Abstract
Hemocyanin (Hc) is an oxygen carrier protein in which oxygen binding is regulated by allosteric effectors such as H+ and l-lactate. Isothermal titration calorimetric measurements showed that l-lactate binds to dodecameric and heterohexameric Hc and to the CaeSS3 homohexamer but not to the CaeSS2 monomer. The binding of lactate caused no change in the optical absorption and x-ray absorption spectra of either oxy- or deoxy-Hc, suggesting that no structural rearrangement of the active site occurred. At pH 6.5, the oxygen binding rate constant kobs obtained by flash photolysis showed a significant increase upon addition of l-lactate, whereas l-lactate addition had little effect at pH 8.3. Lactate binding caused a concentration-dependent shift in the interhexameric distances at pH 6.5 based on small angle x-ray scattering measurements. These results show that l-lactate affects oxygen affinity at pH 6.5 by modulating the global structure of Hc without affecting its binuclear copper center (the active site). In contrast to this, the active site structure of deoxy-Hc is affected by changes in pH (Hirota, S., Kawahara, T., Beltramini, M., Di Muro, P., Magliozzo, R. S., Peisach, J., Powers, L. S., Tanaka, N., Nagao, S., and Bubacco, L. (2008) J. Biol. Chem. 283, 31941–31948). Upon addiction of lactate, the kinetic behavior of oxygen rebinding for Hc was heterogeneous under low oxygen concentrations at pH 6.5 due to changes in the T and R state populations, and the equilibrium was found to shift from the T toward the R state with addition of lactate.
Keywords: Biophysics, Enzymes/Cooperativity, Methods/Spectroscopy, Methods/X-ray Absorption Spectroscopy, Oxygen/Binding, Oxygen/Transport, Protein/Copper, Regulation Allosteric
Introduction
Hemocyanin (Hc)3 is an oxygen carrier protein present in the hemolymph of several arthropod species. Its biological function is based on the reversible binding of molecular oxygen to an active site containing a binuclear copper center (1). Arthropod Hcs are oligomeric proteins comprising 75-kDa subunits, each containing a single active site. In the presence of Ca2+ and Mg2+ in vivo and in vitro, the protein exists as heterohexamers (or multiheterohexamers) in which different subunits interact through specific contact areas. In the case of Carcinus aestuarii Hc, at least three subunit types (CaeSS1, CaeSS2, and CaeSS3) have been isolated and characterized (2). The subunits within the hexamer are arranged into two layers of trimers which are shifted by 60° relative to one another. One 3-fold symmetry axis exists along the axial direction of the molecule, whereas three 2-fold symmetry axes are oriented perpendicularly to the 3-fold axis (1). Subunit-subunit interactions are responsible for formation of hexamers (1 × 6-mers of 450 kDa) and for the interaction of hexameric building blocks to form 2 × 6- (3), 4 × 6- (4), 6 × 6- (5), and 8 × 6-mers (6) with molecular masses up to millions of daltons. These complex quaternary structures are species-specific, but all oligomers can be dissociated into monomers by removing Ca2+ and Mg2+ ions and/or increasing pH (3).
At the active site, each copper ion is bound to the imidazole ϵ-nitrogen of three conserved histidine residues. Reversible oxygen binding occurs at this site, and the protein can exist in two forms: deoxygenated (deoxy-Hc) and oxygenated (oxy-Hc), as a function of the oxygen concentration and the presence of allosteric effectors such as hydrogen ions or l-lactate. In the deoxy form, the active site copper is cuprous Cu(I) and the protein is colorless, whereas for the oxygenated form, the active site is formally a Cu(II)-O22−–Cu(II) complex and has blue color. Oxygen is bound in the μ-η2:η2 mode, and the absorption spectrum of the oxygenated site is characterized by absorption bands at 337 and 560 nm, assigned respectively to πσ*→Cu(II) and πv*→Cu(II) charge transfer transitions (7).
Hcs bind oxygen cooperatively, and as a general rule, the more complex quaternary structures with higher molecular mass exhibit stronger cooperative interactions manifested in increased Hill coefficients. Investigation of the origins of cooperativity has been approached through examination of the three-dimensional crystal structures of deoxy- and oxy-Hc (8) as well as through x-ray absorption spectroscopy (XAS) (9) and small angle x-ray scattering (SAXS) (10, 11), in some cases in the presence of small molecules acting as allosteric modifiers and/or at low pH. The results of these studies indicate conformational changes that occur at the level of the active site as well as the tertiary and quaternary structure of multimers. In particular for the metal site, an important trigger for affinity change is believed to be an alteration in the copper-copper distance reported to be 4.6 Å in deoxy-Hc and 3.6 Å in oxy-Hc (8), whereas the average bond lengths for the Nϵ-to-copper bonds are essentially unchanged in both forms. Such a rearrangement would enable coordination of dioxygen to both metal ions. Little additional structural information concerning tertiary and quaternary changes accompanying oxygen binding comes from comparison of the crystal structures of the oxy and deoxy subunit II homohexamers from Limulus polyphemus (which are noncooperative); in these examples, the tertiary and quaternary structures of oxygenated and deoxygenated hexamers are quite similar (12). Therefore, little insight into the structural origins of cooperativity is currently available. However, structural features at the level of protein subunit interactions must change in native Hcs as a function of ligand binding because there are no direct interactions in the dicopper site with effector molecules such as hydrogen ions or l-lactate, both of which are known to alter oxygen affinity and cooperativity.
Oxygen binding by Hcs is finely regulated by a number of allosteric effectors such as H+ (9, 13), l-lactate (10), and urate (14). Several models (two-state, three-state, and nested Monod-Wyman-Changeux models) have been applied to describe the binding affinity and cooperativity of arthropod Hcs and their dependence on solution conditions (15). Although allosteric regulators are believed to play important roles in vivo, information on the molecular mechanisms that lead to the observed changes in oxygen affinity and cooperativity is scanty. In general, an increase in H+ concentration decreases oxygen affinity as well as cooperativity (3, 16). This phenomenon is referred to as the Bohr effect. The range of pH values for a strong Bohr effect in decapods is 7.4–7.8, which is the physiologically relevant range for the seawater environment (17). The origin of the effect has been recently interpreted in terms of a protonation-dependent change in the active site geometry of copper, resulting in a change in the binding rate of dioxygen to the dinuclear Cu(I) active site in the subunits (9).
Compared with H+, l-lactate has an opposite effect such that increasing the l-lactate concentration increases oxygen affinity and cooperativity of binding. l-Lactate is a metabolite generated as a by-product of glycolysis under low oxygen conditions, and its concentration in cells is directly dependent on the oxygen debt. The physiological concentration of l-lactate under normoxia is very low (0.030–0.1 mm) (18, 19), but it can increase up to ∼30 mm during high activity of the animal (20). Under these conditions of acidosis, the importance of the l-lactate effect increasing the oxygen affinity could reside in counterbalancing the Bohr effect in Hc thus maintaining the oxygen affinity of the carrier. It had been suggested from oxygen affinity measurements on Panulirus interruptus and Callinectes sapidus Hc that l-lactate not only shifts the allosteric equilibrium toward the high affinity form (R state) from the low affinity form (T state) of the protein but also increases the oxygen affinity of both the T and R states (21, 22); in contrast to this, a more recent study on Carcinus maenas Hc suggested that l-lactate increases oxygen affinity by preferentially raising the oxygen affinity in the T state without affecting the affinity in the R (or high affinity) state (18). Concerning the l-lactate interaction site, l-lactate has been recently shown by electrospray ionization mass spectrometry to interact with the lightest subunits of C. maenas Hc (23) at a site or sites only formed in assembled hexamers. Despite the fact that pH and l-lactate affect the oxygen binding characteristics of Hc in a physiologically relevant fashion, their structural effects on Hc are still not defined in detail.
In this paper, we have addressed the issue of the l-lactate effect on oxygen binding mainly in the Hc from the brachyuran crab C. aestuarii. We chose this Hc because its oxygen-binding behavior has been investigated in detail as a function of both pH and l-lactate concentration (9, 18). Isothermal titration calorimetry (ITC) experiments were performed to monitor l-lactate binding to the protein, optical and XAS were applied to characterize structural effects on the active site, and flash photolysis was used to define the rate constants for oxygen binding under various conditions. Finally, SAXS was used to disclose conformational effects at the level of tertiary and quaternary structures. The results provide new insights into the physiology of Hc function and how l-lactate affects oxygen binding affinity by modulating the global structure of the protein without affecting its active site.
EXPERIMENTAL PROCEDURES
Preparation of Hc Samples
Hc was purified from the hemolymph of live animals as described previously (2, 24). The protein was purified to a final concentration of 25 mg/ml in 50 mm Tris-HCl, pH 7.6, with 20 mm CaCl2 and stored at −20 °C with 20% sucrose added as a cryoprotectant. Sucrose was eliminated before the measurements by overnight dialysis against the chosen buffer. The Hc obtained resulted in a mixture of ∼18% hexameric and 82% dodecameric fractions (supplemental Fig.S1). The dodecameric fraction was purified from the mixture by size exclusion chromatography on a Pharmacia fast protein liquid chromatography system (SEC-FPLC) with a prep-grade Hi-Load Superdex 200 26/60 column (Pharmacia) equilibrated with the same buffer. The dodecameric fraction did not dissociate further, and its oxygenation state was estimated from the absorbance ratio A337/A278, assuming 0.21 for a fully oxygenated form (2). The CaeSS2 and CaeSS3 monomers were purified as described previously (2). The isolated CaeSS3 monomers were then converted to homohexamers by addition of 20 mm CaCl2 at pH 7.6. It is worth mentioning that the CaeSS2 monomer does not assemble into higher molecular mass species (2).
ITC Measurements
Microcalorimetric measurements were carried out on purified dodecameric C. aestuarii Hc, its CaeSS2 monomer, and CaeSS3 homohexamer with a VP-ITC isothermal titration calorimeter (MicroCal). Titration curves were obtained at 20 °C by adding 25 aliquots (10 μl each) of l-lactate (100 mm) into Hc (1.453 ml, 16.7 μm hexamer) using buffers of 50 mm MES-NaOH, pH 6.5, or 50 mm Tris-HCl, pH 8.3, each with 20 mm CaCl2. The titration curves were measured several times for each condition and analyzed with the ORIGIN software program.
Optical Absorption Measurements
Optical absorption spectra were measured at 37 °C on a Shimadzu UV-3100PC spectrophotometer. The protein concentration was adjusted to 80 μm (subunit) in 50 mm Tris-HCl or MES-NaOH buffer, each containing 20 mm CaCl2 at the desired pH.
X-ray Absorption Measurements
CuK-edge absorption spectroscopy was carried out on highly concentrated (1 mm subunits, i.e. 2 mm in copper) Hc solutions on beam lines X-9A and X-10C at the National Synchrotron Light Source (NSLS), Brookhaven National Laboratory. Sample handling, radiation flux, scanning, and signal detection as well as data analysis were performed as described previously (9).
Flash Photolysis Measurements
Purified C. aestuarii Hc, CaeSS2 monomer, and CaeSS3 homohexamer in 50 mm Tris-HCl, pH 8.3, each with 20 mm CaCl2, were degassed on a vacuum line and then flushed with nitrogen gas. Dithionite was added anaerobically (final concentration 1 mm) to reduce any cupric copper in the protein. The solution was then dialyzed under aerobic conditions against 50 mm Tris-HCl or MES with 20 mm CaCl2 at the desired pH. After dialysis, the protein concentration was adjusted to 20 μm (subunit), and the sample was equilibrated under an atmosphere of oxygen plus nitrogen, prepared using a gas generator (MX-3S; Crown, Tokyo, Japan), such that the partial pressure of oxygen varied from 5 to 100%. The sample solution was transferred into a sealed quartz cell, which was filled with the same gas mixture. Photolysis of oxy-Hc samples was accomplished using the third harmonic of a Nd:YAG laser (Surelight I-10; Continuum, Santa Clara, CA; pulse energy, 40 mJ; pulse width, 5 ns; pulse frequency, 10 Hz) for excitation. Time-resolved absorbance changes were measured at 20 °C with illumination from a xenon lamp orthogonal to the laser pulse and were recorded on a digital oscilloscope (TDS 3012B; Tektronix, Tokyo, Japan), which received voltage signals from the photomultiplier attached to a monochromator (RSP-601-03; Unisoku, Osaka, Japan). The traces were obtained as averages of 512 or 640 pulses, and least squares exponential fits were performed for the time-resolved absorption data using Igor Pro version 6.0 (WaveMetrics).
SAXS
SAXS measurements were carried out at the SOLEIL synchrotron (St. Aubin, France), beamline SWING. The wavelength of the monochromatic x-ray beam was 1.033 Å, and the scattering pattern was recorded on a two-dimensional CCD camera. The sample-to-detector distance was 2.3 m, giving a scattering vector q range of 0.01 to 0.3Å−1 (q = 4π*sin(θ)/λ with 2θ as scattering angle). C. aestuarii Hc was present in the samples as a mixture of hexamers and dodecamers (supplemental Fig. S1). Fully oxygenated samples were measured at a concentration of 4 mg/ml in 50 mm MES, pH 6.5, and 50 mm Tris-HCl, pH 8.3, with 20 mm CaCl2 in the presence of the reported l-lactate concentrations. Exposure time was 4 s, and typically 15 exposures for protein and 20 exposures for buffer samples were averaged. No radiation damage effects in terms of oxygen binding properties and protein cleavage could be detected subsequent to x-ray exposure. Raw experimental scattering curves were normalized for detector response, photon flux, and capillary thickness. Buffer-subtracted curves were analyzed with GNOM (25) and yielded particle distance distribution functions p(r), maximum particle dimensions, and particle radii of gyration. Ab initio shape determination of C. aestuarii Hc was carried out using the dummy atom (DA) method as implemented in the program DAMMIF (26, 27). Such models are defined as an assembly of densely packed spheres with radii much smaller than the maximum particle dimension. Because of the stochastic nature of the DA optimization algorithm, several runs of the program were performed to obtain an acceptable solution. 700 DA models were obtained from the scattering curves under all six conditions analyzed (at pH 6.5 and 8.3 and l-lactate concentrations of 0, 1, and 40 mm). Of the 700 DA models, approximately 50–60% were not acceptable because they presented small, loosely connected or poorly defined DA “domains.” The selection of the DA model was done by first computing a tight boundary around each DA model using principal components analysis (28) and then removing the DA models that presented an increase in one of the bounding-box lengths of more than 2 to 5% from the average value. For each accepted DA model, dummy atoms were clustered in two groups (each group representing a single hexamer), and the center of each cluster was calculated from the mean of the DA coordinates that belong to the cluster. The interhexameric separation was then calculated as a geometric distance between the cluster centers.
RESULTS
ITC Measurements of Lactate Binding to Hc
To obtain information on the binding of l-lactate to Hc, ITC measurements were performed using purified C. aestuarii Hc at pH 6.5 and 8.3 and CaeSS2 monomer at pH 6.5 (Fig. 1). The titration of purified CaeSS3 homohexamers prepared from reassociated monomers was also obtained (supplemental Fig. S2). Previous l-lactate titration studies of C. sapidus Hc reported 2.2 binding sites/hexamer, and ultrafiltration techniques gave 2.8 sites/hexamer (29). However, the same authors reported approximately one binding site of l-lactate for P. interruptus Hc hexamer based on oxygen equilibrium studies (21). Recently, a mole ratio of 0.35 l-lactate per subunit was reported for C. meanas Hc (hemolymph) at pH 7.0 (18).
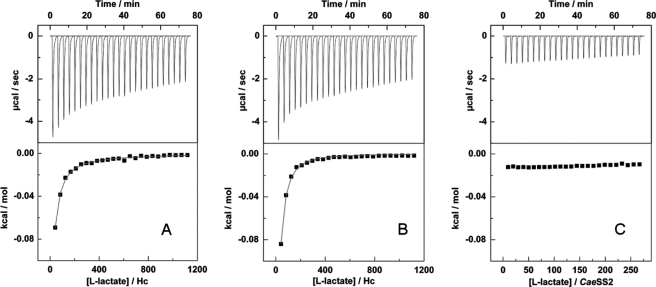
FIGURE 1.
Calorimetric titration of C. aestuarii oxy-Hc (16.7 μm hexamer) with successive injections of l-lactate (100 mm in the syringe, 25 × 10-μl aliquots) at pH 6.5 (A) and 8.3 (B) together with titration of CaeSS2 monomer (70 μm) at pH 6.5 (C). Upper, raw data showing the amount of heat generated after each injection of l-lactate. Lower, plot of the amount of heat generated per injection as a function of the l-lactate/Hc molar ratio. The solid lines represent the best-fitted curves of the data to a model of hexamer:l-lactate = 1:2 (A) and 1:1 (B) stoichiometry, respectively. Titration of the ligand without the protein was performed to subtract the effect of the heat because of ligand dilution.
In the current study, l-lactate binding showed a typical curve for 1:1 binding to the hexameric Hc with a binding constant of about 840 ± 30 m−1 at pH 8.3. The ΔH and ΔS obtained from the fitting were −40 ± 4 kJ/mol and −80 ± 20 J/(K·mol), respectively. For titration of l-lactate at pH 6.5, the fitting of the data with a model of 1:1 stoichiometry was not as successful as that obtained for pH 8.3. A two-site fitting was therefore used that provided two binding constant values of about 840 ± 140 and 65 ± 35 m−1, respectively. The ΔH and ΔS of each phase were −30 ± 5 kJ/mol, −50 ± 10 J/(K·mol) and −32 ± 4 kJ/mol, −75 ± 30 J/(K·mol), respectively. The binding constant for the weak phase was comparable with that obtained by the flash photolysis experiments (described below). No binding of lactate by titration of CaeSS2 monomer subunits at pH 6.5 could be demonstrated whereas binding was found in the case of the CaeSS3 homohexamer; these results are in agreement with flash photolysis data (see below) showing that l-lactate had no effect on the value of kobs for the CaeSS2 monomer, whereas an effect was demonstrated for the CaeSS3 homohexamer.
l-Lactate Binding Does Not Affect the Binuclear Copper Site
After establishing that l-lactate binds to oligomeric Hc in a physiologically relevant range of concentrations, the 560 nm charge transfer band in the optical spectrum of oxy-Hc was used as a spectroscopic probe to explore the effect of l-lactate on the integrity of the binuclear copper center. As shown in supplemental Fig. S3, the optical properties of oxy-Hc are not affected by l-lactate, either at pH 6.5 or 8.3.
The optical results were confirmed by XAS. This technique is a sensitive probe of the electronic structure of the copper ions and the geometry of the active site. The edge spectra of oxygenated P. interruptus Hc in the presence and absence of l-lactate were almost superimposable (supplemental Fig. S4). However, under the same conditions the oxygen-binding rate constant kobs (defined in the following paragraph) at pH 7.3 under oxygen saturation increased from 42.7 ± 1.4 to 47.2 ± 0.5 ms−1. Additionally, XAS provides a unique approach to investigate the deoxy-Hc active site which is not amenable to other spectroscopic tools because of the electronic structure of Cu(I). Clearly resolved changes were reported previously for the effects of hydrogen ions on the structure of the deoxy-Hc copper center (9). However, spectra of deoxy-Hc with and without l-lactate (up to the same amount used for the ITC measurements) were very similar (supplemental Fig. S5). These observations show that the oxy and deoxy forms of Hc do not undergo detectable structural rearrangement of the active site upon l-lactate binding to the protein.
Kinetics of Oxygen Rebinding after Photodissociation of Oxy-Hc
The irradiation of oxy-Hc with laser pulses at 355 nm results in the photolysis of bound oxygen to give the deoxy protein, followed by ligand rebinding after the pulse. Because of the spectroscopic difference between oxy- and deoxy-Hc, the first step (photolysis) decreases the intensity of the 337 nm absorption band. Under the conditions used here for photolysis, this decrease was less than 5% of the change that occurs upon complete deoxygenation, indicating that only a small fraction of the protein was converted. Throughout the photolysis experiment the Hc multimers remain in the high affinity form. After photolysis, oxygen rebinding can be followed by the absorption increase at 337 nm on a time scale of 10 μs to 1 ms, and the oxygen-binding rate constant, kobs, is obtained by single-exponential fitting of the kinetic data. The initial absorption intensity is restored at the end of the rebinding process demonstrating the full reversibility of the photolysis. The correlation of the observed effect with the expected optical changes of the oxy-Hc active site had been demonstrated in a previous study showing that the action spectrum for photolysis strictly follows the absorption band of oxy-Hc (9).
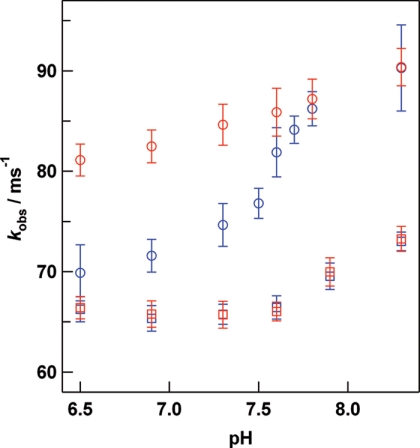
The pH dependence of kobs under oxygen saturated conditions (1.39 mm) in the presence and absence of 40 mm l-lactate is shown in Fig. 2. The sigmoidal behavior of kobs in the absence of l-lactate shows that there are two states for Hc, corresponding to the species present at high and low pH values. The pKa for conversion between these two states was about 7.6 and was not affected by the addition of l-lactate. At low pH (pH 6.5), the kobs value showed a significant increase upon the addition of l-lactate, whereas at high pH (pH 8.3) l-lactate addition had little effect. A pH dependence of kobs was also found in the case of the CaeSS2 monomer, whereas l-lactate had no influence on oxygen binding properties (Fig. 2). On the other hand, the kobs for oxygen rebinding to the CaeSS3 homohexamer at pH 6.5 under oxygen saturated conditions increased from 73.6 ± 0.7 to 82.8 ± 0.7 ms−1 with addition of 40 mm l-lactate.
FIGURE 2.
pH dependence of kobs for oxygen rebinding to the dodecameric form of C. aestuarii Hc (circles) and CaeSS2 monomer (squares) in the presence (red) and absence (blue) of 40 mml-lactate. Experimental conditions: 20 μm (subunit) Hc; 1.39 mm O2; laser pulse, 40 mJ, 10 Hz; 20 °C. Each trace is an average of 64 × 8 or 64 × 10 shots.
Oxygen rebinding kinetic experiments were performed at pH 6.5 and 8.3 as a function of oxygen concentration, yet exploring a range of values that ensure full saturation of Hc with oxygen before photolysis in each case. The kobs values increased linearly as a function of oxygen concentration (supplemental Fig. S5) as expected for a simple rebinding phenomenon. The increase was observed for the oxygen concentrations investigated, both at pH 6.5 and 8.3, as well as in the presence and absence of l-lactate. From these plots, kon and koff can be calculated using Equation 1 (9).
Under our experimental conditions, oxygen is in large excess with respect to Hc (at 20 °C, [O2] = 220 μm, [Hc]total (subunit) = 20 μm; <5% of Hc becomes deoxygenated after photoreaction), hence the term [deoxy-Hc] can be neglected in Equation 1, and the observed rebinding rate depends linearly on the oxygen concentration. The observed oxygen binding rate increased with increasing pH from 6.5 to 8.3 for all oxygen concentrations investigated. At pH 8.3, the kobs values in the presence and absence of l-lactate were essentially the same (supplemental Fig. S6, triangles). These results indicate that kon, koff, and thus the dissociation constant KD are not affected by l-lactate at pH 8.3 within the investigated oxygen concentrations where the protein is largely in the high affinity form.4 In contrast, at pH 6.5 the kobs value increases upon addition of l-lactate. The plot of kobs versus [O2] had a steeper slope and the same intercept, indicating an increase in kon and KD values in the presence of l-lactate (supplemental Fig. S6). The value of kobs for oxygen binding to Hc at pH 6.5 with various l-lactate concentrations under oxygen-saturated conditions showed characteristic saturation behavior (supplemental Fig. S7). The change in kobs as a function of l-lactate concentration at pH 6.5 was fitted well by assuming a 1:1 complex of the hexamer and l-lactate. The binding constant was estimated by least squares fitting to be 160 ± 70 m−1. Because it is known that two lactate molecules can bind per hexamer, as shown by previous reports (21, 29) and here using calorimetry at pH 6.5, the lactate dependence of kobs corresponds to that for the lower affinity binding.
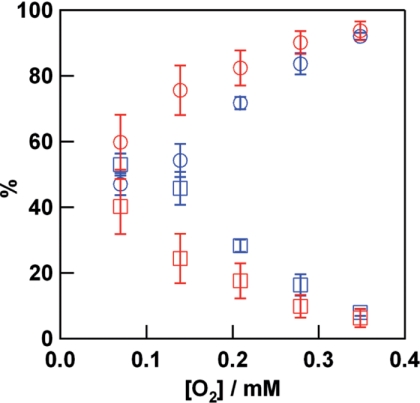
Flash photolysis experiments carried out under low oxygen concentrations (5% oxygen and 95% nitrogen at atmospheric pressure) at pH 6.5 revealed heterogeneity in the kinetic behavior of oxygen rebinding. The protein is still multimeric under these conditions, and thus the recovery of the absorption at 337 nm is fitted by a double, rather than a single exponential (9). The contribution from the slower phase compared with that of the faster phase increases as the oxygen concentration is decreased (Fig. 3). The amplitude of the two phases was comparable under 5% oxygen atmosphere at pH 6.5, whereas the fast phase becomes predominant upon increasing the oxygen concentration. The fast and slow phases may be attributed to oxygen binding to R and T state multimers, respectively. The amplitude of the fast phase increases at all oxygen concentrations in the presence of l-lactate. A shift from the T toward the R state by addition of lactate is consistent with a previous report for C. sapidus Hc (22). In contrast to the behavior of the multimeric protein, the oxygen rebinding for CaeSS2 monomer shows a single phase even at low oxygen and low pH conditions (supplemental Fig. S8).
FIGURE 3.
Percentage of the fast (circles) and slow (squares) phases of oxygen rebinding obtained by flash photolysis of C. aestuarii oxy-Hc in the presence (red) and absence (blue) of 40 mml-lactate under various oxygen concentrations at pH 6.5. Least square fitting of the time course of the absorption change was performed. Experimental conditions are the same as those in Fig. 2, except for a higher Hc concentration (60 μm subunit) for the experiment under 5% oxygen and 95% nitrogen atmosphere, for which results were essentially the same as that obtained for 20 μm (subunit) Hc.
SAXS
SAXS data (supplemental Fig. S9) were analyzed according to standard procedures to generate the distance distribution function p(r), which represents the probability of finding two volume elements separated by distance r within the population of observed scattering centers. The p(r) function represents a histogram of all distances in the sample, thus it gives information about particle shape and changes that the molecule undergoes under different conditions. As previously observed for Homarus americanus Hc (10), the p(r) curve of the 2 × 6-meric C. aestuarii Hc is composed of two overlapping bell-shaped curves. The first one with the maximum around 75 Å reflects the distance distribution of volume elements within the hexamers, whereas the second curve around 140 Å, reflects interhexameric distances.
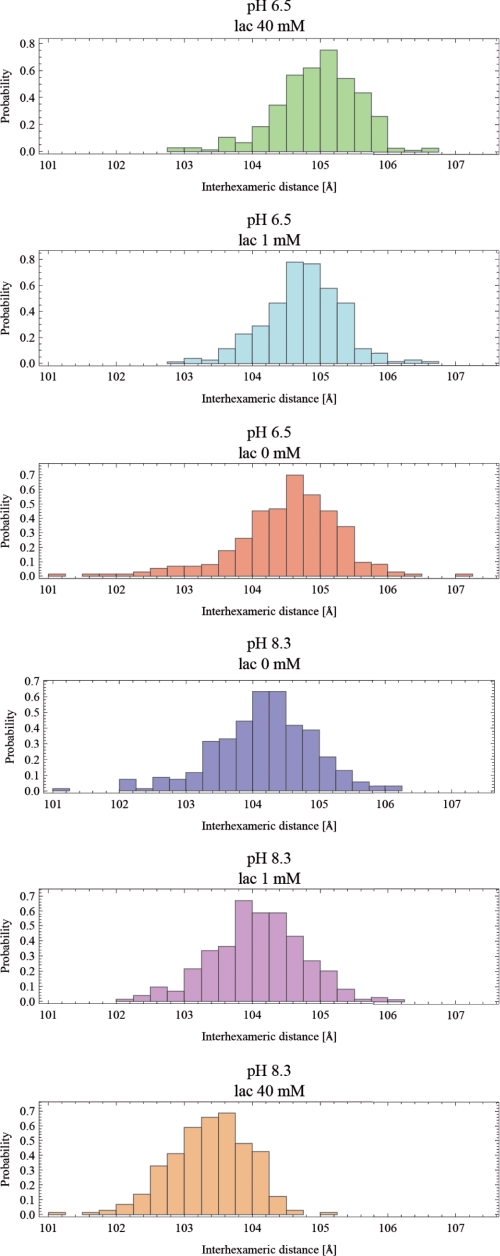
Because only small differences were observed upon the addition of l-lactate, the effect of l-lactate on C. aestuarii Hc was further evaluated by using a novel approach that involves calculating the interhexameric distances of all DA models fitted to the scattering data. Following the removal of poor DA models, interhexameric distances were compared using t test statistics. Interestingly, there was no significant difference in the distribution of interhexameric distances between the samples at pH 6.5 and 8.3. Upon addition of l-lactate, the low pH sample had a lactate concentration-dependent shift toward larger interhexameric distances whereas the high pH sample showed an opposite trend (smaller interhexameric distance with increasing l-lactate). The comparison between histograms of interhexameric distances obtained from DA models is shown in Fig. 4. These results show that the structural changes induced in the quaternary structure of Hc by interaction with l-lactate are opposite at high and low pH.
FIGURE 4.
Histogram of interhexameric distance distributions in C. aestuarii Hc as calculated from DA models generated by multiple runs of the DAMMIF program. Red, pH 6.5, 0 mm l-lactate; light blue, pH 6.5, 1 mm l-lactate; green, pH 6.5, 40 mm l-lactate; blue, pH 8.3, 0 mm l-lactate; purple, pH 8.3, 1 mm l-lactate; orange, pH 8.3, 40 mm l-lactate.
DISCUSSION
pH Effect on Hc Structure
The structural basis of the pH effect on the oxygen binding properties of Hc (the Bohr effect) and the effect on the rate constants obtained in flash photolysis experiments have been reported previously (9). In that study, the kon value increased when the pH was raised from 6.5 to 8.3 (pH 6.5, 49.1 ± 0.6 mm−1s−1; pH 8.3, 63.5 ± 0.5 mm−1s−1). These results show that the oxygen binding rate constant of deoxy-Hc sites in the high affinity form of the multimeric protein, which is present because nearly all sites retain oxygen in the photolysis experiment, increased with increasing pH, whereas no significant change was observed for the koff value. In another related type 3 copper protein, the bacterial phenol and cathecol oxidase tyrosinase which is monomeric, no significant pH dependence was observed in its oxygen binding, which shows that the pH dependence in Hc is a characteristic that emerged in oligomeric Hcs (30). From the oxygen-binding rate constants reported here, the pH dependence in the R state (the high affinity form) exhibited a pKa value of 7.6, which was not affected by the presence of l-lactate. Therefore, at least two substates should exist for the high affinity form of oxy-Hc (at high oxygen concentration), R (low pH) and R* (high pH). This is consistent with a prior report that hydrogen ions cause structural changes in the active site of deoxy-Hc (9).
Beyond the previously reported pH-dependent geometry of copper in the deoxy protein, the Bohr effect could also arise from changes in structural features remote from the active site. The data presented here, however, do not provide any evidence for a role of the quaternary structure in the Bohr effect. This is based on three independent observations. Variation of pH does not affect the equilibrium between hexamers and dodecamers (supplemental Fig. S9). The quaternary structures of the dodecamers at low and high pH values were indistinguishable in the SAXS analysis of the interhexameric distances. Finally, the kobs for the CaeSS2 monomer increased at higher pH (Fig. 2). Taken together, these results indicated that the Bohr effect depends upon local effects on the structure of the active site.
l-Lactate Binding to Hc
Microcalorimetric results showed that l-lactate binds to Hc with an affinity consistent with physiological relevance (binding constant about 840 m−1), but at high pH, the protein exhibits the same oxygen binding properties with or without this effector. A second binding process for l-lactate was found to have a binding constant of 65 ± 35 m−1. This phase was detected by calorimetric experiments at pH 6.5 but not at pH 8.3. The binding constant of 65 ± 35 m−1 was comparable with the value obtained from the modulation of the oxygen binding constant by l-lactate observed in the flash photolysis experiments (160 ± 70 m−1). Here, the low affinity binding constant for lactate was observed only at pH 6.5.
ITC experiments using CaeSS3 homohexamers showed that l-lactate binds (and an increased oxygen binding rate constant was found in photolysis experiments), whereas ITC with CaeSS2 monomers did not demonstrate any binding, nor does lactate affect the rate of oxygenation in photolysis measurements within the pH range investigated. These data indicate that the hexameric quaternary structure is essential for both l-lactate binding and modification of the oxygen binding affinity.
The structure of the active site of Hc either in the oxy or deoxy state is not substantially changed by the addition of l-lactate. These results suggest that l-lactate likely affects the quaternary structure and thereby modulates oxygen binding properties. This interpretation is supported by the SAXS data that identify a rearrangement of the quaternary structure upon l-lactate addition. The nature of the allosteric effect produced by l-lactate is consequentially distinct from that found in the Bohr effect, in which alteration of the active site structure governs the functional effect (9). Hc is shown to bind only one or two l-lactate molecules/hexamer (21, 29). Because l-lactate affected the oxygen binding rate kobs without affecting the active site structure, this effector likely binds at a remote site and has an indirect (or steric) effect on oxygen binding. Relevant to this is a proposal, a specific phenylalanine near the active site and conserved in Hcs could act as the trapdoor for an oxygen entry/exit channel in Hc (8). This “door” is reported to be open in the Panulirus deoxy-Hc x-ray structure and closed in both the oxy and deoxy Limulus structures. The authors go on to say that there is an allosterically controlled gating mechanism underlying the function of this phenylalanine in oxygen binding. This type of structural feature could be relevant to the model proposed here in which polypeptide structural changes are concluded to control ligand access to the active site.
In addition, the kobs value for O2 binding was affected by l-lactate for the CaeSS3 homohexamer, which was competent to bind this ligand presumably at an intersubunit site (supplemental Fig. S2). An intersubunit region is the more likely site for l-lactate binding because such binding could induce changes in quaternary structure of the hexameric and/or dodecameric Hc, which would not be expected were lactate to bind to an exterior site.
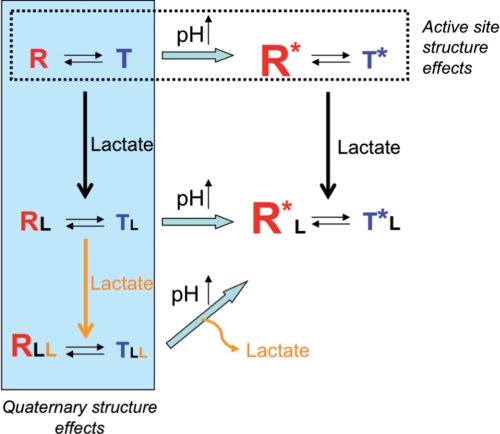
In conclusion, a scheme that explains the Bohr and l-lactate effects can be developed according to the present results and a previous study of arthropod Hc (9) (Fig. 5). Two structural features are incorporated in the scheme. Rearrangement of the active site with increasing pH, represented by R and R*, and a quaternary structural change caused by l-lactate at pH 6.5 (R versus RLL) both produce faster oxygen binding (9) and a shift in the R-T equilibrium toward the R state. Unlike the affect of pH on active site structure of deoxy-Hc, the effector properties of lactate are mediated through structural changes that do not alter the coordination geometry of the metal ions in the active site.
FIGURE 5.
Schematic representation of the pH and l-lactate effects on the structure-function relationships in Hc. The effect of pH is illustrated horizontally across the top of the scheme, whereas the effect of increasing l-lactate concentration is shown vertically on the left side. The size of the letters R and T represents the amount of these forms. The R* and R represent the two high affinity forms at high and low pH, respectively.
Supplementary Material
This work was supported by National Institutes of Health Grant GM40168 (to J. P.). This work was also supported by Grants-in-aid for Scientific Research from Ministry of Education, Culture, Sports, Science and Technology, Japan and Japan Society for the Promotion of Science (to S. H.), Fondo per gli Investimenti della Ricerca di Base, Ministero università e ricerca, and “Progetto di Ateneo” CPDA 051777 from the University of Padova (to L. B. and M. B.) and by the Executive Program of Cooperation in the Fields of Science and Technology between the Government of Italy and the Government of Japan (to L. B. and S. H.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S9.
An effect of l-lactate on the low affinity form has been described at pH 7.85 in Ref. 18, but the effect occurs under low saturation conditions where the amount of oxygenated protein would be below the optical absorbance detection limit in the flash photolysis experiment.
- Hc
- hemocyanin
- deoxy-Hc
- deoxygenated Hc
- oxy-Hc
- oxygenated Hc
- XAS
- x-ray absorption spectroscopy
- SAXS
- small angle x-ray scattering
- ITC
- isothermal titration calorimetry
- MES
- 4-morpholineethanesulfonic acid
- DA model
- dummy atom model.
REFERENCES
- 1.Volbeda A., Hol W. G. (1989) J. Mol. Biol. 209, 249–279 [DOI] [PubMed] [Google Scholar]
- 2.Dainese E., Di Muro P., Beltramini M., Salvato B., Decker H. (1998) Eur. J. Biochem. 256, 350–358 [DOI] [PubMed] [Google Scholar]
- 3.Markl J., Decker H. (1992) Adv. Comp. Environ. Physiol. 13, 325–376 [Google Scholar]
- 4.Cong Y., Zhang Q., Woolford D., Schweikardt T., Khant H., Dougherty M., Ludtke S. J., Chiu W., Decker H. (2009) Structure 17, 749–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Markl J., Moeller A., Martin A. G., Rheinbay J., Gebauer W., Depoix F. (2009) J. Mol. Biol. 392, 362–380 [DOI] [PubMed] [Google Scholar]
- 6.Martin A. G., Depoix F., Stohr M., Meissner U., Hagner-Holler S., Hammouti K., Burmester T., Heyd J., Wriggers W., Markl J. (2007) J. Mol. Biol. 366, 1332–1350 [DOI] [PubMed] [Google Scholar]
- 7.Metz M., Solomon E. I. (2001) J. Am. Chem. Soc. 123, 4938–4950 [DOI] [PubMed] [Google Scholar]
- 8.Magnus K. A., Hazes B., Ton-That H., Bonaventura C., Bonaventura J., Hol W. G. J. (1994) Proteins 19, 302–309 [DOI] [PubMed] [Google Scholar]
- 9.Hirota S., Kawahara T., Beltramini M., Di Muro P., Magliozzo R. S., Peisach J., Powers L. S., Tanaka N., Nagao S., Bubacco L. (2008) J. Biol. Chem. 283, 31941–31948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartmann H., Lohkamp B., Hellmann N., Decker H. (2001) J. Biol. Chem. 276, 19954–19958 [DOI] [PubMed] [Google Scholar]
- 11.Hartmann H., Decker H. (2002) Biochim. Biophys. Acta 1601, 132–137 [DOI] [PubMed] [Google Scholar]
- 12.Hazes B., Magnus K. A., Bonaventura C., Bonaventura J., Dauter Z., Kalk K. H., Hol W. G. (1993) Protein Sci. 2, 597–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Decker H., Sterner R. (1990) J. Mol. Biol. 211, 281–293 [DOI] [PubMed] [Google Scholar]
- 14.Menze M. A., Hellmann N., Decker H., Grieshaber M. K. (2000) Biochemistry 39, 10806–10811 [DOI] [PubMed] [Google Scholar]
- 15.Beltramini M., Colangelo N., Giomi F., Bubacco L., Di Muro P., Hellmann N., Jaenicke E., Decker H. (2005) FEBS J. 272, 2060–2075 [DOI] [PubMed] [Google Scholar]
- 16.Truchot J. P. (1992) Adv. Comp. Environ. Physiol. 12, 377–410 [Google Scholar]
- 17.Mangum C. P., Shick J. M. (1972) Comp. Biochem. Physiol. B. 42A, 693–697 [Google Scholar]
- 18.Weber R. E., Behrens J. W., Malte H., Fago A. (2008) J. Exp. Biol. 211, 1057–1062 [DOI] [PubMed] [Google Scholar]
- 19.Lallier F., Truchot J. P. (1989) Respir. Physiol. 77, 323–336 [DOI] [PubMed] [Google Scholar]
- 20.Dissanayake A., Galloway T. S., Jones M. B. (2008) Marine Environ. Res. 66, 445–450 [DOI] [PubMed] [Google Scholar]
- 21.Johnson B. A., Bonaventura J., Bonaventura C. (1987) Biochim. Biophys. Acta 916, 376–380 [DOI] [PubMed] [Google Scholar]
- 22.Johnson B. A., Bonaventura C., Bonaventura J. (1988) Biochemistry 27, 1995–2001 [DOI] [PubMed] [Google Scholar]
- 23.Bruneaux M., Terrier P., Leize E., Mary J., Lallier F. H., Zal F. (2009) Proteins 77, 589–601 [DOI] [PubMed] [Google Scholar]
- 24.Bubacco L., Magliozzo R. S., Beltramini M., Salvato B., Peisach J. (1992) Biochemistry 31, 9294–9303 [DOI] [PubMed] [Google Scholar]
- 25.Svergun D. I. (1992) J. Appl. Crystallogr. 25, 495–503 [Google Scholar]
- 26.Svergun D. I. (1999) Biophys. J. 76, 2879–2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franke D., Svergun D. I. (2009) J. Appl. Crystallogr. 42, 342–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dimitrov D., Knauer C., Kriegel K., Rote G. (2009) Comp. Geom. Theor. Appl. 42, 772–789 [Google Scholar]
- 29.Johnson B. A., Bonaventura C., Bonaventura J. (1984) Biochemistry 23, 872–878 [DOI] [PubMed] [Google Scholar]
- 30.Hirota S., Kawahara T., Lonardi E., de Waal E., Funasaki N., Canters G. W. (2005) J. Am. Chem. Soc. 127, 17966–17967 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.