac lymph nodes (LNs) were removed, and single cell suspensions were prepared. T cells were purified with nylon wool columns (Wako). In some experiments, CD4+ T cells or CD8+ T cells were negatively selected by MACS. Briefly, nylon wool-purified T cells or LN cells were incubated with bio-anti-B220, CD11b, and CD4 or CD8 antibodies followed by SAv-microbeads (Miltenyi Biotec). Then cells were applied to LS columns or AutoMACS. More than 95% purity was confirmed using flow cytometry.
SAv of various concentrations indicated in the figures or figure legends was preincubated with 1 μg/ml biotinylated (bio)-α-GalNAc-soluble polyacrylamide polymers (PAA) (27), bio-β- GlcNAc-PAA, bio-60-mer MUC1 tandem repeat synthetic peptide (non-glycosylated: AHGVTSAPDTRPAPGSTAPP ×3), or enzymatically glycosylated bio-MUC1 60-mer (9Tn (AHGVT*SAPDTRPAPGS*T*APP × 3, in which the asterisk attached on the right indicates a GalNAc residue) or 14Tn (AHGVT*S* APDT*RPAPGS*T*APP ×3, in which a GalNAc residue of the sixth Ser was observed only with the first and the second repeats), kindly provided by Dr. H. Clausen (University of Copenhagen, Denmark) (28). Immature BM-DCs or CD11c+ BM-DCs were cultured overnight with SAv complexes as antigens in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 10 mm HEPES, 50 mm β-mercaptoethanol, 100 units/ml penicillin, 100 μg/ml streptomycin, and granulocyte-macrophage colony-stimulating factor. Antigen-pulsed BM-DCs were treated with 50 μg/ml mitomycin C (Kyowa Hakko Kirin Co., Tokyo, Japan). SAv-primed T cells (3 × 105 cells) were then co-cultured with antigen-pulsed BM-DCs in a 96-well U-bottom plate for 3 days. [3H]Thymidine (GE Healthcare) was added during the last 16 h. Data are shown as the mean cpm of triplicate cultures. The assays were judged to have failed due to a mismatch of MHC between T cells and DCs when the levels of [3H]thymidine uptake by T cells co-cultured with unpulsed BM-DCs of Mgl2+/+ or Mgl2−/− mice were similar to that of T cells stimulated with concanavalin A.
To determine cytokine production levels, culture supernatants were collected 3 days after co-culturing of SAv-primed T cells with antigen-pulsed BM-DCs. IFN-γ (eBioscience), IL-4, IL-10, and IL-17A (Biolegend) were measured using cytokine enzyme-linked immunosorbent assay kits according to the manufacturer's instructions.
Statistics
Results of carbohydrate binding and internalization assays and antigen presentation assays were statistically evaluated using Student's t test. p values less than 0.05 were considered significant.
RESULTS
Preparation and Characterization of MGL2-specific mAb URA-1
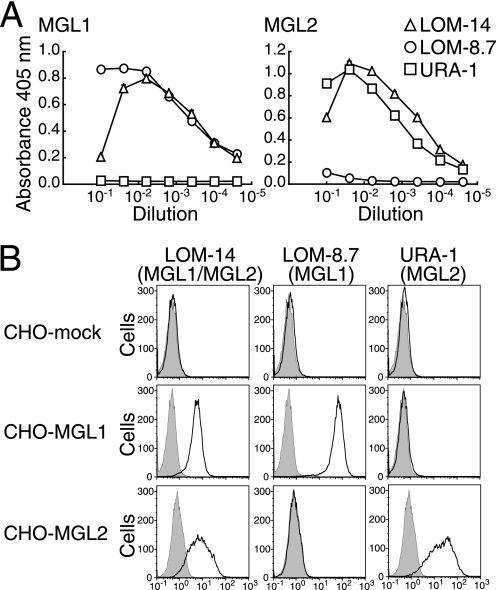
Hybridomas secreting mAb specific for MGL2-ECD, but not for MGL1-ECD, were prepared, and a specific mAb URA-1 (IgG2a, κ) was chosen. The reactivity of this mAb with MGL1-ECD and MGL2-ECD was compared with that of mAb LOM-8.7 (MGL1-specific) and mAb LOM-14 (cross-reactive between MGL1 and MGL2) (Fig. 1A). The mAb URA-1 was shown to be specific for MGL2. The specificity of mAb URA-1 was also confirmed by flow cytometric analysis using CHO cells stably transfected with cDNAs of full-length Mgl1 or Mgl2 (Fig. 1B). Because the stem domains of MGL1 and MGL2 have highly homologous amino acid sequences, the sequence within the CRD was thought to be responsible for the specificity of mAb URA-1. This mAb bound to MGL2-CRD as well as MGL2-ECD (supplemental Fig. 1A). The 61st amino acid within the CRD of MGL2 was previously shown to be important for the carbohydrate recognition of MGL2 (29). When this Leu was mutated to Val, which is the amino acid in MGL1 at the same position, the reactivity of mAb URA-1 to MGL2 diminished (supplemental Fig. 1A). A similarly drastic effect was observed when the 110th Phe was substituted with Ile and when the 113th Asp was substituted with Gly (supplemental Fig. 1A). To compare its blocking activity with that of mAb LOM-8.7 (25), we investigated the inhibition of ligand binding by mAb URA-1 using bio-Lewis X (LeX)-PAA (for MGL1) and bio-β-GalNAc-PAA (for MGL2). As shown in supplemental Fig. 1B, mAb URA-1 inhibited the binding of β-GalNAc-PAA to MGL2-ECD but not LeX-PAA to MGL1-ECD.
FIGURE 1.
Specificity of mAb URA-1 for MGL2 compared with mAb LOM-14 and mAb LOM-8.7. A, binding of mAb LOM-14 (anti-MGL1/MGL2, open triangle), mAb LOM-8.7 (anti-MGL1, open circle), and mAb URA-1 (anti-MGL2, open square) to MGL1-ECD or MGL2-ECD in the enzyme-linked immunosorbent assay is shown. Hybridoma culture supernatants were serially diluted as indicated. Data are shown as the mean ± S.D. of triplicates. B, binding profiles of the same mAbs to CHO cells transfected with Mgl1 or Mgl2 cDNA using flow cytometric analysis are shown. The binding profiles of control rat IgG are shown by gray-filled lines. The binding profiles of these three mAbs indicated above are shown by black lines.
Immunohistochemical Distribution of MGL1 and MGL2 in Normal Mouse Tissues
The binding sites for mAb LOM-8.7 and mAb URA-1 were compared by immunohistochemistry. Both mAbs bound to cells in the connective tissues of almost all organs except for the brain; this profile was similar to the one previously shown using cross-reactive mAb LOM-14 (18). The number of cells that reacted with mAb URA-1 was similar to the number that reacted with mAb LOM-8.7 in most organs, including the thymus, large intestine, esophagus, stomach, heart, trachea, kidney, and skin (Fig. 2A and data not shown). The mAb binding profiles to sections of the thymus, a representative organ, are shown in Fig. 2A. The binding sites of these mAbs were present along the capsule and septa and scattered in the cortex and medulla. In spleens, MGL1+ cells (i.e. cells reactive with mAb LOM-8.7) had a more widespread distribution than MGL2+ cells (i.e. cells reactive with mAb URA-1) (Fig. 2B). The variations and distributions of the mAb-reactive cells in peripheral LNs have been published separately (30). In lung alveolar spaces, the number of MGL2+ cells appeared to be slightly greater than the number of MGL1+ cells (Fig. 2C).
FIGURE 2.
Immunohistochemical localizations of binding sites for mAb LOM-8.7 and mAb URA-1 in various tissues. A, Thymus. B, spleen. C, Lung. The distribution of cells stained with these antibodies indicated by arrowheads was limited within connective tissues in these organs. The apparent relative incidence of mAb LOM-8.7- and mAb URA-1-positive cells was similar in thymi. The apparent relative incidence was greater with mAb LOM-8.7 than with mAb URA-1 in spleens, whereas it was slightly greater with mAb URA-1 than with mAb LOM-8.7 in lungs. Scale bars represent 50 μm for A and C and 100 μm for B.
Identification of DCs Expressing MGL1 and/or MGL2 in BM, Spleens, and Lungs
The surface expression of MGL1 and MGL2 on cells from the BM, spleens, and lungs were analyzed by flow cytometry using mAb LOM-8.7 and mAb URA-1 after excluding dead cells and CD3+ or CD19+ cells. The mAbs were found to bind subsets of CD11c+ cells (Fig. 3, A and B). BM and spleen were populated with more MGL1+ cells than MGL2+ cells. All MGL2+ cells appeared to co-express MGL1 in the BM, spleens, and lungs, whereas a significant portion of MGL1 single-positive cells were observed in the BM and spleens (Fig. 3C). In the BM, MGL1 and MGL2 double-positive cells represented a homogeneous population that was CD11c+CD4−CD8−CD11b+B220−MHCII+F4/80low, whereas MGL1 single-positive cells contained at least two populations that were CD11c+B220+ cells and CD11c+B220− cells (Fig. 3D). In spleens, MGL1 and MGL2 double-positive cells were CD11c+CD4±CD8−CD11b+MHCII+F4/80low, and MGL1 single-positive cells contained at least two populations of CD11clowCD4+CD8±CD11b− B220+MHCIIlow and CD11c+CD4±CD8−CD11b+B220−MHCII+F4/80low cells (Fig. 3E). We also confirmed that CD11clow MGL1 single-positive cells in the spleen expressed mPDCA-1 (data not shown). These cells were also positive for B220 and should be classified as plasmacytoid DCs (pDCs) (31–33). In the lung, alveolar macrophages with high levels of autofluorescence did not express MGL1 or MGL2, as previously reported (17). MGL1 and MGL2 double-positive cells in the lung were CD11c+CD4−CD8−CD11b+MHCII+F4/80low (Fig. 3F). Although the number of cells in this organ with immunohistochemical reactivity with mAb URA-1 appeared to be slightly greater than the number of cells stained with mAb LOM-8.7, flow cytometry revealed a cell population that was reactive to both mAbs. These results collectively indicate that cells reactive to both mAb LOM-8.7 and mAb URA-1 correspond to CD8− conventional DCs (cDCs), and MGL1 single-positive cells are pDCs and cDCs.
FIGURE 3.
Expression of MGL1 and MGL2 on cells from BM, spleens, and lungs analyzed by flow cytometry. A, staining profiles of anti-CD11c and bio-LOM-8.7 followed by PE-Cy7-SAv are shown. B, staining profiles of anti-CD11c and APC-URA-1 are shown. C, staining profiles of bio-LOM-8.7 followed by PE-Cy7-SAv and APC-URA-1 are shown. In panels D–F, MGL1 and MGL2 double-positive cells and MGL1 single-positive cells gated in C were further analyzed for the expression of CD11c and other surface markers: D, cells from BM; E, cells from spleens; F, cells from lungs. In all panels cells were analyzed after excluding dead cells and CD3+ or CD19+ cells. The data are representative of three or more independent experiments.
Cell Surface Expression of MGL1 and MGL2 on Induced Macrophages and BM-DCs
The reactivity of these mAbs to BM-macrophages and PEC-macrophages was examined using flow cytometry. The mAb LOM-8.7 bound to BM-macrophages (Fig. 4A) and PEC-macrophages (Fig. 4B) at significant levels. In contrast, the levels of mAb URA-1 binding to BM-macrophages (Fig. 4A) and PEC-macrophages (Fig. 4B) were very low. As Raes et al. (34) reported, the expression of Mgl1 and Mgl2 mRNA may be induced by alternative activation of macrophages, PEC-macrophages were cultured overnight in the presence of IL-4 or IFN-γ, and mAb binding was examined. Classical activation of macrophages by IFN-γ was confirmed by the observed increase of MHC class II surface expression using flow cytometry (data not shown), and alternative activation of macrophages by IL-4 was confirmed by the induction of mRNA expression of Mgl1, Mgl2, Arginase-1, and MR (supplemental Fig. 2). Surface MGL1 expression was slightly elevated after an overnight culture of PEC-macrophages with IL-4, but mAb URA-1 binding slightly decreased compared with cells cultured without cytokines (Fig. 4C). When intracellular bindings of these antibodies were examined by flow cytometry or cytostaining after permeabilization, the induction of intracellular proteins corresponding to these lectins was not observed (data not shown).
FIGURE 4.
Expression of MGL1 and MGL2 on surface of macrophages analyzed by flow cytometry. A, BM-macrophages. B, PEC-macrophages. C, PEC-macrophages cultured overnight in the presence or absence of indicated cytokines. In all panels, the profiles obtained with isotype controls are shown as gray-filled lines, and the profiles with mAbs LOM-8.7 and URA-1 are shown as black lines. Geometric MFI for the mAb binding and isotype control are shown in each panel and at the right of each panel, respectively.
Both mAb LOM-8.7 and mAb URA-1 bound at significant levels to the surface of immature BM-DCs expressing low to moderate levels of MHC class II, whereas their bindings to mature BM-DCs expressing high levels of MHC class II were low (Fig. 5, A and C). The mRNA level of Mgl1 examined by reverse transcription-PCR was previously reported to correlate with the surface binding of mAb LOM-14 to BM-DCs; the bindings were high at immature stages and low at mature stages (19). A similar mRNA expression pattern was observed for Mgl2 (data not shown).
FIGURE 5.
Expression of MGL1 and MGL2 on surface of CD11c+ BM-DCs. A, BM-DCs were prepared from BM cells of WT littermate of Mgl1−/− mice (Mgl1+/+). B, BM-DCs were prepared from BM cells of Mgl1−/− mice. C, BM-DCs were prepared from BM cells of WT littermate of Mgl2−/− mice (Mgl2+/+). D, BM-DCs were prepared from BM cells of Mgl2−/− mice. In all panels CD11c+ cells were obtained by MACS, and bindings of mAbs LOM-8.7 and URA-1 were determined by flow cytometry. The data are representative of three or more independent experiments. E, MGL2 expression profiles of Mgl1+/+ BM-DCs (black line derived from the right panel in A) and Mgl1−/− BM-DCs (gray-filled line derived from the right panel in B) are shown as overlaid histograms. F, MGL1 expression profiles of Mgl2+/+ BM-DCs (black line derived from the middle panel in C) and Mgl2−/− BM-DCs (gray-filled line derived from the middle panel in D) are shown as overlaid histograms. In panels E and F, MFI for the mAb binding is shown under each panel.
MGL2 Is Required for the Binding and Uptake of GalNAc-PAA by BM-DCs
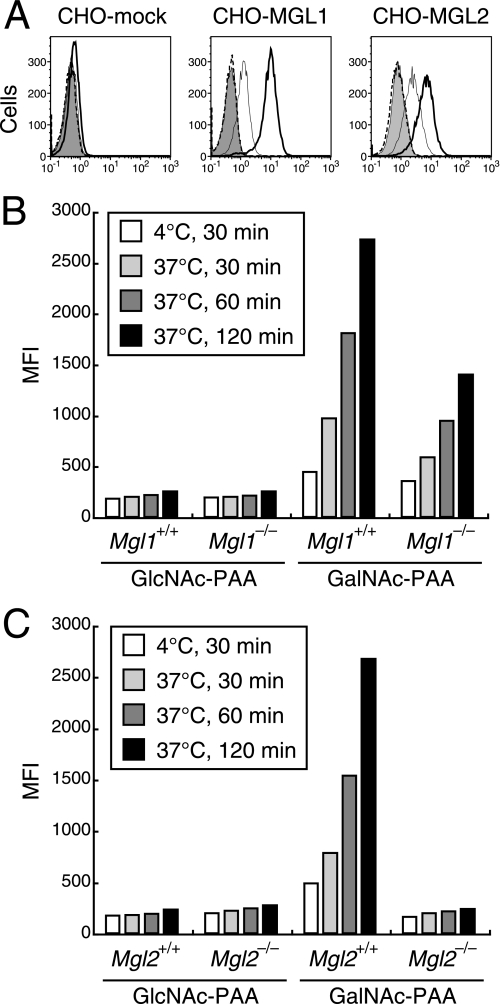
We previously reported that GalNAc-PAA was efficiently bound and internalized by immature BM-DCs (19). To understand whether MGL1 or MGL2 alone was sufficient for the recognition of GalNAc-PAA, the binding and uptake of GalNAc-PAA was examined using CHO cells transfected with Mgl1 or Mgl2 cDNA. FITC-GalNAc-PAA, but not FITC-GlcNAc-PAA, was bound and internalized by both cell types (Fig. 6A). These results demonstrated that MGL1 or MGL2 alone on CHO cells was sufficient to bind and internalize GalNAc-PAA.
FIGURE 6.
Binding and uptake of GalNAc-PAA by CHO cells transfected with Mgl1 or Mgl2 cDNA and by CD11c+ BM-DCs. A, flow cytometric profiles of CHO transfectants with FITC-PAA are shown. The cells were incubated with FITC-GlcNAc-PAA (dotted lines) or FITC-GalNAc-PAA (thin lines) on ice for 30 min to estimate the amount bound to cell surfaces. The relative amounts of FITC-GalNAc-PAA internalized by the cells were estimated as follows. The cells were incubated with FITC-GalNAc-PAA at 37 °C for 30 min, treated with EDTA to remove cell surface-associated FITC-GalNAc-PAA, and then analyzed (bold lines). The cells incubated with FITC-GalNAc-PAA on ice for 30 min and then treated with EDTA were also analyzed as a control (gray-filled). B, BM-DCs were prepared from BM cells of Mgl1−/− or WT littermate (Mgl1+/+) mice. C, BM-DCs were prepared from BM cells of Mgl2−/− or WT littermate (Mgl2+/+) mice. In panels B and C, CD11c+ cells were obtained by MACS. Binding and uptake of FITC-PAA were examined using flow cytometry by the same methods as in A. Differences in the geometric MFI of FITC after uptake at 37 °C and binding on ice are shown. In all panels the data are representative of three independent experiments.
To determine whether the binding and uptake of FITC-GalNAc-PAA was mediated by MGL1 or MGL2 expressed on DCs, BM-DCs were prepared from the BM cells of Mgl1−/−, Mgl2−/−, or WT littermate mice. Mgl2−/− mice were generated as described in supplemental Fig. 3. The mice bred normally and were healthy under specific pathogen-free conditions at least up to 6 months. There was no remarkable phenotype in the development of immune cells according to the examination of peripheral blood smears and flow cytometric analysis of thymocytes (data not shown). The absence of MGL1 or MGL2 was confirmed on BM-DCs of Mgl1−/− or Mgl2−/− mice, respectively (Fig. 5, B and D). Unexpectedly, MGL2 expression on Mgl1−/− BM-DCs (geometric mean fluorescence intensity (MFI) = 1679) was approximately half of WT littermate (Mgl1+/+) BM-DCs (MFI = 2690) (Fig. 5, A, B, and E), and MGL1 expression on Mgl2−/− BM-DCs (MFI = 399) was significantly lower than that of BM-DCs from WT littermate (Mgl2+/+) mice (geometric MFI = 1717) (Fig. 5, C, D, and F). The relative mRNA expression levels of Mgl1 in Mgl2−/− mice or Mgl2 in Mgl1−/− mice normalized to β-actin were lower than that of WT littermates, as measured by real-time reverse transcription-PCR (data not shown). However, the mechanism of reduced mRNA expression levels of Mgl1 or Mgl2 on Mgl2−/− or Mgl1−/− cells, respectively, is currently unknown.
BM-DCs prepared from Mgl1+/+ (Fig. 6B) or Mgl2+/+ mice (Fig. 6C) bound and internalized FITC-GalNAc-PAA but not FITC-GlcNAc-PAA. The level of binding and the rate of uptake of FITC-GalNAc-PAA by Mgl1−/− BM-DCs were compared with those of Mgl1+/+ BM-DCs (Fig. 6B). FITC-GalNAc-PAA binding was slightly lower than Mgl1+/+ BM-DCs, and FITC-GalNAc-PAA uptake was approximately half that of Mgl1+/+ BM-DCs at each time point. These results appear to correlate with the cell surface levels of MGL2 on Mgl1−/− BM-DCs. In contrast to the MFI of FITC-GalNAc-PAA binding and uptake by Mgl1−/− BM-DCs, that of Mgl2−/− BM-DCs was reduced to almost the same levels of the controls (Fig. 6C). These results strongly suggest that cell surface expression of MGL2 on BM-DCs is required for the binding and internalization of FITC-GalNAc-PAA.
Presentation of Antigens Linked to GalNAc Residues Internalized through MGL2 on BM-DCs
To investigate whether antigens containing terminal GalNAc residues were presented by MHC molecules to T cells after internalization, we developed an antigen presentation assay using bio-GalNAc-PAA conjugated with SAv as a model antigen. Mice were immunized with SAv, and SAv-primed T cells were harvested. T cell proliferation was efficiently induced in a dose-dependent manner when the cells were co-cultured with BM-DCs preincubated with bio-GalNAc-PAA conjugated with SAv (Fig. 7A). BM-DCs preincubated with SAv alone or bio-GlcNAc-PAA conjugated with SAv did not induce efficient proliferation of SAv-primed T cells. Because BM-DCs preincubated with bio-GalNAc-PAA alone did not induce efficient proliferation of SAv-primed T cells (supplemental Fig. 4A) and because BM-DCs preincubated with GalNAc-SAv did not induce efficient proliferation of ovalbumin-primed T cells (supplemental Fig. 4B), we concluded that the efficient proliferation of SAv-primed T cells after co-culturing with GalNAc-SAv-pulsed BM-DCs was antigen-specific.
FIGURE 7.
T cell activation through antigen presentation by BM-DCs pulsed with bio-GalNAc-PAA conjugated to SAv. A, SAv-primed T cells were co-cultured with immature BM-DCs pulsed with bio-GalNAc-PAA conjugated to SAv (GalNAc-SAv, closed circle), bio-GlcNAc-PAA conjugated to SAv (GlcNAc-SAv, closed triangle), SAv (open square), or no antigen (open circle). The DC to T cell ratio was 4:1. T cell proliferation was measured by [3H]thymidine uptake. Data are shown as the mean cpm ± S.D. of triplicate cultures. **, p < 0.01 and ***, p < 0.001 for GalNAc-SAv compared with GlcNAc-SAv at different SAv concentrations. B, production of cytokines was measured with culture supernatants of SAv-primed T cells co-cultured with CD11c+ BM-DCs. IFN-γ and IL-17A were detected and quantified. BM-DCs were pulsed with GalNAc-SAv (black bar), GlcNAc-SAv (dark gray bar), or no antigen (open bar). The DC to T cell ratio was 10:1. Data are shown as the mean concentration (pg/ml) ± S.D. of triplicate cultures. *, p < 0.05 and ***, p < 0.001 for GalNAc-SAv compared with GlcNAc-SAv. C, SAv-primed T cells were co-cultured with immature BM-DCs pulsed with bio-14Tn-MUC1 and SAv (14Tn-MUC1-SAv, closed diamond), bio-9Tn-MUC1 and SAv (9Tn-MUC1-SAv, closed circle), bio-MUC1 and SAv (MUC1-SAv, closed triangle), SAv (open square), or no antigen (open circle). The DC to T cell ratio was 4:1. T cell proliferation was measured by [3H]thymidine uptake, and the data are shown as the mean cpm ± S.D. of triplicate cultures. **, p < 0.01 and ***, p < 0.001 for 14Tn-MUC1-SAv or 9Tn-MUC1-SAv compared with MUC1-SAv, respectively. D, nylon wool (NW)-purified, CD4+, and CD8+ T cells obtained from SAv-immunized mice were compared for their proliferation response to GalNAc-PAA-mediated antigen presentation by co-culturing them with CD11C+ BM-DCs pulsed with GalNAc-SAv (black bar), GlcNAc-SAv (dark gray bar), SAv (light gray bar), or no antigen (open bar). The DC to T cell ratio was 4:1. T cell proliferation was measured by [3H]thymidine uptake, and the data are shown as the mean cpm ± S.D. of triplicate cultures. **, p < 0.01 and ***, p < 0.001 and not significant (n.s.) for GalNAc-SAv compared with no antigen, GlcNAc-SAv, or SAv. E, SAv-primed T cells were co-cultured with CD11c+ BM-DCs from Mgl1−/− or WT littermate (Mgl1+/+) mice. F, SAv-primed T cells were co-cultured with CD11c+ BM-DCs from Mgl2−/− or WT littermate (Mgl2+/+) mice. In panels E and F, CD11c+ BM-DCs were pulsed with GalNAc-SAv (black bar), GlcNAc-SAv (dark gray bar), SAv (light gray bar), or no antigen (open bar). The DC to T cell ratio was 75:1. T cell proliferation was measured by [3H]thymidine uptake, and the data are shown as the mean cpm ± S.D. of triplicate cultures. *, p < 0.05, **, p < 0.01, ***, p < 0.001, and for GalNAc-SAv compared with no antigen, GlcNAc-SAv, or SAv.
SAv-primed T cells were shown to produce IFN-γ and IL-17A when co-cultured with BM-DCs preincubated with GalNAc-SAv (Fig. 7B). IL-4 and IL-10 were below the detectable levels (data not shown).
To test whether glycoproteins containing terminal GalNAc residues were able to induce efficient antigen-specific T cell proliferation, bio-MUC1 peptides with enzymatically attached GalNAc residues were used. Flow cytometry confirmed that immature BM-DCs bound and internalized complexes of bio-9Tn-MUC1 peptides and FITC-SAv (data not shown). SAv conjugated with MUC1 peptides containing 9 or 14 GalNAc residues, but not with non-glycosylated MUC1 peptides, induced efficient proliferation of SAv-primed T cells in a dose-dependent manner (Fig. 7C).
When SAv-primed T cells were separated as CD4+ cells and CD8+ cells and used for the antigen presentation assays, only SAv-primed CD4+ T cells were able to proliferate and secrete IL-17A (Fig. 7D and supplemental Fig. 5). This CD4+-specific antigen presentation was not likely due to the immunization method; BM-DCs were able to cross-present SAv to CD8+ T cells when treated with CpG-oligodeoxynucleotide (35) (supplemental Fig. 5B).
The involvement of MGL1 or MGL2 in antigen presentation was directly examined using BM-DCs prepared from Mgl1−/−, Mgl2−/−, or WT littermate mice. Mgl1−/− BM-DCs pulsed with bio-GalNAc-PAA conjugated with SAv induced efficient T cell proliferation compared with SAv alone or bio- GlcNAc-PAA conjugated with SAv (Fig. 7E). In contrast, Mgl2−/− BM-DCs pulsed with bio-GalNAc-PAA conjugated with SAv did not induce T cell proliferation (Fig. 7F). As indicated by the surface expression levels of CD40 and CD86 analyzed using flow cytometry, the lack of MGL1 or MGL2 did not influence the maturation of BM-DCs after antigen uptake (data not shown); the increased antigen uptake and presentation due to GalNAc residues having high affinity to MGL2 is likely the basis for the efficient CD4+ T cell response.
DISCUSSION
The organ distribution of cells expressing mouse MGL2, a novel isolectin of MGL, was examined using a specific mAb URA-1, and the role of this lectin in the uptake and presentation of glycosylated antigens was assessed using DCs from Mgl2−/− mice. Site-directed mutagenesis of the MGL2-CRD clearly demonstrated that the Leu-61, Phe-110, and Asp-113 amino acid residues were required for mAb URA-1 binding (supplemental Fig. 1A). Also, the results indicated that this antibody recognized a conformational epitope. As a consequence, denaturation of MGL2 by SDS treatment during Western blotting analysis abolished the mAb reactivity (data not shown). Leu-61, one of the amino acid residues required for antibody binding, was previously shown to be involved in ligand binding (29), supporting our finding that mAb URA-1 was capable of blocking the binding of ligands to MGL2 (supplemental Fig. 1B).
The combined use of MGL2-specific mAb URA-1 and MGL1-specific mAb LOM-8.7 enabled us to examine the protein expression patterns of MGL1 and MGL2, respectively, for the first time. Both MGL1 and MGL2 were detected in the connective tissues of almost all organs; previous studies using cross-reactive mAb LOM-14 reported similar observations (18). However, cells expressing MGL2 were limited to a portion of MGL1-expressing cells, mostly cDCs. In contrast, MGL1 was expressed on macrophages, cDCs, and pDCs. The fact that the distribution of MGL2 is more limited to cDCs than MGL1 strongly implies that the function of this molecule is closely linked to the role of cDCs. MGL2-positive cells were rare in BMs, and spleens but were present in peripheral organs, including skin, LNs (30), large intestine (36), and lungs. It is known that splenic cDCs are mainly derived from precursor cells residing in the spleen and are formed independently of BM-derived or blood precursor cells under steady-state conditions (37, 38). Therefore, the expression of MGL2 was likely to be induced after precursor cells migrated into peripheral tissues. Auffray et al. (39) reported that when Gr-1−CX3CR1+ resting monocytes extravasated and invaded surrounding tissues in response to tissue damage, Mgl2 mRNA expression was up-regulated. The expression of MGL1, but not MGL2, was observed on pDCs, which is consistent with the report by Cisse et al. (40) showing that Mgl1 is predominantly expressed on pDCs compared with other cells in the spleen. Human MGL was reported to be expressed on CD11c+DR+Lin− DCs from fresh blood but not on CD11c−DR+Lin− pDCs (41). Considering that the primary sequence and the carbohydrate specificity of mouse MGL2 are similar to those of human MGL, mouse MGL2 should represent the counterpart to human MGL.
It was previously reported that Mgl1 and Mgl2 gene expression was induced upon alternative activation of macrophages by Th2-associated cytokines such as IL-4 and/or IL-13 (34). Based on these observations, MGL1 and MGL2 were expected to serve as surface markers identifying alternatively activated macrophages. However, we clearly demonstrated in the present paper that MGL1 and MGL2 protein expression was not significantly up-regulated on thioglycollate-induced PEC-macrophages after stimulation with IL-4 in vitro, even though the mRNA levels were elevated. It is possible that MGL1 and MGL2 protein expression may be up-regulated under certain situations in vivo, including allergy and helminth infection, but this possibility remains to be further elucidated.
In the present study, we demonstrated that complexes of protein antigens having GalNAc residues were efficiently internalized through MGL2 on immature BM-DCs and presented to antigen-specific CD4+ T cells. Such uptake and presentation were not likely to be mediated by MGL1, as shown by the comparison between BM-DCs from Mgl1−/− and Mgl2−/− mice. Recombinant MGL1 was previously shown to have affinity for GalNAc residues linked to a peptide (23). Furthermore, MGL1 or MGL2 alone on CHO cells was sufficient to bind and internalize GalNAc-PAA to a similar extent (Fig. 6A). However, when BM-DCs from Mgl1−/− and Mgl2−/− mice were compared for their binding and uptake of GalNAc-PAA, the decrease in GalNAc-PAA binding and uptake (Fig. 6, B and C) corresponded to the surface levels of MGL2 but not those of MGL1. It appeared that cell surface expression of MGL2 on BM-DCs was required for the internalization of GalNAc-PAA. Because LeX-PAA, which is expected to mimic the ligand for MGL1, showed very low binding to MGL1 expressed on cell surfaces (data not shown), the involvement of MGL1 expressed on BM-macrophages or BM-DCs with the binding and uptake of carbohydrate ligands remains unclear.
It is widely accepted that DCs are capable of presenting antigens to both CD4+ and CD8+ T cells. However, BM-DCs pulsed with GalNAc-SAv stimulated SAv-primed CD4+ but not CD8+ T cells (Fig. 7D and supplemental Fig. 5). Thus, we attempted to confirm that such a cross-presentation did occur in the experimental system we employed. CpG-oligodeoxynucleotide treatment before antigen uptake was previously shown to induce cross-presentation by BM-DCs (35). Based on this report, BM-DCs were treated overnight with CpG- oligodeoxynucleotide and then incubated with SAv at a relatively high concentration (100 μg/ml) for 2 h. The CpG-treated and SAv-pulsed BM-DCs induced IL-17A production from both CD4+ and CD8+ SAv-primed T cells (supplemental Fig. 5B). The antigen presentation by CpG-treated BM-DCs pulsed with GalNAc-SAv was not examined because CpG treatment induced maturation of BM-DCs and decreased the expression levels of MGL2. However, these results strongly suggest that antigens internalized through MGL2 are efficiently presented on MHC class II but not on MHC class I.
MGL2 binds ligands at neutral pH, and the affinity was significantly lowered under acidic conditions (a half-maximal ligand binding pH 5.7 for MGL2) (16). Because the GalNAc-PAA internalized by immature BM-DCs has previously been shown to co-localize with LAMP-1 and MHC class II (19), ligands internalized through MGL2 should be transported and released in the late endosomes/lysosomes. These processes might be mediated by the tyrosine-based uptake motif (YENF) in the cytoplasmic tail. The role of the cytoplasmic motif in uptake and intracellular trafficking of MGL2 remains to be elucidated. Ovalbumin conjugated to anti-DEC-205 mAb was targeted to DCs in vivo and presented on MHC class I as well as MHC class II (42, 43). On the other hand, antigens targeted to DEC-205 on BM-DCs were not presented on MHC class I (42). The possibility of antigen cross-presentation as a result of uptake through MGL2 should further be examined in vivo or by using cells isolated from mouse tissue, although MGL2 was not expressed on CD8+ cDCs (Fig. 3), which is thought to be an important subset for cross-presentation.
In vivo targeting is an important for achieving therapeutic immunization. To explore this possibility, we injected GalNAc-SAv subcutaneously and attempted to examine antigen-specific T cell proliferation in vitro. However, SAv itself inhibited T cell proliferation in vitro by unknown reasons, and this system was found unsuited to study the enhancement of immune responses.4 Novel strategies without SAv should be developed to investigate the use of glycoforms in immunotherapy.
To date only a limited number of reports have demonstrated that glycosylated antigens are efficiently taken up for presentation by DCs through C-type lectins. Mannosylated-bovine serum albumin or mannosylated peptides were presented more efficiently than non-mannosylated forms by monocyte-derived DCs (44). Such efficient uptake of mannosylated antigens was likely to be mediated by MR and/or DC-SIGN. Soluble ovalbumin, known to induce a T cell response, was reported to be efficiently internalized by MR for cross-presentation (45, 46). In the present study we demonstrated that tumor antigen MUC1 with GalNAc residues was recognized and internalized by immature BM-DCs, and SAv conjugated to MUC1 with GalNAc was presented to T cells. Furthermore, human MGL on monocyte-derived DCs is involved in the binding and uptake of Tn-MUC1 (13). Altered O-glycans, such as the exposed GalNAc residues on this mucin, are known to be associated with tumor cells. MUC1 that has a sialylated TF or sialylated Tn, both of which are also expressed by carcinoma cells, is known to impair maturation of DCs and induce IL-10highIL-12low regulatory DCs; however, the receptor required for the recognition of sialylated MUC1 is unknown (47, 48). On the other hand, antigens having GalNAc residues did not impair the maturation of BM-DCs (data not shown) and did not seem to induce regulatory DCs; we did not observe IL-10 production after co-culturing T cells and GalNAc-SAv-pulsed BM-DCs in the present study. Thus, the question of whether MGL might be involved in tumor surveillance or induction of self-tolerance in the absence/presence of adequate stimuli in vivo should be answered through further investigation.
In conclusion, we demonstrated that MGL2 was preferentially expressed on cDCs and involved in the efficient uptake and presentation of antigens with GalNAc residues. Targeting of MGL by the addition of GalNAc residues to antigens should contribute to novel vaccine development.
Supplementary Material
Acknowledgments
We thank Kyoko Sakai and Miki Noji for assistance in the preparation of this manuscript and Miho Mori and Satomi Yoshinaga for animal care. We thank Dr. Kayo Inaba for advice in generating BM-DCs and antigen presentation assays, Dr. Henrik Clausen for providing biotinylated MUC1 peptides, Dr. Stephan Hedrick for providing Mgl1−/− mice, and the Kirin Brewery Co., Ltd., for supplying the granulocyte-macrophage colony-stimulating factor. We also thank Dr. Gen Yamada, Center for Animal Resources and Development, Kumamoto University, for kind help in generating Mgl2−/− mice.
This work was supported in part by grants-in-aid from the Ministry of Education, Science, Sports, and Culture of Japan, the Research Association for Biotechnology, and the Program for Promotion of Fundamental Studies in Health Sciences of the Pharmaceutical and Medical Device Agency.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Experimental Procedures and Figs. 1–5.
K. Denda-Nagai, unpublished data.
- DC
- dendritic cell
- APC
- allophycocyanin
- BM
- bone marrow
- cDC
- conventional DC
- pDC
- plasmacytoid DC
- CRD
- carbohydrate recognition domain
- DPBS
- Dulbecco's modified phosphate-buffered saline
- ECD
- extracellular domain
- FITC
- fluorescein isothiocyanate
- LeX
- Lewis X
- LN
- lymph node
- mAb
- monoclonal antibody
- MFI
- mean fluorescence intensity
- MGL
- macrophage galactose-type calcium-type lectin
- MHC
- major histocompatibility complex
- MR
- mannose receptor
- PAA
- polyacrylamide polymer
- PE
- phycoerythrin
- PEC
- peritoneal exudate cells
- TF
- Thomsen-Friedenreich
- SAv
- streptavidin
- WT
- wild type
- bio
- biotinylated
- IL
- interleukin
- IFN
- interferon.
REFERENCES
- 1.Apweiler R., Hermjakob H., Sharon N. (1999) Biochim. Biophys. Acta 1473, 4–8 [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J., Steinman R. M. (1998) Nature 392, 245–252 [DOI] [PubMed] [Google Scholar]
- 3.Cambi A., Figdor C. G. (2003) Curr. Opin. Cell Biol. 15, 539–546 [DOI] [PubMed] [Google Scholar]
- 4.Figdor C. G., van Kooyk Y., Adema G. J. (2002) Nat. Rev. Immunol. 2, 77–84 [DOI] [PubMed] [Google Scholar]
- 5.Robinson M. J., Sancho D., Slack E. C., LeibundGut-Landmann S., Reis e Sousa C. (2006) Nat. Immunol. 7, 1258–1265 [DOI] [PubMed] [Google Scholar]
- 6.Carter R. W., Thompson C., Reid D. M., Wong S. Y., Tough D. F. (2006) Cell. Immunol. 239, 87–91 [DOI] [PubMed] [Google Scholar]
- 7.Idoyaga J., Cheong C., Suda K., Suda N., Kim J. Y., Lee H., Park C. G., Steinman R. M. (2008) J. Immunol. 180, 3647–3650 [DOI] [PubMed] [Google Scholar]
- 8.Tacken P. J., de Vries I. J., Torensma R., Figdor C. G. (2007) Nat. Rev. Immunol. 7, 790–802 [DOI] [PubMed] [Google Scholar]
- 9.van Vliet S. J., Aarnoudse C. A., Broks-van den Berg V. C., Boks M., Geijtenbeek T. B., van Kooyk Y. (2007) Eur. J. Immunol. 37, 2075–2081 [DOI] [PubMed] [Google Scholar]
- 10.Springer G. F. (1997) J. Mol. Med. 75, 594–602 [DOI] [PubMed] [Google Scholar]
- 11.Denda-Nagai K., Irimura T. (2000) Glycoconj. J. 17, 649–658 [DOI] [PubMed] [Google Scholar]
- 12.von Mensdorff-Pouilly S., Verstraeten A. A., Kenemans P., Snijdewint F. G., Kok A., Van Kamp G. J., Paul M. A., Van Diest P. J., Meijer S., Hilgers J. (2000) J. Clin. Oncol. 18, 574–583 [DOI] [PubMed] [Google Scholar]
- 13.Napoletano C., Rughetti A., Agervig Tarp M. P., Coleman J., Bennett E. P., Picco G., Sale P., Denda-Nagai K., Irimura T., Mandel U., Clausen H., Frati L., Taylor-Papadimitriou J., Burchell J., Nuti M. (2007) Cancer Res. 67, 8358–8367 [DOI] [PubMed] [Google Scholar]
- 14.Suzuki N., Yamamoto K., Toyoshima S., Osawa T., Irimura T. (1996) J. Immunol. 156, 128–135 [PubMed] [Google Scholar]
- 15.Sato M., Kawakami K., Osawa T., Toyoshima S. (1992) J. Biochem. 111, 331–336 [DOI] [PubMed] [Google Scholar]
- 16.Tsuiji M., Fujimori M., Ohashi Y., Higashi N., Onami T. M., Hedrick S. M., Irimura T. (2002) J. Biol. Chem. 277, 28892–28901 [DOI] [PubMed] [Google Scholar]
- 17.Imai Y., Akimoto Y., Mizuochi S., Kimura T., Hirano H., Irimura T. (1995) Immunology 86, 591–598 [PMC free article] [PubMed] [Google Scholar]
- 18.Mizuochi S., Akimoto Y., Imai Y., Hirano H., Irimura T. (1997) Glycobiology 7, 137–146 [DOI] [PubMed] [Google Scholar]
- 19.Denda-Nagai K., Kubota N., Tsuiji M., Kamata M., Irimura T. (2002) Glycobiology 12, 443–450 [DOI] [PubMed] [Google Scholar]
- 20.Higashi N., Fujioka K., Denda-Nagai K., Hashimoto S., Nagai S., Sato T., Fujita Y., Morikawa A., Tsuiji M., Miyata-Takeuchi M., Sano Y., Suzuki N., Yamamoto K., Matsushima K., Irimura T. (2002) J. Biol. Chem. 277, 20686–20693 [DOI] [PubMed] [Google Scholar]
- 21.Dupasquier M., Stoitzner P., Wan H., Cerqueira D., van Oudenaren A., Voerman J. S., Denda-Nagai K., Irimura T., Raes G., Romani N., Leenen P. J. (2006) J. Leukoc. Biol. 80, 838–849 [DOI] [PubMed] [Google Scholar]
- 22.Iida S., Yamamoto K., Irimura T. (1999) J. Biol. Chem. 274, 10697–10705 [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto K., Ishida C., Shinohara Y., Hasegawa Y., Konami Y., Osawa T., Irimura T. (1994) Biochemistry 33, 8159–8166 [DOI] [PubMed] [Google Scholar]
- 24.Onami T. M., Lin M. Y., Page D. M., Reynolds S. A., Katayama C. D., Marth J. D., Irimura T., Varki A., Varki N., Hedrick S. M. (2002) Mol. Cell. Biol. 22, 5173–5181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimura T., Imai Y., Irimura T. (1995) J. Biol. Chem. 270, 16056–16062 [DOI] [PubMed] [Google Scholar]
- 26.Corti M., Brody A. R., Harrison J. H. (1996) Am. J. Respir. Cell Mol. Biol. 14, 309–315 [DOI] [PubMed] [Google Scholar]
- 27.Bovin N. V. (1998) Glycoconj. J. 15, 431–446 [DOI] [PubMed] [Google Scholar]
- 28.Wandall H. H., Hassan H., Mirgorodskaya E., Kristensen A. K., Roepstorff P., Bennett E. P., Nielsen P. A., Hollingsworth M. A., Burchell J., Taylor-Papadimitriou J., Clausen H. (1997) J. Biol. Chem. 272, 23503–23514 [DOI] [PubMed] [Google Scholar]
- 29.Oo-Puthinan S., Maenuma K., Sakakura M., Denda-Nagai K., Tsuiji M., Shimada I., Nakamura-Tsuruta S., Hirabayashi J., Bovin N. V., Irimura T. (2008) Biochim. Biophys. Acta 1780, 89–100 [DOI] [PubMed] [Google Scholar]
- 30.Kumamoto Y., Denda-Nagai K., Aida S., Higashi N., Irimura T. (2009) PLoS One 4, e5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asselin-Paturel C., Boonstra A., Dalod M., Durand I., Yessaad N., Dezutter-Dambuyant C., Vicari A., O'Garra A., Biron C., Brière F., Trinchieri G. (2001) Nat. Immunol. 2, 1144–1150 [DOI] [PubMed] [Google Scholar]
- 32.Krug A., French A. R., Barchet W., Fischer J. A., Dzionek A., Pingel J. T., Orihuela M. M., Akira S., Yokoyama W. M., Colonna M. (2004) Immunity 21, 107–119 [DOI] [PubMed] [Google Scholar]
- 33.Nakano H., Yanagita M., Gunn M. D. (2001) J. Exp. Med. 194, 1171–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raes G., Brys L., Dahal B. K., Brandt J., Grooten J., Brombacher F., Vanham G., Noël W., Bogaert P., Boonefaes T., Kindt A., Van den Bergh R., Leenen P. J., De Baetselier P., Ghassabeh G. H. (2005) J. Leukoc. Biol. 77, 321–327 [DOI] [PubMed] [Google Scholar]
- 35.Datta S. K., Redecke V., Prilliman K. R., Takabayashi K., Corr M., Tallant T., DiDonato J., Dziarski R., Akira S., Schoenberger S. P., Raz E. (2003) J. Immunol. 170, 4102–4110 [DOI] [PubMed] [Google Scholar]
- 36.Saba K., Denda-Nagai K., Irimura T. (2009) Am. J. Pathol. 174, 144–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naik S. H., Metcalf D., van Nieuwenhuijze A., Wicks I., Wu L., O'Keeffe M., Shortman K. (2006) Nat. Immunol. 7, 663–671 [DOI] [PubMed] [Google Scholar]
- 38.Varol C., Landsman L., Fogg D. K., Greenshtein L., Gildor B., Margalit R., Kalchenko V., Geissmann F., Jung S. (2007) J. Exp. Med. 204, 171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Auffray C., Fogg D., Garfa M., Elain G., Join-Lambert O., Kayal S., Sarnacki S., Cumano A., Lauvau G., Geissmann F. (2007) Science 317, 666–670 [DOI] [PubMed] [Google Scholar]
- 40.Cisse B., Caton M. L., Lehner M., Maeda T., Scheu S., Locksley R., Holmberg D., Zweier C., den Hollander N. S., Kant S. G., Holter W., Rauch A., Zhuang Y., Reizis B. (2008) Cell 135, 37–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clark G., Munster D., Yusuf S., Hart D. N. (2005) Cell. Immunol. 236, 21–28 [DOI] [PubMed] [Google Scholar]
- 42.Bonifaz L., Bonnyay D., Mahnke K., Rivera M., Nussenzweig M. C., Steinman R. M. (2002) J. Exp. Med. 196, 1627–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonifaz L. C., Bonnyay D. P., Charalambous A., Darguste D. I., Fujii S., Soares H., Brimnes M. K., Moltedo B., Moran T. M., Steinman R. M. (2004) J. Exp. Med. 199, 815–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan M. C., Mommaas A. M., Drijfhout J. W., Jordens R., Onderwater J. J., Verwoerd D., Mulder A. A., van der Heiden A. N., Scheidegger D., Oomen L. C., Ottenhoff T. H., Tulp A., Neefjes J. J., Koning F. (1997) Eur. J. Immunol. 27, 2426–2435 [DOI] [PubMed] [Google Scholar]
- 45.Burgdorf S., Kautz A., Böhnert V., Knolle P. A., Kurts C. (2007) Science 316, 612–616 [DOI] [PubMed] [Google Scholar]
- 46.Burgdorf S., Lukacs-Kornek V., Kurts C. (2006) J. Immunol. 176, 6770–6776 [DOI] [PubMed] [Google Scholar]
- 47.Monti P., Leone B. E., Zerbi A., Balzano G., Cainarca S., Sordi V., Pontillo M., Mercalli A., Di Carlo V., Allavena P., Piemonti L. (2004) J. Immunol. 172, 7341–7349 [DOI] [PubMed] [Google Scholar]
- 48.Rughetti A., Pellicciotta I., Biffoni M., Bäckström M., Link T., Bennet E. P., Clausen H., Noll T., Hansson G. C., Burchell J. M., Frati L., Taylor-Papadimitriou J., Nuti M. (2005) J. Immunol. 174, 7764–7772 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.