Abstract
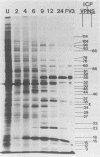
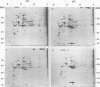
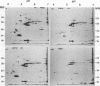
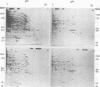
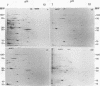
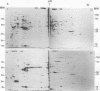
Analysis of frog virus 3-infected BHK cells by two-dimensional, acidic and basic gel electrophoresis showed that at least 90 infected cell-specific polypeptides could be detected. These polypeptides represent between 70 and 85% of the coding capacity of the viral genome. The polypeptides were sequentially induced in at least three phases. The virus gradually suppressed host cell polypeptide synthesis during infection, although the synthesis of a few cell polypeptides may be “switched off” early in infection.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aubertin A. M., Hirth C., Travo C., Nonnenmacher H., Kirn A. Preparation and properties of an inhibitory extract from frog virus 3 particles. J Virol. 1973 May;11(5):694–701. doi: 10.1128/jvi.11.5.694-701.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Drillien R., Spehner D., Kirn A. Cell killing by frog virus 3: evidence for cell killing by single viral particles or single viral subunits. Biochem Biophys Res Commun. 1977 Nov 7;79(1):105–111. doi: 10.1016/0006-291x(77)90066-3. [DOI] [PubMed] [Google Scholar]
- Elliott R. M., Bateson A., Kelly D. C. Phosphonoacetic Acid inhibition of frog virus 3 replication. J Virol. 1980 Jan;33(1):539–542. doi: 10.1128/jvi.33.1.539-542.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R. M., Kelly D. C. Frog virus 3 replication: induction and intracellular distribution of polypeptides in infected cells. J Virol. 1980 Jan;33(1):28–51. doi: 10.1128/jvi.33.1.28-51.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick M. L., Walker M. J. Suppression of the synthesis of cellular macromolecules by herpes simplex virus. J Gen Virol. 1978 Oct;41(1):37–51. doi: 10.1099/0022-1317-41-1-37. [DOI] [PubMed] [Google Scholar]
- Goorha R., Willis D. B., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. VI. Frog virus 3 replication is dependent on the cell nucleus. J Virol. 1977 Feb;21(2):802–805. doi: 10.1128/jvi.21.2.802-805.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granoff A. Viruses of amphibia. Curr Top Microbiol Immunol. 1969;50:107–137. doi: 10.1007/978-3-642-46169-9_4. [DOI] [PubMed] [Google Scholar]
- Kelly D. C., Avery R. J. Frog virus 3 deoxyribonucleic acid. J Gen Virol. 1974 Aug;24(2):339–348. doi: 10.1099/0022-1317-24-2-339. [DOI] [PubMed] [Google Scholar]
- Kelly D. C., Robertson J. S. Icosahedral cytoplasmic deoxyriboviruses. J Gen Virol. 1973 Jun;20(Suppl):17–41. doi: 10.1099/0022-1317-20-Supplement-17. [DOI] [PubMed] [Google Scholar]
- Moore N. F., Kelley J. M., Wagner R. R. Envelope proteins of vesicular stomatitis virions: accessibility to iodination. Virology. 1974 Sep;61(1):292–296. doi: 10.1016/0042-6822(74)90264-5. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Willis D. B., Goorha R., Miles M., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. VII. Transcriptional and post-transcriptional regulation of virus gene expression. J Virol. 1977 Oct;24(1):326–342. doi: 10.1128/jvi.24.1.326-342.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]