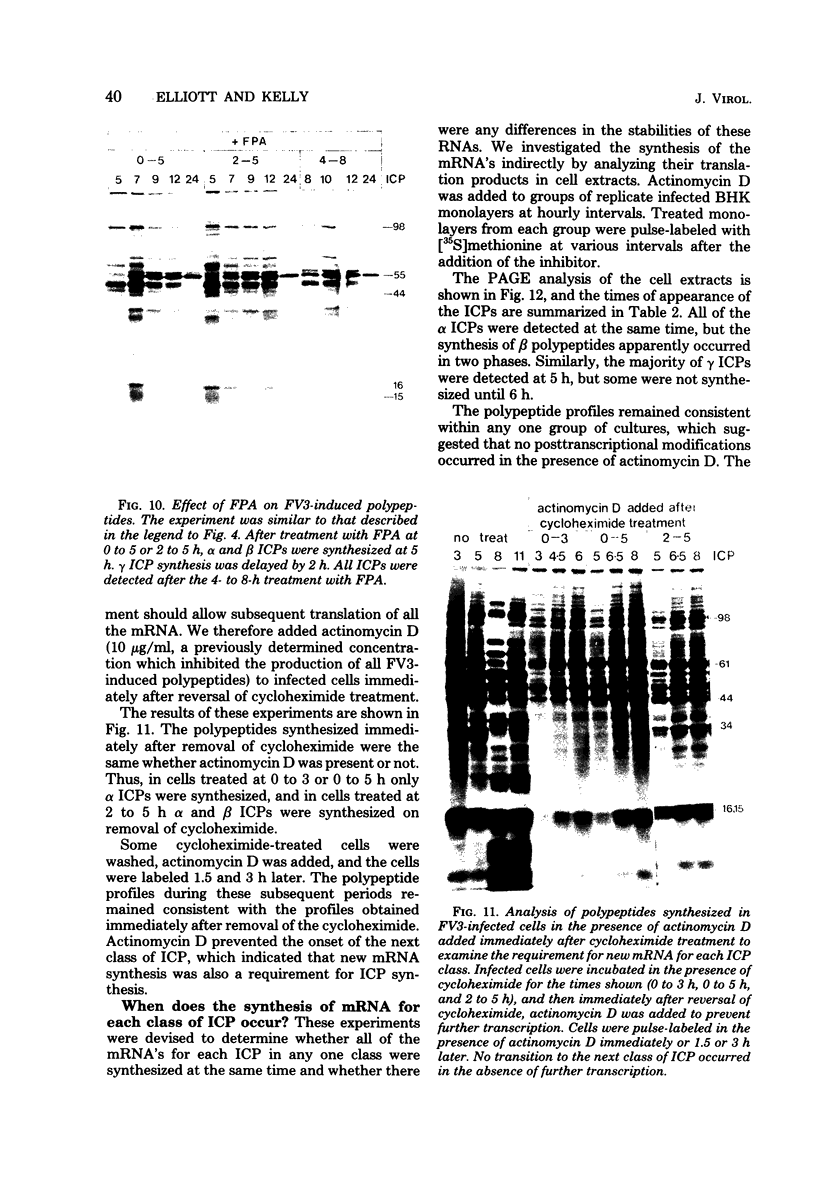
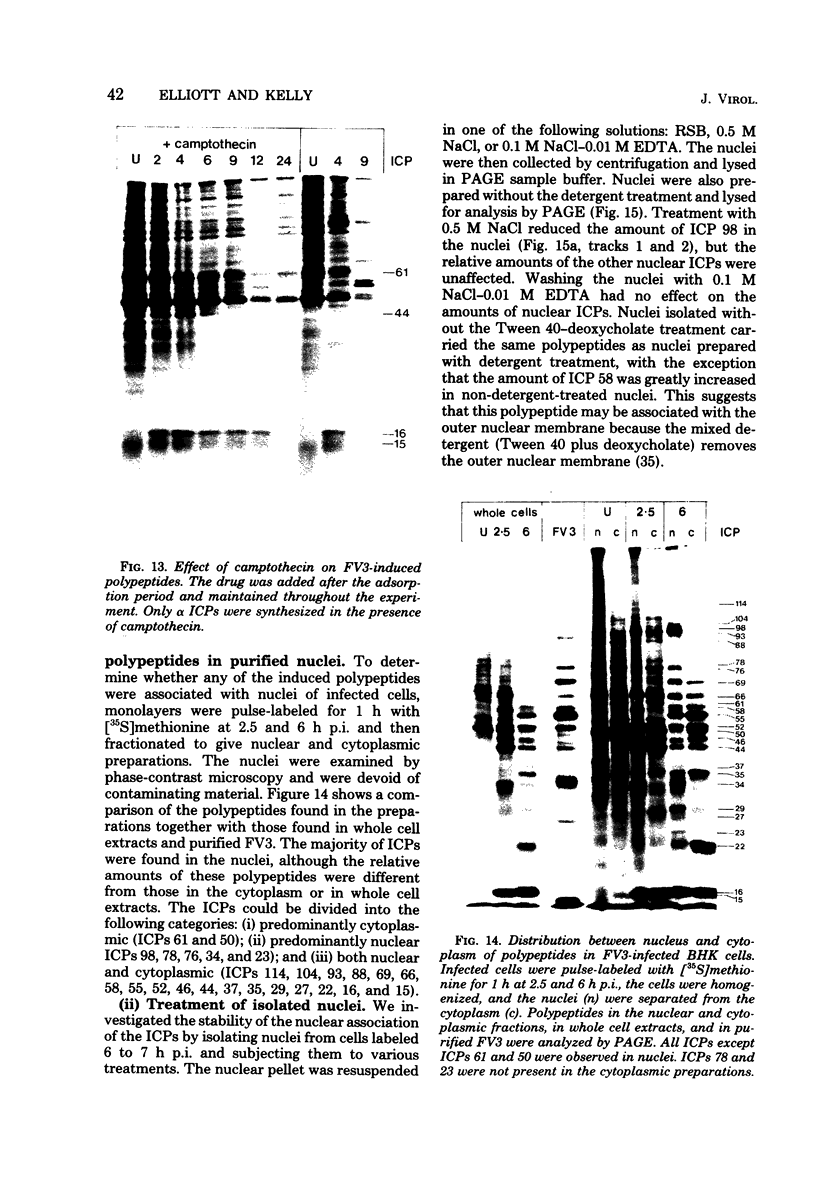
Abstract
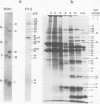
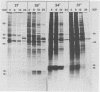
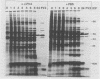
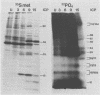
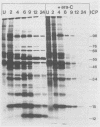
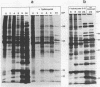
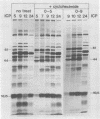
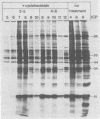
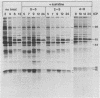
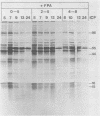
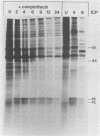
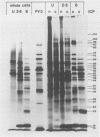
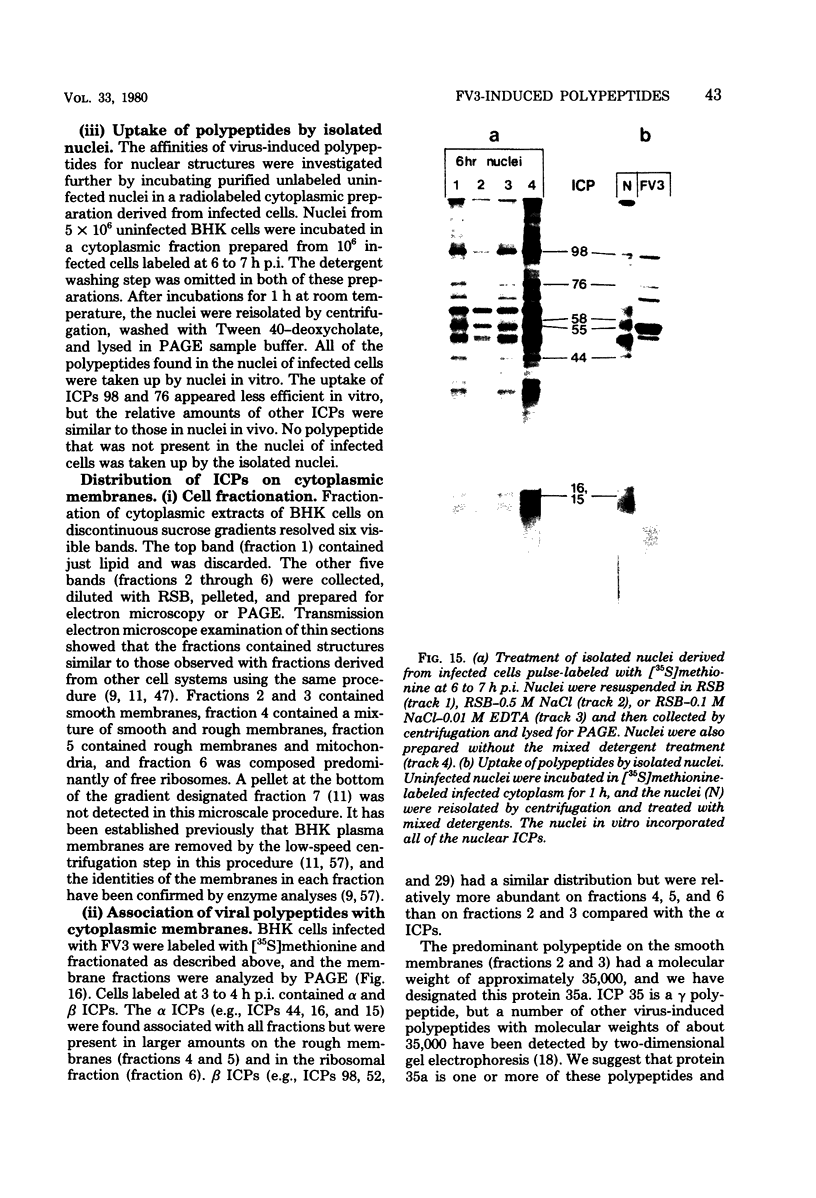
The synthesis of the polypeptides induced in frog virus 3-infected cells was analyzed by high-resolution sodium dodecyl sulfate-polyacrylamide gel electrophoresis of radiolabeled cell extracts. Purified frog virus 3 contained 22 polypeptides, with molecular weights in the range 9 × 103 to 114 × 103. All of the structural and an additional seven nonstructural polypeptides were detected in infected cell lysates. The following three classes of induced polypeptides (under temporal control) were observed in BHK cells: at 2 h, four α polypeptides; at 4 h, 13 β polypeptides; and at 6 h, the remaining 12 γ polypeptides. The total molecular weight of the infected cell-specific polypeptides (ICPs) was ≃ 1.5 × 106, which accounts for about 30% of the coding capacity of the viral genome. At least 10 of the induced polypeptides were phosphorylated, but none was glycosylated or sulfated. No evidence for posttranslation cleavage of polypeptides in pulse-chase and inhibition experiments was obtained. The synthesis of γ polypeptides was not detected in the presence of the viral DNA replication inhibitors cytosine arabinoside and hydroxyurea, but halogenated nucleotides apparently had no effect. These results suggest that α and β polypeptides are “early” events and that detectable γ polypeptide synthesis is dependent on the production of progeny viral DNA. The regulation of frog virus 3-induced polypeptide synthesis in infected BHK cells was examined by using inhibitors of protein and RNA synthesis and amino acid analogs. These experiments confirmed the existence of three sequentially synthesized, coordinately regulated classes of polypeptides, designated α, β, and γ. The requirements for the synthesis of each class were as follows: (i) α polypeptides did not require previous cell protein synthesis; (ii) β polypeptides required a prescribed period of α polypeptide synthesis and new mRNA synthesis; and (iii) γ polypeptides required prior synthesis of functional β polypeptides and new mRNA synthesis. α polypeptide synthesis was controlled by β and γ polypeptides, and α and β polypeptides were involved in the suppression of host cell polypeptide synthesis. Indirect evidence was obtained for the temporal regulation of frog virus 3 transcription. The intracellular distribution of virus-induced polypeptides in cells infected with frog virus 3 was investigated by using standard cell fractionation techniques. Most of the 29 induced polypeptides were bound to structures within the nucleus, and only two ICPs were not associated with purified nuclei. When isolated nuclei were incubated in an infected cell cytoplasm preparation, all of the nuclear ICPs were incorporated in vitro. All of the ICPs were associated with ribosomal and rough endoplasmic reticulum fractions of infected cells, and a number of ICPs were found on smooth intracellular membranes. Most of the ICPs were also associated with purified plasma membranes of infected cells, and one polypeptide (ICP 58) was highly enriched in the plasma membrane compared with whole cell extracts or purified frog virus 3.
Full text
PDF




















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armentrout R. W., McAuslan B. R. RNA synthesis in cells infected with an icosahedral cytoplasmic deoxyvirus (frog virus 3). J Virol. 1974 May;13(5):1083–1092. doi: 10.1128/jvi.13.5.1083-1092.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubertin A. M., Decker C., Kirn A. Effect of gamma-rays on infectivity and capacity for nuclear-associated and cytoplasmic DNA replication of FV 3 in chick embryo fibroblasts. Radiat Res. 1970 Oct;44(1):178–186. [PubMed] [Google Scholar]
- Aubertin A. M., Hirth C., Travo C., Nonnenmacher H., Kirn A. Preparation and properties of an inhibitory extract from frog virus 3 particles. J Virol. 1973 May;11(5):694–701. doi: 10.1128/jvi.11.5.694-701.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai R., Lachmi B. E. The effect of frog virus 3 on the biological activity of various RNA viruses. J Gen Virol. 1973 Oct;21:201–204. doi: 10.1099/0022-1317-21-1-201. [DOI] [PubMed] [Google Scholar]
- Bingen A., Kirn A. Fibrillar bodies in hepatocyte nuclei during the course of the toxic hepatitis produced by frog virus 3 in mice. J Ultrastruct Res. 1975 Feb;50(2):167–173. doi: 10.1016/s0022-5320(75)80047-5. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bosmann H. B. Camptothecin inhibits macromolecular synthesis in mammalian cells but not in isolated mitochondria of E. coli. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1412–1420. doi: 10.1016/0006-291x(70)90544-9. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., Tamm I. The role of cytoplasmic membranes in poliovirus biosynthesis. Virology. 1970 Sep;42(1):100–111. doi: 10.1016/0042-6822(70)90242-4. [DOI] [PubMed] [Google Scholar]
- Campadelli-Fiume G., Costanzo F., Mannini-Palenzona A., La Placa M. Impairment of host cell ribonucleic acid polymerase II after infection with frog virus 3. J Virol. 1972 Apr;9(4):698–700. doi: 10.1128/jvi.9.4.698-700.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo F., Campadelli-Fiume G., Foà-Tomasi L., La Placa M. Effect of non-permissive temperature on protein synthesis of frog virus 3 infected cells. Boll Ist Sieroter Milan. 1976 Sep 30;55(4):267–271. [PubMed] [Google Scholar]
- Costanzo F., La Placa M., Palenzoma A. M., Novello F., Stirpe F. Selective inhibition of a nuclear RNA polymerase in cells infected with frog virus. Nature. 1970 Jul 18;227(5255):294–295. doi: 10.1038/227294a0. [DOI] [PubMed] [Google Scholar]
- Dales S., Mosbach E. H. Vaccinia as a model for membrane biogenesis. Virology. 1968 Aug;35(4):564–583. doi: 10.1016/0042-6822(68)90286-9. [DOI] [PubMed] [Google Scholar]
- Darlington R. W., Granoff A., Breeze D. C. Viruses and renal carcinoma of Rana pipiens. II. Ultrastructural studies and sequential development of virus isolated from normal and tumor tissue. Virology. 1966 May;29(1):149–156. doi: 10.1016/0042-6822(66)90204-2. [DOI] [PubMed] [Google Scholar]
- Elliott R. M., Bateson A., Kelly D. C. Phosphonoacetic Acid inhibition of frog virus 3 replication. J Virol. 1980 Jan;33(1):539–542. doi: 10.1128/jvi.33.1.539-542.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R. M., Bravo R., Kelly D. C. Frog Virus 3 Replication: Analysis of Structural and Nonstructural Polypeptides in Infected BHK Cells by Acidic and Basic Two-Dimensional Gel Electrophoresis. J Virol. 1980 Jan;33(1):18–27. doi: 10.1128/jvi.33.1.18-27.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R. M., Lescott T., Kelly D. C. Serological relationships of an iridescent virus (type 25) recently isolated from Tipula sp. with two other iridescent viruses (types 2 and 22). Virology. 1977 Sep;81(2):309–316. doi: 10.1016/0042-6822(77)90147-7. [DOI] [PubMed] [Google Scholar]
- Erickson J. S., Kaplan A. S. Synthesis of proteins in cells infected with herpesvirus. IX. Sulfated proteins. Virology. 1973 Sep;55(1):94–102. doi: 10.1016/s0042-6822(73)81011-6. [DOI] [PubMed] [Google Scholar]
- Esteban M., Metz D. H. Early virus protein synthesis in vaccinia virus-infected cells. J Gen Virol. 1973 May;19(2):201–206. doi: 10.1099/0022-1317-19-2-201. [DOI] [PubMed] [Google Scholar]
- Fenwick M. L., Walker M. J., Petkevich J. M. On the association of virus proteins with the nuclei of cells infected with herpes simplex virus. J Gen Virol. 1978 Jun;39(3):519–529. doi: 10.1099/0022-1317-39-3-519. [DOI] [PubMed] [Google Scholar]
- Franklin R. M. Structure and synthesis of bacteriophage PM2, with particular emphasis on the viral lipid bilayer. Curr Top Microbiol Immunol. 1974;(68):107–159. doi: 10.1007/978-3-642-66044-3_5. [DOI] [PubMed] [Google Scholar]
- Gaby N. S., Kucera L. S. DNA-dependent RNA polymerase activity associated with subviral particles of polyhedral cytoplasmic dexoyribovirus. J Virol. 1974 Aug;14(2):231–238. doi: 10.1128/jvi.14.2.231-238.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafford L. G., Randall C. C. Virus-specific RNA and DNA in nuclei of cells infected with fowlpox virus. Virology. 1976 Jan;69(1):1–14. doi: 10.1016/0042-6822(76)90189-6. [DOI] [PubMed] [Google Scholar]
- Gibson W., Roizman B. Proteins specified by herpes simplex virus. Staining and radiolabeling properties of B capsid and virion proteins in polyacrylamide gels. J Virol. 1974 Jan;13(1):155–165. doi: 10.1128/jvi.13.1.155-165.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goorha R., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. I. Virus-specific protein synthesis and its regulation. Virology. 1974 Jul;60(1):237–250. doi: 10.1016/0042-6822(74)90381-x. [DOI] [PubMed] [Google Scholar]
- Goorha R., Murti G., Granoff A., Tirey R. Macromolecular synthesis in cells infected by frog virus 3. VIII. The nucleus is a site of frog virus 3 DNA and RNA synthesis. Virology. 1978 Jan;84(1):32–50. doi: 10.1016/0042-6822(78)90216-7. [DOI] [PubMed] [Google Scholar]
- Goorha R., Willis D. B., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. VI. Frog virus 3 replication is dependent on the cell nucleus. J Virol. 1977 Feb;21(2):802–805. doi: 10.1128/jvi.21.2.802-805.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granoff A., Came P. E., Breeze D. C. Viruses and renal carcinoma of Rana pipiens. I. The isolation and properties of virus from normal and tumor tissue. Virology. 1966 May;29(1):133–148. doi: 10.1016/0042-6822(66)90203-0. [DOI] [PubMed] [Google Scholar]
- Granoff A. Viruses of amphibia. Curr Top Microbiol Immunol. 1969;50:107–137. doi: 10.1007/978-3-642-46169-9_4. [DOI] [PubMed] [Google Scholar]
- Guir J., Braunwald J., Kirn A. Inhibition of host-specific DNA and RNA synthesis in KB cells following infection with frog virus 3. J Gen Virol. 1971 Sep;12(3):293–301. doi: 10.1099/0022-1317-12-3-293. [DOI] [PubMed] [Google Scholar]
- Hardy C. L., Randall C. C., Gafford L. G. A viral-induced protein in the nuclei of cells infected with fowlpox virus. Virology. 1978 Jan;84(1):236–241. doi: 10.1016/0042-6822(78)90242-8. [DOI] [PubMed] [Google Scholar]
- Harrison S. C., Caspar D. L., Camerini-Otero R. D., Franklin R. M. Lipid and protein arrangement in bacteriophage PM2. Nat New Biol. 1971 Feb 17;229(7):197–201. doi: 10.1038/newbio229197a0. [DOI] [PubMed] [Google Scholar]
- Holtzman E., Smith I., Penman S. Electron microscopic studies of detergent-treated HeLa cell nuclei. J Mol Biol. 1966 May;17(1):131–135. doi: 10.1016/s0022-2836(66)80099-2. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Proteins specified by herpes simplex virus. XI. Identification and relative molar rates of synthesis of structural and nonstructural herpes virus polypeptides in the infected cell. J Virol. 1973 Dec;12(6):1347–1365. doi: 10.1128/jvi.12.6.1347-1365.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. S., Horwitz S. B. Intracellular degradation of HeLa and adenovirus type 2 DNA induced by camptothecin. Biochem Biophys Res Commun. 1971 Nov 5;45(3):723–727. doi: 10.1016/0006-291x(71)90476-1. [DOI] [PubMed] [Google Scholar]
- Kang H. S., McAuslan B. R. Virus-associated nucleases: location and properties of deoxyribonucleases and ribonucleases in purified frog virus 3. J Virol. 1972 Aug;10(2):202–210. doi: 10.1128/jvi.10.2.202-210.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A. S., Ben-Porat T. Synthesis of proteins in cells infected with herpesvirus. XI. Sulfated, structural glycoproteins. Virology. 1976 Apr;70(2):561–563. doi: 10.1016/0042-6822(76)90300-7. [DOI] [PubMed] [Google Scholar]
- Kelly D. C., Atkinson M. A. Frog virus 3 replication: electron microscope observations on the terminal stages of infection in chronically infected cell cultures. J Gen Virol. 1975 Sep;28(3):391–407. doi: 10.1099/0022-1317-28-3-391. [DOI] [PubMed] [Google Scholar]
- Kelly D. C., Avery R. J. Frog virus 3 deoxyribonucleic acid. J Gen Virol. 1974 Aug;24(2):339–348. doi: 10.1099/0022-1317-24-2-339. [DOI] [PubMed] [Google Scholar]
- Kelly D. C. Frog virus 3 replication: electron microscope observations on the sequence of infection in chick embryo fibroblasts. J Gen Virol. 1975 Jan;26(1):71–86. doi: 10.1099/0022-1317-26-1-71. [DOI] [PubMed] [Google Scholar]
- Kelly D. C., Robertson J. S. Icosahedral cytoplasmic deoxyriboviruses. J Gen Virol. 1973 Jun;20(Suppl):17–41. doi: 10.1099/0022-1317-20-Supplement-17. [DOI] [PubMed] [Google Scholar]
- Kelly D. C., Vance D. E. The lipid content of two iridescent viruses. J Gen Virol. 1973 Nov;21(2):417–423. doi: 10.1099/0022-1317-21-2-417. [DOI] [PubMed] [Google Scholar]
- Kessel D., Dysard R. Effects of camptothecin on RNA synthesis in L-1210 cells. Biochim Biophys Acta. 1973 Jul 27;312(4):716–721. doi: 10.1016/0005-2787(73)90075-0. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Choppin P. W. Lipids of plasma membranes of monkey and hamster kidney cells and of parainfluenza virions grown in these cells. Virology. 1969 Jun;38(2):255–268. doi: 10.1016/0042-6822(69)90367-5. [DOI] [PubMed] [Google Scholar]
- Korant B. D. Cleavage of viral precursor proteins in vivo and in vitro. J Virol. 1972 Oct;10(4):751–759. doi: 10.1128/jvi.10.4.751-759.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucera L. S. Effects of temperature on frog polyhedral cytoplasmic deoxyribovirus multiplication: thermosensitivity of initiation, replication, and encapsidation of viral DNA. Virology. 1970 Nov;42(3):576–589. doi: 10.1016/0042-6822(70)90304-1. [DOI] [PubMed] [Google Scholar]
- Kucera L. S., Granoff A. Viruses and renal carcinoma of Rana pipiens. VI. Interrelationships of macromolecular synthesis and infectious virus production in frog virus 3-infected BHK 21/13 cells. Virology. 1968 Feb;34(2):240–249. doi: 10.1016/0042-6822(68)90233-x. [DOI] [PubMed] [Google Scholar]
- LaColla P., Weissbach A. Vaccinia virus infection of HeLa cells. I. Synthesis of vaccinia DNA in host cell nuclei. J Virol. 1975 Feb;15(2):305–315. doi: 10.1128/jvi.15.2.305-315.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maes R., Granoff A. Viruses and renal carcinoma of Rana pipiens. IV. Nucleic acid synthesis in frog virus 3-infected BHK 21/13 cells. Virology. 1967 Nov;33(3):491–502. doi: 10.1016/0042-6822(67)90125-0. [DOI] [PubMed] [Google Scholar]
- McAuslan B. R., Armentrout R. W. The biochemistry of icosahedral cytoplasmic deoxyviruses. Curr Top Microbiol Immunol. 1974;(68):77–105. doi: 10.1007/978-3-642-66044-3_4. [DOI] [PubMed] [Google Scholar]
- McAuslan B. R., Smith W. R. Deoxyribonucleic acid synthesis in FV-3-infected mammalian cells. J Virol. 1968 Oct;2(10):1006–1015. doi: 10.1128/jvi.2.10.1006-1015.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser A. G., Caliguiri L. A., Scheid A. S., Tamm I. Chemical and enzymatic characteristics of cytoplasmic membranes of poliovirus-infected HeLa cells. Virology. 1972 Jan;47(1):30–38. doi: 10.1016/0042-6822(72)90235-8. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Compans R. W. Effects of glucosamine, 2-deoxyglucose, and tunicamycin on glycosylation, sulfation, and assembly of influenza viral proteins. Virology. 1978 Feb;84(2):303–319. doi: 10.1016/0042-6822(78)90250-7. [DOI] [PubMed] [Google Scholar]
- Nevins J. R., Joklik W. K. Poly (A) sequences of vaccinia virus messenger RNA: nature, mode of addition and function during translation in vitra and in vivo. Virology. 1975 Jan;63(1):1–14. doi: 10.1016/0042-6822(75)90365-7. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ortin J., Vińuela E. Requirement of cell nucleus for African swine fever virus replication in Vero cells. J Virol. 1977 Mar;21(3):902–905. doi: 10.1128/jvi.21.3.902-905.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington T. H., Follett E. A. Vaccinia virus replication in enucleate BSC-1 cells: particle production and synthesis of viral DNA and proteins. J Virol. 1974 Feb;13(2):488–493. doi: 10.1128/jvi.13.2.488-493.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington T. H. Vaccinia virus polypeptide synthesis: sequential appearance and stability of pre- and post-replicative polypeptides. J Gen Virol. 1974 Dec;25(3):433–444. doi: 10.1099/0022-1317-25-3-433. [DOI] [PubMed] [Google Scholar]
- Pinter A., Compans R. W. Sulfated components of enveloped viruses. J Virol. 1975 Oct;16(4):859–866. doi: 10.1128/jvi.16.4.859-866.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell W. C., Blair G. E. Polypeptide phosphorylation in adenovirus-infected cells. J Gen Virol. 1977 Jan;34(1):19–35. doi: 10.1099/0022-1317-34-1-19. [DOI] [PubMed] [Google Scholar]
- Sawicki S. G., Jelinek W., Darnell J. E. 3'-Terminal addition to HeLa cell nuclear and cytoplasmic poly (A). J Mol Biol. 1977 Jun 15;113(1):219–235. doi: 10.1016/0022-2836(77)90051-1. [DOI] [PubMed] [Google Scholar]
- Siev M., Weinberg R., Penman S. The selective interruption of nucleolar RNA synthesis in HeLa cells by cordycepin. J Cell Biol. 1969 May;41(2):510–520. doi: 10.1083/jcb.41.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberstein H., August J. T. Characterization of a virion protein kinase as a virus-specified enzyme. J Biol Chem. 1976 May 25;251(10):3185–3190. [PubMed] [Google Scholar]
- Silberstein H., August J. T. Phosphorylation of animal virus proteins by a virion protein kinase. J Virol. 1973 Sep;12(3):511–522. doi: 10.1128/jvi.12.3.511-522.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberstein H., August J. T. Purification and properties of a virion protein kinase. J Biol Chem. 1976 May 25;251(10):3176–3184. [PubMed] [Google Scholar]
- Stanley P., Gandhi S. S., White D. O. The polypeptides of influenza virus. VII. Synthesis of the hemagglutinin. Virology. 1973 May;53(1):92–106. doi: 10.1016/0042-6822(73)90468-6. [DOI] [PubMed] [Google Scholar]
- Summers D. F., Shaw E. N., Stewart M. L., Maizel J. V., Jr Inhibition of cleavage of large poliovirus-specific precursor proteins in infected HeLa cells by inhibitors of proteolytic enzymes. J Virol. 1972 Oct;10(4):880–884. doi: 10.1128/jvi.10.4.880-884.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K. B., McAuslan B. R. Proteins of polyhedral cytoplasmic deoxyviruses. I. The structural polypeptides of FV 3 . Virology. 1971 Jul;45(1):200–207. doi: 10.1016/0042-6822(71)90127-9. [DOI] [PubMed] [Google Scholar]
- Tripier F., Braunwald J., Markovic L., Kirn A. Budding of frog virus 3 studied by immunological and cytochemical methods in electron microscopy. Intervirology. 1974;3(5-6):305–318. doi: 10.1159/000149768. [DOI] [PubMed] [Google Scholar]
- Tripier F., Markovic L., Braunwald J., Kirn A. Données nouvelles sur la structure et le bourgeonnement du FV3 (frog virus 3) Ann Microbiol (Paris) 1975 Dec;126(4):447–460. [PubMed] [Google Scholar]
- Vilagines R., McAuslan B. R. Proteins of polyhedal cytoplasmic deoxyvirus. II. Nucleotide phosphohydrolase activity associated with frog virus 3. J Virol. 1971 May;7(5):619–624. doi: 10.1128/jvi.7.5.619-624.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis D. B., Goorha R., Miles M., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. VII. Transcriptional and post-transcriptional regulation of virus gene expression. J Virol. 1977 Oct;24(1):326–342. doi: 10.1128/jvi.24.1.326-342.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis D. B., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. IV. Regulation of virus-specific RNA synthesis. Virology. 1976 Apr;70(2):399–410. doi: 10.1016/0042-6822(76)90281-6. [DOI] [PubMed] [Google Scholar]
- Willis D. B., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. IX. Two temporal classes of early viral RNA. Virology. 1978 May 15;86(2):443–453. doi: 10.1016/0042-6822(78)90084-3. [DOI] [PubMed] [Google Scholar]
- Willis D. B., Granoff A. Macromolecular synthesis in cells infected with frog virus 3. V. The absence of polyadenylic acid in the majority of frog virus 3-specific mRNA species. Virology. 1976 Sep;73(2):543–547. doi: 10.1016/0042-6822(76)90417-7. [DOI] [PubMed] [Google Scholar]
- Willis D., Granoff A. Lipid composition of frog virus 3. Virology. 1974 Sep;61(1):256–269. doi: 10.1016/0042-6822(74)90260-8. [DOI] [PubMed] [Google Scholar]